Apomixis is the natural ability of more than 400 plant species to reproduce asexually through seed (Nogler, 1984a). Sexual embryos result from the union of male and female gametes, which produces genetically varied offspring. In contrast, apomictic embryos are formed without paternal contribution. Therefore, apomictic offspring carry the full genetic constitution of the mother and form a stable clone, a feature of great value for plant breeding and seed production.
For many years, apomixis was studied only by a small group of interested botanists (Nogler, 1984a; Asker and Jerling, 1992) and visionary plant breeders (Petrov et al., 1979; Hanna and Bashaw, 1987; Savidan, 1992). However, because of its tremendous potential for agriculture, apomixis research has attracted much more attention during the last few years (Koltunow et al., 1995; Vielle-Calzada et al., 1996; Grossniklaus et al., 1998). If apomixis could be introduced into sexual crops, it would greatly simplify breeding schemes and allow the fixation of any genotype (however complex), including that of F1 hybrids. Apomixis technology could play a major role in feeding the growing population of our planet (Jefferson, 1994; Thoenissen, 2001) provided that it will be freely accessible to all users, especially resource-poor farmers in developing countries, requiring innovative approaches for technology generation, patenting, and licensing (http://billie.btny.purdue.edu/apomixis).
Current apomixis research focuses on elucidating the genetic basis and molecular mechanisms that control apomictic reproduction (see accompanying Meeting Report). Two major complementary approaches are being pursued: (1) to identify genes controlling individual elements of apomixis in well-defined sexual model species (reviewed in Grossniklaus, 2001), and (2) to unravel the genetic control of apomixis in natural apomicts (reviewed in Savidan, 2000). For nearly two decades, the genetic control of apomixis had been elucidated in very few species. Recently, however, inheritance studies for several natural apomicts have been published that shed new light on the genetic control of this important developmental process (van Dijk et al., 1999; Bicknell et al., 2000; Matzk et al., 2000; Noyes and Rieseberg, 2000; Pupilli et al., 2001; Quarin et al., 2001).
DEVELOPMENTAL ASPECTS OF APOMIXIS
Apomixis occurs in many species from more than 40 plant families and is thought to have evolved multiple times from sexual ancestors. Therefore, it is likely that the controls of sexual and apomictic reproduction are closely interrelated. Sexual reproduction and apomixis are not mutually exclusive, and both processes can occur in parallel, as it is typical of facultative apomicts, which produce a mixture of apomictic and sexual progeny. Although the mechanisms leading to apomictic reproduction are diverse (Koltunow, 1993; Crane, 2001), they share the common feature that ancestral sexual processes are deregulated in space and time (Grossniklaus, 2001). Two distinct types of apomixis have been described (Gustafsson, 1947a, 1947b): (1) sporophytic apomixis (adventitious embryony), in which an embryo forms directly from an unreduced sporophytic cell, and (2) gametophytic apomixis, which involves the formation of an unreduced embryo sac (female gametophyte). Although no less interesting, the genetics of sporophytic apomixis has not been investigated in great detail, which is why we focus on gametophytic apomixis in this article.
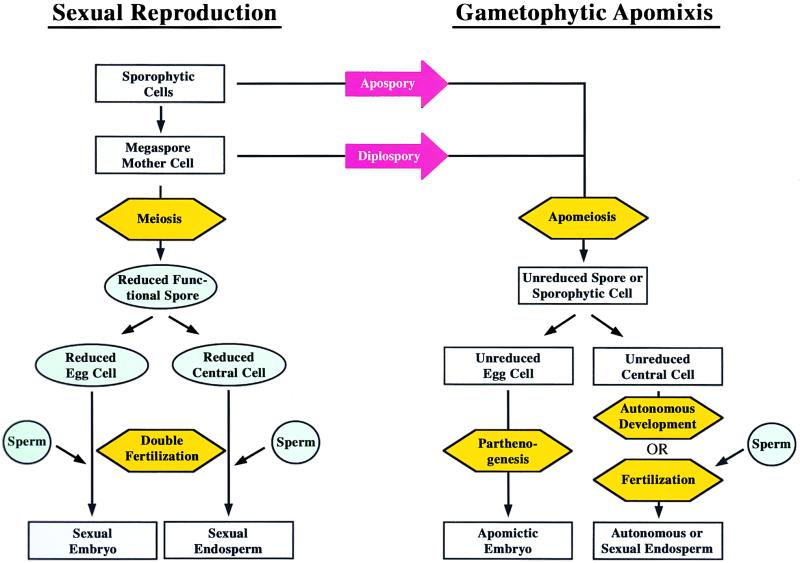
Sexual reproduction involves the generation and fusion of reduced gametes (Figure 1). Female gametogenesis and double fertilization occur within the ovule, a specialized reproductive organ (reviewed in Drews et al., 1998; Grossniklaus and Schneitz, 1998; Yang and Sundaresan, 2000). Usually a single cell within the ovule, the megaspore mother cell (MMC) becomes committed to the sexual pathway, undergoes meiosis, and forms a tetrad of four reduced spores. Only one of these will divide and ultimately form the mature embryo sac containing the female gametes. Double fertilization involves two pairs of gametic cells: the egg cell fuses with one sperm to form the embryo and give rise to the next generation, and the central cell fuses with a second sperm to form the endosperm, a nutritive tissue important for seed development and/or germination.
