High light (beyond what is needed for maximum photosynthesis) is a major plant stress. Under extreme high-light conditions, the photosynthetic apparatus can be damaged irreversibly, but plants and algae have devised various strategies to protect themselves (photoprotection) (Björkman and Demmig-Adams, 1994). One of the strategies for survival in high light is to eliminate the excess absorbed energy as heat (thermal dissipation), which can be measured as nonphotochemical quenching (NPQ) of chlorophyll fluorescence. The process of NPQ involves acidification of the lumen of the photosynthetic membranes, operation of the xanthophyll cycle, and specific components of the antenna of photosystem II (PSII).
In this issue of The Plant Cell, Elrad et al. (pages 1801–1816) characterize a mutant of Chlamydomonas reinhardtii, designated npq5, that is defective in its ability to establish rapid, reversible NPQ of chlorophyll a fluorescence. This mutant lacks a major light-harvesting polypeptide (Lhcbm1), suggesting that Lhcbm1 is required for the cells to elicit NPQ. The relationship between specific antenna complexes and NPQ has been, and still is, hotly debated. The article by Elrad et al. (2002), which shows a strong correlation between a protein of the trimeric complexes of light-harvesting complex IIb (LHCIIb) and NPQ, adds to this excitement.
PHOTOSYNTHESIS AND CHLOROPHYLL FLUORESCENCE
The first act of photosynthesis is the absorption of photons by antenna molecules (within femtoseconds), leading to the formation of excited chlorophyll molecules (Chl*). These Chl* decay to ground state by (1) excitation energy transfer to reaction centers, leading to photochemistry, (2) light emission as fluorescence, and (3) heat loss. The production of oxygen, NADPH, and ATP requires the four major multiprotein complexes of PSI and PSII, the cytochrome b6/f complex (Cyt bf), and ATP synthase, plus a multitude of antenna complexes whose major function is to transfer excitation energy to the photosynthetic reaction centers. Another crucial function of antenna complexes is to serve as a safety valve for the thermal dissipation of excess absorbed light energy.
The primary charge separations of photosynthesis occur simultaneously in the reaction center chlorophylls P700 (of PSI) and P680 (of PSII). Photochemistry is over within picoseconds, and all further reactions can proceed in darkness. The positive charges produced by PSII oxidize water to molecular O2, and the negative charges reduce plastoquinone to plastoquinol. Protons of water are released into the lumen. Plastoquinol also releases protons into the lumen of the thylakoids as it transfers electrons to Cyt bf.
The positive charges produced by PSI ultimately are reduced by electrons available from Cyt bf, and the negative charges reduce NADP+ to NADPH. The energy stored in the proton gradient across the thylakoid membranes is used to synthesize ATP (by the ATP synthase complex). However, when photosynthesis becomes saturated and excess light is absorbed, NADPH and ATP are not made rapidly enough to dissipate the proton gradient, and the pH of the thylakoid lumen decreases. Acidification of the lumen initiates reactions leading to photoprotection events that involve changes in the deexcitation of Chl*. One path for this deexcitation is fluorescence.
Chlorophyll fluorescence of antenna molecules has been used over the years as one of the most powerful noninvasive tools to probe photosynthesis and the ways in which excitation energy is dissipated within the light-harvesting and reaction center complexes (Govindjee, 1995). An important caveat for the analysis of chlorophyll fluorescence measurements is that changes in fluorescence intensity are not necessarily correlated with changes in the quantum yield of fluorescence (the number of photons emitted as fluorescence divided by the number of photons absorbed). Chlorophyll fluorescence of PSI and its light-harvesting complex, LHCI, is very weak and is relatively unaffected as the intensity of light in the environment changes. However, chlorophyll fluorescence from the antenna of PSII is much stronger than that of PSI, and it changes dramatically as environmental conditions change.
Errors in interpretation are possible, for example, if light-harvesting complexes move from the strongly fluorescent PSII region of the photosynthetic apparatus to the weakly fluorescent PSI regions. This movement of antennae between PSII and PSI is designated “state changes” (Allen and Forsberg, 2001). When oxygenic photosynthetic organisms are exposed to excess PSII light, plastoquinol accumulates and, through a series of biochemical events, may stimulate the phosphorylation of LHCIIb polypeptides, which then can move physically from the PSII region to the PSI region of the photosynthetic membranes. This reorganization, designated state II, causes a decrease in fluorescence intensity but not fluorescence yield. If plants are exposed to light absorbed predominantly by PSI, the process is reversed and the organism enters state I, which is characterized by higher fluorescence intensity (but not yield).
