Abstract
BACKGROUND AND OBJECTIVES:
Cystic fibrosis (CF) is known for its impact on the lung and pancreas of individuals; however, impaired growth is also a common complication. We hypothesized that targeting the biological defect in the CF transmembrane conductance regulator (CFTR) protein may affect growth outcomes.
METHODS:
In this post hoc analysis, we assessed linear growth and weight in 83 children (aged 6–11 years) enrolled in 2 clinical trials, the longitudinal-observation GOAL study and the placebo-controlled ENVISION study, to evaluate the effects of ivacaftor, a CFTR potentiator. We calculated height and weight z scores and height and weight growth velocities (GVs).
RESULTS:
In ivacaftor-treated children in GOAL, height and weight z scores increased significantly from baseline to 6 months (increases of 0.1 [P < .05] and 0.26 [P < .0001], respectively); height GV increased significantly from 3 to 6 months (2.10-cm/year increase; P < .01). In ivacaftor-treated children in ENVISION, height and weight z scores increased significantly from baseline to 48 weeks (increases of 0.17 [P < .001] and 0.35 [P < .001], respectively). Height and weight GVs from baseline to 48 weeks were also significantly higher with ivacaftor than with placebo (differences of 1.08 cm/year [P < .05] and 3.11 kg/year [P < .001], respectively).
CONCLUSIONS:
Ivacaftor treatment in prepubescent children may help to address short stature and altered GV in children with CF; results from these analyses support the existence of an intrinsic defect in the growth of children with CF that may be ameliorated by CFTR modulation.
What’s Known on This Subject:
Impaired growth is a common complication in patients with cystic fibrosis (CF), both manifesting and contributing to the reduced lung function seen in patients with CF. This poor growth may be mediated by the CF transmembrane conductance regulator (CFTR) protein.
What This Study Adds:
Treatment with ivacaftor, a CFTR potentiator, in prepubescent children with CF and at least 1 copy of the G551D-CFTR mutation was associated with improvements in multiple growth variables, including linear growth.
The life expectancy of patients with cystic fibrosis (CF) continues to increase, leading to clinical challenges not previously encountered in this patient population. With a prevalence of ∼1 in 3500 live births, CF is the leading life-limiting genetic disorder in the white population.1–3 Although CF is a multisystem disorder characterized by recurrent lung and intestinal disease, different constellations of symptoms are associated with different CF mutations, and thus phenotype varies greatly from patient to patient.4 The disease is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene that lead to deficient or defective CFTR protein5 and reduced anion transport5 and that are passed on through an autosomal recessive inheritance.6 There are >2000 different mutations in the CFTR gene.7
The CFTR potentiator ivacaftor has been shown to improve anion transport and CFTR function in individuals with CF and a G551D-CFTR mutation.8,9 Ivacaftor functions as a potentiator of the defective gating (class III) of this mutation5,10 and has been shown to improve lung function in addition to weight and BMI.11,12 However, studies have yet to report the effects of ivacaftor on linear growth in children with CF.
Although the exact mechanisms of growth restriction are not known, the impact of CF on linear growth and body weight is well documented. Poor growth is both a manifestation of the disease and a contributor to poor lung function through its impact on chest size and lung growth. Because insulin-like growth factor I (IGF-I) signaling is mediated by CFTR, CFTR dysfunction may directly affect linear growth, adding to indirect effects due to poor nutrition.13 Optimal BMI during the first 6 years of life is associated with better lung function at ages 6–7,14,15 and the Cystic Fibrosis Foundation recommends that children and young adults aged 2 to 20 years maintain a BMI greater than or equal to the 50th percentile.14 Furthermore, stunting (defined as height-for-age below the fifth percentile based on National Center for Health Statistics criteria) was found to be an independent risk factor for mortality in CF.16 Konstan et al17 reported that deficits in linear growth were evident before the detection of significant lung disease in children with CF. At 3 years of age, the children studied were at the 33rd percentile for height relative to National Center for Health Statistics benchmarks. Poor growth at 3 years and relative weight loss over time also predicted lung health at age 6.17
Clinical strategies for improving growth (both weight gain and linear growth) for patients with CF have predominantly focused on maximizing nutrition (eg, increasing caloric intake and ensuring proper use of pancreatic enzymes).14 Increasing the anabolic drive to grow (through supplemental growth hormone [GH] therapy) has also been proposed as a potential intervention.18,19 Appetite stimulants are a mainstay of increased caloric consumption20 but should be used with caution, because agents such as megestrol acetate are also associated with increasing fat mass to the exclusion of lean body mass.21,22 GH increases growth and lean body mass21,23,24 but likely does not address the underlying reasons for poor growth. Until recently, the potential impact on growth outcomes of targeting the CFTR defect itself had not been tested. We hypothesized that targeting the biological defect in the CFTR protein may affect growth outcomes. Here we present growth outcomes data from an observational study of ivacaftor treatment (G551D Observational Study [GOAL]) and from the placebo-controlled, randomized Evaluation of Efficacy and Safety of VX-770 in Children Six to Eleven Years Old with CF (ENVISION) trial, both of which evaluated prepubescent children with 1 copy of the G551D-CFTR mutation.
