Abstract
BACKGROUND:
Lower socioeconomic status (SES) and environmental tobacco smoke (ETS) exposure are both associated with poorer disease outcomes in cystic fibrosis (CF), and children with low SES are disproportionately exposed to ETS. We analyzed a large cohort of young children with CF to distinguish the impact of SES and ETS on clinical outcomes.
METHODS:
The Early Pseudomonas Infection Control Observational study enrolled Pseudomonas-negative young children with CF <13 years of age. An enrollment survey assessed SES and ETS exposures. Forced expiratory volume in 1 second (FEV1), crackles and wheezes, and weight-for-age percentile were assessed at each clinical encounter over at least 4 years. Repeated measures analyses estimated the association of SES and ETS exposures with longitudinal clinical outcomes, adjusting for confounders.
RESULTS:
Of 1797 participants, 1375 were eligible for analysis. Maternal education was high school or less in 28.1%, 26.8% had household income <$40 000, and 43.8% had Medicaid or no insurance. Maternal smoking after birth was present in 24.8%, more prevalent in household with low SES. In separate models, lower SES and ETS exposure were significantly associated with lower FEV1% predicted, presence of crackles or wheezes, and lower weight percentile. In combined models, effect estimates for SES changed minimally after adjustment for ETS exposures, whereas estimates for ETS exposures were attenuated after adjusting for SES.
CONCLUSIONS:
ETS exposure was disproportionately high in low SES families in this cohort of children with CF. Lower SES and ETS exposure had independent adverse effects on pulmonary and nutritional outcomes. Estimated effect of SES on FEV1 decreased minimally after ETS adjustment, suggesting health disparity risks independent of ETS exposure.
What’s Known on This Subject:
Among patients with cystic fibrosis, low socioeconomic status (SES) and environmental tobacco smoke (ETS) exposures have both been associated with poorer outcomes. However, limited studies have assessed the role of ETS exposure in health outcomes associated with low SES.
What This Study Adds:
In a large US cohort of children with cystic fibrosis, we confirm disproportionate ETS exposure in low SES families. The estimated effect of SES on pulmonary and nutritional outcomes decreased minimally after adjustment for ETS, suggesting disparity risks independent of smoke exposure.
Disparities in health outcomes due to socioeconomic status (SES) are well recognized in many chronic diseases of childhood, including asthma and diabetes. Among patients with cystic fibrosis (CF), refinements in the treatment of CF have resulted in dramatic improvements in nutritional status, lung function, and longevity in the overall population. Nonetheless, there is increased awareness of variation in the severity and progression of CF lung disease among individuals afflicted with this disease.1 Although CF transmembrane conductance regulator (CFTR) mutations and genetic modifiers play an important role in the heterogeneity of CF outcomes, the role of environmental exposures, such as low SES and environmental tobacco smoke (ETS) exposure, are also of great importance.2–6 The course of disease in any individual with CF depends on how these influences interact to promote and mitigate the importance of each other.7
Studies from both the United Kingdom and the United States have defined a strong association between SES and CF disease outcomes.2,3,8,9 Low SES is associated with a twofold to threefold higher mortality rate and significantly worse lung function and nutritional status.3 Regional median family income shows an exposure-response relationship with worsened mortality, lung function, and nutrition, demonstrating that the association is incremental rather than a dichotomous one that affects only the impoverished.2
The reason for the SES association with CF outcomes is multifactorial. One link appears to be the correlation between SES and ETS exposure. In 2013, monthly or more frequent tobacco smoke exposure was reported in 23.5% of individuals with CF in the United States.1 There is a clear relationship between smoking and SES in the general population, and this extends to families of children with CF.10–12 A dose-dependent association of ETS exposure with poor lung function and growth in children with CF was initially reported in 1990 among attendees of a summer camp and has been confirmed in most (although not all) subsequent reports.13–16 A recent publication exploring this relationship confirmed the continued high prevalence of ETS exposure in patients with CF, especially those with low SES, and the association with decreased lung function.17 That analysis found that the relationship between SES and CF outcomes was significantly attenuated by adjustment for ETS exposure, suggesting that the observed association between low SES and lower lung function was in large part due to the greater exposure to ETS among children with low SES. These findings have important implications in understanding health disparities, so we sought to evaluate them further by using a highly characterized, large, contemporary US cohort of patients with CF assembled specifically to study longitudinal outcomes in young children with CF. Specifically, our objective was to evaluate the association of SES and ETS both independently and jointly on longitudinal measures of lung function, presence of crackles or wheezes, and weight-for-age percentile in the Early Pseudomonas Infection Control Observational (EPIC OBS) cohort. We hypothesized that we would observe adverse effects of both SES and ETS exposure on clinical status, but that the effect of SES would be significantly attenuated by ETS exposure.
Methods
Study Participants
The design of the EPIC OBS Study has been described previously.18,19 Children with an established diagnosis of CF who were 0 to 12 years of age and had no previous lifetime isolation of Pseudomonas aeruginosa from respiratory cultures or who were P aeruginosa negative for ≥2 years if previously P aeruginosa positive were enrolled at 59 accredited US CF care centers between 2004 and 2006.20 EPIC OBS participants were included in the current analysis if they were enrolled by the end of 2006, had a baseline family survey (see Data Collection section) completed within 1 month of enrollment, and had follow-up data for a minimum of 4 years. Written informed consent was obtained from the family of each participant, and the study was approved by the institutional review board at each participating site.
