Abstract
Background
Lipids, which are associated with atherogenesis, clotting, and the fibrinolytic pathway, may be important prognostic indicators of recurrent myocardial infarction. The aim of this study was to determine the predictive value of baseline lipid fractions for nonfatal recurrent myocardial infarction in patients with ST segment elevation myocardial infarction 2 years after primary percutaneous coronary intervention in China.
Methods
Cox proportional-hazards models were used to evaluate the association between potential risk factors, including lipid fractions, and the occurrence of nonfatal recurrent myocardial infarction in 2402 consecutive patients who underwent primary percutaneous coronary intervention for ST segment elevation myocardial infarction.
Results
The cumulative incidence of recurrent myocardial infarction was 2.7% at 1 year, 3.8% at 2 years, and 5.8% at 3 years after percutaneous coronary intervention. The effects of collinearity of lipids were investigated. In concerning the principal components analysis, composing factor 1 (scoring factors were 0.689 for non-HDL, 0.702 for LDL, 0.182 for HDL) which had eigenvalues of 1.86 and explained 62% of the variability among lipid cholesterols was significantly associated with recurrent MI in the final adjusted analysis of the lipid cholesterols principal components. Non-high-density lipoprotein cholesterol was the strongest independent predictor of nonfatal recurrent myocardial infarction. The adjusted hazards ratios for nonfatal recurrent myocardial infarction were 1.26 (95% confidence interval (CI): 1.05–1.51) for non-high-density lipoprotein cholesterol, 1.17 (95% CI: 0.99–1.39) for low-density lipoprotein and 1.15 (95% CI: 0.95–1.40) for HDL. After adjusting for gender and age, the odds ratio for patients in the highest non-high-density lipoprotein cholesterol quartile was 2.10 (95% CI: 1.19–3.72).
Conclusions
Non-high-density lipoprotein cholesterol value is a stronger predictor of nonfatal recurrent myocardial infarction than other lipid risk factors in patients with ST segment elevation myocardial infarction. Moreover, the occurrence of reinfarction after percutaneous coronary intervention was highest for patients in the highest non-high-density lipoprotein cholesterol quartile.
Trial registration
http://www.chictr.org.cn/edit.aspx?pid=13583&htm=4, registration number: ChiCTR-EPC-16008199, date of registration:2013.01.01.
Electronic supplementary material
The online version of this article (doi:10.1186/s12944-017-0418-5) contains supplementary material, which is available to authorized users.
Keywords: Recurrent myocardial infarction, ST segment elevation myocardial infarction, Serum transaminase, Primary percutaneous coronary intervention, Lipid
Background
Primary percutaneous coronary intervention (PCI), the current standard treatment for ST segment elevation myocardial infarction (STEMI), reduces the risk of recurrent myocardial infarction (MI). However, MI reoccurs in 2–6% patients after successful PCI and is associated with poor clinical outcomes [1–3]. Survivors of acute coronary syndromes have a high risk of recurrent events [4], the reasons for which are complex and multifaceted. For instance, stent thrombosis can lead to recurrent MI, although newer durable polymer-based drug-eluting stents have antithrombogenic properties, resulting in a lesser degree of thrombus adhesion [5–7]. Other events that can lead to recurrent MI are the progression of atherosclerosis and lipid-rich plaque rupture [4, 8, 9].
Lipids, which are associated with atherogenesis, clotting, and the fibrinolytic pathway, are important prognostic indicators of recurrent MI [8, 10]. Low-density lipoprotein (LDL) cholesterol is a key risk factor for cardiovascular disease, and current guidelines indicate that first-line therapy should focus on lowering LDL [11–13]. However, prospective cohort studies show that non-high-density lipoprotein (non-HDL) cholesterol is an independent risk factor for mortality among individuals predominately free of coronary heart disease [14, 15], and recently revised National Cholesterol Education Program guidelines recommend that non-HDL and LDL be equally targeted in patients with coronary heart disease [16]. Non-HDL, which is calculated by subtracting high-density lipoprotein (HDL) cholesterol from total cholesterol (TC) [13], includes triglyceride (TG)-rich lipoprotein, LDL, very low-density lipoprotein (VLDL), chylomicron remnants, and intermediate-density lipoprotein (IDL) [17, 18].
Recently, Non-HDL has arose considerable interest and been shown to be an effective predictor of cardiovascular disease, including cardiovascular mortality, in patients with [19–21] and without [15, 22, 23] cardiovascular disease. However, few studies have explored the predictive value of non-HDL after adjusting for collinearity among lipid variables and directly compared varying baseline lipid fractions for predicting nonfatal recurrent MI for coronary artery stenosis among patients with STEMI treated with stents or angioplasty, especially in China. In China, home to one-fifth of the world’s people, there is a rising burden of cardiovascular disease. Therefore, previously studies from Western countries may not be applicable. It is known, racial differences, including genetic factors, life style, and the environmental circumstances will affect long-term outcomes in STEMI patients. Consequently, the aim of this study was to determine whether baseline TG, HDL, non-HDL, or LDL values predict nonfatal recurrent MI in patients with STEMI 2 years after primary PCI in China.