Methods
Study Designs
GOAL was a longitudinal, observational cohort study in children (≥6 years) with CF and at least 1 G551D-CFTR mutation who were initiating ivacaftor treatment.25 Data were obtained at baseline and at 1, 3, and 6 months postinitiation. Data before study enrollment were obtained from the Cystic Fibrosis Foundation Patient Registry,26 which collects information on the health status of people with CF who receive care in an accredited care center in the United States and who agree to participate in the registry to calculate baseline growth velocity (GV).
ENVISION (NCT00909727) was a phase 3, randomized, placebo-controlled, double-blind trial evaluating the pharmacokinetics, efficacy, and safety of ivacaftor in children aged 6–11 years with CF who possessed at least 1 copy of the G551D-CFTR mutation. Primary results of pulmonary function improvements have previously been published.12 Other inclusion requirements included a forced expiratory volume in 1 second (FEV1) of 40% to 105% of that predicted on the basis of the patient’s age, sex, and height, as well as a body weight of ≥15 kg. After enrollment, patients were evaluated on day 15 and at weeks 8, 16, 24, 32, 40, and 48.
Participants
For both data sets, we evaluated prepubertal children (aged 6–11 years) to avoid changes in growth due to pubertal influences. Tanner staging as an indicator of pubertal status was not assessed in either study.
Study Assessments
In GOAL, height and weight were used to calculate z scores at baseline, 3 months, and 6 months. Height and weight GVs at baseline were calculated by using registry data collected in the previous year. Standard weight and height z scores were compared in ENVISION at baseline, 24 weeks, and 48 weeks. Height z score was calculated by using Centers for Disease Control and Prevention Clinical Growth Charts.27 Overall GVs for height (cm/year) and weight (kg/year) were assessed for the entire 48 weeks of the study, with GVs calculated by using the following formula: GV (units/year) = (value measured at time 2 – value measured at time 1)/(number of months between time 2 and time 1) × 12. Data on weight change from baseline at 48 weeks for ENVISION have previously been published.12
Statistical Analyses
Each comparison was made for the combined prepubertal group (boys and girls), as well as for boys and girls separately. Data from the GOAL study were analyzed by using analysis of variance, with the last observation carried forward for missing data. Unless otherwise indicated, results are expressed in means and SEMs. Missing data points were not imputed in the analysis of the ENVISION data. Paired t tests were used to analyze change from baseline within each treatment group. Analyses of covariance were used to estimate the treatment effect overall and within each sex. The covariates used in this model were percent predicted FEV1 (ppFEV1) at day 1 and weight or height z score at day 1, as applicable. This was a post hoc analysis of data collected from previously published studies and was not powered to detect significant treatment effects.
Results
Study designs and baseline characteristics for patients in GOAL and ENVISION are presented in Table 1. GOAL evaluated 35 prepubertal children with a mean enrollment age of 8.7 years (SD = 1.6 years). ENVISION evaluated 48 prepubertal children (25 in the placebo arm and 23 in the ivacaftor arm) with a mean enrollment age of 8.8 years (SD = 1.8) in the placebo arm and 8.5 years (SD = 1.8 years) in the ivacaftor arm.
TABLE 1.