Data Collection
Study data were collected at enrollment, annually, and at each clinical encounter via study-specific forms and the Cystic Fibrosis Foundation National Patient Registry, as previously reported.18 This analysis used self-reported (by parent or guardian) data from enrollment on ETS exposures, maternal education, annual household income and insurance status, and encounter-based data on forced expiratory volume in 1 second (FEV1) (ages 6 and older) and other demographic and clinical characteristics. FEV1 was expressed as a percentage of the predicted value based on the reference equations of Wang et al21 and Hankinson et al.22 The data cutoff for this analysis was December 31, 2010.
Statistical Analysis
SES predictors included mother’s education (high school or less, college or more), annual household income (<$40 000, $40 000–$80 000, ≥$80 000), and insurance status (Medicaid, other insurance, no insurance). ETS predictors included history of mother smoking during pregnancy, mother smoking any time after birth of child, household member smoking, and child ever around people who smoked in the past 3 months. The primary outcome was encounter-based measures of FEV1% predicted during follow-up through 2010, which was year 4 to 6 of the study depending on the year of enrollment.
Repeated measures analyses based on linear mixed models were used to evaluate the effect of potential ETS and SES predictors reported at baseline on longitudinal measures of FEV1% predicted. A time variable, age in years at each visit, was included in all models to capture the change in FEV1% predicted over time. A first-order autoregressive autocorrelation working correlation structure was adopted to account for the within-subject correlation among repeated measurements of FEV1. All models were adjusted for demographic and disease-specific covariates (age, sex, race, ethnicity, CFTR genotype risk group, and diagnosis after pre- or neonatal screening).23,24
First, SES and ETS exposures were evaluated separately as predictors of lung function. SES exposures were modeled together to assess their collective effects. Due to concerns for collinearity of ETS exposure variables, the a priori decision was made to evaluate each ETS exposure in separate models. Then, SES and ETS exposures were evaluated in combined models. The estimates of the effect of each SES and ETS exposure from the separate and combined models were compared. Similar repeated measures analyses were conducted to assess the association of SES and ETS exposures with occurrence of crackles or wheezes on chest examination at clinical encounters during follow-up and with longitudinal measures of weight-for-age percentile. For the outcomes of crackles and wheezes, we fitted multivariable longitudinal logistic regression models based on a generalized estimating equations approach with a first-order autoregressive autocorrelation working correlation structure. For the outcome of weight-for-age percentile, we used linear mixed models that included age and a quadratic term of age to accommodate for potentially nonlinear changes of weight percentile over time. Adjusted coefficient or odds ratio (OR) with corresponding 95% confidence interval (CI) estimates were reported for each model, and a P < .05 was considered statistically significant. All analyses were performed by using SAS 9.3 (SAS Institute, Cary, NC).
Results
Figure 1 describes selection of the study cohort. A total of 1797 participants were enrolled in EPIC OBS; 109 were excluded from this analysis cohort because their CF diagnosis was subsequently reversed, they were P aeruginosa positive at enrollment, or they were enrolled after 2006; 85 participants were excluded because the baseline family survey was not completed; 228 were excluded because they were not followed for at least 4 years. The resulting cohort for this analysis comprised 1375 participants.
FIGURE 1.
Selection of the study cohort.
Characteristics of the study cohort at enrollment are summarized in Table 1.
TABLE 1.
Characteristics of the Study Cohort at Enrollment (n = 1375)
| n | % | |
|---|---|---|
| Sex | ||
| Girls | 687 | 50.0 |
| Boys | 688 | 50.0 |
| Age at enrollment,a y | ||
| 0–<3 | 364 | 26.5 |
| 3–<6 | 381 | 27.7 |
| 6–<9 | 341 | 24.8 |
| ≥9 | 289 | 21.0 |
| Race | ||
| Not White | 61 | 4.4 |
| White | 1314 | 95.6 |
| Ethnicity | ||
| Not Hispanic | 1280 | 93.1 |
| Hispanic | 46 | 3.3 |
| Missing | 49 | 3.6 |
| Genotype risk groupb | ||
| High risk | 1074 | 78.1 |
| Low risk | 99 | 7.2 |
| Unclassified | 150 | 10.9 |
| Missing | 52 | 3.8 |
| Diagnosis by screeningc | ||
| No | 1046 | 76.1 |
| Yes | 297 | 21.6 |
| Missing | 32 | 2.3 |
| Mother’s education | ||
| High school or less | 387 | 28.1 |
| Some college or more | 951 | 69.2 |
| Missing | 37 | 2.7 |
| Annual household income | ||
| <$40 000 | 368 | 26.8 |
| $40 000-$79 000 | 437 | 31.8 |
| ≥$80 000 | 363 | 26.4 |
| Missing | 207 | 15.1 |
| Insurance statusd | ||
| Medicaid or no insurance | 602 | 43.8 |
| Private or other insurance | 761 | 55.3 |
| Missing | 12 | 0.9 |
| Mother smoked during pregnancy | ||
| No | 1083 | 78.8 |
| Yes | 189 | 13.7 |
| Unknown | 103 | 7.5 |
| Mother smoked any time after birth of child | ||
| No | 974 | 70.8 |
| Yes | 341 | 24.8 |
| Missing | 60 | 4.4 |
| Household member smokes cigarettes | ||
| No | 966 | 70.3 |
| Yes | 400 | 29.1 |
| Missing | 9 | 0.6 |
| Child around people smoking in past 3 mo | ||
| Ever | 720 | 52.4 |
| Never | 631 | 45.9 |
| Missing | 24 | 1.7 |
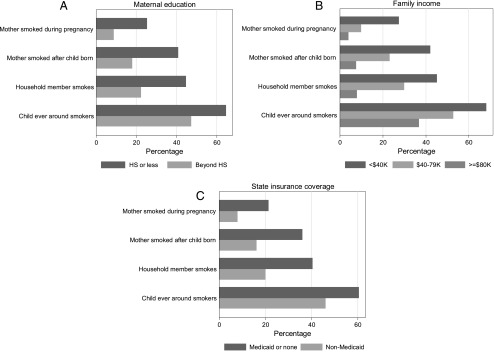
Maternal education was reported to be high school or less in 28.1%, annual household income <$40 000 in 26.8%, and Medicaid or no insurance in 43.8% of participants. Figure 2 shows that children from low SES households characterized by low maternal education, low household income, and/or Medicaid or no insurance had higher percentages with each of the ETS exposures than children from higher SES households.