Methods
Study population
Data were analyzed from patients with a first STEMI who were admitted to the cardiology department at the First Hospital of Jilin University between January 1, 2013 and December 31, 2014. In accordance with the European Society of Cardiology/American College of Cardiology consensus document [24], we included patients who met at least two of the following criteria: characteristic severe chest pain lasting more than 30 min, electrocardiographic changes, and/or elevation of serum cardiac biomarkers. We excluded patients who had a previous MI, were currently receiving lipid-lowering treatment or drugs, or had insufficient baseline lipid measurement. In total, we included 2402 consecutive STEMI patients who underwent PCI without thrombolysis or conservative therapy. Baseline demographic data, medical history, laboratory data, angiographic results, and clinical variables during hospitalization were retrieved from the department’s electronic database (Table 1). The study protocol was approved by the ethics review board of the First Hospital of Jilin University (No. 2016–263).
Table 1.
Baseline characteristics of participants
| Characteristic | Recurrent MI | P-value | |
|---|---|---|---|
| Yes (n = 103) n (%) or mean ± SD |
No (n = 2299) n (%) or mean ± SD |
||
| Demographic data | |||
| Age (years) | 58 ± 12 | 59 ± 11 | 0.817 |
| Male | 69 (67.0) | 1642 (71.4) | 0.331 |
| Hospital stay (days) | 6.9 ± 2.9 | 7.2 ± 3.5 | 0.946 |
| Medical history | |||
| Diabetes mellitus | 45 (43.7) | 1044 (45.4) | 0.152 |
| Hypertension | 29 (28.2) | 509 (22.1) | 0.731 |
| Previous PCI | 3 (2.9) | 66 (2.9) | 0.980 |
| Atrial fibrillation | 1 (1.0) | 90 (3.9) | 0.126 |
| Peripheral vascular disease | 3 (2.9) | 35 (1.5) | 0.261 |
| Arrhythmia (VT/VF) | 9 (8.7) | 166 (7.2) | 0.562 |
| Infarct location by ECG | 0.032 | ||
| Inferior | 38 (36.9) | 1114 (48.5) | |
| Anterior | 60 (58.3) | 1131 (49.2) | |
| Lateral | 5 (4.9) | 54 (2.4) | |
| Killip classification | 0.925 | ||
| I | 82 (79.6) | 1796 (78.1) | |
| II | 14 (13.6) | 312 (13.6) | |
| III | 3 (2.9) | 66 (2.9) | |
| IV | 4 (3.9) | 125 (5.4) | |
| Laboratory data | |||
| K+ (mmol/L) | 3.9 ± 0.5 | 3.9 ± 0.5 | 0.181 |
| Na+ (mmol/L) | 138.6 ± 5.8 | 139.2 ± 4.1 | 0.685 |
| Non-HDL (mmol/L) | 3.8 ± 1.2 | 3.5 ± 1.0 | 0.003 |
| HDL (mmol/L) | 1.2 ± 0.3 | 1.1 ± 0.3 | 0.053 |
| LDL (mmol/L) | 3.1 ± 1.0 | 2.8 ± 0.8 | 0.004 |
| TG (mmol/L) | 1.8 ± 1.1 | 1.7 ± 1.2 | 0.309 |
| Glucose (mmol/L) | 7.6 ± 3.2 | 7.4 ± 3.3 | 0.368 |
| ALT (unit/L) | 46.4 ± 33.1 | 58.4 ± 114.4 | 0.171 |
| AST (unit/L) | 162.2 ± 162.9 | 176.4 ± 297.3 | 0.932 |
| Alkaline phosphatase (unit/L) | 71.1 ± 23.1 | 73.6 ± 28.1 | 0.309 |
| ɤ-Glutamyl transpeptidase (unit/L) | 46.8 ± 43.3 | 49.1 ± 73.8 | 0.980 |
| Cardiac troponin I (ng/mL) | 23.4 ± 67.7 | 22.2 ± 62.0 | 0.646 |
| Creatine kinase MB (ng/ml) | 31.7 ± 53.4 | 40.8 ± 77.7 | 0.415 |
| NT-proBNP (pg/mL) | 1595.7 ± 2259.3 | 1889.2 ± 4908.0 | 0.967 |
| Number of stents | 1.2 (0.8) | 1.2 (0.8) | 0.605 |
| Method of reperfusion | 0.046 | ||
| Balloon angioplasty | 18 (17.5) | 255 (11.1) | |
| Drug-eluting stent implantation | 85 (82.5) | 2044 (88.9) | |
| Thrombus aspiration | 9 (8.7) | 280 (12.2) | 0.294 |
| Temporary pacemaker | 3 (2.9) | 137 (6.0) | 0.197 |
| Late PCI (>12 h after symptom onset) | 65 (63.1) | 1352 (58.8) | 0.386 |
| Discharge medications | |||
| Aspirin | 100(97.1) | 2263(98.4) | 0.290 |
| Platelet P2Y12 inhibitor | 69(67.0) | 1502(65.3) | 0.729 |
| Statins | 101(98.1) | 2276(99.0) | 0.357 |
| Beta-blocker | 71(68.9) | 1477(64.3) | 0.331 |
| ACE inhibitor or ARB | 68(66.0) | 1317(57.3) | 0.079 |
| Calcium blockers | 6(5.8) | 128(5.6) | 0.911 |
| Diuretic | 34(33.0) | 750(32.6) | 0.935 |
| All-cause mortality at 2 years | 7 (6.8) | 119 (5.2) | 0.471 |
SD standard deviation, PCI percutaneous coronary intervention, VT/VF ventricular tachycardia/fibrillation, ECG electrocardiogram, non-HDL non-high-density lipoprotein, HDL high-density lipoprotein, LDL low-density lipoprotein, ALT alanine aminotransferase, AST aspartate aminotransferase, NT-proBNP N-terminal pro-brain natriuretic peptide, ACE angiotensin-converting enzyme, ARB angiotensin receptor blocker
Primary PCI protocol
Before PCI, patients were administered aspirin (loading dose of 300 mg and maintenance dose of 100 mg/day), clopidogrel (loading dose of 600 mg and maintenance dose of 75 mg/day), and intravenous unfractionated heparin (70 U/kg bolus). Coronary angiography was performed using standard techniques. Thrombus aspiration, a temporary pacemaker, and/or an intra-aortic balloon pump were used at the surgeon’s discretion. Standard management was provided by responsible physicians. Generally, patients received aspirin, atorvastatin/rosuvastatin, clopidogrel, a β-blocker, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers and diuretics. Dural antiplatelet therapy was prescribed for at least 1 year in all patients with successful drug-eluting stents implantation.
