Photosynthesis in oxygen-evolving organisms is driven by electron transport through the photochemical reaction centers photosystem I (PSI) and photosystem II (PSII), two large protein complexes located in the chloroplast thy-lakoid membrane. PSI and PSII each contain an array of light-harvesting antenna pigments that absorb light energy and transfer it to a reaction center core complex, where electron transport drives the conversion of energy into the stable chemical products NADPH and ATP. PSII absorbs predominantly red light and produces a strong oxidant that extracts electrons from water and releases oxygen (the water-splitting reaction of photosynthesis) and a reductant that transfers electrons on to PSI. PSI absorbs predominantly far-red light plus electrons from PSII to produce a strong reductant capable of reducing NADP+ to NADPH.
Light-driven ATP synthesis (photophosphorylation) is tightly coupled to electron transport through PSII and PSI and occurs within an ATP synthase complex embedded in the thylakoid membrane. Two other major complexes involved in photosynthetic energy conversion are cytochrome b6f, which controls the shuttle of electrons from PSII to PSI via the mobile electron carriers plastoquinone and plastocyanin, and LHCII, a light-harvesting protein complex that functions as an auxiliary antenna for PSII and also appears to influence energy distribution between PSI and PSII.
Most of the proteins of these complexes, which together make up the photosynthetic apparatus, are encoded by the chloroplast genome. On the basis of the high degree of conservation of structure and composition of photosystems in all organisms examined to date, it is thought that all photosystems evolved from a single ancestor. In fact, there is a high degree of conservation among most of the ∼120 genes encoded by numerous plastid genomes from plants and algae that have been sequenced completely (reviewed in Rochaix, 1997).
Suguira (1992) divided plastid genes into three major categories on the basis of their functions: plastome expression and maintenance (such as genes encoding rRNA, tRNA, and ribosomal protein subunits), photosynthesis, and unknown function. A total of 26 to 34 unidentified open reading frames were found in various chloroplast genomes, and at least 12 of these were highly conserved in size and sequence among the chloroplast genomes examined to date. Hallic and Bairoch (1994) introduced the term ycf (hypothetical chloroplast open reading frame) for chloroplast open reading frames of unknown function.
TARGETED INACTIVATION OF ycf9
Chloroplast transformation is possible via recombination between plastid genome sequences and homologous sequences that flank a gene expression cassette (which produces “transplastomic” plants), in contrast to nuclear transformation, in which an uncontrolled number of foreign gene copies are integrated into the genome at random sites. Thus far, most plastid transformations have been performed in Chlamydomonas reinhardtii and tobacco. Technical limitations have hindered plastid transformation in other systems, although progress in recent years holds promise for routine application in other higher plant species, and the technique has been used successfully in rice (Heifetz, 2000).
Targeted gene inactivation via chloroplast transformation has been used to characterize the function of several of the ycf genes in Chlamydomonas and tobacco. Thus, ycf6 (Hager et al., 1999) and ycf7 (Takahashi et al., 1996) were found to be essential components of the cytochrome b6f complex in tobacco and Chlamydomonas, respectively. In this issue of The Plant Cell, Swiatek et al. (pages 1347–1367) describe the use of targeted gene inactivation of the chloroplast ycf9 gene in both Chlamydomonas and tobacco to provide strong evidence that the ycf9 product, named PsbZ, is a subunit of the PSII core complex.
Previous research on this gene has yielded ambiguous results. Because plant cells contain numerous chloroplasts, and thus numerous copies of the chloroplast genome, it can be difficult to obtain transplastomic plants that are homoplasmic for foreign transgenes. Mäenpää et al. (2000) were unable to obtain transplastomic tobacco plants homoplasmic for an inactivated ycf9 gene and concluded that the ycf9 gene product was likely essential for chloroplast function. Furthermore, the use of a polyclonal antibody raised against a portion of the ycf9 gene product on thylakoid fractions separated by standard techniques suggested that the ycf9 product was not associated with PSII or LHCII but possibly was associated with PSI. On the other hand, Ruf et al. (2000) produced homoplasmic ycf9 knockout tobacco plants that displayed no apparent phenotype under normal growth conditions. However, the ycf9 mutant plants showed impaired growth and oxygen-evolving capacity under low light, suggesting that they had reduced light-harvesting efficiency relative to wild-type plants. These ycf9 mutant plants also were found to have reduced levels of CP26, a minor antenna protein of LHCII, and in wild-type plants the ycf9 gene product was found to co-purify with LHCII fractions, suggesting that the Ycf9 protein formed part of the LHC complex (Ruf et al., 2000).
