Abstract
BACKGROUND AND OBJECTIVE:
Congenital cytomegalovirus (cCMV) infection remains a leading cause of childhood hearing loss. Currently universal CMV screening at birth does not exist in the United States. An alternative approach could be testing infants who do not pass their newborn hearing screening (NHS) for cCMV. This study was undertaken to evaluate whether a targeted approach will identify infants with CMV-related sensorineural hearing loss (SNHL).
METHODS:
Infants born at 7 US medical centers received NHS and were also screened for cCMV while in the newborn nursery. Infants who tested positive for CMV received further diagnostic audiologic evaluations to identify or confirm hearing loss.
RESULTS:
Between 2007 and 2012, 99 945 newborns were screened for both hearing impairment and cCMV. Overall, 7.0% of CMV-positive infants did not pass NHS compared with 0.9% of CMV-negative infants (P < .0001). Among the cCMV infants who failed NHS, diagnostic testing confirmed that 65% had SNHL. In addition, 3.6% of CMV-infected infants who passed their NHS had SNHL confirmed by further evaluation during early infancy. NHS in this cohort identified 57% of all CMV-related SNHL that occurred in the neonatal period.
CONCLUSIONS:
A targeted CMV approach that tests newborns who fail their NHS identified the majority of infants with CMV-related SNHL at birth. However, 43% of the infants with CMV-related SNHL in the neonatal period and cCMV infants who are at risk for late onset SNHL were not identified by NHS.
What’s Known on This Subject:
Congenital cytomegalovirus (CMV) infection is a leading cause of childhood hearing loss. Although CMV saliva screening of newborns for CMV identifies infected infants for monitoring and early intervention, routine CMV screening does not occur in the United States.
What This Study Adds:
A targeted CMV testing approach identifies infants with CMV-related hearing loss at birth. However, 43% of the infants with CMV-related hearing loss and congenital CMV infants who are at risk for late onset hearing loss will not be identified.
Congenital cytomegalovirus (cCMV) infection is found worldwide and contributes to thousands of children each year being born with or developing permanent disability such as hearing loss, vision loss, cerebral palsy, cognitive impairment, and developmental delay. In the United States, Canada, Western Europe, and Australia, cCMV is estimated to occur in ∼0.5% to 0.7% of all live births.1–3 In other parts of the world, such as Latin America, Africa, and most countries in Asia, cCMV rates are even higher at ∼1% to 2% of all births.4–8 Approximately 10% of infants with cCMV will have clinical findings at birth (symptomatic infection). The vast majority of infected infants (≈90%), however, will have no clinical manifestations present during the newborn period (asymptomatic infection).9 Approximately 40% to 60% of symptomatic infants will manifest permanent sequelae, with sensorineural hearing loss (SNHL) being the most common, followed by cognitive impairment, retinitis, and cerebral palsy.2,10–12 Asymptomatic infants are also at risk for CMV-related disabilities, and ∼10% to 15% of asymptomatic infants will develop SNHL.2,11–15 Disabilities from symptomatic and asymptomatic cCMV infection are more common in children in the United States than other more recognized diseases such as Down syndrome, fetal alcohol syndrome, or spina bifida.16
cCMV infection significantly contributes to permanent childhood hearing loss, with CMV-related SNHL being second only to genetic causes both at birth and during the early years of life.14,17 SNHL after cCMV may be present at birth or occur later in childhood (late onset). Children with SNHL after cCMV may also have further worsening or progression of their losses.11–13,15
Although cCMV is a leading cause of SNHL in children and is more common than any of the other screened newborn conditions in the United States, routine newborn CMV screening does not occur in the United States. Limited CMV awareness by both providers and parents, the difficulty in confirming the diagnosis of cCMV after the newborn period, the inability to predict which children with cCMV will have sequelae, the lack of effective treatments to prevent or ameliorate the effects of the virus, and the absence of an inexpensive and rapid screening test have been some of the obstacles preventing the implementation of widespread CMV screening in the past. Recent advances in the development of a rapid, high-throughput method for detecting CMV in saliva,18 success with antiviral treatment in symptomatic infants,19 and the recognition that early identification for targeted monitoring and intervention during critical stages of speech and language acquisition improves outcomes20,21 have led to renewed interest in both targeted and universal approaches to screening newborns for cCMV. As part of the CMV and Hearing Multicenter Screening (CHIMES) study, ∼100 000 infants were tested for CMV and received a newborn hearing screening (NHS) while in the hospital nursery, thus allowing us to examine the effectiveness of a targeted approach in identifying infants with CMV-related hearing loss where only newborns who did not pass NHS would be tested for cCMV.