To guard against misinterpretations of fluorescence measurements, both the lifetimes of fluorescence, which directly reflect quantum yields, and the state changes must be measured. A convenient way to measure state changes is through low-temperature (77K) fluorescence spectra in which both PSII and PSI are strongly fluorescent, but with emission peaks at specific wavelengths. In state II, the ratio of fluorescent bands at 685 and 695 nm (both from PSII) to those at 720 and 735 nm (both from PSI) are much lower than in state I.
PHOTOPROTECTION: THE ROLE OF PROTONATION AND THE XANTHOPHYLL CYCLE
A role of xanthophylls in photoprotective heat dissipation was demonstrated by Demmig et al. (1988)(reviewed by Demmig- Adams et al., 1996). The xanthophyll cycle (Yamamoto et al., 1999) involves the conversion of violaxanthin (V) to zeaxanthin (Z) via antheraxanthin (A). Thermal dissipation is correlated with the disappearance of V and the appearance of A and/or Z, and the cycle is reversed when excess light is removed.
The hypothesized molecular mechanism can be summarized as follows. Excess protons that accumulate in the lumen (see above) can (1) stimulate the deepoxidation of V, and (2) protonate one or more of the antenna complexes of PSII. Process 2 may (a) promote a close association of Z with Chl*, or (b) cause a conformational change in the antenna complexes that leads to increased heat loss from Chl*. In mechanism a, excitation energy would be transferred from Chl* to Z, and a dissipation of energy as heat would take place in Z. There is a great deal of debate regarding whether mechanism a or b is correct, but it is possible that both mechanisms operate, depending on the species and/or environ-mental conditions.
THE DIMMER SWITCH
When plants are exposed to excess light, the quantum yield of chlorophyll a fluorescence (from the antenna of PSII) is decreased (i.e., there is a quenching of chlorophyll a fluorescence). The possibility of a photochemical component of fluorescence quenching at saturating light, involving a photoprotective role for O2 as an electron sink, has been discussed recently by Ort and Baker (2002). However, the nonphotochemical character of this process is demonstrated by the observation that it occurs even when photochemistry is saturated and all QA molecules (the first quinone electron acceptor of PSII) are in the reduced state (QA−). Gilmore et al. (1995)(1998) have shown by measuring the lifetime of chlorophyll a fluorescence in several higher plants that as V is converted to Z during thermal dissipation and NPQ, the fraction of a 2-ns component of the chlorophyll fluorescence lifetime decreases, along with a concomitant increase in a 0.4-ns component.
This is a reversible phenomenon that has been referred to as the “dimmer switch.” It represents not only reduced fluorescence but also a means of reducing the excitation energy that is directed from the antennae to the reaction center, because the energy stored in Chl* is lost rapidly via processes that promote thermal dissipation within the antennae. As fluorescence yield decreases, the yield of thermal dissipation increases.
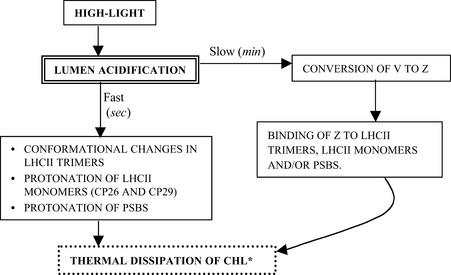
Figure 1 is a generalized but all-inclusive scheme of some of the possibilities involved in the chemistry and components of the dimmer switch. Excess light leads to overacidification of the thylakoid lumen that promotes the conversion of V to Z and also the protonation of lumenal polypeptides, perhaps the minor chlorophyll–protein complexes (CP26 and CP29) and/or PsbS. Alternatively, conformation changes may occur in LHCIIb trimers. On the other hand, binding of Z to PsbS, to minor antenna (CP26 and CP29), or to LHCIIb trimers may lead to NPQ, either directly by heat loss in Z or by “self-quenching” by chlorophyll a itself. More research is needed to clearly define the mechanistic aspects of this process.
Figure 1.
Model of Thermal Dissipation in Vascular Plants and Green Alga.
High light results in lumen acidification, which in turn stimulates changes in the antenna of PSII that promote thermal dissipation. The exact nature of these changes has not been defined, but they may include conformational changes in LHCII trimers, protonation of and conformational changes in the LHCII monomers and/or minor antenna (CP26, CP29, etc.), and/or protonation of and conformational changes in PsbS. These complexes all have been implicated as sites of thermal dissipation. The acidification also stimulates the deepoxidation of V to Z. Z acts either “directly” (by accepting excitation energy from Chl* and dissipating it as heat) or “indirectly” (by altering antenna conformation) to further increase the rate of thermal dissipation. (Figure courtesy of Dafna Elrad.)