Biochemical analysis
Baseline TC, TG, HDL, glucose, and LDL values were measured directly in plasma after fasting. All blood samples were obtained at the time of hospital admission and were analyzed in the certified laboratory department of the First Hospital of Jilin University.
Clinical follow-up
A diagnosis of recurrent MI required two of the following criteria: ischemic symptoms for at least 30 min, electrocardiographic changes and creatine kinase-MB value at least twice the upper limit of normal, or troponin I value at least twice the upper limit of normal. Troponin I levels were not used to diagnose recurrent MI within 10 days of the index MI. Follow-up data were collected from hospital records and telephone interviews after discharge until death or April 1, 2016, whichever occurred first. Mortality data for patients who were lost to telephone follow-up were obtained from computerized records of the population registry bureau.
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD), and categorical variables are presented as frequency and percentage. Baseline continuous and categorical variables for patients with and without recurrent MI during follow-up were compared using Wilcoxon’s rank-sum tests and Chi-square tests, respectively. Cox proportional hazard models were used to evaluate associations between baseline lipid levels and clinical characteristics and recurrent MI at 2-year follow-up. Potential confounding factors were entered into the multivariate predictors of recurrent MI if they were clinical relevant or showed univariate differences between groups. Included confounders were lipid, infarct location, diabetes mellitus, atrial fibrillation, peripheral vascular disease, method of reperfusion, thrombus aspiration, temporary pacemaker and number of stents. The lipid variables were correlated in multivariate analysis and potentially resulting in multicollinearity. Principle component analysis was performed in order to investigate the effects of collinearity expected to occur among lipid cholesterol and to determine weights for the included variables. The variables which contributed most to the variance were then selected to be included in the regression analysis. Multivariate models were constructed for lipid risk factor adjusting for other predictors in univariate analyses to calculate multiple-adjusted Hazard ratios (HRs) and their 95% confidence intervals (CIs). According to common practice in principal component analysis, principal composing factors 1 and 2 were retained as covariates in the multivariate models of recurrent MI. Regression coefficients were calculated to estimate the HRs associated with 1-SD higher baseline values of each baseline lipid fraction: 1.20 mmol/L TG, 1.03 mmol/L non-HDL, 0.83 mmol/L LDL, and 0.30 mmol/L HDL. Model 1 was adjusted for age and gender, and model 2 was adjusted for age, gender, Killip classification, prior PCI, diabetes mellitus, hypertension, stroke, atrial fibrillation, thrombus aspiration, arrhythmia (ventricular tachycardia/fibrillation (VT/VF)), and method of reperfusion. Model 3 was with variables in Model 2, as well as with additional lipid fraction (per SD change) of non-collinearity. Lipid values were categorized into quartiles (≤25th, 25th to <50th, 50th to <75th, and ≥75th percentiles). Patients with lipid values in the lowest quartile were used as a reference to assess associations between lipid fractions and recurrent MI, adjusting for covariates. For all analyses, a two-sided P-value <0.05 was considered statistically significant. All analyses were conducted using Stata software, version 12 (Stata Corp., College Station, TX).
Results
Incidence of nonfatal recurrent MI
Of the 2402 patients included in this study, there were 129 deaths during the median 2.2 years of follow-up (range: 30–1226 days). A total of 102 patients suffered recurrent nonfatal MI. The incidence of recurrent MI was 2.7% at 1 year, 3.8% at 2 years, and 5.8% at 3 years (Fig. 1).
Fig. 1.