Study Design and Baseline Characteristics for GOAL and ENVISION Studies
| Characteristic | GOAL | ENVISION | |
|---|---|---|---|
| Ivacaftor (n = 35) | Placebo (n = 25) | Ivacaftor (n = 23) | |
| Trial design | Observational | Randomized | |
| Control | Predrug data | Placebo arm | |
| Study visits analyzed | Baseline and 3 and 6 months postbaseline | Baseline and 24 and 48 weeks postbaseline | |
| Sex (male/female), n | 19/16 | 16/9 | 9/14 |
| Age, y | |||
| Mean (SD) | 8.7 (1.6) | 8.8 (1.8) | 8.5 (1.8) |
| Range | 6–11 | 6–11 | 6–11 |
| Height, mean (SD), cm | 131.1 (11.3) | 131.9 (12.0) | 133.2 (14.3) |
| Height z score, mean (SD) | −0.15 (1.3) | −0.34 (0.9) | 0.00 (1.0) |
| Weight, mean (SD), kg | 29.8 (7.2) | 29.6 (7.0) | 31.2 (10.3) |
| Weight z score, mean (SD) | 0.01 (1.2) | −0.16 (0.8) | 0.08 (1.0) |
| BMI, mean (SD), kg/m2 | 17.1 (2.4) | 16.8 (1.8) | 17.2 (2.7) |
| FEV1, mean (SD), % predicted | 106.4 (14.6) | 83.8 (20.8) | 87.3 (14.6) |
| Pseudomonas positive,a n (%) | 15 (42.9) | 12b (57.1) | 8c (40.0) |
| Sweat chloride, mean (SD), mmol/L | 107.3 (9.9) | 104.3 (8.7) | 104.2 (15.2) |
Within 12 months of starting the study.
Of 21 patients with available data.
Of 20 patients with available data.
GOAL
Height and Weight z Scores
At baseline, patients with CF were below average in height z score on the basis of the Centers for Disease Control and Prevention growth curves (Table 2). After ivacaftor use, a significant increase in height z score was observed at 6 months (P < .05). Boys and girls exhibited a similar improvement (Supplemental Table 1); however, changes in height z score by sex did not achieve significance, likely because of small sample sizes. At baseline, patients with CF had a weight z score that was similar to the national average (Table 2). Ivacaftor treatment was associated with a statistically significant increase in body weight z score at both 3 and 6 months (P < .05 and P < .0001, respectively). A significant improvement was observed in boys at 3 and 6 months (P < .05 and P < .001, respectively; Supplemental Table 3); the improvement seen in girls was not significant.
TABLE 2.
Weight and Height Outcomes From the GOAL Study
| Outcome | Mean (SEM) | P (Compared With Baseline) |
|---|---|---|
| Height z score | ||
| Baseline | −0.15 (0.21) | — |
| 3 months | −0.12 (0.21) | NS |
| 6 months | −0.05 (0.22) | <.05 |
| Weight z score | ||
| Baseline | 0.01 (0.20) | — |
| 3 months | 0.14 (0.19) | <.05 |
| 6 months | 0.27 (0.20) | <.0001 |
| Height GV, cm/y | ||
| Before baseline | 5.44 (0.31) | — |
| Baseline to 3 months | 6.27 (0.57) | NSa |
| 3 to 6 months | 7.54 (0.66) | <.01a |
| Weight GV, kg/y | ||
| Before baseline | 2.61 (0.43) | — |
| Baseline to 6 months | 7.15 (0.98) | <.0001a |
NS, not significant; —, not applicable.
Calculated GV was compared with mean GV before baseline.
Height and Weight GVs
A significant increase in height GV was observed between 3 and 6 months for the full population (P < .01; Table 2). Height GV improved significantly in girls from 3 to 6 months (P < .05) but not in boys (Supplemental Table 3). After 6 months of ivacaftor treatment, weight GV was improved compared with the results seen before enrollment (P < .0001; Table 2). This improvement in weight GV was observed in boys and girls (P < .001 and P < 0.05 respectively; Supplemental Table 3).
ENVISION
Height z Scores
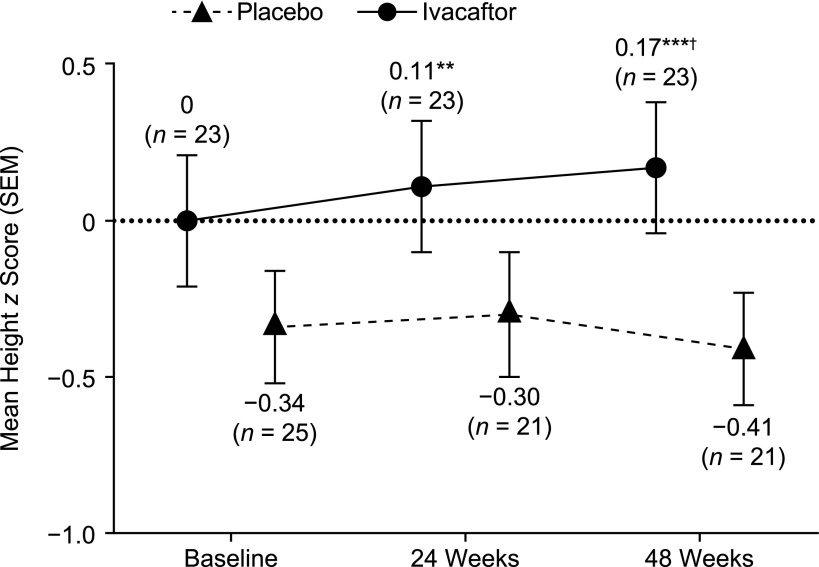
At baseline, height z score was average in the ivacaftor arm and below average in the placebo arm (Fig 1). In the ivacaftor arm, height z score increased significantly from baseline at 24 and 48 weeks (P < .01 and P < .001, respectively). A significant improvement was seen in boys at 24 and 48 weeks (P < .05 and P < .01, respectively) and in girls at 48 weeks (P < .01; Supplemental Fig 4). In the placebo arm, the change from baseline in height z score was slightly positive but nonsignificant at 24 weeks and was slightly negative but nonsignificant at 48 weeks (Fig 1). Boys and girls exhibited similar nonsignificant changes (Supplemental Fig 4). In the overall group, change from baseline in height z score was significantly higher in the ivacaftor arm than in the placebo arm at 48 weeks (P < .05).