Swiatek et al. show quite convincingly that PsbZ is in fact a genuine subunit of PSII. Evidence is presented from both Chlamydomonas and tobacco, using specific antibodies against PsbZ on thylakoid fractions from wild-type psbZ-deficient mutant preparations as well as preparations from various other mutants lacking other components of the photosynthetic apparatus. Thus, PsbZ was found to comigrate precisely with PSII core subunits in wild-type Chlamydomonas and was present in mutants lacking PSI, ATP synthase, chlorophyll a/b antenna proteins, or the cytochrome b6f complex but was absent in other mutants lacking PSII cores. It was also found to be associated with PSII core fractions from wild-type tobacco plants, but it was missing from PSII core fractions from the psbZ-deficient mutant tobacco.
One of the great strengths of this study is the use of the two different systems, Chlamydomonas and tobacco, to investigate protein function. Somewhat different experiments were performed in each system, depending on the availability of mutants and techniques that were applicable to each, yet the results were in good agreement, making the conclusions far more robust than if only one of the systems had been used. This is perhaps a particularly important point in this case, because others had obtained somewhat different results concerning the function of ycf9 (Mäenpää et al., 2000; Ruf et al., 2000). This work also demonstrates the potential usefulness of Chlamydomonas as a model system for higher plant processes.
PSII SUBUNIT ORGANIZATION
PSII is made up of at least 17 subunits, most of which are embedded within the thylakoid membrane: the reaction center proteins D1 (PsbA) and D2 (PsbD); the chlorophyll-containing antennae CP47 (PsbB) and CP43 (PsbC); the cytochrome b559 α- and β-subunits (PsbE and PsbF); and a number of smaller subunits of largely unknown function (PsbH, PsbI, PsbJ, PsbK, PsbL, PsbM, PsbN, and PsbX). Several other subunits, including cytochrome c550 (PsbV), PsbU, and PsbO, extend into the thylakoid lumen from PSII (Zouni et al., 2001).
PSII probably exists as a dimer in higher plants and algae (Santini et al., 1994; Shi et al., 2000; Zouni et al., 2001), and the dimer forms a PSII-LHCII supercomplex with LHCII subunits. There is evidence that some of the low molecular weight proteins, such as PsbH, PsbK, and PsbL, are involved in PSII dimer stabilization. Shi et al. (2000) showed that another low molecular weight protein named PsbW is involved in the stabilization of dimeric PSII complexes in Arabidopsis. In contrast to wild-type plants, the dimeric form of PSII could not be isolated from antisense psbW plants with more than a 96% reduction in PsbW protein content. Furthermore, the antisense plants exhibited a 50% reduction in oxygen evolution and a 40% loss of PSII reaction center proteins relative to wild-type plants, suggesting that the dimerization of PSII is important to maintain the stability of the photosynthetic apparatus. PsbW is a nucleus-encoded protein, leading to the interesting speculation that it represents a higher plant mechanism for providing a degree of nuclear control over photosynthetic activity in the partly autonomous chloroplast (Shi et al., 2000).
Swiatek et al. present evidence that the PsbZ protein is involved in maintaining the stability of PSII-LHCII supercomplexes. After membrane solubilization with an appropriate detergent, sucrose gradient sedimentation results in the separation of PSII core subunits into several different fractions corresponding to PSII-LHCII supercomplexes, PSII dimers, or PSII monomers. PSII-LHCII supercomplexes were readily identified from wild-type tobacco preparations but were completely absent in preparations from the psbZ-deficient mutant. Mutant preparations also failed to accumulate other PSII- and LHCII-associated proteins at the positions of PSII supercomplexes.
It is known that interactions between the PSII core and the LHCII antenna are controlled by phosphorylation (Allen, 1992). Swiatek et al. found that, consistent with a role in PSII-LHCII interactions, the phosphorylation status of PSII cores and LHCII antennae was altered markedly in the psbZ-deficient mutants of both Chlamydomonas and tobacco.