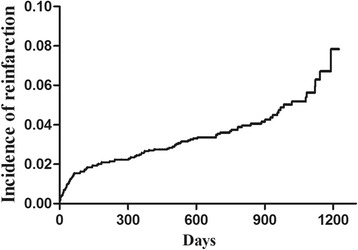
Incidence of recurrent MI during long-term follow-up. The incidence of recurrent MI was 2.7% at 1 year, 3.8% at 2 years, and 5.8% at 3 years
Risk factors for recurrent MI
Baseline data from patients with and without recurrent MI, including demographic characteristics, medical history, laboratory data, culprit vessel, and PCI procedure, are shown in Table 1. In concerning the principal components analysis, composing factor 1 had eigenvalues of 1.86 and explained 62% of the variability among lipid cholesterols and composing factor 2 had eigenvalues of 0.98 and explained 33%. Table 2 presented the scoring factors for each variable composing factor 1 and 2. Composing factor 1 was significantly associated with recurrent MI in the final adjusted analysis of the lipid cholesterols principal components (Table 3). Compared with patients without recurrent MI, patients with recurrent MI had significantly higher non-HDL and LDL values.
Table 2.
Scoring factors for principle components
| Variable | Scoring factor | |
|---|---|---|
| Composing Factor 1 | Composing Factor 2 | |
| Non-HDL | 0.689 | −0.204 |
| LDL | 0.702 | −0.053 |
| HDL | 0.182 | 0.978 |
non-HDL non-high-density lipoprotein, LDL low-density lipoprotein
Table 3.
Predictors of recurrent MI
| Category | Multivariate Analysis | ||
|---|---|---|---|
| Adjusted HR | 95% CI | P-value | |
| Composing Factor 1 | 1.19 | 1.05–1.35 | 0.008 |
| Composing Factor 2 | 1.13 | 0.94–1.36 | 0.202 |
| Age | 1.00 | 0.98–1.01 | 0.585 |
| Gender | 0.81 | 0.52–1.26 | 0.356 |
| Infarct location by ECG | 1.48 | 1.04–2.11 | 0.031 |
| Diabetes mellitus | 1.45 | 0.93–2.24 | 0.100 |
| Atrial fibrillation | 0.28 | 0.04–2.01 | 0.204 |
| Peripheral vascular disease | 1.94 | 0.60–6.21 | 0.266 |
| Method of reperfusion | 0.45 | 0.23–0.88 | 0.020 |
| Thrombus aspiration | 0.81 | 0.40–1.64 | 0.559 |
| Temporary pacemaker | 0.70 | 0.21–2.31 | 0.554 |
| Number of stents | 1.15 | 0.86–1.55 | 0.344 |
HR hazard ratio, CI confidential interval, non-HDL non-high-density lipoprotein, LDL low-density lipoprotein. ECG electrocardiogram
Patients with anterior MI, as detected by electrocardiogram (ECG), had a significantly higher rate of MI recurrence than those with inferior or lateral MI. The method of reperfusion also differed significantly between patients with and without recurrent MI. Reinfarction rates did not differ by gender or age. No associations were observed between recurrent MI and diabetes mellitus, hypertension, previous PCI, atrial fibrillation, peripheral vascular disease, arrhythmia (VT/VF), Killip classification, number of stents, thrombus aspiration, late PCI (>12 h after symptom onset), a temporary pacemaker or discharge medications. Multivariate analysis showed that lipid cholesterols, infarct location by ECG, and successful drug-eluting stent implantation were significant independent baseline predictors of nonfatal recurrent MI at 2-year follow-up (Table 3).
Lipid stratification and recurrent MI
Multivariate regression models examining baseline lipid risk factors showed that nonfatal recurrent MI was most strongly associated with non-HDL followed by LDL (Table 4) after adjusting for age and gender (model 1) or diabetes mellitus, hypertension, infarct location, method of reperfusion, previous PCI, atrial fibrillation, arrhythmia (ventricular tachycardia/fibrillation), Killip classification, and thrombus aspiration (model 2) and variable in model 2 as well as with additional lipid fractions (per SD change) of non-collinearity (model 3).
Table 4.
Comparison of lipoprotein cholesterol levels in predicting non-fatal recurrent MI
| Variable | Model 1 HR (95% CI) |
P-value | Model 2 HR (95% CI) |
P-value | Model 3 HR (95% CI) |
P-value |
|---|---|---|---|---|---|---|
| Non-HDL | 1.27 (1.08–1.50) | 0.032 | 1.26 (1.06–1.49) | 0.005 | 1.26 (1.05–1.51) | 0.006 |
| LDL | 1.22 (1.03–1.44) | 0.087 | 1.20 (1.02–1.42) | 0.010 | 1.17 (0.99–1.39) | 0.013 |
| HDL | 1.17 (1.00–1.40) | 0.175 | 1.18 (0.99–1.40) | 0.016 | 1.15 (0.95–1.40) | 0.006 |
HR hazards radio, CI confidence interval, non-HDL non-high-density lipoprotein cholesterol, HDL high-density lipoprotein cholesterol, LDL low-density lipoprotein cholesterol, TG triglyceride. Adjusted HRs with 95% CIs of lipid fraction per SD change (1.20 mmol/L TG, 1.03 mmol/L non-HDL, 0.83 mmol/L LDL, 0.30 mmol/L HDL) interval for different models
In the Adult Treatment Panel III report of the National Cholesterol Education Program [13], non-HDL as a secondary target should be limited to patients with elevated serum TG values (>200 mg/dl). When our analysis was restricted to patients with TG values ≤200 mg/dl, the adjusted ORs were 1.49 (95% CI: 0.83–2.70) for non-HDL and 0.88 (95% CI: 0.45–1.72) for LDL. Thus, non-HDL was a stronger predictor of nonfatal recurrent MI than other lipid risk factors in all models.