Figure 1.
Scheme of Sexual and Apomictic Reproduction.
Reduced stages of the life cycle are shown in shaded ovals, and unreduced stages are shown in rectangular boxes. The key developmental events that are affected or altered in apomictic species are highlighted in hexagons.
During gametophytic apomixis, several of these developmental steps are bypassed or altered (Figure 1; Koltunow, 1993; Vielle-Calzada et al., 1996). (1) Chromosome reduction is circumvented (apomeiosis) such that unreduced cells initiate embryo sac development. These unreduced cells can originate from an aberrant or missing meiosis of the MMC (diplospory). Alternately, the unreduced embryo sac forms directly from a cell within the ovule other than the MMC (apospory) and the sexual products degenerate. (2) The unreduced egg cell initiates embryogenesis in the absence of fertilization (parthenogenesis). (3) The central cell either develops autonomously or, in the majority of apomicts, requires fertilization to initiate development (pseu-dogamy). The development of normal endosperm in apomicts is important for seed viability and often requires special adaptations of embryo sac development or fertilization (reviewed in Grossniklaus et al., 2001). Recent studies suggest that some of these developmental steps are under independent genetic control, at least in some apomicts (van Dijk et al., 1999; Matzk et al., 2000; Noyes and Rieseberg, 2000).
THE INHERITANCE OF APOMIXIS
Genetic studies largely depend on crosses and recombination events neither of which is easily obtained in apomicts. However, because most apomicts produce normal, reduced pollen, the inheritance of apomixis can be investigated by analyzing the segregation ratios in crosses with related sexuals. Such analyses are difficult because gametophytic apomicts are almost without exception polyploids, causing complex modes of inheritance. An assessment of the breeding system in the hybrids requires cytological observations or, at least, time-consuming progeny tests. Variation in the expressivity of apomixis may create an additional complication. Moreover, much of the earlier work was done on the Rosaceae, which are extremely difficult to analyze because the multiple MMCs formed in sexuals make the distinction between reduced and unreduced embryo sacs difficult. As a consequence, for many years the genetics of apomixis seemed unclear, complex, and idiosyncratic. However, since the end of the 1970s, a clear general pattern in the inheritance of various types of gametophytic apomixis has emerged, first in the Ranunculaceae and Poaceae and later in the Compositae.
Using more suitable apomictic species and focusing on one element of apomixis, apomeiosis, pioneer studies by Nogler in the buttercup species Ranunculus auricomus and by Savidan in the grass Panicum maximum indicated that apospory in these two species segregated as a single dominant mendelian factor (Savidan, 1982; Nogler, 1984b, and references therein). Subsequent investigations showed that both apospory and diplospory in other species also fitted this segregation model (Table 1). Although a dominant mendelian factor can represent any genetic constitution from a single gene to an entire chromosome (e.g., mammalian sex determination), these observations often were taken as evidence for monogenic inheritance. According to this model, apomictic plants possess the simplex genotype Aaaa, carrying in addition to the dominant apomeiosis allele A several recessive alleles for sexual reproduction. Apomictic plants thus carry the potential for sexual reproduction, but in a more or less repressed state, because of the presence of the dominant apomixis factor. Limited penetrance of the apomixis factor explains the occurrence of facultative apomixis. The presence of recessive sexual alleles explains how a cross between two facultative apomicts can generate abundant purely sexual offspring. Although the occurrence of apomixis fits this model, the degree of apomixis often is dependent on environmental conditions (Nogler, 1984a) and/or on modifier genes (Bicknell et al., 2000). These as yet unspecified factors need further investigation in the future. Moreover, it remains to be determined whether this general model also applies to the many apomicts in the Rosaceae.