FIGURE 2.
Bar charts for each SES variable: A, maternal education; B, family income; C, state insurance coverage, showing the percentage of participants exposed to ETS (as measured by 4 different questions). HS, high school.
Impact of SES and ETS on Lung Function
During year 4 of the study, the best value of FEV1% predicted averaged 101.8 (SD 16.0) among 1065 children ages 6 and older. In models evaluating ETS and SES exposures separately, all SES exposures had significant negative associations with mean absolute FEV1% predicted during the study period (Table 2, SES model). Similarly, ETS exposures had significant negative associations with mean absolute FEV1% predicted (Table 2, ETS models 1–4); the most negative association was observed for the mother smoking after the child’s birth, which was associated with mean FEV1% predicted 6.0% lower (95% CI −7.4 to −4.5; P < .0001) compared with children whose mothers did not report smoking after birth.
TABLE 2.
Separate Models to Assess Impact of SES and ETS Predictors on FEV1% Predicted
| Exposure Variable | n/Observationa | Coefficientb | 95% CI | P |
|---|---|---|---|---|
| SES modelc | 1050/18 416 | |||
| High school education or less | −1.90 | −3.60 to −0.19 | .03 | |
| Household income <$40 000 | −5.82 | −7.94 to −3.71 | <.0001 | |
| Household income $40 000–79 000 | −3.14 | −4.83 to −1.44 | .0003 | |
| Medicaid or no insurance | −2.49 | −4.09 to −0.89 | .0024 | |
| Age, y | −1.11 | −1.46 to −0.76 | <.0001 | |
| ETS model 1 | 1160/20 056 | |||
| Mother smoked during pregnancy | −4.59 | −6.43 to −2.74 | <.0001 | |
| Age, y | −1.10 | −1.44 to −0.77 | <.0001 | |
| ETS model 2 | 1197/20 743 | |||
| Mother smoked after child’s birth | −5.98 | −7.44 to −4.52 | <.0001 | |
| Age, y | −1.11 | −1.44 to −0.78 | <.0001 | |
| ETS model 3 | 1240/21 607 | |||
| Household member smokes | −2.60 | −4.03 to −1.16 | .0004 | |
| Age, y | −1.11 | −1.43 to −0.78 | <.0001 | |
| ETS model 4 | 1224/21 321 | |||
| Child ever around smokers in past 3 mo | −3.21 | −4.48 to −1.93 | <.0001 | |
| Age, y | −1.11 | −1.43 to −0.78 | <.0001 |
The numbers of participants and longitudinal observations for each model.
The interpretation of the coefficient estimate for each SES or ETS exposure variable is the difference in mean absolute FEV1% predicted between those with the exposure and those in the reference group. Each model included age in years at each visit to capture the change in FEV1% predicted over time. Each model also included covariate adjustment for prespecified demographic and disease characteristics (enrollment age, sex, race, ethnicity, genotype risk group, and diagnosis by screening).
Reference groups for the SES variables include any education beyond high school, income ≥$80 000, and non-Medicaid insurance.
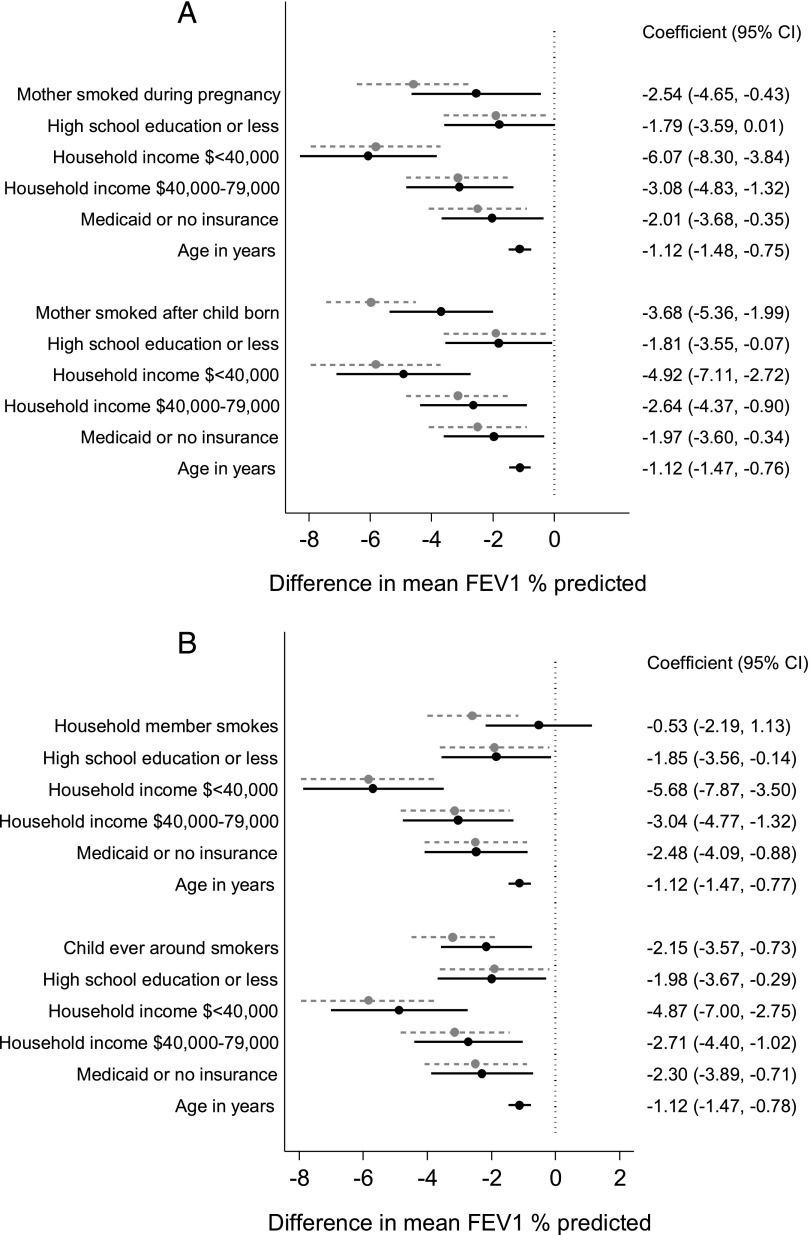
Associations between SES predictors and FEV1% predicted were minimally changed when SES predictor variables and ETS exposures were evaluated simultaneously in multivariable models. In contrast, the associations between ETS exposures and FEV1 % predicted were attenuated to a greater degree. Figure 3 shows the estimates of mean difference in absolute FEV1% predicted between those exposed and unexposed from the final multivariable models, and for comparison, estimates from the models evaluating SES and ETS exposures separately (from Table 2). The separation between the estimates from the combined models (dark balls and solid lines) and the models evaluating ETS and SES separately (gray balls and dashed lines) indicate the degree to which the effect of the exposure was attenuated in the combined model. All measures of SES and 3 of the 4 measures of ETS exposures (Fig 3B) remained significant independent predictors of lung function in the multivariable models.
FIGURE 3.