FIGURE 1.
Height z scores over time in ENVISION. n indicates number of patients at each time point. **P < .01 versus baseline; ***P < .001 versus baseline; †P < .05 versus placebo.
Weight z Scores
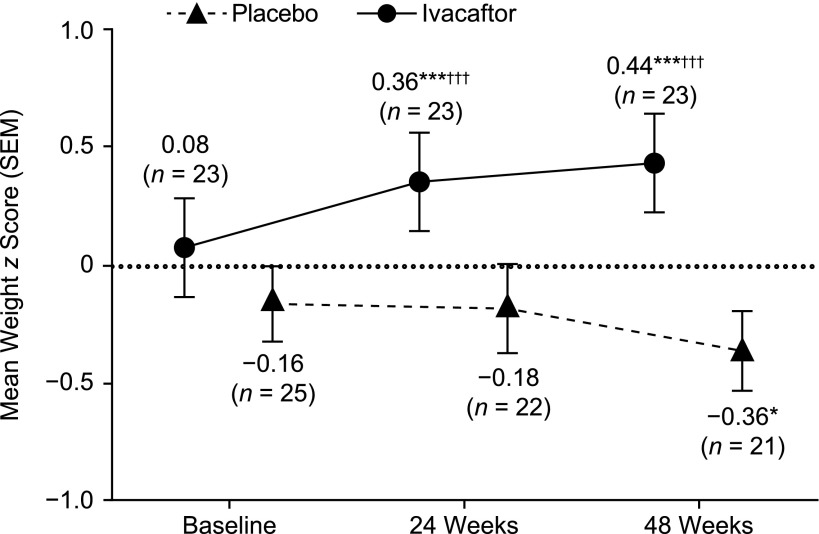
At baseline, weight z score was average in the ivacaftor arm and below average in the placebo arm (Fig 2). Ivacaftor treatment was associated with a statistically significant increase in weight z score at 24 and 48 weeks (P < .001 for both). Similar results were observed in girls (P < .001 for 24 weeks and P < .01 for 48 weeks) and boys (P < .05 for both time points) (Supplemental Fig 5). Treatment with placebo was associated with a statistically significant decrease in weight z score at 48 weeks compared with baseline (P < .05; Fig 2); boys and girls exhibited similar findings as the combined population, but differences in weight z score did not achieve significance in these subgroups (Supplemental Fig 5). The change from baseline in weight z score was significantly higher in the ivacaftor arm than in the placebo arm at 24 and 48 weeks (P < .001 for both). The change from baseline in weight z score was significantly higher in the ivacaftor arm than in the placebo arm in boys at 24 and 48 weeks (P < .05 and P < .01, respectively); the change from baseline in weight z score was also significantly higher in the ivacaftor arm than in the placebo arm in girls at 24 and 48 weeks (P < .01 for both).
FIGURE 2.
Weight z scores over time in ENVISION. n indicates number of patients at each time point. *P < .05 versus baseline; ***P < .001 versus baseline; †††P < .001 versus placebo.
Height and Weight GVs
Patients had a significantly greater height GV in the ivacaftor arm than in the placebo arm (P < .05; Fig 3). Both boys and girls exhibited nonsignificant improvements compared with the placebo arm (Supplemental Fig 6). Patients also had a significantly higher weight GV in the ivacaftor arm than in the placebo arm (P < .001; Fig 3). Boys and girls exhibited similar statistically significant improvements compared with placebo (P < .05 for both; Supplemental Fig 6).
FIGURE 3.
Height and weight GVs from baseline to 48 weeks in ENVISION. n indicates number of patients with measurements at baseline and 48 weeks. †P < .05 versus placebo; †††P < .001 versus placebo. LS, least squares.