Table 1.
Inheritance of Elements of Gametophytic Apomixis (Apomeiosis and Parthenogenesis) in Members of the Ranunculaceae, Poaceae, and Compositae
| Species | Apomeiosis Type |
Family | Inferred Genotype |
Most Closely Linked Molecular Marker |
Evidence for Suppression Recombination |
Reference |
|---|---|---|---|---|---|---|
| Apomeiosis | ||||||
| Ranunculus auricomus | Apospory | Ranunculaceae | Aaaa | – | – | Nogler, 1984b |
| Panicum maximum | Apospory | Poaceae | Aaaa | – | – | Savidan, 1982 |
| Pennisetum squamulatum | Apospory | Poaceae | Aaaa | 0 cM | Yes | Ozias-Akins et al.,1998 |
| Brachiaria decumbens | Apospory | Poaceae | Aaaa | 1.2 cM | ? | Pessino et al., 1998 |
| Paspalum simplex | Apospory | Poaceae | Aaaa | 0 cM | Yes | Pupilli et al., 2001 |
| Hieracium piloselloides | Apospory | Compositae | Aaa | – | – | Bicknell et al., 2000 |
| Hieracium aurantiacum | Apospory | Compositae | Aaa | – | – | Bicknell et al., 2000 |
| Tripsacum dactyloides | Diplospory | Poaceae | Aaaa | 0 cM | Yes | Grimanelli et al., 1998a, 1998b |
| Erigeron annuus | Diplospory | Compositae | Aaa | 0 cM | Yes | Noyes and Rieseberg, 2000 |
| Taraxacum officinale | Diplospory | Compositae | Aaa | 4.4 cM | ? | van Dijk et al., unpublished |
| Parthenogenesis | ||||||
| Poa pratensis | Apospory | Poaceae | Pppp | 6.6 cM | ? | Barcaccia et al., 1998 |
| Erigeron annuus | Diplospory | Compositae | Ppp | 7.3 cM | No | Noyes and Rieseberg, 2000 |
The genetic models are based on segregation analyses of sexual × apomictic crosses and in most cases supported by cosegregation of closely linked molecular markers. No distinction is made between disomic (Aa/aa) and tetrasomic (Aaaa) inheritance. The distance between the apomixis locus and the most closely linked marker is indicated in centimorgans (cM). (–), not investigated; (?), no conclusive data.
THE APOMEIOSIS LOCUS IS LOCATED IN A RECOMBINATIONALLY SUPPRESSED REGION
The segregation model described above has been supported and refined by the isolation of molecular markers that are linked to the presumed apomixis loci in several species (Table 1). In all cases in which it has been critically tested to date, a strong suppression of recombination around the apomeiosis locus has been found. For instance, strict cosegregation with apomeiosis of many more molecular markers than expected was found in aposporous Pennisetum squamulatum (Ozias-Akins et al., 1998) and diplo-sporous Erigeron annuus (Noyes and Rieseberg, 2000). In Brachiaria decumbens (Pessino et al., 1998), Tripsacum dactyloides (Grimanelli et al., 1998a), and Paspalum simplex (Pupilli et al., 2001), comparative mapping with maize or rice markers showed a lack of recombination in the region associated with the apospory locus. Markers that were spread over a region ranging from 15 to 40 centimorgans in the sexual relatives strictly cosegregated in these apomicts. Repression of recombination could frustrate map-based cloning efforts because closely linked markers may be at great physical distances from the apomixis loci.