We further categorized lipid values into quartiles (Fig. 2, Additional file 1: Table S1). Patients in the highest non-HDL quartile had the highest OR for recurrent MI in both univariate and multivariate models. Similar results were observed for LDL quartiles, whereas no associations were found between HDL and TG values and risk of recurrent MI. Furthermore, there was a linear increase in the log OR for recurrent MI with increasing non-HDL and LDL quartiles, suggesting a linear relationship between non-HDL and LDL values and recurrent MI.
Fig. 2.
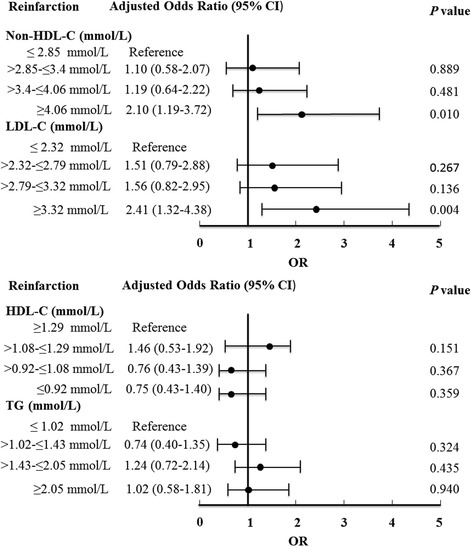
Association between baseline lipid values and incidence of nonfatal recurrent MI. non-HDL, non-high-density lipoprotein; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglyceride; CI, confidence interval; OR, odds radio
Discussion
Within this cohort analysis of STEMI patients with baseline lipid measurements, lipid cholesterols were an independent predictor of nonfatal recurrent MI during long-term follow-up after PCI in China, even when controlling for several demographic and clinical variables known to influence patient outcome. The occurrence of reinfarction was highest for patients with STEMI undergoing primary PCI in the highest non-HDL cholesterol quartile and non-HDL independently predicted nonfatal recurrent MI in a dose-dependent manner.
Incidence of recurrent MI and prognostic implication
The 1-year MI recurrence rate for our patients was lower than that previously reported for patients with acute MI treated with primary PCI (6%) [25]. However, in a meta-analysis of 23 trials, including patients with STEMI who underwent primary angioplasty, the 1-year MI recurrence rate was 3%, which is closer to our findings [26]. Our results also support an increasing risk of recurrent MI over time, with an incidence 2.15 times higher at 3 years than at 1 year after the index MI. Although MI recurrence rates vary among studies employing different definitions, sample sizes, and follow-up periods, our results are consistent with those of previous studies indicating that the risk of recurrent MI is not eliminated after PCI despite rapid advances in percutaneous therapy over the past decades due to improvements in stent platforms and implantation techniques, reductions in stent thrombosis, and the use of adjunctive medical therapy [27–30]. However, prior studies had demonstrated the superiority of stent implantation over balloon angioplasty alone in the treatment of acute MI [31–33]. Especially, drug-eluting stents further decreased repeat revascularization of target lesion or stent thrombosis and target-vascular infarction than bare-metal stents in patients with STEMI [28, 30]. Our results were consistent with those of others. We found that successful drug-eluting stent implantation reduced the risk of recurrent MI compared with balloon angioplasty alone, as patients with balloon angioplasty alone usually had complicated or irregular coronary lumen or diffuse atherosclerotic lesions [33].
Baseline lipid values and nonfatal recurrent MI
We found that non-HDL value was an independent predictor of nonfatal recurrent MI in patients with STEMI after PCI. In comparing with other lipid fractions, non-HDL value reflects the TG-rich lipoprotein content of several proatherogenic subfractions, including VLDL, IDL, and chylomicron remnants. Several studies report that IDL and small VLDL values, but not LDL value, correlates with the progression of atherosclerosis [34, 35]. In the National Heart, Lung, and Blood Institute Type II Coronary Intervention Study, TG-rich lipoproteins were associated with a faster progression of atherosclerotic lesions [36]. This previous study reports that the change in IDL at 2 years predicted cardiovascular disease progression at 5 years; this association remained significant after adjusting for treatment assignment. Although the molecular mechanisms of this association are not fully understood, they are becoming increasingly clear. Small TG-rich lipoproteins can be directly taken up by macrophages to produce foam cells and are intimately associated with clotting and the fibrinolytic pathway, thereby contributing to lesion progression, plaque rupture, and clinical coronary events [8, 37]. Our results confirmed that the risk of recurrent MI was highest for patients in the highest non-HDL quartile.
By contrast, non-HDL was not a significant independent risk factor for subsequent all-cause mortality. This might be explained in part because patients with lipoprotein measurements undergoing primary angiography are less sick than those with surgical contraindications or who died before lipid measurements could be obtained. In addition, the nutraceuticals and functional substances contained in food had already been considered to influence lipids levels beyond common clinical treatment [38]. Moreover, the influences of genetics and environmental factors on the lipid levels of individuals, the dosage of statin or other prescription drug, adherence to therapy and cardiac rehabilitation participation have important roles on the overall cardiovascular risk [39–41]. Our results are in accord with those of a previous study that did not detect a relationship between non-HDL and cardiovascular disease outcomes in a cohort of patients with prevalent coronary artery disease [42]. However, a recent systematic review of studies, including patients with multiple risk factors for cardiovascular disease, found no evidence that non-HDL markers predict the occurrence of cardiovascular events [43]. Therefore, there is a need for more research in this field.