Methods
Study Population
Between March 2007 and March 2012, 100 607 infants born at 7 US medical centers (University of Alabama at Birmingham Hospital, Birmingham, AL; The University of Mississippi Medical Center, Jackson, MS; Saint Peter’s University Hospital, New Brunswick, NJ; Carolinas Medical Center, Charlotte, NC; Good Samaritan Hospital, Cincinnati, OH; Magee Womens Hospital, Pittsburgh, PA; and Parkland Memorial Hospital, Dallas, TX) were consented and enrolled prospectively in the CHIMES Study. All live-born infants were eligible for participation. Mothers were approached postpartum to obtain written informed consent for their infant’s enrollment in the study. Upon enrollment, saliva specimens were collected from the newborn and additional dried blood spots were obtained at the time of routine newborn metabolic screening and tested for CMV as previously described.18,22 Infants with positive saliva or dried blood spots screening specimens were enrolled in the follow-up component of the study to confirm cCMV and to monitor their hearing outcome. Newborn medical records were reviewed for infants with cCMV to determine if the infants had clinically apparent disease. An a priori definition of symptomatic cCMV was established at the beginning of the CHIMES study by study investigators. Infants were considered to have symptomatic cCMV if they had any of the following symptoms in the newborn period: generalized petechial rash, purpuric rash, hepatomegaly, splenomegaly, jaundice with direct bilirubin of 3 mg/dL or greater, unexplained neurologic/CNS abnormalities (eg, microcephaly, seizures, focal or generalized neurologic deficits), or chorioretinitis. Clinical decisions about further evaluations and possible treatment of the CMV-infected infants were made by the physicians at each study site. The CHIMES study did not include treatment of cCMV infants. Local institutional review board approval was obtained at each site.
NHS
NHS results and any additional outpatient hearing screens or diagnostic follow-up audiologic testing results were collected from the individual hospital’s audiology program for each infant enrolled in the study. Each study site followed the NHS protocol designed for their hospital. Most of the hospitals used a 2-stage protocol where infants who did not pass in the hospital were scheduled for an additional outpatient hearing screen, and infants not passing their outpatient hearing screen were scheduled for a follow-up diagnostic audiologic evaluation. Infants with cCMV, regardless of hearing screen status, received a diagnostic audiologic assessment at 3 to 8 weeks of age as part of the CHIMES study. The CHIMES study diagnostic audiology protocol included a tone burst Auditory Brainstem Response with thresholds at 0.5, 1.0, 2.0, and 4.0 kHz and Distortion Product Otoacoustic Emissions for each ear. Bone conduction, tympanometry, and ipsilateral acoustic reflexes were performed with a 1000-Hz probe tone if hearing loss was suspected. CMV-negative infants who referred (ie, did not pass) on NHS were audiologically managed per their hospital’s and state’s recommendations for a diagnostic audiologic assessment by 3 months of age for the identification of possible hearing loss in the infants.21 CMV-negative infants did not receive their audiological assessments as part of the CHIMES study.
Statistical Analysis
All statistical analyses were performed by using SAS software, version 9.3 (SAS Institute, Inc, Cary, NC).
The results of CMV screening were compared with the newborn hearing results. Binomial 95% confidence intervals (CIs) were calculated for point estimates. Statistical significance was determined by using a 2-tailed χ2 or Fisher’s exact test with a 5% level of significance, where appropriate.