Estimates and 95% CIs (black dots, solid lines) for the difference in mean FEV1 % predicted from multivariable models, including both SES and ETS exposures, and prespecified clinical characteristics. For comparison, gray dots, dashed lines show estimates and 95% CIs from models evaluating SES and ETS exposures separately (Table 2).
Impact of SES and ETS on Chest Examination Findings
Crackles or wheezes on chest auscultation were reported for 22% to 24% of the participants during each of years 1 to 4 on study. We found an increased risk of crackles or wheezes among children from households with lower income, relative to households with the highest level of income (OR 1.4; 95% CI 1.0 to 2.0; P = .03 for income <$40 000; and OR 1.3; 95% CI 1.0 to 1.8; P = .05 for income $40 000–$79 000). There was also an increased risk of crackles or wheezes among children whose mothers reported smoking after the child’s birth (OR 1.4; 95% CI 1.1 to 1.8; P = .003) or if the child was ever around smokers (OR 1.3; 95% CI 1.0 to 1.6; P = .02). None of these associations remained significant in the multivariate models that simultaneously evaluated SES and ETS.
Impact of SES and ETS on Weight-for-Age Percentile
During year 4 of the study, mean weight percentile was 50.3 (SD 27.3) among 1371 children. In separate models evaluating the effect of SES and ETS exposures, mother’s education level of high school or less was associated with a mean 4.5% lower weight-for-age percentile (95% CI −7.9 to −1.2; P = .008) compared with college or more, and household income <$40 000 was associated with a mean 5.7% lower weight percentile (95% CI −9.8 to −1.6; P = .007) compared with the highest level of annual household income (Supplemental Table 3). Maternal smoking during pregnancy (−4.3%; 95% CI −7.9 to −0.65; P = .02) and after birth (−4.0%; 95% CI −6.9 to −1.1; P = .007) were also associated with lower mean weight-for-age percentiles (Supplemental Table 3).
Similar to findings for lung function, the associations between SES predictors and weight-for-age percentile were minimally changed when SES predictor variables and ETS exposures were evaluated simultaneously in multivariable models (Supplemental Table 4). ETS exposures did not remain significant predictors of weight percentile in the combined models.
Discussion
This study examined a large US multicenter longitudinal cohort of young children with CF, by using robust measures of SES and ETS exposure, and confirmed previous reports of the disproportionate exposure to ETS among children in families with low SES as well as the independent association of low SES and ETS exposure with poorer lung function. In multivariable mixed models that simultaneously evaluated the impact of SES and ETS exposures, we found, contrary to our hypothesis, that the strong association between SES and lung function was only minimally affected by adjustment for ETS exposure. The associations between ETS exposures and lung function were more strongly attenuated after adjustment for SES. Our findings suggest that there are SES-related disparities in lung function independent of ETS exposure, and similarly, that ETS has impacts on lung function even after taking into account the effects of SES.
We also found a higher risk of crackles and wheezes among children with lower SES or exposure to ETS. The independent effects of SES and ETS on detection of crackles or wheezes did not persist in our combined model, likely due to the high degree of collinearity (overlap) of these characteristics. Finally, we found low SES and exposure to ETS to be associated with poorer weight. Similar to lung function, the strong association between SES and weight percentile was minimally affected by adjustment for ETS exposure, suggesting that there are SES-related disparities in nutrition that are independent of ETS exposure.