Because suppressed recombination occurs in both dicot and monocot species, it may be a general characteristic of apomeiosis loci. This could be related to their function as observed in other complex loci containing several genes involved in a common process (coadapted gene complexes), such as the heterostyly supergene in Primula (Ernst, 1936), the self-incompatibility (S) loci in Brassica (Lewis, 1962; Awadalla and Charlesworth, 1999), the mating-type locus in Chlamydo-monas (Ferris and Goodenough, 1994), and the major histocompatibility locus in humans (O'hUigin et al., 2000). Alternately, it could be an evolutionary by-product of long term asexual reproduction (Judson and Normark, 1996; Welch and Meselson, 2000). In Pennisetum species, markers that are linked to apospory in the apomicts could not be detected by hybridization in sexual relatives (Ozias-Akins et al., 1998; Roche et al., 1999), indicating that the apomicts were either hemizygous for the apomixis locus (A---) or that the alleles were highly divergent (A a′ a′ a′), as was observed for the Brassica S locus (Boyes et al., 1997; Suzuki et al., 1999).
ONE MASTER APOMIXIS GENE OR SEVERAL INDEPENDENT APOMIXIS GENES?
Apomictic development deviates from the sexual pathway in apomeiosis, parthenogenesis, and often endosperm development (autonomy, altered embryo sac development, or altered fertilization). Are these elements of apomixis all controlled by a single gene or by several genes? In the pioneering studies on R. auricomus and P. maximum, parthenogenesis was strictly associated with apospory. Hence, apomixis as a whole was inherited as a single mendelian trait (Savidan, 1982; Nogler, 1984b). Similarly, in Hieracium piloselloides, all three elements are inherited as a single genetic trait (Bicknell et al., 2000). In these species, apomixis could be regulated by a single master regulatory gene controlling all elements or by a gene complex of several tightly linked genes that are recombinationally locked. In other species, however, crosses between sexuals and apomicts have yielded progeny combining elements of both the sexual and the apomictic developmental pathways. In Taraxacum officinale, hybrids were recovered that displayed diplospory and autonomous endosperm development but that lacked parthenogenesis (van Dijk et al., 1999). Such “apomixis recombinants” also have been reported in Poa pratensis (Matzk et al., 2000), which suggests that the elements of apomixis in these species are regulated by different genes. In Erigeron annuus, separate genes for diplospory (A) and parthenogenesis (P) have been mapped genetically (Noyes and Rieseberg, 2000). Apomicts in this species carry the simplex genotype for both genes (Aaa and Ppp). In contrast to the diplospory A locus, no suppression of recombination was observed around the parthenogenesis P locus (Table 1). In light of these new findings, it is likely that coadapted gene complexes are present in those species in which all elements of apomixis cosegregate.
SEGREGATION DISTORTION OF APOMIXIS LOCI
As mentioned above, gametophytic apomicts are usually polyploid, whereas related sexuals are diploid. Is gametophytic apomixis incompatible with diploidy? Again, the pioneering work on R. auricomus by Nogler appears to have general relevance. Nogler showed that diploid offspring that developed parthenogenetically from reduced diploid egg cells of tetraploid apomicts (dihaploids) or diploids produced through anther culture were able to reproduce apomictically (Nogler, 1982). This shows that apomixis and diploidy are not incompatible, a finding that has been confirmed in several other species (Bicknell, 1997; Kojima and Nagato, 1997). However, what matters is the origin of the diploid offspring, because zygotic diploids derived from the fusion of haploid egg cells and haploid sperm never reproduced apomictically in Ranunculus. Nogler hypothesized that the apospory (A) locus was recessive lethal in the gametes. Consequently, the A locus could be transmitted via diploid gametes to generate polyploid apomicts but not via haploid gametes to generate diploid apomicts. It is also possible that mutations closely linked to the A locus cause haploid gamete nonfunctionality. The net result is that haploid gametes carrying the A locus do not contribute to offspring production, resulting in segregation distortion of the A locus.
More recently, additional evidence has been obtained for segregation distortion of apomixis loci in other plant species, such as Tripsacum dactyloides, Pennisetum squamulatum, and E. annuus (Grimanelli et al., 1998b; Ozias-Akins et al., 1998; Noyes and Rieseberg, 2000). Transmission studies of markers linked to apomixis loci in E. annuus indicate different causes of nontransmission: the parthenogenesis locus P in E. annuus was not transmitted because of selection against haploid gametes, as was observed for the A locus in R. auricomus. The diplospory locus A, in contrast, was not transmitted because of meiotic drive. In these triploid apomicts, the nondiplospory chromosomes seem to pair preferentially, leaving the diplospory chromosome as a univalent that always ends up in a diploid pollen grain (Noyes and Rieseberg, 2000). In Hieracium piloselloides, different crossing schemes indicate that apomixis can be transmitted via both haploid and diploid gametes, but post-zygotic lethality rather than segregation distortion causes the absence of apomixis in diploids (Bicknell et al., 2000).