Results
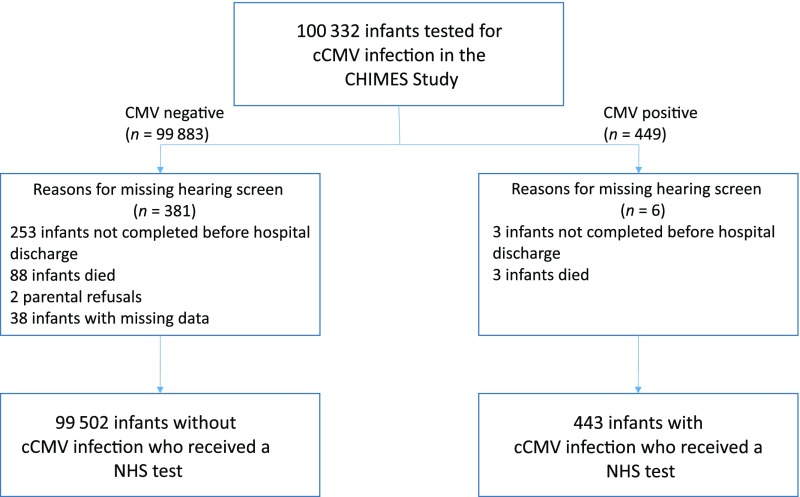
Of the 100 332 enrolled infants with a CMV test result, 99 945 (99.6%) had an NHS result (Fig 1). Reasons for not having NHS results included the following: hearing screen not completed before discharge from the nursery; infant death; or parental refusal. Of the 6 CMV-positive infants who did not have an NHS result, 3 symptomatic preterm infants died before a hearing screen was obtained, and 2 infants did not enroll in the follow-up component, so no follow-up information is available about whether these infants had hearing loss or normal hearing. The other infant did not have any evidence of hearing loss at birth confirmed by a diagnostic audiologic assessment when the infant enrolled in the follow-up component of the CMV study. Of the 99 945 infants who received an NHS, 443 (0.4%) were diagnosed with cCMV infection. Study characteristics of the 99 945 infants are seen in Table 1.
FIGURE 1.
Study cohort for the CHIMES study.
TABLE 1.
Study Characteristics for the 99 945 Newborns Who Underwent NHS and CMV Testing at the 7 Sites
| Characteristic | % (no.) |
|---|---|
| Infant sex | |
| Girl | 49.2 (49 160) |
| Boy | 50.8 (50 784) |
| Infant race/ethnicity | |
| Asian | 4.1 (4160) |
| Black | 24.0 (23 946) |
| White, Hispanic | 32.3 (32 269) |
| White, non-Hispanic | 37.1 (37 048) |
| Multiracial | 2.5 (2527) |
| Insurance status for hospital stay | |
| Private | 35.2 (35 156) |
| Public or no insurance | 64.8 (64 783) |
| Maternal age, mean (SD), y | 27.4 (6.1) |
| Hospital site | |
| Birmingham, Alabama | 12.0 (12 015) |
| Jackson, Mississippi | 6.3 (6346) |
| New Brunswick, New Jersey | 10.7 (10 706) |
| Charlotte, North Carolina | 15.1 (15 081) |
| Cincinnati, Ohio | 14.1 (14 071) |
| Pittsburgh, Pennsylvania | 19.1 (19 103) |
| Dallas, Texas | 22.6 (22 623) |
| Hospital nursery | |
| Well-infant | 96.5 (96 735) |
| NICU | 3.5 (3209) |
The NHS referral (did not pass) rate for the study population was 1.0% (95% CI, 0.9%–1.0%). However, 7.0% of CMV-positive infants did not pass their hearing screen compared with 0.9% of CMV-negative infants who did not pass their hearing screen (P < .0001). The same pattern remained in both the well-infant and the NICU nurseries, where the CMV-positive infants were significantly more likely to fail their hearing screen compared with CMV-negative infants (Table 2). Among infants with asymptomatic cCMV, 20/403 (5%, 95% CI, 3.1%–7.6%) failed NHS. In the well-infant and the NICU nurseries, 15/375 (4%, 95% CI, 2.3%–6.5%) and 5/28 (18%, 95% CI, 6%–37%) asymptomatic infants did not pass NHS, respectively. Symptomatic cCMV infants had a much higher referral rate of 11/40 (28%, 95% CI, 15%–44%), and had similar referral rates in both the well-infant (7/25; 28%, 95% CI, 12%–49%) and the NICU (4/15; 27%, 95% CI, 8%–55%) nurseries.