The relationship between SES and tobacco use in our cohort mirrored these relationships in the general population.25 Twenty-five percent of children had exposure to maternal smoking in our cohort, and the prevalence was higher among families with lower SES. These data reflect national trends estimating tobacco use in 26% of adults living below the poverty level compared with 15% among those living at or above this level.26
Our analysis supports past reports of associations between both SES and ETS and low FEV1.2,3,16,17,25,27,28 The adverse association of low SES on poor lung function in CF is likely driven by multiple factors, including poorer health literacy and adherence and higher prevalence of depression and anxiety.3,29–31 Decreased access to hospital and CF center care, although an important cause of SES-related adverse health outcomes in other chronic diseases, has not been shown to have a major influence in previous studies in CF.3,32,33 Nonetheless, lack of access or impaired ability to navigate community resources, such as exercise activities, school advocacy for frequent absences, mental health counseling, parenting and child care support, and nutritional supplements, may limit the effectiveness of referrals generated by the CF team, impeding care delivery downstream.
Our findings differ slightly from those of Collaco and colleagues,17 who found in their cohort study of 812 twins and siblings with CF that the association between SES and lung function was no longer significant after adjustment for ETS exposure, but that the relationship between ETS and lung function was unaffected by adjustment for SES. Potential explanations for our slightly differing results are differences in our cohorts (ours was younger and Pseudomonas-negative at enrollment), different measures of SES and ETS, and different sets of covariates in our multivariable models. Nonetheless, both studies report a similar prevalence of home ETS exposure and support the important adverse effects of low SES and exposure to ETS on health outcomes in CF.
To our knowledge, the detection of crackles or wheezes on chest examination in association with SES or ETS exposures has not been previously examined in CF. The presence of daily respiratory symptoms in CF have been associated with worse health outcomes, including low FEV1 % predicted and frequency of antibiotic courses.34–38
SES and ETS impact on growth and nutrition in children with CF has been reviewed.3,7,39 In our cohort, both SES and ETS exposures were associated with lower weight percentile. Our analyses suggest that the disparities in nutritional outcomes associated with low SES, specifically maternal education level and household income, persist even after taking into account the effects of ETS. Maternal nutritional knowledge specific to CF has previously been reported to predict children’s dietary adherence score.40 Food insecurity, although not assessed in the CF population, has been associated with worse health outcomes in the general population.41
A strength of our study is that it includes a range of measures of both SES and ETS. SES disparities vary in degree of health-damaging exposures, including early life conditions, inadequate nutrition, poor housing, air pollution, stress, and health access.25,28,42 Income, education level, and health insurance status as markers of SES reflect different aspects of the complex relationship of SES disparities and respiratory health. Similarly, ETS exposures may have differential impact based on source, timing, and exposure, including prenatally.43 Our analysis supports developmental implications of smoke exposure in pregnancy and early childhood. Dose-dependent in utero smoke exposure has been associated with decreased lung function measures in healthy newborns, and infants with CF exposed to ETS have been reported to have more bronchial hyperreactivity.44,45 Assessment of multiple SES and ETS exposures may better delineate the source of disparities to help target interventions.
An important limitation of our study is self-report of exposures, particularly ETS. Smoking may be underreported due to its social stigma, particularly around children with chronic respiratory disease. The misclassification introduced by self-report would likely attenuate the observed effect size. In addition, our cohort was selected for an absence of P aeruginosa infection at enrollment, so our findings may not be generalizable to children with chronic P aeruginosa infection. Given the observational nature of this study, we cannot determine the causal nature of relationships between SES and ETS and health outcomes.
Conclusions
Our study explored prospectively collected data as part of EPIC OBS, a large multicenter longitudinal study, which allowed examination of the effects of SES and ETS exposures on clinical outcomes in young children with CF. Our results indicate that low SES and exposure to ETS are independent risk factors for lower lung function, crackles and wheezes, and lower weight percentile in young children with CF. In addition, the effects of SES on these outcomes are not accounted for simply by disproportionate exposure to ETS among children from families with lower SES. Our findings reinforce the importance of addressing the interrelationship of SES and ETS in CF clinical outcomes analyses and suggest that health care policies addressing SES disparities and fostering smoking cessation could prove beneficial to the lung health of individuals with CF.
Acknowledgments
The authors thank the Cystic Fibrosis Foundation for the use of the CF Foundation National Patient Registry data to conduct this study. Additionally, we thank the patients, care providers, and clinic coordinators at CF Centers throughout the United States for their contributions to the CF Foundation National Patient Registry.