THE DEBATE: LHCIIb VERSUS MINOR CHLOROPHYLL COMPLEXES
There is general agreement that the xanthophyll cycle–dependent photoprotection mechanism involves the thermal dissipation of excitation in the antenna of PSII. The debate that is highlighted by Elrad et al. (2002) concerns the nature of the antenna complexes that are critical for this dissipation. Is it LHCIIb, the major peripheral antenna, the minor antenna (e.g., CP26 and CP29), or another component? There are 10 genes in Chlamydomonas that encode the major Lhcb polypeptides that form the trimeric domain of LHCII. There are also minor, monomeric Lhcb polypeptides that may link the trimeric domain of the complex to the core of PSII.
One hypothesis is that the peripheral LHCIIb complex is involved through structural/conformational changes (reviewed by Horton et al., 1999); another hypothesis suggests that the internal minor antenna plays a primary role (Bassi et al., 1993; Crofts and Yerkes, 1994). It is likely that nature has invented various ways to do the same thing depending on the detailed architecture of the photosynthetic apparatus in the different organisms and the different environments in which these organisms are grown.
Bassi et al. (1993) suggested the involvement of CP29 and CP26 in NPQ as a result of preferential binding of xanthophylls to these proteins. Furthermore, Frank et al. (2001) and Crimi et al. (2001) showed that the binding of Z leads to a shorter lifetime for the chlorophyll fluorescence component in vitro in CP26 and CP29, respectively. On the other hand, Andersson et al. (2001), using antisense Arabidopsis plants that lacked CP26 or CP29, showed that the loss of the minor antenna complexes did not alter NPQ. Obviously, additional research is necessary before a final decision can be made regarding the role of CP26 or CP29 in photoprotection.
Gilmore et al. (1996), using the chlorina f104 mutant of barley grown over a broad range of light intensities, have shown that PSII-related activities, including NPQ, do not change even when a major change occurs in the chlorophyll a/b ratios and the antenna size. Because this mutant is known to have reduced levels of the trimeric Lhcb, it was concluded that this domain of the antennae is not involved in NPQ. On the other hand, Chow et al. (2000) observed, in intermittent-light-grown plants transferred to continuous light, a correlation between increasing levels of chlorophyll b and LHCIIb and NPQ, suggesting a role of LHCIIb in thermal dissipation. To clarify these relationships, experiments are needed in which both the major and minor LHCII polypeptides, as well as PsbS, are quantified in the same samples that are used to measure NPQ.
NPQ MUTANTS OF ARABIDOPSIS AND CHLAMYDOMONAS
Higher plants are closely related to green algae; thus, it is natural to use single-celled green algae as model systems for higher plants. In the early days of Otto Warburg and Robert Emerson, Chlorella was the chosen green alga. However, the current alga of choice is Chlamydomonas, because it can grow heterotrophically (using acetate as a source of fixed carbon in the absence of photosynthesis) and is amenable to manipulation using sophisticated molecular techniques (Rochaix et al., 1998; Grossman, 2000).
Niyogi et al. (1997) made a major advance when they isolated several mutants of Chlamydomonas, especially those blocked in the conversion of V to Z (npq1) and Z to V (npq2), among others. Subsequently, it was found that the npq2 mutant accumulates Z and has a higher true NPQ than wild-type cells (Govindjee and Seufferheld, 2002). Holub et al. (2000) have demonstrated a lower lifetime of chlorophyll a fluorescence in single cells of the npq2 mutant of Chlamydomonas, confirming that the change is in the quantum yield of fluorescence and thus in thermal dissipation.
Similarly, research on Arabidopsis mutants has established a central role of the xanthophyll cycle in NPQ and thus in photoprotection (Niyogi et al., 1998; reviewed by Niyogi, 1999). However, it is clear that there are some differences in the characteristics of NPQ in Arabidopsis and Chlamydomonas. For example, 80% of NPQ that develops in Arabidopsis requires the xanthophyll cycle, whereas only ∼30% of the Chlamydomonas NPQ is xanthophyll cycle dependent. Thus, caution is advised when extrapolating data from one organism to the other.
HIGHLIGHTING THE npq5 MUTANT: THE ROLE OF THE Lhcbm1 GENE PRODUCT
The npq5 mutant isolated by Elrad et al. (2002) clearly establishes a relationship between the generation of NPQ and trimeric LHCIIb. First, there is a correlation between reduced NPQ and the absence of the Lhcbm1 gene in the mutant. Elrad et al. (2002) show that the lesion in Lhcbm1 is responsible for the decrease in NPQ, because transformation of the mutant strain with the Lhcbm1 gene led to the restoration of normal NPQ. Furthermore, by measuring fluorescence intensities and ratios of fluorescence emitted at 680 and 710 nm (which reflect the ratio of antenna chlorophyll associated with PSII relative to PSI) after experimentally induced state changes, they show convincingly that there are no differences in the state changes between the wild type and the mutant strain.