TABLE 2.
Newborn Hearing Screen Referral Rates for Infants by CMV Status, Overall and by Nursery
| CMV Screen | No. Screened | No. Referred | Hearing Screen Referral Rates, % (95% CI) | P |
|---|---|---|---|---|
| CMV positive | 443 | 31 | 7.0% (4.8%–9.8%) | <.0001 |
| CMV negative | 99 502 | 930 | 0.9% (0.8%–1.0%) | |
| Well-Infant Nursery | ||||
| CMV positive | 400 | 22 | 5.5% (3.5%–8.2%) | <.0001 |
| CMV negative | 96 336 | 768 | 0.8% (0.7%–0.9%) | |
| NICU | ||||
| CMV positive | 43 | 9 | 20.9% (10.0%–36.0%) | <.001 |
| CMV negative | 3166 | 162 | 5.1% (4.4%–5.9%) |
Of the 31 (7%) CMV-positive infants who did not pass NHS, 20 (65%) were confirmed to have SNHL by diagnostic audiologic evaluations. The other 11 (35%) who failed NHS were confirmed to have normal hearing by diagnostic evaluation. An additional 15 (3.6%) CMV-positive infants who passed NHS had SNHL confirmed by a diagnostic hearing evaluation in the first 3 to 8 weeks of life. The severity of the hearing loss in cCMV infants is seen in Table 3. Those infants who failed NHS were more likely diagnosed with bilateral loss (60%) and also were diagnosed with at least moderate hearing loss (65%). Of the 15 CMV-positive infants who passed their hearing screen but were diagnosed with SNHL during infancy, 9 (60%) had mild loss and 4 of these 9 infants had bilateral loss. The other 6 (40%) of 15 infants were diagnosed with at least a moderate to severe SNHL and 3 of these 6 infants had bilateral loss. None of the 31 CMV-positive infants who failed NHS nor the 15 additional infants who had SNHL were diagnosed as having syndromes or other malformations associated with hearing loss, or had a family history of hearing loss.
TABLE 3.
SNHL Severity by Newborn Hearing Screen Status for Infants With cCMV Infection
| Did Not Pass Hearing Screen, No. (%) | Passed Hearing Screen, No. (%) | Total, No. (%) | |
|---|---|---|---|
| Unilateral loss | 8 (40) | 8 (53) | 16 (46) |
| Bilateral loss | 12 (60) | 7 (47) | 19 (54) |
| Mild loss (21–40 dB HL) | 7 (35) | 9 (60) | 16 (46) |
| Moderate or greater loss (>40 dB HL) | 13 (65) | 6 (40) | 19 (54) |
| Total SNHL | 20 (57) | 15 (43) | 35 (100) |
Overall, NHS identified 20/35 (57%, 95% CI, 39%–74%) infants who had CMV-related SNHL in the newborn period leaving 43% not identified with hearing loss. In asymptomatic infants, NHS identified only 9/19 (47%, 95% CI, 24%–71%) of the CMV-related SNHL in these infants, missing 53% with hearing loss. Among symptomatic infants, NHS identified CMV-related hearing loss in 11/16 (69%, 95% CI, 41%–89%) infants.