Glossary
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- CI
confidence interval
- EPIC OBS
Early Pseudomonas Infection Control Observational
- ETS
environmental tobacco smoke
- FEV1
forced expiratory volume in 1 second
- OR
odds ratio
- SES
socioeconomic status
Appendix: The EPIC Study Group
Albany Medical College, Albany, NY, Paul Comber, MD, PhD and Terese Evens; All Children’s Hospital CF Center, St Petersburg, FL, Magdalen Gondor, MD and Kathy Hosler; Baylor College of Medicine, Houston, TX, Peter Hiatt, MD and Omalee Lopez; Brown University Medical School, Rhode Island Hospital, Providence, RI, Karen Daigle, MD; Cardinal Glennon Children’s Medical Center, St Louis, MO, Blakeslee Noyes, MD and Freda Branch; Children’s Hospital, Boston, MA, Henry Dorkin, MD, Nicolas Fenton, and Kelsey Hill; Children’s Hospital & Clinics, Minneapolis, MN, John McNamara, MD and Mary Sachs; Children’s Hospital Colorado, Aurora, CO, Stacey Martiniano, MD and Meg Anthony; Children’s Hospital of Los Angeles, Los Angeles, CA, Arnold Platzker, MD; Children’s Hospital Medical Center of Akron, Akron, OH, Gregory Omlor, MD and Deborah Ouellette; Children’s Hospital of Michigan, Detroit, MI, Ibrahim Abdulhamid, MD and Catherine Van Wagnen; Children’s Hospital of Milwaukee, Milwaukee, WI, Diana Quintero, MD and Tami Miller; Children’s Hospital, University of Birmingham, Birmingham, AL, Hector Gutierrez, MD and Susan Keeling; Children’s Hospital of Pittsburgh, Pittsburgh, PA, David Orenstein, MD and Sandra Hurban; Children’s Hospital at Westchester Medical Center, Valhalla, NY, Allen Dozor, MD and Armando Ramirez; Children’s Medical Center of Dayton, Dayton, OH, Gary Mueller, MD and Sandy Bartosik; Children’s Mercy Hospital, Kansas City, MO, Philip Black, MD and Rose Thompson; Children’s Specialty Center, Fletcher Allen Health Care, Burlington, VT, Thomas Lahiri, MD and Rose Bergeron; Cohen Children’s Hospital, Great Neck, NY, Joan DeCelie-Germana, MD, Lynn Bonitz, and Susan Galvin; Cook Children’s Medical Center, Fort Worth, TX, Maynard Dyson, MD and Heather Urbanek; Dartmouth Hitchcock Clinic-Lebanon, Lebanon, NH, H. Alix Ashare, MD and Mary Griffin; Alfred I. DuPont Hospital for Children, Wilmington, DE, Aaron Chidekel, MD and Dana Geiser; Emory University School of Medicine, Atlanta, GA, Hector Gutierrez, MD and Susan Keeling; Helen DeVos Women & Children’s Center, Grand Rapids, MI, Susan Millard, MD and Cynthia Gile; Intermountain CF Center, Salt Lake City, UT, Barbara Chatfield, MD and Taylor Block; Johns Hopkins University, Baltimore, MD, Peter Mogayzel, MD, PhD and Carolyn Chapman; Kaiser Permanente Medical Care Program, Oakland, CA, Byron Quick, MD and Julie Lee; Le Bonheur Children’s Medical Center, Memphis, TN, Robert Schoumacher, MD and Catherine Horobetz; Massachusetts General Hospital, Boston, MA, Allen Lapey, MD and Henry Dorkin, MD; Medical College of Georgia, Augusta, GA, Kathleen McKie, MD and Heidi Stapp; Memorial Miller Children’s Hospital, University of California, Irvine, Long Beach, CA, Felice Adler-Shohet, MD and Jay Lieberman, MD; Milton S. Hershey Medical Center, Penn State University, Hershey, PA, Gavin Graff, MD, Lisa Allwein, and Diane Kitch; Maine Medical Center Cystic Fibrosis Center, Portland, ME, AnneMarie Cairns, DO, Carrie Milliard, and Harmony Renna; Monmouth Medical Center, Long Branch, NJ, Robert Zanni, MD and Bridget Marra; Nationwide Children’s Hospital, Columbus, OH, Karen McCoy, MD and Diana Gilmore; Nemours Children’s Clinic, Jacksonville, FL, David Schaeffer, MD, Elizabeth DeLuca, and Roslyn Whitley; Northwestern University, Chicago, IL, Adrienne Savant, MD and Zainub Ashrafi; Oregon Health Sciences University, Portland, OR, Alexandra Cornell, MD and Brendan Klein; Rainbow Babies and Children’s Hospital, Cleveland, OH, Michael Konstan, MD and Ashlee Parsons; Riley Hospital for Children, Indianapolis, IN, Clement Ren, MD and Lisa Bendy; Seattle Children’s Hospital, Seattle, WA, Margaret Rosenfeld MD, MPH, Alan Genatossio, and Sharon McNamara; St Christopher’s Hospital for Children, Philadelphia, PA, Laurie Varlotta, MD and Diana Figueroa; St Louis Children’s Hospital, St Louis, MO, Thomas Ferkol, MD and Sarah Smith; Stanford University Medical Center, Palo Alto, CA, Richard Moss, MD and Colleen Dunn; SUNY Upstate Medical University, Syracuse, NY, Christopher Fortner, MD and Donna Lindner; UMass Memorial Health Care, Worcester, MA, Evan Bailey, MD and Karen Longtine; University of California at San Francisco, San Francisco, CA, Dennis Nielson, MD and Courtney Moreno; University of Florida College of Medicine, Gainesville, FL, Terry Spencer, MD; University of Iowa Hospital and Clinics, Iowa City, IA, Richard Ahrens, MD and Mary Teresi; University of Kentucky, Lexington, KY, Jamshed Kanga, MD and Viktoria Bulkley; University of Michigan Health System, Ann Arbor, MI, Samya Nasr, MD and Dawn Kruse; University of Mississippi Medical Center, Jackson, MS, Joseph Maguire, MD; University of Nebraska Medical Center, Omaha, NE, John Colombo, MD and Jill Fahner; University of North Carolina at Chapel Hill, Chapel Hill, NC, George Retsch-Bogart, MD and Rose Cunnion; University of Rochester Medical Center, Rochester, NY, Karen Voter, MD and Judy Sroka; University of Virginia Health System, Charlottesville, VA, Deborah Froh, MD, Mary Alice Blackwell, Patricia Moss, and Christie Aderholt; University of Wisconsin, Madison, WI, Michael Rock, MD and Linda Makholm; Vanderbilt Children’s Hospital, Nashville, TN, Rebekah Brown, MD and Stephanie Steen; Women & Children’s Hospital of Buffalo, Buffalo, NY, Danielle Goetz, MD and Christine Roach.