Discussion
For many years, the treatment of growth restriction in CF has focused on increasing oral intake of a high-calorie, high-protein diet, partially through appetite stimulation, and on optimizing pancreatic enzyme replacement therapy. Multiple clinical trials have also reported improvements in growth through the use of anabolic stimulants, primarily GH.18,23 However, neither of these approaches targets the basic molecular defect in CF. Our study presents the first evidence that correction of the abnormally functioning CFTR channel is associated with improved linear growth and supports the hypothesis that defective CFTR function directly contributes to impaired linear growth in CF.
Caloric intake is needed for adequate growth, and meeting increased energy and nutrient needs can undeniably help growth in CF. Individuals with CF have caloric requirements 1.5 to 2 times those of average individuals.14 Thus, the intake necessary to sustain good linear growth may not be met. Evidence to support the impact of nutritional intake can be ascertained from the use of newborn screens. Children and young adults with CF identified at birth show improved linear growth and final adult height, findings that are attributed to earlier interventions with nutritional intake and improved growth in the early years of life.28,29
Lung disease severity has been shown to correlate with prepubertal growth.30 We did not assess the correlation between linear growth and improved lung function here due to the small sample size. However, patients enrolled in GOAL showed a statistically significant improvement in ppFEV1 from baseline to 6 months (P < .001),25 and ivacaftor-treated patients in ENVISION showed a statistically significant improvement in ppFEV1 compared with placebo-treated patients at 48 weeks (P < .001).12
In animal models of CF, the absence of clinical confounders such as pancreatic insufficiency and lung disease does not translate into normalized growth. The mouse CF model does not develop human-analog CF-type diseases of the lung or pancreas31; however, affected animals show reduced growth (length and weight) relative to wild-type animals.32 Although it has been argued that these differences reflect inadequate nutritional intake in the mouse model, newborn CF piglets also have shorter humeral lengths than their wild-type littermates, before the contribution of altered nutritional intake.13 Because all piglets would have had the same nutritional intake in utero, this finding implies that CFTR deficiency has an intrinsic and early impact on linear growth.
How might CFTR modulation improve growth in CF through a CFTR-dependent pathway? One proposed mechanism for growth restriction in CF is decreased levels of IGF-I, an effector agent of GH.13 Increasing IGF-I concentrations with exogenous GH likely contributes to the improved growth seen in clinical trials, because it has been established that IGF-I concentration is an indicator of nutritional status.33 IGF-I has both endocrine and autocrine/paracrine functions and effects on growth. Measurable levels of IGF-I in the serum are produced primarily in the liver, and production is reduced by nutritional as well as insulin deficiency, 2 problems often seen in CF. It is IGF-I produced at the target tissues that has autocrine/paracrine effects, and multiple studies in CF showed reduced concentrations of IGF-I in both humans and animal models.13,32,34 Interestingly, decreased IGF-I concentrations can be detected despite controlling for nutritional differences or malnutrition. This finding is evident, for example, in newborn CF pigs, whose nutritional intake is dependent on maternal nutrient transfer in utero. Newborn CF pigs have reduced IGF-I concentrations,13 implying an intrinsic effect of CFTR deficiency on IGF-I production and potentially a direct influence on bone growth. Together, these results suggest that correction of the underlying defect in CFTR could increase IGF-I levels, resulting in improved nutritional status.
Another mechanism potentially contributing to poor growth in the setting of CFTR impairment is the Wnt signal transduction cascade. Linear growth of long bones results from endochondral bone formation, in which cartilaginous growth is replaced by ossified bone at the growth plate. This process is governed by a complex set of interactions among GH, IGF-I,35 thyroid hormone,35 sex steroids,36 and glucocorticoids, as well as promotion by Wnt signaling.37 Our laboratory recently reported decreased canonical Wnt signaling in CFTR−/− murine osteoblasts,38 suggesting another intrinsic mechanism for poor growth and osteopenia in CF.
Data presented here from both observational and placebo-controlled studies provide evidence that improvements in linear growth and GV are seen after therapeutic intervention with ivacaftor in children with the G551D-CFTR mutation. However, these studies were not specifically designed or powered to address the association between ivacaftor and linear growth/GV. We also recognize that these improvements are seen in conjunction with weight gain, as well as improved pulmonary function, which could confound the influence of CFTR on linear growth; nevertheless, the consistency of the data and the strength of preclinical data indicating a role for CFTR suggest that a CFTR-mediated pathway likely has at least some impact. Given the complexity of growth, it may be difficult to prove this definitively in the clinic.