CMV-positive infants with SNHL identified by NHS and those who passed their hearing screen but had SNHL in the neonatal period comprised 7.9% (95% CI, 5.6%–10.8%) of all infants with cCMV. As expected when infants were categorized by the presence of clinical findings at birth, those with symptomatic infection had a significantly higher rate of hearing loss than those with asymptomatic cCMV at birth. SNHL occurred in 38.1% (95% CI, 23.6%–54.4%) of the symptomatic infants compared with 4.7% (95% CI, 2.9%–7.3%) of the asymptomatic infants (P < .0001).
Discussion
Our large study of almost 100 000 infants revealed that a targeted CMV screening approach that only tests newborns who do not pass NHS identified the majority of infants with CMV-related SNHL at birth. However, this approach failed to identify a significant number of infants with CMV-related SNHL (43%) during infancy. Among infants with asymptomatic cCMV, 53% of those with CMV-related SNHL at birth will not be identified by a targeted approach. In addition, only testing infants who failed their hearing screen will miss the CMV-positive infants who are without symptoms at birth, pass NHS, and who go on to develop late onset hearing loss. A previous retrospective study in Texas revealed 6% of hearing impairment in newborns was attributable to CMV when they used a targeted CMV screening approach.23 Another study in Italy revealed that 10% of infants with SNHL detected <2 months of age had cCMV infection.24 Although these studies indicated that testing infants who fail NHS for CMV could identify CMV-related SNHL, both studies were retrospective and did not include CMV screening of all infants. Our study included both CMV and hearing screening of all infants and provides reliable estimates of the effectiveness of a targeted CMV screening approach in identifying infants with CMV-related SNHL.
An important finding of our study is that newborns with cCMV have a significantly higher NHS referral rate (7%) than CMV-negative infants. These results indicate that newborns who do not pass their hearing screen and have no other known etiology for their possible hearing loss should be screened for CMV infection. In fact, existing clinical guidelines from the 2007 Statement by the Joint Committee on Infant Hearing recommend that infants with confirmed hearing loss and an uncertain etiology after an initial medical evaluation should have an expanded multidisciplinary evaluation protocol that includes testing for CMV.21 However, by the time permanent hearing loss is confirmed by the diagnostic audiologic evaluation and the initial medical evaluation is completed, it will be too late to confirm cCMV. Testing of infants who refer on NHS for CMV by saliva or urine polymerase chain reaction before hospital discharge or by 2 to 3 weeks of age by the pediatric medical home provider will provide confirmation of CMV as the cause of any suspected congenital hearing loss. After 3 weeks of age, cCMV cannot be reliably diagnosed as the etiology for infants with SNHL.
NHS programs have been successful in identifying congenital hearing loss but do have some limitations because of the sensitivity and specificity of hearing screen tools and testing protocols.25 In many programs, the majority of infants who fail NHS will not have permanent hearing loss.26,27 Although it would be expected that more infants with cCMV who failed their hearing screen would have permanent loss, our finding that 64% of the infants had SNHL was a higher confirmation rate than expected on the basis of other studies.26,27
It is unclear why 43% of all CMV-positive infants and 53% of asymptomatic cCMV infants passed NHS but were confirmed to have CMV-related SNHL in the newborn period. A previous multicenter study estimated that ∼23% of infants who passed a 2-stage hospital screening protocol had permanent hearing loss at 9 months of age; however, it is estimated that their protocol missed up to 70% of all cases of mild unilateral and bilateral hearing loss.28,29 At another center, one-third of the pediatric cochlear implant population had previously passed NHS.30 The percentage of CMV-positive infants with SNHL who passed their hearing screen was higher than these previously reported studies. It is possible that some of the infants who passed NHS but were confirmed to have SNHL were missed because of limitations of the NHS algorithms that were unable to reliably detect mild or isolated frequency region hearing losses. However, this does not explain the infants who had moderate to severe hearing loss identified on their diagnostic evaluation. It is also possible that the hearing loss occurred after the first week after birth or progressed to a measurable level by 6 to 8 weeks after birth. However, this is speculative and no previous data exist to suggest that CMV-related hearing loss is unstable in the neonatal period.
