Multidrug transporters form a large class of membrane proteins present in the cells of most organisms. These proteins bind to a variety of potentially cytotoxic compounds and remove them from the cell in an ATP- or proton-dependent process (Zhelenova et al., 2000). Traditionally, multidrug transporters have been divided into four superfamilies: the ATP binding cassette (ABC) superfamily, the major facilitator superfamily, the small multidrug resistance family, and the resistance-nodulation-cell division family. Brown et al. (1999) defined a fifth family, called the multidrug and toxic compound extrusion (MATE) family of transporters. The MATE family is characterized by the presence of 12 putative transmembrane segments and by the absence of “signature sequences” specific to the other multidrug transporter superfamilies. MATE proteins are believed to function as proton-dependent efflux transporters, based on the genetic characterization of two family members, NorM from Vibrio parahaemolyticus and its homolog YdeH from Escherichia coli. Expression of these proteins in E. coli confers resistance to various antibiotics and antimicrobial agents that is dependent on the maintenance of a proton gradient across the plasma membrane. MATE genes are abundant in bacteria and plants—the Arabidopsis genome contains at least 54 MATE family members—but have not been found in mammals. Aside from NorM and YdeH, very little functional information is available on these proteins.
In this issue of The Plant Cell, Diener et al. (pages 1625–1637) describe the functional analysis of the MATE gene ALF5 in Arabidopsis. The alf5 mutant exhibits greatly inhibited formation of lateral roots when grown in commercial Bacto agar (Figure 1). Interestingly, it was found that the mutant produced roots that were indistinguishable from wild-type roots when grown in soil, in a different brand of agar, or in extensively washed Bacto agar, suggesting that the alf5 mutation caused sensitivity to a soluble contaminant present in the Bacto agar. The alf5 locus was cloned and found to contain a 29-bp deletion in a gene, ALF5, that encodes a MATE family integral membrane protein. Gene expression analysis using AFL5 fused to the β-glucuronidase reporter gene in transgenic plants indicated that the ALF5 gene is highly expressed in the root epidermis and cortex. In addition, expression of ALF5 in yeast conferred resistance to the toxic cation tetramethylammonium, supporting the conclusion that ALF5 is a functional MATE efflux transporter.
Figure 1.
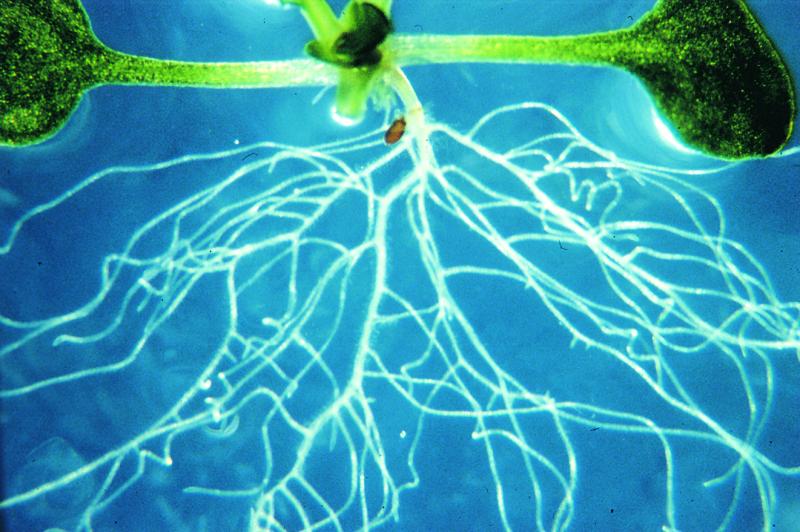
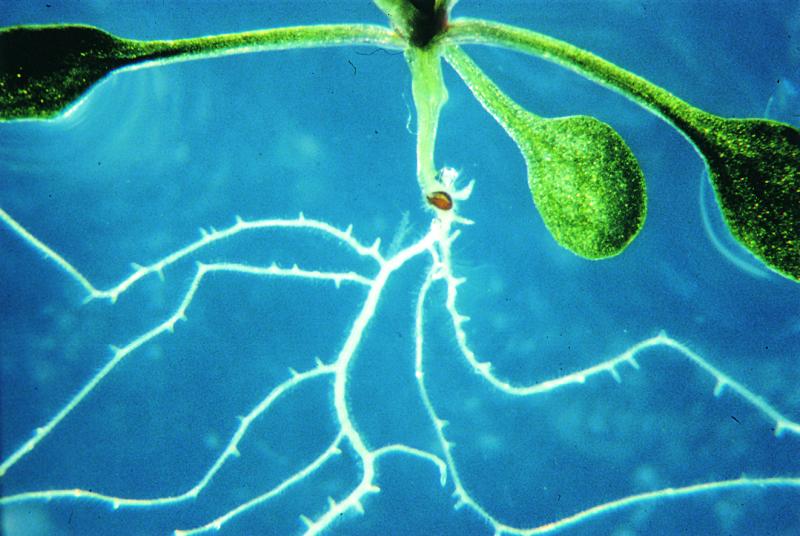
Roots of Wild-Type (Top) and alf5 Mutant Plants Grown in a Commercial Agar.