CONCLUSIONS
Apomixis is a complex trait involving the modification of several steps of normal sexual development. Recent reports on the inheritance of apomixis have revealed several common features. (1) The genetic control of apomixis is dominant; this is true for all elements of apomixis studied so far. This observation is often taken as evidence for apomixis being caused by a mutated gene, but it is also compatible with the misexpression of wild-type genes playing key regulatory roles in sexual development (Koltunow, 1993; Grossniklaus, 2001). The latter view is supported by the fact that both sexual and apomictic reproduction coexist in facultative apomicts and that apomictic plants can be obtained from sexual progenitors through hybridization (reviewed in Carman, 2001) or simple chromosome doubling (Quarin et al., 2001). (2) The apomeiosis locus resides in a recombinationally suppressed region, suggesting that even in species in which the elements of apomixis are under common control, a complex of several coadapted genes may be present. (3) The apomixis locus is usually associated with gametic or zygotic lethality or its transmission is reduced by some other mechanism, with the result that haploid gametes do not produce progeny, thereby maintaining or driving the polyploidization associated with apomictic species.
As many reproductive mutants in sexual species indicate, disturbances of sexual development toward elements of apomixis can easily cause abortion and sterility. Selection has perfected natural apomicts, refining the developmental modifications and integrating them into a functional developmental system with high fertility that is likely controlled by coadapted gene complexes. Although many of the features discussed in this article pose obstacles to understanding the molecular basis of natural apomixis, a complementary approach using both sexual and apomictic model systems bears great promise that apomixis can eventually be harnessed to contribute to sustained agricultural production.
Acknowledgments
We thank Margaret Collinge, Christoph Ringli, and three anonymous reviewers for comments on the manuscript, and Peter van Baarlen and Daniel Grimanelli for helpful discussions.
References
- Asker, S.E., and Jerling, L. (1992). Apomixis in Plants. (Boca Raton, FL: CRC Press).
- Awadalla, P., and Charlesworth, D. (1999). Recombination and selection at Brassica self-incompatibility loci. Genetics 152, 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcaccia, G., Mazzucato, A., Albertini, E., Zethof, J., Gerats, A., Pezotti, M., and Falcinelli, M. (1998). Inheritance of parthenogenesis in Poa pratensis L.: Auxin test and AFLP linkage analysis support monogenic control. Theor. Appl. Genet. 97, 74–82. [Google Scholar]
- Bicknell, R.A. (1997). Isolation of a diploid, apomictic plant of Hieracium aurantiacum. Sex. Plant Reprod. 10, 168–172. [Google Scholar]
- Bicknell, R.A., Borst, N.K., and Koltunow, A.M. (2000). Monogenic inheritance of apomixis in two Hieracium species with distinct developmental mechanisms. Heredity 84, 228–237. [DOI] [PubMed] [Google Scholar]
- Boyes, D.C., Nasrallah, M.E., Vrebalov, J., and Nasrallah, J.B. (1997). The self-incompatibility (S) haplotypes of Brassica contain highly divergent and rearranged sequences of ancient origin. Plant Cell 9, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman, J.G. (2001). The gene effect: Genome collision and apomixis. In The Flowering of Apomixis: From Mechanisms to Genetic Engineering, Y. Savidan, J.G. Carman, and T. Dresselhaus, eds (Mexico, D.F.: CIMMYT, IRD, European Commission DG VI), pp. 95–110.
- Crane, C.F. (2001). Classification of apomictic mechanisms. In The Flowering of Apomixis: From Mechanisms to Genetic Engineering, Y. Savidan, J.G. Carman, and T. Dresselhaus, eds (Mexico, D.F.: CIMMYT, IRD, European Commission DG VI), pp. 24–43.