Footnotes
Dr Ong contributed to data interpretation and drafted the initial manuscript; Drs Schechter, Emerson, and Rosenfeld conceptualized and designed the study, carried out the initial analysis, interpreted the data, and reviewed and revised the manuscript; Drs Gibson and Morgan contributed to data interpretation and critically reviewed the manuscript; Ms Yang and Dr Peng carried out data analysis, contributed to data interpretation, and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by Cystic Fibrosis Foundation Therapeutics grants OBSERV13K0 and EPIC0K0 to Dr Rosenfeld, Seattle Children’s Hospital, Seattle, WA; Nemours Children’s Clinic Research Program, Jacksonville, FL. Dr Peng is supported by National Institutes of Health grant R01HL113548. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Morgan is a consultant to Genentech in his role as Co-Chair of the Epidemiologic Study of Cystic Fibrosis. Dr Rosenfeld is a consultant to Genentech as a member of the North American Scientific Advisory Group of the Epidemiologic Study of Cystic Fibrosis. The other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Cystic Fibrosis Foundation Patient Registry Annual data report to the center directors, 2013. Available at: https://www.cff.org/2013_CFF_Annual_Data_Report_to_the_Center_Directors.pdf. Accessed September 10, 2015
- 2.Taylor-Robinson DC, Smyth RL, Diggle PJ, Whitehead M. The effect of social deprivation on clinical outcomes and the use of treatments in the UK cystic fibrosis population: a longitudinal study. Lancet Respir Med. 2013;1(2):121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schechter MS, Shelton BJ, Margolis PA, Fitzsimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med. 2001;163(6):1331–1337 [DOI] [PubMed] [Google Scholar]
- 4.Goss CH, Newsom SA, Schildcrout JS, Sheppard L, Kaufman JD. Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. Am J Respir Crit Care Med. 2004;169(7):816–821 [DOI] [PubMed] [Google Scholar]
- 5.Boyle MP, Sabadosa KA, Quinton HB, Marshall BC, Schechter MS. Key findings of the US Cystic Fibrosis Foundation’s clinical practice benchmarking project. BMJ Qual Saf. 2014;23(suppl 1):i15–i22 [DOI] [PubMed] [Google Scholar]
- 6.Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet. 2015;16(1):45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schechter MS. Nongenetic influences on cystic fibrosis outcomes. Curr Opin Pulm Med. 2011;17(6):448–454 [DOI] [PubMed] [Google Scholar]
- 8.O’Connor GT, Quinton HB, Kneeland T, et al. Median household income and mortality rate in cystic fibrosis. Pediatrics. 2003;111(4 pt 1). Available at: www.pediatrics.org/cgi/content/full/111/4pt1/e333 [DOI] [PubMed] [Google Scholar]
- 9.Barr HL, Britton J, Smyth AR, Fogarty AW. Association between socioeconomic status, sex, and age at death from cystic fibrosis in England and Wales (1959 to 2008): cross sectional study. BMJ. 2011;343:d4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson DE, Emont SL, Brackbill RM, Cameron LL, Peddicord J, Fiore MC. Cigarette smoking prevalence by occupation in the United States. A comparison between 1978 to 1980 and 1987 to 1990. J Occup Med. 1994;36(5):516–525 [PubMed] [Google Scholar]
- 11.Schuster MA, Franke T, Pham CB. Smoking patterns of household members and visitors in homes with children in the United States. Arch Pediatr Adolesc Med. 2002;156(11):1094–1100 [DOI] [PubMed] [Google Scholar]
- 12.King K, Martynenko M, Bergman MH, Liu YH, Winickoff JP, Weitzman M. Family composition and children’s exposure to adult smokers in their homes. Pediatrics. 2009;123(4). Available at: www.pediatrics.org/cgi/content/full/123/4/e559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin BK. Exposure of children with cystic fibrosis to environmental tobacco smoke. N Engl J Med. 1990;323(12):782–788 [DOI] [PubMed] [Google Scholar]
- 14.Campbell PW III, Parker RA, Roberts BT, Krishnamani MR, Phillips JA III. Association of poor clinical status and heavy exposure to tobacco smoke in patients with cystic fibrosis who are homozygous for the F508 deletion. J Pediatr. 1992;120(2 pt 1):261–264 [DOI] [PubMed] [Google Scholar]
- 15.Kovesi T, Corey M, Levison H. Passive smoking and lung function in cystic fibrosis. Am Rev Respir Dis. 1993;148(5):1266–1271 [DOI] [PubMed] [Google Scholar]
- 16.Smyth A, O’Hea U, Williams G, Smyth R, Heaf D. Passive smoking and impaired lung function in cystic fibrosis. Arch Dis Child. 1994;71(4):353–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collaco JM, Vanscoy L, Bremer L, et al. Interactions between secondhand smoke and genes that affect cystic fibrosis lung disease. JAMA. 2008;299(4):417–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treggiari MM, Rosenfeld M, Mayer-Hamblett N, et al. ; EPIC Study Group . Early anti-pseudomonal acquisition in young patients with cystic fibrosis: rationale and design of the EPIC clinical trial and observational study. Contemp Clin Trials. 2009;30(3):256–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenfeld M, Emerson J, McNamara S, et al. ; EPIC Study Group Participating Clinical Sites . Baseline characteristics and factors associated with nutritional and pulmonary status at enrollment in the cystic fibrosis EPIC observational cohort. Pediatr Pulmonol. 2010;45(9):934–944 [DOI] [PubMed] [Google Scholar]