As a result of the disruption of the ALF5 gene, which encodes a MATE family efflux transporter, the roots of the mutant are sensitive to a contaminant in the agar. The figure was provided by Gerald Fink.
PLANTS AS “GREEN LIVERS”
Plant cells, like the cells of most organisms, are capable of removing a large number of potentially toxic compounds from the cytoplasm. In plants, these compounds are either sequestered in vacuoles or transported to the cell wall. Toxic compounds may be of xenobiotic origin or produced endogenously (e.g., phenolics, flavonoids, and phytoalexins). The bronze-colored phenotype of the Bronze2 mutation in maize, for example, is caused by the inability of the mutant to transport anthocyanin from the cytosol to the vacuoles. Bronze2 encodes a glutathione transferase (Marrs et al., 1995), and the mutant is unable to carry out conjugation of anthocyanin with glutathione, a necessary step before transport of conjugated glutathione to the vacuole.
Sandermann (1992) likened plant metabolism of toxic compounds to that of the mammalian liver because of the presence and activity of cytochrome P450 monooxygenases and glutathione transferases, which resemble the two major enzyme systems of the liver. Plant cytochrome P450s and glutathione transferases are involved in the first two phases of detoxification of a number of polychlorinated and polycyclic hydrocarbons and related xenobiotic compounds as well as endogenous toxins. In phase I, cytochrome P450s prepare a substrate for phase II via hydroxylation, and phase II glutathione transferases carry out the conjugation of the hydroxylated compound to reduced glutathione. Subsequently, phase III involves the transport of the glutathione conjugate out of the cytoplasm to the vacuole or cell wall. There is good evidence that multidrug transporters of the ABC superfamily are involved in the transport of glutathione conjugates in plant cells (Rea et al., 1998; Theodoulou, 2000). Sandermann (1992) described plants as “green livers” that might act as a global sink for environmental pollutants of this nature. The presence of MATE efflux proteins in plants, which are presumed to carry out transport of lipophilic cations and related compounds that are not glutathione conjugates, broadens the scope of this concept and opens up more possibilities for plant biotechnology.
EVOLUTIONARY PLANT TRICKS
Despite the wide range of chemically and structurally distinct substrates for multidrug transporters, transporters of all five superfamilies show a preference for hydrophobic (lipophilic) cations, such as quaternary ammonium antiseptics (Stermitz et al., 2000). Lipophilic cations, such as berberine alkaloids, are commonly produced by plants. Lewis (1999) proposed that berberine alkaloids represent a larger group of cationic toxins that fueled the evolution of microbial multidrug transporters. Interestingly, Stermitz et al. (2000) found that several plant species in the genus Berberis, which produce berberine, also produce an inhibitor of multidrug transporters, identified as 5′-methoxyhydnocarpin. Berberine exhibited relatively weak antimicrobial action, presumably because of its efflux from bacterial cells by multidrug transporters. 5′-Methoxyhydnocarpin had no antimicrobial activity alone, but it strongly potentiated the action of berberine and other toxins against the growth of Staphylococcus aureus. This finding suggests that the main function of some microbial multidrug transporters is resistance against plant-produced antimicrobial compounds.