- Drews, G.N., Lee, D., and Christensen, C.A. (1998). Genetic analysis of female gametophyte development and function. Plant Cell 10, 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst, A. (1936). Heterostylie-Forschung: Versuche zur genetischen Analyse eines Organisations und “Anpassungs” Merkmales. Z. Indukt. Abstammungs. Vererbungsl. 71, 156–230. [Google Scholar]
- Ferris, P.J., and Goodenough, U. (1994). The mating-type locus of Chlamydomonas reinhardtii contains highly rearranged DNA sequences. Cell 76, 1135–1145. [DOI] [PubMed] [Google Scholar]
- Grimanelli, D., LeBlanc, O., Espinosa, E., Perotti, E., Gonzalez de Leon, D., and Savidan, Y. (1998. a). Mapping diplo-sporous apomixis in tetraploid Tripsacum: One gene or several genes? Heredity 80, 33–39. [DOI] [PubMed] [Google Scholar]
- Grimanelli, D., LeBlanc, O., Espinosa, E., Perotti, E., Gonzalez de Leon, D., and Savidan, Y. (1998. b). Non-mendelian transmission of apomixis in maize-Tripsacum hybrids caused by a transmission ratio distortion. Heredity 80, 40–47. [DOI] [PubMed] [Google Scholar]
- Grossniklaus, U. (2001). From sexuality to apomixis: Molecular and genetic ap-proaches. In The Flowering of Apomixis: From Mechanisms to Genetic Engineering, Y. Savidan, J.G. Carman, and T. Dresselhaus, eds (Mexico, D.F.: CIMMYT, IRD, European Commission DG VI), pp. 168–211.
- Grossniklaus, U., and Schneitz, K. (1998). Genetic and molecular control of ovule development and megagametogenesis. Semin. Cell Dev. Biol. 9, 227–238. [DOI] [PubMed] [Google Scholar]
- Grossniklaus, U., Koltunow, A., and van Lookeren Campagne, M. (1998). A bright future for apomixis. Trends Plant Sci. 3, 415–416. [Google Scholar]
- Grossniklaus, U., Spillane, C., Page, D.R., and Köhler, C. (2001). Genomic imprinting and seed development: Endosperm formation with and without sex. Curr. Opin. Plant Sci. 4, 21–27. [DOI] [PubMed] [Google Scholar]
- Gustafsson, A. (1947. a). Apomixis in angio-sperms. II. Lunds Univ. Årsskr. NF II 42, 71–179. [Google Scholar]
- Gustafsson, A. (1947. b). Apomixis in angio-sperms. III. Lunds Univ. Årsskr. NF II 43, 183–370. [Google Scholar]
- Hanna, W.W., and Bashaw, E.C. (1987). Apomixis: Its identification and use in plant breeding. Crop Sci. 27, 1136–1139. [Google Scholar]
- Jefferson, R.A. (1994). Apomixis: A social revolution for agriculture? Biotechnology and Development Monitor 19, 14–16. [Google Scholar]
- Judson, O.P., and Normark, B.B. (1996). Ancient asexual scandals. Trends Ecol. Evol. 11, 41–46. [DOI] [PubMed] [Google Scholar]
- Kojima, A., and Nagato, Y. (1997). Discovery of highly apomictic and highly amphimictic dihaploids in Allium tuberosum. Sex. Plant Reprod. 10, 8–12. [Google Scholar]
- Koltunow, A.M. (1993). Apomixis: Embryo sacs and embryos formed without meiosis or fertilization in ovules. Plant Cell 5, 1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltunow, A.M., Bicknell, R.A., and Chaudhury, A.M. (1995). Apomixis: Molecular strategies for the generation of genetically identical seeds without fertilization. Plant Physiol. 108, 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, D. (1962). The generation of self-incompatibility alleles. J. Theor. Biol. 2, 69–71. [Google Scholar]
- Matzk, F., Meister, A., and Schubert, I. (2000). An efficient screen for reproductive pathways using mature seeds of monocots and dicots. Plant J. 21, 97–108. [DOI] [PubMed] [Google Scholar]
- Nogler, G.A. (1982). How to obtain diploid apomictic Ranunculus auricomus plants not found in the wild state. Bot. Helv. 92, 13–22. [Google Scholar]
- Nogler, G.A. (1984a). Gametophytic apomixis. In Embryology of Angiosperms, B.M. Johri, ed (Berlin: Springer-Verlag), pp. 475–518.