In addition to the fact that NHS failed to detect 43% of CMV-positive infants who had SNHL in the newborn period, the progressive nature of CMV-related hearing loss in ∼50% of children with SNHL underscores the limitations of the targeted CMV screening approach.12,13 The rate of hearing loss progression in cCMV infection seems to be similar regardless of whether a child has an asymptomatic or a symptomatic infection, although the symptomatic infants have a greater degree of severity and also earlier progression of their hearing loss.12 With current pediatric newborn screening practices, CMV-positive infants who pass NHS but have CMV-related SNHL, whether stable or progressive loss, will be missed by any targeted screening program and otherwise will remain unidentified because routine CMV screening does not occur.
There are limitations in our study in that although all live-born infants were eligible to participate at the hospitals not all were enrolled in the study. Infants who were in the NICU were less likely to be approached by study staff because of the fragility of the infant and to not place any additional burdens on their families. Infants who were discharged early or who were delivered on weekends or evenings may have been missed if study personnel were not available to obtain consent. It is possible that we missed cCMV infants, especially asymptomatic infants, and underestimated the rate of cCMV infection for our hospital sites. Our study revealed a 0.4% cCMV rate that is lower than some previously reported studies,2,3 although not lower for other large studies of cCMV.31,32 However, the lower cCMV rate should not impact the observed difference in the hearing referral rates between CMV-positive and CMV-negative infants, because there is no evidence to suggest the missed cCMV infants would have had a different hearing referral rate than those infants diagnosed. Also, the rates of CMV-related SNHL in the study were similar to previous reports, so it is not likely that the study missed a significant number of CMV-positive infants at the sites.11,12
Targeted CMV screening will minimize the diagnostic etiology odyssey for some of the infants with suspected hearing loss because cCMV can only be reliably diagnosed within the first few weeks after birth. Also, infants identified with CMV-related hearing loss through targeted screening will have the opportunity for more focused audiologic monitoring, early intervention, and antiviral treatment. However, the limitations of a targeted CMV screening approach are the failure to identify all CMV-related SNHL in the newborn period and missing the cCMV infants who pass NHS but are at risk for late onset hearing loss.
Conclusions
A targeted CMV screening approach does identify the majority of infants with CMV-related SNHL in the newborn period. However, this method fails to identify a significant number of infants with CMV-related SNHL during infancy highlighting the need to develop approaches to improve detection of CMV-related hearing loss at birth. Strategies to identify all infants with cCMV who remain at risk for late onset and progressive hearing losses are needed.
Acknowledgments
We are indebted to our medical, nursing, and audiology colleagues, and the infants and their parents who agreed to take part in this study.
Glossary
- cCMV
congenital cytomegalovirus
- CHIMES
CMV and Hearing Multicenter Screening
- CI
confidence interval
- CMV
cytomegalovirus
- NHS
newborn hearing screening
- SNHL
sensorineural hearing loss
Footnotes