Swiatek et al. present strong evidence that PsbZ is associated with the PSII core. In light of the previous results of Ruf et al. (2000) that suggest the protein is associated with LHCII and the evidence from Swiatek et al. that PsbZ is involved intimately with PSII-LHCII interactions, it seems likely that PsbZ occupies a position in the PSII core near the PSII-LHCII interface. Figure 1 portrays a model for the structure of PSII-LHCII supercomplexes showing the possible location of PsbZ subunits and adjacent LHCII subunits and minor antenna proteins.
Figure 1.
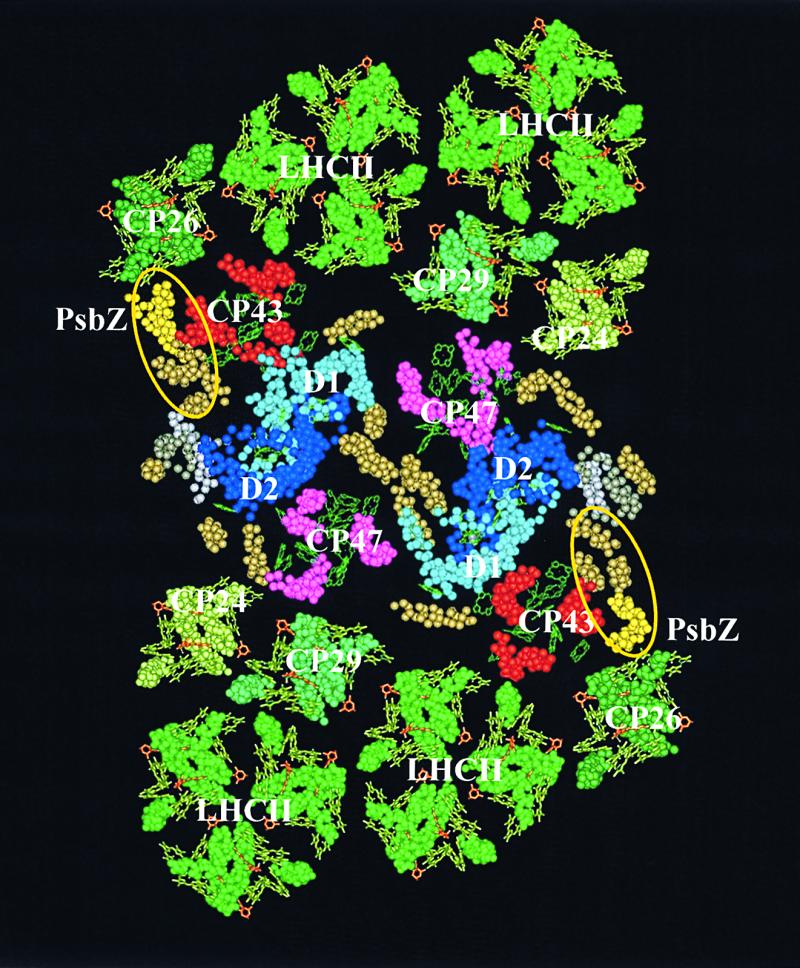
Molecular Reconstruction of the PSII-LHCII Supercomplex Showing the Putative Location of PsbZ.
The representation of PSII cores is based on the atomic coordinates (PDB accession number 1Fe1) published by Zouni et al. (2001). The representation of LHCII proteins is based on Kühlbrandt et al. (1994). The location and orientation of the peripheral antenna complexes with respect to the PSII core are based on Harrer et al. (1998), Boekema et al. (1999), and Nield et al. (2000). Protein moieties are shown by space fill models of the C-α carbon atoms, and chlorophylls are shown as green wire frame models. The helices from the PSII core that have no attribution by Zouni et al. (2001) are shown in dark yellow. The putative location of PsbZ, as suggested by Swiatek et al., is among the five “unidentified” helices circled in yellow. The most likely candidates for PsbZ are shown in light yellow and stand close to CP26 and CP43.