Aside from plant–pathogen interactions, one habitat in which bacteria are likely to encounter toxic plant compounds is in root nodules. In Rhizobium etli, multidrug transporters have been found to play an important role in nodulation of bean (Phaseolus vulgaris). RmrA encodes an R. etli multidrug efflux pump gene that is induced by flavonoids released from the roots of P. vulgaris, and mutations in this gene were found to reduce nodulation in the bean by an average of 40% (Gonzalez-Pasayo and Martinez-Romero, 2000).
Another habitat in which bacteria might commonly encounter plant toxins is in the stomachs of herbivores. In E. coli, the transcription repressor MarR binds various phenolic compounds such as salicylate and regulates the expression of two multidrug transporters to produce a more effective efflux pump system. Sulavik et al. (1995) suggested that drug resistance in E. coli is thus enhanced when the bacteria reside in an omnivore gut rich in plant antimicrobial compounds.
DIVERSITY OF FUNCTIONS FOR MULTIDRUG TRANSPORTERS
It is apparent that multidrug transporters constitute large superfamilies in plants, as in other organisms. The Arabidopsis genome contains at least 60 open reading frames for ABC transporters (Davies and Coleman, 2000). As with the less well known MATE family, the function of the majority of these genes is unknown, but characterization of a few family members suggests a multiplicity of functions in plant growth and development within the superfamily, in addition to their role in the transport of xenobiotic compounds.
Sidler et al. (1998) showed that an Arabidopsis ABC transporter, AtPGP1, is involved in the regulation of hypocotyl elongation during photomorphogenesis. Under certain light conditions, plants overexpressing PGP1 developed longer hypocotyls, whereas plants with inhibited expression of PGP1 produced shorter hypocotyls compared with the wild type. Hypocotyl elongation in the dark was unaffected by alterations in PGP1 expression. In wild-type plants, the AtPGP1 gene was found to be expressed in the plasma membrane of root and shoot apices, and the authors proposed that AtPGP1 is involved in the transport of a signal molecule, such as a peptide hormone, from the shoot apical region.
Some mammalian ABC transporters, such as the cystic fibrosis transmembrane conductance regulator (CFTR) and the sulfonylurea receptor (SUR), have been shown to act as ion channels and/or channel regulators. CFTR functions as an outwardly rectifying Cl− channel that also regulates other ion channels, and the SUR acts as an ATP- dependent K+ channel (Theodoulou, 2000). Some researchers have begun to look for such activity among ABC transporters in plants and have found evidence that ABC proteins may function as ion channel regulators in guard cells. Gaedeke et al. (2001) and Leonhardt et al. (1999) have investigated a slow ion channel in Arabidopsis guard cells that shows CFTR-like characteristics and that may coordinate the efflux of K+ and other ions during stomatal closure.
The MATE transporter superfamily also may cover a diverse range of functions in plant growth and development. Debeaujon et al. (2001) recently reported that the TRANSPARENT TESTA12 (TT12) gene encodes another MATE family member in Arabidopsis. The function of TT12 appears to be in controlling the vacuolar sequestration of flavonoids in the seed coat (testa) endothelium. Because of their high chemical reactivity, flavonoids are toxic endogenous compounds that must be removed from the cytoplasm after their synthesis and sequestered in the vacuole or cell wall. There is evidence that they function as protectants against UV light damage, oxidative stress, and pathogen attack. The mutant seeds, lacking the function of the TT12 MATE protein, appear to be unable to transport and accumulate flavonoids in the vacuoles of the seed coat endothelium. The seeds are pale in color and also show reduced seed dormancy, supporting the idea that flavonoids play an important role in seed biology (Winkel-Shirley, 1998). Thus, it appears that we can expect a multiplicity of functions in growth and development for the many other plant MATE family members. Surprisingly, Diener et al. found a second open reading frame at the alf5 locus, LAL5, which lies immediately downstream of ALF5 and encodes a polypeptide with 83% identity to ALF5. It is not known if LAL5 is expressed, and this needs to be determined, but the authors reported that the gene appeared to be intact in the alf5 mutant. If the gene were expressed, it would appear to be functionally distinct from ALF5.