Some limitations of this study should be taken into consideration. First, this is a retrospective analysis of a consecutive cohort of patients treated with primary angioplasty from a single center which located in Northeast China and the data may not reflect the general population of STEMI patients. Other P2Y12 were not available except for clopidogrel during the study period. However, patient data were imputed electronically by a relatively constant group of attending physicians; the overall strategic management of patients, including PCI techniques and devices used during the procedure, was more homogeneous than would be in a multi-centered study. Second, sudden death that may have been caused by fatal recurrent MI, and cases of non-symptomatic recurrent MI, may have been overlooked, meaning that the actual incidence of nonfatal recurrent MI was probably higher than that observed in our study. However, these limitations are balanced by our continuous admission and avoidance of ascertainment bias that occurs in clinical studies using selected patients. Third, although we used multivariate models to adjust for covariates, it is possible that unmeasured confounding variables may have affected the relationship between lipid values and the incidence of nonfatal recurrent MI.
Conclusions
To our knowledge, this is the first study to investigate the relationship between baseline lipid values and nonfatal recurrent MI in patients with STEMI who were treated with primary PCI. Of all lipid fractions, non-HDL was the strongest predictor of recurrent MI. Moreover, the occurrence of reinfarction after PCI was highest for patients in the highest non-HDL quartile.
Acknowledgements
Not applicable.
Funding
This work was supported by funding from the National Natural Science Foundation of China (81573230).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on request.
Authors’ contributions
LQ and MG designed the study, performed statistical analysis, and drafted the manuscript. YC and YZ collected data and assisted with statistical analysis and manuscript drafting. WHZ and LW assisted with study design and coordination, and data collection. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study protocol was approved by the ethics review board of the First Hospital of Jilin University (No. 2016–263). All patients gave written informed consent.
Abbreviations
- CK
Creatine kinase
- ECG
Electrocardiogram
- HDL
High-density lipoprotein
- IDL
Intermediate density lipoprotein
- LDL
Low-density lipoprotein
- MI
Myocardial infarction
- non-HDL
Non-high-density lipoprotein
- PCI
Percutaneous coronary intervention
- STEMI
ST segment elevation myocardial infarction
- TC
Total cholesterol
- TG
Triglyceride
- VLDL
Very low-density lipoprotein
- VT/VF
Ventricular tachycardia/fibrillation
Additional file
Associations between baseline lipid values with recurrent MI. (DOC 74 kb)
Contributor Information
Ming Gao, Email: gaoming13@mails.jlu.edu.cn.
Yang Zheng, Email: zhengyanghappy07@sina.com.
Weihua Zhang, Email: whcyzb@qq.com.
Yi Cheng, Email: hengyi@jlu.edu.cn.
Lin Wang, Email: hristina.wl@foxmail.com.
Ling Qin, Phone: +86-15804301762, Email: 1071927028@qq.com.
References
- 1.Kernis SJ, Harjai KJ, Stone GW, Grines LL, Boura JA, Yerkey MW, O’Neill W, Grines CL. The incidence, predictors, and outcomes of early reinfarction after primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2003;42:1173–1177. doi: 10.1016/S0735-1097(03)00920-3. [DOI] [PubMed] [Google Scholar]
- 2.Sabatine MS, Cannon CP, Gibson CM, Lopez-Sendon JL, Montalescot G, Theroux P, Lewis BS, Murphy SA, McCabe CH, Braunwald E. Clopidogrel as adjunctive reperfusion therapy -thrombolysis in myocardial infarction I. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. Jama. 2005;294:1224–1232. doi: 10.1001/jama.294.10.1224. [DOI] [PubMed] [Google Scholar]
- 3.Van’t Hof AW, Ten Berg J, Heestermans T, Dill T, Funck RC, van Werkum W, Dambrink JH, Suryapranata H, van Houwelingen G, Ottervanger JP, Stella P, Giannitsis E, Hamm C. Ongoing Tirofiban in myocardial infarction evaluation 2 study g. Prehospital initiation of Tirofiban in patients with ST-elevation myocardial infarction undergoing primary angioplasty (on-TIME 2): a multicentre, double-blind, randomised controlled trial. Lancet. 2008;372:537–546. doi: 10.1016/S0140-6736(08)61235-0. [DOI] [PubMed] [Google Scholar]
- 4.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vlaar PJ, Svilaas T, van der Horst IC, Diercks GF, Fokkema ML, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES, Suurmeijer AJ, Zijlstra F. Cardiac death and reinfarction after 1 year in the thrombus aspiration during percutaneous coronary intervention in acute myocardial infarction study (TAPAS): a 1-year follow-up study. Lancet. 2008;371:1915–1920. doi: 10.1016/S0140-6736(08)60833-8. [DOI] [PubMed] [Google Scholar]