- 20.Farrell PM, Rosenstein BJ, White TB, et al. ; Cystic Fibrosis Foundation . Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153(2):S4–S14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Dockery DW, Wypij D, et al. Pulmonary function growth velocity in children 6 to 18 years of age. Am Rev Respir Dis. 1993;148(6 pt 1):1502–1508 [DOI] [PubMed] [Google Scholar]
- 22.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187 [DOI] [PubMed] [Google Scholar]
- 23.McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet. 2003;361(9370):1671–1676 [DOI] [PubMed] [Google Scholar]
- 24.McKone EF, Goss CH, Aitken ML. CFTR genotype as a predictor of prognosis in cystic fibrosis. Chest. 2006;130(5):1441–1447 [DOI] [PubMed] [Google Scholar]
- 25.Celedón JC, Roman J, Schraufnagel DE, Thomas A, Samet J. Respiratory health equality in the United States. The American Thoracic Society perspective. Ann Am Thorac Soc. 2014;11(4):473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamal A, Homa DM, O’Connor E, et al. Current cigarette smoking among adults - United States, 2005-2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–1240 [DOI] [PubMed] [Google Scholar]
- 27.Schechter MS, Margolis PA. Relationship between socioeconomic status and disease severity in cystic fibrosis. J Pediatr. 1998;132(2):260–264 [DOI] [PubMed] [Google Scholar]
- 28.Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–252 [DOI] [PubMed] [Google Scholar]
- 29.Oates GR, Stepanikova I, Gamble S, Gutierrez HH, Harris WT. Adherence to airway clearance therapy in pediatric cystic fibrosis: socioeconomic factors and respiratory outcomes. Pediatr Pulmonol. 2015;50(12):1244–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quittner AL, Goldbeck L, Abbott J, et al. Prevalence of depression and anxiety in patients with cystic fibrosis and parent caregivers: results of The International Depression Epidemiological Study across nine countries. Thorax. 2014;69(12):1090–1097 [DOI] [PubMed] [Google Scholar]
- 31.Smith BA, Georgiopoulos AM, Quittner AL. Maintaining mental health and function for the long run in cystic fibrosis. Pediatr Pulmonol. 2016;51(S44):S71–S78 [DOI] [PubMed] [Google Scholar]
- 32.Schechter MS, McColley SA, Silva S, et al. . Association of socioeconomic status with the use of chronic therapies and healthcare utilization in children with cystic fibrosis. J Pediatr. 2009;155(5):634–639.e1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buu MC, Milla CE, Wise PH. Response. Chest. 2016;150(3):753. [DOI] [PubMed] [Google Scholar]
- 34.Sanders DB, Emerson J, Ren CL, et al. ; EPIC Study Group . Early childhood risk factors for decreased FEV1 at age six to seven years in young children with cystic fibrosis. Ann Am Thorac Soc. 2015;12(8):1170–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konstan MW, Butler SM, Wohl ME, et al. ; Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis . Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr. 2003;142(6):624–630 [DOI] [PubMed] [Google Scholar]
- 36.Byrnes CA, Vidmar S, Cheney JL, et al. ; ACFBAL Study Investigators . Prospective evaluation of respiratory exacerbations in children with cystic fibrosis from newborn screening to 5 years of age. Thorax. 2013;68(7):643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.VanDevanter DR, Wagener JS, Pasta DJ, et al. Pulmonary outcome prediction (POP) tools for cystic fibrosis patients. Pediatr Pulmonol. 2010;45(12):1156–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren CL, Konstan MW, Rosenfeld M, Pasta DJ, Millar SJ, Morgan WJ; Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis . Early childhood wheezing is associated with lower lung function in cystic fibrosis. Pediatr Pulmonol. 2014;49(8):745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schechter MS. Non-genetic influences on cystic fibrosis lung disease: the role of sociodemographic characteristics, environmental exposures, and healthcare interventions. Semin Respir Crit Care Med. 2003;24(6):639–652 [DOI] [PubMed] [Google Scholar]
- 40.Anthony H, Paxton S, Bines J, Phelan P. Psychosocial predictors of adherence to nutritional recommendations and growth outcomes in children with cystic fibrosis. J Psychosom Res. 1999;47(6):623–634 [DOI] [PubMed] [Google Scholar]
- 41.Pruitt SL, Leonard T, Xuan L, et al. Who is food insecure? Implications for targeted recruitment and outreach, National Health and Nutrition Examination Survey, 2005-2010. Prev Chronic Dis. 2016;13:E143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kerem E, Cohen-Cymberknoh M. Disparities in cystic fibrosis care and outcome: socioeconomic status and beyond. Chest. 2016;149(2):298–300 [DOI] [PubMed] [Google Scholar]
- 43.Leone FT, Carlsen KH, Folan P, et al. ; ATS Tobacco Action Committee; American Thoracic Society . An official American Thoracic Society research statement: current understanding and future research needs in tobacco control and treatment. Am J Respir Crit Care Med. 2015;192(3):e22–e41 [DOI] [PubMed] [Google Scholar]
- 44.Lødrup Carlsen KC, Jaakkola JJ, Nafstad P, Carlsen KH. In utero exposure to cigarette smoking influences lung function at birth. Eur Respir J. 1997;10(8):1774–1779 [DOI] [PubMed] [Google Scholar]
- 45.Kopp BT, Sarzynski L, Khalfoun S, et al. Detrimental effects of secondhand smoke exposure on infants with cystic fibrosis. Pediatr Pulmonol. 2015;50(1):25–34 [DOI] [PubMed] [Google Scholar]