Dr Fowler conceptualized and designed the study, assisted in the development of the CMV and Hearing Multicenter Screening (CHIMES) audiology protocols, carried out the data analyses, drafted the initial manuscript, and reviewed and revised the manuscript; Dr McCollister assisted in the development of the CHIMES audiology protocols and reviewed and revised the manuscript; Dr Sabo assisted in the development of the CHIMES audiology protocols, coordinated and supervised audiology data collection at the Pennsylvania site, and reviewed and revised the manuscript; Dr Shoup assisted in the development of the CHIMES audiology protocols, participated in collection, coordination, and supervision of audiology data collection at the Texas site, and reviewed and revised the manuscript; Dr Owen assisted in the development of the CHIMES audiology protocols, assisted in collecting the audiology data, and coordinated and completed study schedule and study forms for the Texas site; Dr Woodruff collected audiology data and coordinated and supervised audiology data collection at the Alabama site and critically reviewed the manuscript; Dr Cox assisted in the development of the CHIMES audiology protocols, collected audiology data at the North Carolina site, and coordinated and supervised the collection of audiology data; Dr Mohamed collected audiology data at the North Carolina site and critically reviewed the manuscript; Dr Choo contributed in the development of the CHIMES audiologic protocols, advised on the collection of audiologic data at the Ohio site, and critically reviewed the manuscript; Dr Boppana conceptualized and designed the study, actively participated in the conduct of the study, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: All phases of this study were funded by the National Institute on Deafness and Other Communication Disorders (NIDCD) grants N01-DC-5-0008 and HHS-N-263-2012-00010-C. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Fowler KB, Stagno S, Pass RF. Maternal age and congenital cytomegalovirus infection: screening of two diverse newborn populations, 1980-1990. J Infect Dis. 1993;168(3):552–556 [DOI] [PubMed] [Google Scholar]
- 2.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17(5):355–363 [DOI] [PubMed] [Google Scholar]
- 3.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253–276 [DOI] [PubMed] [Google Scholar]
- 4.Britt WJ. Cytomegalovirus. In: Remington J, Klein J, Wilson C, Nizet V, Maldonado Y, eds. Infectious Diseases of the Fetus and Newborn Infant. 7th ed. Philadelphia, PA: Elsevier Saunders; 2011:706–755 [Google Scholar]
- 5.Dar L, Pati SK, Patro AR, et al. Congenital cytomegalovirus infection in a highly seropositive semi-urban population in India. Pediatr Infect Dis J. 2008;27(9):841–843 [DOI] [PubMed] [Google Scholar]
- 6.Kaye S, Miles D, Antoine P, et al. Virological and immunological correlates of mother-to-child transmission of cytomegalovirus in The Gambia. J Infect Dis. 2008;197(9):1307–1314 [DOI] [PubMed] [Google Scholar]
- 7.van der Sande MA, Kaye S, Miles DJ, et al. Risk factors for and clinical outcome of congenital cytomegalovirus infection in a peri-urban West-African birth cohort. PLoS One. 2007;2(6):e492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto AY, Mussi-Pinhata MM, Cristina P, Pinto G, Moraes Figueiredo LT, Jorge SM. Congenital cytomegalovirus infection in preterm and full-term newborn infants from a population with a high seroprevalence rate. Pediatr Infect Dis J. 2001;20(2):188–192 [DOI] [PubMed] [Google Scholar]
- 9.Pass RF, Fowler KB, Boppana S. Clinical importance of cytomegalovirus infection: an overview. In: Landini MP, ed. Progress in Cytomegalovirus Research. New York, NY: Elsevier Science Publishers; 1991:3–10 [Google Scholar]
- 10.Fowler KB, Stagno S, Pass RF, Britt WJ, Boll TJ, Alford CA. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326(10):663–667 [DOI] [PubMed] [Google Scholar]
- 11.Fowler KB, Dahle AJ, Boppana SB, Pass RF. Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J Pediatr. 1999;135(1):60–64 [DOI] [PubMed] [Google Scholar]
- 12.Dahle AJ, Fowler KB, Wright JD, Boppana SB, Britt WJ, Pass RF. Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J Am Acad Audiol. 2000;11(5):283–290 [PubMed] [Google Scholar]
- 13.Fowler KB, McCollister FP, Dahle AJ, Boppana S, Britt WJ, Pass RF. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr. 1997;130(4):624–630 [DOI] [PubMed] [Google Scholar]
- 14.Fowler KB, Boppana SB. Congenital cytomegalovirus (CMV) infection and hearing deficit. J Clin Virol. 2006;35(2):226–231 [DOI] [PubMed] [Google Scholar]