The discovery of multidrug sensors that regulate the expression of some microbial multidrug transporters suggests that the main function of these transporters is the efflux of xenobiotic toxins (Lewis, 1999). At least three multidrug sensors have been identified. BmrR is a transcription factor in Bacillus subtilis that activates the expression of the multidrug transporter gene Bmr in response to binding a number of hydrophobic cations, many of which are also substrates of the Bmr protein. In S. aureus, the QacA multidrug transporter gene is repressed by QacR, and binding of QacR to various cations induces QacA expression. And in E. coli, repression of the EmrAB transporter gene is relieved by binding of the EmrR repressor to various neutral compounds. It is interesting to speculate that multidrug sensors also will be found to control the expression of plant transporter genes such as ALF5, whose principal function appears to be the efflux of toxic compounds.
References
- Brown, M.H., Paulsen, I.T., and Skurray, R.A. (1999). The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol. Microbiol. 31, 393–395. [DOI] [PubMed] [Google Scholar]
- Davies, T.G.E., and Coleman, J.O.D. (2000). The Arabidopsis thaliana ATP-binding cassette proteins: An emerging superfamily. Plant Cell Environ. 23, 431–443. [Google Scholar]
- Debeaujon, I., Peeters, A.J.M., Léon-Kloosterziel, K.M., and Koornneef, M. (2001). The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 13, 853–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener, A.C., Gaxiola, R.A., and Fink, G.R. (2001). Arabidopsis ALF5, a multidrug efflux transporter gene family member, confers resistance to toxins. Plant Cell 13, 1625–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaedeke, N., Klein, M., Kolukisaoglu, U., Forestier, C., Muller, A., Ansorge, M., Becker, D., Mamnun, Y., Kuchler, K., Schulz, B., Mueller-Roeber, B., and Martinoia, E. (2001). The Arabidopsis thaliana ABC transporter AtMRP5 controls root development and stomata movement. EMBO J. 20, 1875–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Pasayo, R., and Martinez-Romero, E. (2000). Multiresistance genes of Rhizobium etli CFN42. Mol. Plant-Microbe Interact. 13, 572–577. [DOI] [PubMed] [Google Scholar]
- Leonhardt, N., Vavasseur, A., and Forestier, C. (1999). ATP binding cassette modulators control abscisic acid–regulated slow anion channels in guard cells. Plant Cell 11, 1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, K. (1999). In search of natural substrates and inhibitors of MDR pumps. J. Mol. Microbiol. Biotechnol. 3, 247–254. [PubMed] [Google Scholar]
- Marrs, K.A., Alfenito, M.R., Lloyd, A.M., and Walbot, V. (1995). A glutathione S-conjugate transferase involved in vacuolar transfer encoded by the maize Bronze-2. Nature 375, 397–400. [DOI] [PubMed] [Google Scholar]
- Rea, P.A., Li, Z.-S., Lu, Y.-P., and Drozdowicz, Y.M. (1998). From vacuolar GS-X pumps to multispecific ABC transporters. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 727–760. [DOI] [PubMed] [Google Scholar]
- Sandermann, H. (1992). Plant metabolism of xenobiotics. Trends Biochem. Sci. 17, 82–84. [DOI] [PubMed] [Google Scholar]
- Sidler, M., Hassa, P., Hasan, S., Ringli, C., and Dudler, R. (1998). Involvement of an ABC transporter in a developmental pathway regulating hypocotyl cell elongation in the light. Plant Cell 10, 1623–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stermitz, F.R., Lorenz, P., Tawara, J.N., Zenewicz, L.A., and Lewis, K. (2000). Synergy in a medicinal plant: Antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. USA 97, 1433–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulavik, M.C., Gambino, L.F., and Miller, P.F. (1995). The MarR repressor of the multiple antibiotic resistance (mar) operon in Escherichia coli: A prototypic member of a family of bacterial regulatory proteins involved in sensing phenolic compounds. Mol. Med. 1, 436–446. [PMC free article] [PubMed] [Google Scholar]
- Theodoulou, F.L. (2000). Plant ABC transporters. Biochim. Biophys. Acta 1465, 79–103. [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley, B. (1998). Flavonoids in seeds and grains: Physiological function, agronomic importance and the genetics of biosynthesis. Seed Sci. Res. 8, 415–422. [Google Scholar]
- Zhelenova, E.E., Markham, P., Edgar, R., Bibi, E., Neyfakh, A.A., and Brennan, R.G. (2000). A structure-based mechanism for drug binding by multidrug transporters. Trends Biochem. Sci. 25, 39–43. [DOI] [PubMed] [Google Scholar]