- 6.Sabate M, Cequier A, Iñiguez A, Serra A, Hernandez-Antolin R, Mainar V, Valgimigli M, Tespili M, den Heijer P, Bethencourt A, Vazquez N, Gómez-Hospital JA, Baz JA, Martin-Yuste V, van Geuns R-J, Alfonso F, Bordes P, Tebaldi M, Masotti M, Silvestro A, Backx B, Brugaletta S, van Es GA, Serruys PW. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. The Lancet;380:1482–1490. [DOI] [PubMed]
- 7.Kolandaivelu K, Swaminathan R, Gibson WJ, Kolachalama VB, Nguyen-Ehrenreich KL, Giddings VL, Coleman L, Wong GK, Edelman ER. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123:1400–1409. doi: 10.1161/CIRCULATIONAHA.110.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodis HN. Triglyceride-rich lipoprotein remnant particles and risk of atherosclerosis. Circulation. 1999;99:2852–2854. doi: 10.1161/01.CIR.99.22.2852. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein JA, Demetriou D, Grines CL, Pica M, Shoukfeh M, O’Neill WW. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 2000;343:915–922. doi: 10.1056/NEJM200009283431303. [DOI] [PubMed] [Google Scholar]
- 10.Rana JS, Boekholdt SM, Kastelein JJ, Shah PK. The role of non-HDL cholesterol in risk stratification for coronary artery disease. Current Atheroscl Erosis Reports. 2012;14:130–134. doi: 10.1007/s11883-011-0224-x. [DOI] [PubMed] [Google Scholar]
- 11.National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 12.Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL, Authors/Task Force M, Additional C. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis. 2016;253:281–344. [DOI] [PubMed]
- 13.Expert Panel on Detection E, Treatment of High Blood Cholesterol in A Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) Jama. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 14.Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. Jama. 1996;276:875–881. doi: 10.1001/jama.1996.03540110029028. [DOI] [PubMed] [Google Scholar]
- 15.Cui Y, Blumenthal RS, Flaws JA, Whiteman MK, Langenberg P, Bachorik PS, Bush TL. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med. 2001;161:1413–1419. doi: 10.1001/archinte.161.11.1413. [DOI] [PubMed] [Google Scholar]
- 16.Expert Dyslipidemia Panel of the International Atherosclerosis Society Panel m An international atherosclerosis society position paper: global recommendations for the management of dyslipidemia--full report. J Clin Lipidol. 2014;8:29–60. doi: 10.1016/j.jacl.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Lauer MS, Fontanarosa PB. Updated guidelines for cholesterol management. JAMA. 2001;285:2508–2509. doi: 10.1001/jama.285.19.2508. [DOI] [PubMed] [Google Scholar]
- 18.Robinson JG. Are you targeting non-high-density lipoprotein cholesterol? J Am Coll Cardiol. 2009;55:42–44. doi: 10.1016/j.jacc.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 19.Fukushima Y, Ohmura H, Mokuno H, Kajimoto K, Kasai T, Hirayama S, Miyauchi K, Miida T, Amano A, Daida H. Non-high-density lipoprotein cholesterol is a practical predictor of long-term cardiac death after coronary artery bypass grafting. Atherosclerosis. 2012;221:206–211. doi: 10.1016/j.atherosclerosis.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Liting P, Guoping L, Zhenyue C. Apolipoprotein B/apolipoprotein A1 ratio and non-high-density lipoprotein cholesterol. Predictive value for CHD severity and prognostic utility in CHD patients. Herz. 2015;40(Suppl 1):1–7. doi: 10.1007/s00059-014-4147-5. [DOI] [PubMed] [Google Scholar]
- 21.Benderly M, Boyko V, Goldbourt U. Apolipoproteins and long-term prognosis in coronary heart disease patients. Am Heart J. 2009;157:103–110. doi: 10.1016/j.ahj.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Sempos CT, Donahue RP, Dorn J, Trevisan M, Grundy SM. Non-high-density lipoprotein and very-low-density lipoprotein cholesterol and their risk predictive values in coronary heart disease. Am J Cardiol. 2006;98:1363–1368. doi: 10.1016/j.amjcard.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 23.Rallidis LS, Pitsavos C, Panagiotakos DB, Sinos L, Stefanadis C, Kremastinos DT. Non-high density lipoprotein cholesterol is the best discriminator of myocardial infarction in young individuals. Atherosclerosis. 2005;179:305–309. doi: 10.1016/j.atherosclerosis.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Hochholzer W, Neumann FJ. The new 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Dtsch Med Wochenschr. 2016;141:782–785. doi: 10.1055/s-0042-107115. [DOI] [PubMed] [Google Scholar]
- 25.Smit JJ, Van’t Hof AW, de Boer MJ, Hoorntje JC, Dambrink JH, Gosselink AT, Ottervanger JP, Kolkman JJ, Suryapranata H. Incidence and predictors of subacute thrombosis in patients undergoing primary angioplasty for an acute myocardial infarction. Thromb Haemost. 2006;96:190–195. [PubMed] [Google Scholar]