One possible weakness of this analysis is that we did not include an assessment of puberty by Tanner staging or another method as a criterion for inclusion, but used age-based inclusion criteria instead. The literature on pubertal development/progression and pubertal peak height velocity suggests a significant delay in children with CF.39,40 Similar delays are reported in CF mice.41 By using the upper limit of age 11, we believe we selected for prepubertal children with CF with comparable mean ages. However, it is possible that some of the older girls included may have entered puberty during the study. The ratios of boys to girls in the ivacaftor and placebo arms of ENVISION were skewed, and although one would expect the treatment effects to be similar in prepubertal boys and girls, this discrepancy could have affected the results.
Another weakness is the inconsistency of height/weight data before enrollment in the clinical trials. To control for this weakness, we used the placebo group in the ENVISION study and used pre-enrollment data at least 3 months before baseline for the GOAL study. The GOAL study is limited because it was an observational study without a placebo arm; therefore, we can only compare results before and after treatment. We examined the placebo-controlled ENVISION study to account for this limitation. Because of the small sample size in ENVISION, there were discrepancies in height and weight z scores between the ivacaftor and placebo groups; however, these differences were not statistically significant (data not shown). Height and weight z scores at baseline were used as covariates in the analysis of corresponding GV; therefore, differences in growth between placebo and ivacaftor arms were not due to these differences at baseline.
Conclusions
In this post hoc analysis of results from the GOAL and ENVISION studies, treatment with ivacaftor significantly improved multiple growth variables (including height and weight z scores) compared with placebo in prepubescent children with CF and the G551D-CFTR mutation. These results suggest that ivacaftor may help to address short stature and altered GV in children with CF, and they support the existence of an intrinsic defect in the growth of children with CF that may be ameliorated by CFTR modulation, which raises further questions for research study. Improving growth with CFTR potentiator therapy, either directly or indirectly, should be a goal of all CF health care providers, which reinforces the need for early initiation of CFTR restoration therapy when possible.
Acknowledgments
The GOAL study was conducted with the participation of Cystic Fibrosis Foundation Therapeutics Development Network study site principal investigators and coordinators. We thank the Cystic Fibrosis Foundation for the use of Cystic Fibrosis Foundation Patient Registry data to conduct this study. In addition, we thank the patients, care providers, and clinic coordinators at CF centers throughout the United States for their contributions to the Cystic Fibrosis Foundation Patient Registry. We acknowledge assistance from Dr Bruce Marshall and Alex Elbert and support for study planning and execution from Dr Preston Campbell. Statistical programming and data review support were provided by Bonnie Bielefeld, MS, an employee of BioBridges. Editorial coordination and support were provided by Dhrupad Patel, PharmD, and Stephen Parker, ELS. Dr Patel and Mr Parker are employees of Vertex Pharmaceuticals Incorporated, and may own stock or stock options in that company. Medical writing and editorial support were provided by Stephanie Vadasz, PhD, and Joshua Safran. Dr Vadasz and Mr Safran are employees of Ashfield Healthcare Communications, which received funding from Vertex Pharmaceuticals Incorporated.
Glossary
- CF
cystic fibrosis
- CFTR
CF transmembrane conductance regulator
- ENVISION
Evaluation of Efficacy and Safety of VX-770 in Children Six to Eleven Years Old with CF
- GH
growth hormone
- GOAL
G551D Observational Study
- GV
growth velocity
- IGF-I
insulin-like growth factor I
- ppFEV1
percent predicted forced expiratory volume in 1 second
Footnotes
Dr Stalvey conceptualized and designed the study, carried out the initial analyses, and drafted the initial manuscript; Ms Pace, Dr Niknian, Ms Tarn, Ms Davis, Dr Heltshe, and Dr Rowe conceptualized and designed the study, carried out the initial analyses, and reviewed and revised the manuscript; Dr Higgins conceptualized and designed the study and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifiers: NCT00909727, NCT01521338).