- 15.Goderis J, De Leenheer E, Smets K, Van Hoecke H, Keymeulen A, Dhooge I. Hearing loss and congenital CMV infection: a systematic review. Pediatrics. 2014;134(5):972–982 [DOI] [PubMed] [Google Scholar]
- 16.Cannon MJ. Congenital cytomegalovirus (CMV) epidemiology and awareness. J Clin Virol. 2009;46(suppl 4):S6–S10 [DOI] [PubMed] [Google Scholar]
- 17.Morton CC, Nance WE. Newborn hearing screening—a silent revolution. N Engl J Med. 2006;354(20):2151–2164 [DOI] [PubMed] [Google Scholar]
- 18.Boppana SB, Ross SA, Shimamura M, et al. ; National Institute on Deafness and Other Communication Disorders CHIMES Study . Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. N Engl J Med. 2011;364(22):2111–2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimberlin DW, Jester PM, Sánchez PJ, et al. ; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group . Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med. 2015;372(10):933–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korver AM, Konings S, Dekker FW, et al. ; DECIBEL Collaborative Study Group . Newborn hearing screening vs later hearing screening and developmental outcomes in children with permanent childhood hearing impairment. JAMA. 2010;304(15):1701–1708 [DOI] [PubMed] [Google Scholar]
- 21.American Academy of Pediatrics, Joint Committee on Infant Hearing . Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120(4):898–921 [DOI] [PubMed] [Google Scholar]
- 22.Boppana SB, Ross SA, Novak Z, et al. ; National Institute on Deafness and Other Communication Disorders CMV and Hearing Multicenter Screening (CHIMES) Study . Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA. 2010;303(14):1375–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stehel EK, Shoup AG, Owen KE, et al. Newborn hearing screening and detection of congenital cytomegalovirus infection. Pediatrics. 2008;121(5):970–975 [DOI] [PubMed] [Google Scholar]
- 24.Barbi M, Binda S, Caroppo S, Ambrosetti U, Corbetta C, Sergi P. A wider role for congenital cytomegalovirus infection in sensorineural hearing loss. Pediatr Infect Dis J. 2003;22(1):39–42 [DOI] [PubMed] [Google Scholar]
- 25.American Speech-Language-Hearing Association Expert panel recommendations on newborn hearing screening. Available at: www.asha.org/Topics/Expert-Panel-Recommendations-on-Newborn-Hearing-Screening/. Accessed November 28, 2016
- 26.Nelson HD, Bougatsos C, Nygren P; 2001 US Preventive Services Task Force . Universal newborn hearing screening: systematic review to update the 2001 US Preventive Services Task Force Recommendation. Pediatrics. 2008;122(1). Available at: www.pediatrics.org/cgi/content/full/122/1/e266 [DOI] [PubMed] [Google Scholar]
- 27.Kennedy C, McCann D, Campbell MJ, Kimm L, Thornton R. Universal newborn screening for permanent childhood hearing impairment: an 8-year follow-up of a controlled trial. Lancet. 2005;366(9486):660–662 [DOI] [PubMed] [Google Scholar]
- 28.Johnson JL, White KR, Widen JE, et al. A multicenter evaluation of how many infants with permanent hearing loss pass a two-stage otoacoustic emissions/automated auditory brainstem response newborn hearing screening protocol. Pediatrics. 2005;116(3):663–672 [DOI] [PubMed] [Google Scholar]
- 29.Ross DS, Holstrum WJ, Gaffney M, Green D, Oyler RF, Gravel JS. Hearing screening and diagnostic evaluation of children with unilateral and mild bilateral hearing loss. Trends Amplif. 2008;12(1):27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young NM, Reilly BK, Burke L. Limitations of universal newborn hearing screening in early identification of pediatric cochlear implant candidates. Arch Otolaryngol Head Neck Surg. 2011;137(3):230–234 [DOI] [PubMed] [Google Scholar]
- 31.Larke RP, Wheatley E, Saigal S, Chernesky MA. Congenital cytomegalovirus infection in an urban Canadian community. J Infect Dis. 1980;142(5):647–653 [DOI] [PubMed] [Google Scholar]
- 32.Ahlfors K, Ivarsson SA, Harris S, et al. Congenital cytomegalovirus infection and disease in Sweden and the relative importance of primary and secondary maternal infections. Preliminary findings from a prospective study. Scand J Infect Dis. 1984;16(2):129–137 [DOI] [PubMed] [Google Scholar]