PsbZ AND NONPHOTOCHEMICAL QUENCHING
Figure 1 shows PsbZ lying adjacent to the CP26 subunit, which is a minor antenna subunit of LHCII. The positioning is based on the location of unidentified PSII subunits from Zouni et al. (2001) and the observation by Swiatek et al. that psbZ-deficient mutants accumulated significantly less CP26 protein than did wild-type plants. CP26 and CP29 are violaxanthin binding proteins and appear to play a role in nonphotochemical quenching (NPQ) of chlorophyll fluorescence, which involves interconversions between violaxanthin and zeaxanthin (the so-called xanthophyll cycle). Exposure of the photosynthetic apparatus to excess light energy induces a reversible decrease in the efficiency of photosynthetic energy conversion. The excess excitation energy can result in damage to the thylakoid membrane (photoinhibition) or it can be dissipated through a controlled mechanism (photoprotection) that is associated intimately with the xanthophyll cycle in many plant species (reviewed in Demmig-Adams, 1990; Horton, 1996).
In the xanthophyll cycle, zeaxanthin is formed from deepoxidation of violaxanthin via the intermediate antheraxanthin in a reaction catalyzed by a deepoxidase, and an epoxidase catalyzes the reconversion of zeaxanthin to antheraxanthin and violaxanthin. There is strong evidence that zeaxanthin functions in the dissipation of excess excitation energy, which protects the photosynthetic apparatus from the damaging effects of photoinhibition by preventing the accumulation of toxic reactive oxygen species. Demmig-Adams (1990) showed that there is a close correlation between zeaxanthin content and the capacity for quenching of chlorophyll a fluorescence and photoprotective energy dissipation (NPQ) in many different species and under a wide range of conditions. However, the mechanism of energy dissipation involving zeaxanthin remains largely unknown.
Swiatek et al. found that psbZ-deficient tobacco plants showed a greatly reduced capacity for NPQ under adverse growth conditions such as increased light intensity and/or decreased temperature. The mutant plants also showed a dramatically altered xanthophyll cycle, as measured by HPLC analysis of various pigments, including violaxanthin, zeaxanthin, and antheraxanthin. When transferred from dim light to high light, wild-type plants showed an increase in the proportion of zeaxanthin from 4 to 33% of total xanthophylls, followed by a rapid decrease to 14% after a 10-min dark recovery period. In contrast, zeaxanthin content in the psbZ-deficient plants increased from 5 to 63% when transferred from dim light to high light and did not recover appreciably when subsequently placed in darkness for 10 min. The total xanthophyll pool also was significantly higher in the mutant plants relative to the wild type. Nonetheless, the mutant plants apparently were unable to use zeaxanthin as a photoprotectant as effectively as did the wild-type plants, as shown by a reduced capacity for NPQ and greater photoinhibition under stress conditions.
Horton (1996) discussed the possibility that zeaxanthin functions only indirectly in NPQ, perhaps by bringing about structural changes within LHCII. There is evidence for a significant role for minor LHCII components (i.e., the violaxanthin binding proteins CP26 and CP29) in NPQ. Swiatek et al. show that PsbZ plays a critical role in interactions between PSII and LHCII and in the formation of NPQ under conditions that give rise to photoinhibition. There are a number of organisms that lack a xanthophyll cycle and/or do not accumulate zeaxanthin yet maintain the capacity for NPQ and photoprotection (Demmig-Adams, 1990; Horton, 1996). In this regard, it is interesting that PsbZ appears to be highly conserved among all photosynthetic organisms, even in those that lack a xanthophyll cycle. Further investigation of the function of PsbZ may provide the key to the mechanism of NPQ and the photoprotective dissipation of excess light energy.