- 26.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 27.Serruys PW, Farooq V, Kalesan B, de Vries T, Buszman P, Linke A, Ischinger T, Klauss V, Eberli F, Wijns W, Morice MC, Di Mario C, Corti R, Antoni D, Sohn HY, Eerdmans P, Rademaker-Havinga T, van Es GA, Meier B, Juni P, Windecker S. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) randomized, noninferiority trial. J Am Coll Cardiol Intv. 2013;6:777–789. doi: 10.1016/j.jcin.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Brar SS, Leon MB, Stone GW, Mehran R, Moses JW, Brar SK, Dangas G. Use of drug-eluting stents in acute myocardial infarction: a systematic review and meta-analysis. J Am Coll Cardiol. 2009;53:1677–1689. doi: 10.1016/j.jacc.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Sabate M, Raber L, Heg D, Brugaletta S, Kelbaek H, Cequier A, Ostojic M, Iniguez A, Tuller D, Serra A, Baumbach A, von Birgelen C, Hernandez-Antolin R, Roffi M, Mainar V, Valgimigli M, Serruys PW, Juni P, Windecker S. Comparison of newer-generation drug-eluting with bare-metal stents in patients with acute ST-segment elevation myocardial infarction: a pooled analysis of the EXAMINATION (clinical Evaluation of the Xience-V stent in Acute Myocardial INfArcTION) and COMFORTABLE-AMI (Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST-Elevation Myocardial Infarction) trials. J Am Coll Cardiol Intv. 2014;7:55–63. doi: 10.1016/j.jcin.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Kalesan B, Pilgrim T, Heinimann K, Raber L, Stefanini GG, Valgimigli M, da Costa BR, Mach F, Luscher TF, Meier B, Windecker S, Juni P. Comparison of drug-eluting stents with bare metal stents in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:977–987. doi: 10.1093/eurheartj/ehs036. [DOI] [PubMed] [Google Scholar]
- 31.Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, Emanuelsson H, Marco J, Legrand V, Materne P, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent study group. N Engl J Med. 1994;331:489–495. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- 32.Berger JS, Fridman V, Brown DL. Comparison of outcomes in acute myocardial infarction treated with coronary angioplasty alone versus coronary stent implantation. Am J Cardiol. 2006;97:977–980. doi: 10.1016/j.amjcard.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 33.Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, Detre K, Veltri L, Ricci D, Nobuyoshi M, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent restenosis study investigators. N Engl J Med. 1994;331:496–501. doi: 10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- 34.Mack WJ, Krauss RM, Hodis HN. Lipoprotein subclasses in the monitored atherosclerosis regression study (MARS). treatment effects and relation to coronary angiographic progression. Arterioscler Thromb Vasc Biol. 1996;16:697–704. doi: 10.1161/01.ATV.16.5.697. [DOI] [PubMed] [Google Scholar]
- 35.Hodis HN, Mack WJ, Dunn M, Liu C, Liu C, Selzer RH, Krauss RM. Intermediate-density lipoproteins and progression of carotid arterial wall intima-media thickness. Circulation. 1997;95:2022–2026. doi: 10.1161/01.CIR.95.8.2022. [DOI] [PubMed] [Google Scholar]
- 36.Krauss RM, Lindgren FT, Williams PT, Kelsey SF, Brensike J, Vranizan K, Detre KM, Levy RI. Intermediate-density lipoproteins and progression of coronary artery disease in hypercholesterolaemic men. Lancet. 1987;2:62–66. doi: 10.1016/S0140-6736(87)92734-6. [DOI] [PubMed] [Google Scholar]
- 37.Huff MW, Evans AJ, Sawyez CG, Wolfe BM, Nestel PJ. Cholesterol accumulation in J774 macrophages induced by triglyceride-rich lipoproteins. Comparison of very low density lipoprotein from subjects with type III, IV, and V hyperlipoproteinemias. Arterioscler Thromb Vasc Biol. 1991;11:221–233. doi: 10.1161/01.ATV.11.2.221. [DOI] [PubMed] [Google Scholar]
- 38.Scicchitano P, Cameli M, Maiello M, Modesti PA, Muiesan ML, Novo S, Palmiero P, Saba PS, Pedrinelli R, Ciccone MM. Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J Funct Foods. 2014;6:11–32. doi: 10.1016/j.jff.2013.12.006. [DOI] [Google Scholar]
- 39.Heller DA, de Faire U, Pedersen NL, Dahlen G, McClearn GE. Genetic and environmental influences on serum lipid levels in twins. N Engl J Med. 1993;328:1150–1156. doi: 10.1056/NEJM199304223281603. [DOI] [PubMed] [Google Scholar]
- 40.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116:1653–1662. doi: 10.1161/CIRCULATIONAHA.107.701466. [DOI] [PubMed] [Google Scholar]
- 42.Bittner V, Hardison R, Kelsey SF, Weiner BH, Jacobs AK, Sopko G, Bypass Angioplasty Revascularization I. Non-high-density lipoprotein cholesterol levels predict five-year outcome in the Bypass Angioplasty Revascularization Investigation (BARI) Circulation. 2002;106:2537–2542. doi: 10.1161/01.CIR.0000038496.57570.06. [DOI] [PubMed] [Google Scholar]
- 43.Sandhu PK, Musaad SMA, Remaley AT, Buehler SS, Strider S, Derzon JH, Vesper HW, Ranne A, Shaw CS, Christenson RH. Lipoprotein biomarkers and risk of cardiovascular disease: a laboratory medicine best practices (LMBP) systematic review. J Appl Lab Med. 2016;1:214–229. doi: 10.1373/jalm.2016.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on request.