- Nogler, G.A. (1984. b). Genetics of apospory in apomictic Ranunculus auricomus. V. Conclusion. Bot. Helv. 94, 411–422. [Google Scholar]
- Noyes, R.D., and Rieseberg, L.H. (2000). Two independent loci control agamospermy (apomixis) in the triploid flowering plant Erigeron annuus. Genetics 155, 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'hUigin, C., Satta, Y., Hausmann, A., Dawkins, R.L., and Klein, J. (2000). The implications of intergenic polymorphism for major histocompatibility complex evolution. Genetics 156, 867–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozias-Akins, P., Roche, D., and Hanna, W.W. (1998). Tight clustering and hemizygosity of apomixis-linked molecular markers in Pennisetum squamulatum implies genetic control of apospory by a divergent locus that may have no allelic form in sexual genotypes. Proc. Natl. Acad. Sci. USA 95, 5127–5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessino, S.C., Evans, C., Ortiz, J.P.A., Armstead, I., Do Valle, C.B., and Hayward, M.D. (1998). A genetic map of the apospory region in Brachiaria hybrids: Identification of two markers closely associated with the trait. Hereditas 128, 153–158. [Google Scholar]
- Petrov, D.F., Belousova, N.I., and Fokina, E.S. (1979). Inheritance of apomixis and its elements in corn-Tripsacum hybrids. Genetika 15, 1827–1836. [Google Scholar]
- Pupilli, F., Labombarda, P., Caceres, M.E., Quarin, Q.L., and Arcioni, S. (2001). The chromosome segment related to apomixis in Paspalum simplex is homoeologous to the telomeric region of the long arm of rice chromosome 12. Mol. Breed., in press.
- Quarin, C.L., Espinoza, F., Martinez, E.J., Pessino, S.C., and Bovo, O.A. (2001). A rise of ploidy level induces the expression of apomixis in Paspalum notatum. Sex. Plant Reprod. 13, 243–249. [Google Scholar]
- Roche, D., Cong, P., Chen, Z., Hanna, W.W., Gustine, D.L., Sherwood, R.T., and Ozias-Akins, P. (1999). An apospory-specific genomic region is conserved between buffelgrass (Cenchrus ciliaris L.) and Pennisetum squamulatum Fresen. Plant J. 19, 203–208. [DOI] [PubMed] [Google Scholar]
- Savidan, Y. (1982). Nature et hérédité de l' apomixie chez Panicum maximum Jacq. Trav. & Doc. Orstom 153, 1–159. [Google Scholar]
- Savidan, Y. (1992). Progress in research on apomixis and its transfer to major grain crops. In Reproductive Biology and Plant Breeding, Y. Dattée, C. Dumas, and A. Gallais, eds (Berlin: Springer-Verlag), pp. 269–279.
- Savidan, Y. (2000). Apomixis: Genetics and breeding. Plant Breed. Rev. 18, 13–86. [Google Scholar]
- Suzuki, G., Kai, N., Hirose, T., Fukui, K., Nishio, T., Takayama, S., Isogai, A., Watanabe, M., and Hinata, K. (1999). Genomic organization of the S locus: Identification and characterization of genes in SLG/SRK region of S9 haplotype of Brassica campestris (syn. rapa). Genetics 153, 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenissen, G.H. (2001). Feeding the world in the 21st century: Plant breeding, biotechnology, and the potential role of apomixis. In The Flowering of Apomixis: From Mechanisms to Genetic Engineering, Y. Savidan, J.G. Carman, and T. Dresselhaus, eds (Mexico, D.F.: CIMMYT, IRD, European Commission DG VI), pp. 1–7.
- van Dijk, P.J., Tas, I.C.Q., Falque, M., and Bakx-Schotman, J.M.T. (1999). Crosses between sexual and apomictic dandelions (Taraxacum). II. The breakdown of apomixis. Heredity 83, 715–721. [DOI] [PubMed] [Google Scholar]
- Vielle-Calzada, J.-P., Crane, C.F., and Stelly, D.M. (1996). Apomixis: The asexual revolution. Science 274, 1322–1323. [Google Scholar]
- Welch, D.M., and Meselson, M. (2000). Evidence for the evolution of bdelloid rotifers without sexual reproduction or genetic exchange. Science 288, 1211–1215. [DOI] [PubMed] [Google Scholar]
- Yang, W.-C., and Sundaresan, V. (2000). Genetics of gametophyte biogenesis in Arabidopsis. Curr. Opin. Plant Sci. 3, 53–57. [DOI] [PubMed] [Google Scholar]