References
- Allen, J.F. (1992). Protein phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta 1098, 275–335. [DOI] [PubMed] [Google Scholar]
- Boekema, E., van Roon, H., Calkoen, F., Bassi, R., and Dekker, J. (1999). Multiple types of association of photosystem II and its light-harvesting antenna in partially solubilized photosystem II membranes. Biochemistry 38, 2233–2239. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams, B. (1990). Carotenoids and photoprotection in plants: A role for the xanthophyll zeaxanthin. Biochim. Biophys. Acta 1020, 1–24. [Google Scholar]
- Hager, M., Biehler, K., Illerhaus, J., Ruf, S., and Bock, R. (1999). Targeted inactivation of the smallest plastid genome-encoded open reading frame reveals a novel and essential subunit of the cytochrome b6f complex. EMBO J. 18, 5834–5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallic, R.B., and Bairoch, A. (1994). Proposal for the naming of chloroplast genes. III. Nomenclature for open reading frames encoded in chloroplast genomes. Plant Mol. Biol. Rep. 12, S29–S30. [Google Scholar]
- Harrer, R., Bassi, R., Testi, M.-G., and Shaefer, C. (1998). Nearest-neighbor analysis of a photosystem II complex from Marchantia polymorpha L. (liverwort), which contains reaction center and antenna proteins. Eur. J. Biochem. 255, 196–205. [DOI] [PubMed] [Google Scholar]
- Heifetz, P.B. (2000). Genetic engineering of the chloroplast. Biochimie 82, 655–666. [DOI] [PubMed] [Google Scholar]
- Horton, P. (1996). Nonphotochemical quenching of chlorophyll fluorescence. In Light as Energy Source and Information Carrier in Plant Physiology, R.C. Jennings, G. Zucchelli, F. Ghetti, and G. Colombetti, eds (New York: Plenum Press), pp. 99–112.
- Kühlbrandt, W., Wang, D.N., and Fujiyoshi, Y. (1994). Atomic model of plant light-harvesting complex by electron crystallography. Nature 367, 614–621. [DOI] [PubMed] [Google Scholar]
- Mäenpää, P., Gonzalez, E.B., Chen, L., Khan, M.S., Gray, J.C., and Aro, E.-M. (2000). The ycf9 (orf 62) gene in the plant chloroplast genome encodes a hydrophobic protein of stromal thylakoid membranes. J. Exp. Bot. 51, 375–382. [DOI] [PubMed] [Google Scholar]
- Nield, J., Funk, C., and Barber, J. (2000). Supermolecular structure of photosystem II and location of the PsbS protein. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochaix, J.-D. (1997). Chloroplast reverse genetics: New insights into the function of plastid genes. Trends Plant Sci. 2, 419–425. [Google Scholar]
- Ruf, S., Biehler, K., and Bock, R. (2000). A small chloroplast-encoded protein as a novel architectural component of the light-harvesting antenna. J. Cell Biol. 149, 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini, C., Tidu, V., Tognon, G., Ghiretti-Magaldi, A., and Bassi, R. (1994). Three dimensional structure of higher plants photosystem II reaction centre: Evidences for its dimeric organization in vivo. Eur. J. Biochem. 221, 307–315. [DOI] [PubMed] [Google Scholar]
- Shi, L.-X., Lorković, Z.J., Oelmüller, R., and Schröder, W.P. (2000). The low molecular mass PsbW protein is involved in the stabilization of the dimeric photosystem II complex in Arabidopsis thaliana. J. Biol. Chem. 275, 37945–37950. [DOI] [PubMed] [Google Scholar]
- Suguira, M. (1992). The chloroplast genome. Plant Mol. Biol. 19, 149–168. [DOI] [PubMed] [Google Scholar]
- Swiatek, M., Kuras, R., Sokolenko, A., Higgs, D., Olive, J., Cinque, G., Müller, B., Eichacker, L.A., Stern, D.B., Bassi, R., Herrmann, R.G., and Wollman, F.-A. (2001). The chloroplast gene ycf9 encodes a photosystem II core subunit, PsbZ, that participates in photosystem II supramolecular architecture. Plant Cell 13, 1347–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, Y., Rahire, M., Breyton, C., Popot, J.L., Joliot, P., and Rochaix, J.D. (1996). The chloroplast ycf7 (petL) open reading frame of Chlamydomonas reinhardtii encodes a small functionally important subunit of the cytochrome b6f complex. EMBO J. 15, 3498–3506. [PMC free article] [PubMed] [Google Scholar]
- Zouni, A., Witt, H.-T., Kern, J., Fromme, P., Krauss, N., Saenger, W., and Orth, P. (2001). Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution. Nature 409, 739–743. [DOI] [PubMed] [Google Scholar]