FINANCIAL DISCLOSURE: Dr Niknian and Dr Higgins are employees of Vertex Pharmaceuticals Incorporated, and may own stocks or stock options in that company; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by Vertex Pharmaceuticals Incorporated, Cystic Fibrosis Foundation Therapeutics (GOAL11K1), and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (P30 DK089507/DK/NIDDK NIH HHS). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Stalvey serves on the Vertex Disease Education Advisory Board. Dr Niknian and Dr Higgins are employees of Vertex Pharmaceuticals Incorporated, and may own stocks or stock options in that company. Dr Rowe has served as an investigator for Bayer HealthCare, Novartis, PTC Therapeutics, and Vertex Pharmaceuticals Incorporated, and has received research grants from Bayer HealthCare, Galápagos, and Novartis Research Institute. The other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Salvatore D, Buzzetti R, Baldo E, et al. An overview of international literature from cystic fibrosis registries. Part 3. Disease incidence, genotype/phenotype correlation, microbiology, pregnancy, clinical complications, lung transplantation, and miscellanea. J Cyst Fibros. 2011;10(2):71–85 [DOI] [PubMed] [Google Scholar]
- 2.Hamosh A, FitzSimmons SC, Macek M Jr, Knowles MR, Rosenstein BJ, Cutting GR. Comparison of the clinical manifestations of cystic fibrosis in black and white patients. J Pediatr. 1998;132(2):255–259 [DOI] [PubMed] [Google Scholar]
- 3.Southern KW, Munck A, Pollitt R, et al. ; ECFS CF Neonatal Screening Working Group . A survey of newborn screening for cystic fibrosis in Europe. J Cyst Fibros. 2007;6(1):57–65 [DOI] [PubMed] [Google Scholar]
- 4.Bonadia LC, de Lima Marson FA, Ribeiro JD, et al. CFTR genotype and clinical outcomes of adult patients carried as cystic fibrosis disease. Gene. 2014;540(2):183–190 [DOI] [PubMed] [Google Scholar]
- 5.Pettit RS. Cystic fibrosis transmembrane conductance regulator-modifying medications: the future of cystic fibrosis treatment. Ann Pharmacother. 2012;46(7–8):1065–1075 [DOI] [PubMed] [Google Scholar]
- 6.Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066–1073 [DOI] [PubMed] [Google Scholar]
- 7.Cystic Fibrosis Centre at the Hospital for Sick Children in Toronto Cystic Fibrosis Mutation Database. Published 2010. Updated April 25, 2011. Available at: www.genet.sickkids.on.ca/app. Accessed November 22, 2015
- 8.Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363(21):1991–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsey BW, Davies J, McElvaney NG, et al. ; VX08-770-102 Study Group . A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18):1663–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA. 2009;106(44):18825–18830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKone EF, Borowitz D, Drevinek P, et al. ; VX08-770-105 (PERSIST) Study Group . Long-term safety and efficacy of ivacaftor in patients with cystic fibrosis who have the Gly551Asp-CFTR mutation: a phase 3, open-label extension study (PERSIST). Lancet Respir Med. 2014;2(11):902–910 [DOI] [PubMed] [Google Scholar]
- 12.Davies JC, Wainwright CE, Canny GJ, et al. ; VX08-770-103 (ENVISION) Study Group . Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med. 2013;187(11):1219–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogan MP, Reznikov LR, Pezzulo AA, et al. Pigs and humans with cystic fibrosis have reduced insulin-like growth factor 1 (IGF1) levels at birth. Proc Natl Acad Sci USA. 2010;107(47):20571–20575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H; Clinical Practice Guidelines on Growth and Nutrition Subcommittee, Ad Hoc Working Group . Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108(5):832–839 [DOI] [PubMed] [Google Scholar]
- 15.Sanders DB, Fink A, Mayer-Hamblett N, et al. Early life growth trajectories in cystic fibrosis are associated with pulmonary function at age 6 years. J Pediatr. 2015;167(5):1081–1088, e1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beker LT, Russek-Cohen E, Fink RJ. Stature as a prognostic factor in cystic fibrosis survival. J Am Diet Assoc. 2001;101(4):438–442 [DOI] [PubMed] [Google Scholar]
- 17.Konstan MW, Butler SM, Wohl ME, et al. ; Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis . Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr. 2003;142(6):624–630 [DOI] [PubMed] [Google Scholar]
- 18.Hardin DS. GH improves growth and clinical status in children with cystic fibrosis—a review of published studies. Eur J Endocrinol. 2004;151(suppl 1):S81–S85 [DOI] [PubMed] [Google Scholar]
- 19.Thaker V, Haagensen AL, Carter B, Fedorowicz Z, Houston BW. Recombinant growth hormone therapy for cystic fibrosis in children and young adults. Cochrane Database Syst Rev. 2013;6:CD008901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chinuck R, Dewar J, Baldwin DR, Hendron E. Appetite stimulants for people with cystic fibrosis. Cochrane Database Syst Rev. 2014;7:CD008190. [DOI] [PubMed] [Google Scholar]
- 21.Homnick DN, Homnick BD, Reeves AJ, Marks JH, Pimentel RS, Bonnema SK. Cyproheptadine is an effective appetite stimulant in cystic fibrosis. Pediatr Pulmonol. 2004;38(2):129–134 [DOI] [PubMed] [Google Scholar]
- 22.Oster MH, Enders SR, Samuels SJ, et al. Megestrol acetate in patients with AIDS and cachexia. Ann Intern Med. 1994;121(6):400–408 [DOI] [PubMed] [Google Scholar]
- 23.Schnabel D, Grasemann C, Staab D, Wollmann H, Ratjen F; German Cystic Fibrosis Growth Hormone Study Group . A multicenter, randomized, double-blind, placebo-controlled trial to evaluate the metabolic and respiratory effects of growth hormone in children with cystic fibrosis. Pediatrics. 2007;119(6). Available at: www.pediatrics.org/cgi/content/full/119/6/e1230 [DOI] [PubMed] [Google Scholar]
- 24.Homnick DN, Marks JH, Hare KL, Bonnema SK. Long-term trial of cyproheptadine as an appetite stimulant in cystic fibrosis. Pediatr Pulmonol. 2005;40(3):251–256 [DOI] [PubMed] [Google Scholar]
- 25.Rowe SM, Heltshe SL, Gonska T, et al. ; GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network . Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190(2):175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knapp EA, Fink AK, Goss CH, et al. The Cystic Fibrosis Foundation Patient Registry: design and methods of a national observational disease registry. Ann Am Thorac Soc. 2016;13(7):1173–1179 [DOI] [PubMed] [Google Scholar]
- 27.Center for Disease Control Growth and Prevention Clinical growth charts. Updated August 4, 2009. Available at: www.cdc.gov/growthcharts/clinical_charts.htm. Accessed December 6, 2015
- 28.Dijk FN, Fitzgerald DA. The impact of newborn screening and earlier intervention on the clinical course of cystic fibrosis. Paediatr Respir Rev. 2012;13(4):220–225 [DOI] [PubMed] [Google Scholar]
- 29.Farrell PM, Lai HJ, Li Z, et al. Evidence on improved outcomes with early diagnosis of cystic fibrosis through neonatal screening: enough is enough! J Pediatr. 2005;147(3 suppl):S30–S36 [DOI] [PubMed] [Google Scholar]
- 30.Assael BM, Casazza G, Iansa P, Volpi S, Milani S. Growth and long-term lung function in cystic fibrosis: a longitudinal study of patients diagnosed by neonatal screening. Pediatr Pulmonol. 2009;44(3):209–215 [DOI] [PubMed] [Google Scholar]
- 31.Wilke M, Buijs-Offerman RM, Aarbiou J, et al. Mouse models of cystic fibrosis: phenotypic analysis and research applications. J Cyst Fibros. 2011;10(suppl 2):S152–S171 [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg LA, Schluchter MD, Parlow AF, Drumm ML. Mouse as a model of growth retardation in cystic fibrosis. Pediatr Res. 2006;59(2):191–195 [DOI] [PubMed] [Google Scholar]
- 33.Livingstone C. The insulin-like growth factor system and nutritional assessment. Scientifica (Cairo). 2012;2012:768731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor AM, Thomson A, Bruce-Morgan C, et al. The relationship between insulin, IGF-I and weight gain in cystic fibrosis. Clin Endocrinol (Oxf). 1999;51(5):659–665 [DOI] [PubMed] [Google Scholar]
- 35.Egawa S, Miura S, Yokoyama H, Endo T, Tamura K. Growth and differentiation of a long bone in limb development, repair and regeneration. Dev Growth Differ. 2014;56(5):410–424 [DOI] [PubMed] [Google Scholar]
- 36.Karsenty G. The mutual dependence between bone and gonads. J Endocrinol. 2012;213(2):107–114 [DOI] [PubMed] [Google Scholar]
- 37.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116(5):1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stalvey MS, Clines KL, Havasi V, et al. Osteoblast CFTR inactivation reduces differentiation and osteoprotegerin expression in a mouse model of cystic fibrosis-related bone disease. PLoS One. 2013;8(11):e80098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Umławska W, Sands D, Zielińska A. Age of menarche in girls with cystic fibrosis. Folia Histochem Cytobiol. 2010;48(2):185–190 [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z, Lindstrom MJ, Lai HJ. Pubertal height velocity and associations with prepubertal and adult heights in cystic fibrosis. J Pediatr. 2013;163(2):376–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin R, Hodges CA, Drumm ML, Palmert MR. The cystic fibrosis transmembrane conductance regulator (Cftr) modulates the timing of puberty in mice. J Med Genet. 2006;43(6):e29. [DOI] [PMC free article] [PubMed] [Google Scholar]