Because mobile LHCIIb is responsible for state changes, these results suggest that the mobile components of LHCIIb are not affected by the loss of Lhcbm1. This demonstrates that there is at least some functional distinction between the components needed for NPQ and those needed for LHCIIb mobility. Further research is needed to determine whether Lhcbm1 is or is not also part of the mobile species.
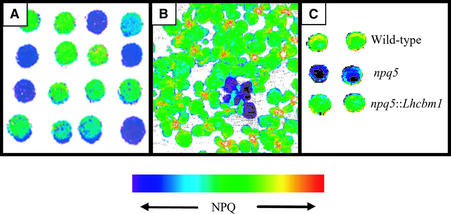
Figure 2 shows false-color images of NPQ in colonies of wild-type Chlamydomonas and a mutant defective for thermal dissipation (A) and in normal Arabidopsis plants and an npq mutant (B). Figure 2C shows two colonies each of wild-type Chlamydomonas cells, the npq5 mutant (which has an incomplete pJD67 insert in the first exon of the Lhcbm1 gene; the insertion event led to a deletion of 320 bp), and a complemented strain (a transformant harboring an ectopic Lhcbm1 gene). These results beautifully depict the differences in NPQ among these strains.
Figure 2.
False-Color Image of NPQ Generated by Video Imaging of Chlorophyll Fluorescence.
(A) Wild-type Chlamydomonas cells and a mutant strain defective in thermal dissipation.
(B) Wild-type Arabidopsis seedlings and seedlings of a mutant defective in thermal dissipation.
(C) Wild-type Chlamydomonas, the npq5 mutant strain, and the npq5 mutant strain complemented with the Lhcbm1 gene.
In (A) and (B), the mutant cells and seedlings appear dark blue, indicating a decrease in NPQ relative to the wild type, which appear green with some yellow-orange specks (as indicated on the false-color scale). (Figure courtesy of Dafna Elrad.)
The npq5 mutant showed the following differences from wild type cells:
(1) A much lower (∼45% of the wild-type level) NPQ of chlorophyll a fluorescence when exposed to 350 μmol·m−2·s−1. It remains to be determined how these differences would change when cells are placed in different conditions (to achieve higher NPQ values than in the samples used here) and when different concentrations of DTT (an inhibitor of V deepoxidase) are used to vary the concentration of Z that is formed.
(2) Fifty percent far-red light reversal of NPQ in the mutant compared with 85% in wild-type cells.
(3) Reversal of NPQ of only 10% after treatment with 10 μM nigericin, compared with 75% in wild-type cells. This shows that the proton gradient across the thylakoid membranes is important for eliciting NPQ. Although small differences in the extent of this gradient between mutant and wild-type cells could cause differences in the final level of NPQ (Govindjee and Spilotro, 2002), none of the photosynthetic measurements made by Elrad et al. (2002) suggest that the mutant is abnormal for the generation of the proton gradient. However, it would be useful to measure the proton gradient precisely in the npq5 mutant; the kinetics of the “P-to-S” chlorophyll a fluorescence decay (at varying nigericin concentrations) can be used to evaluate proton accumulation in the lumen.
(4) Lower NPQ in the mutant at all light intensities relative to wild-type cells, suggesting that there are intensity-dependent differences in the level of NPQ between the npq5 mutant and wild-type cells.
(5) A significantly greater degree of photoinhibition after 10 min of exposure with ∼1000 μmol·m−2·s−1 light and treatment with lincomycin (which limits the repair of PSII by inhibiting translation on chloroplast ribosomes).
(6) Decreases in the pigments in the mutant strain: chlorophyll a (by 18%); chlorophyll b (by 27%); xanthophyll pool (by 15%); and neoxanthin and loroxanthin (together by 34%). The chlorophyll a/b ratio is slightly higher (10%) in the mutant, which could be ascribed to decreases in an LHCIIb component. These changes in pigments need to be understood more fully through the isolation, characterization, and quantification of pigment protein complexes in mutant and wild-type cells.
Nevertheless, the npq5 mutant and wild-type cells are similar in many respects. The ratio of Z+A/Z+A+V (deepoxidation state of the cells) at various light intensities and the kinetics of Z formation in high light are essentially identical in mutant and wild-type cells. Elrad et al. (2002) concluded from this observation that the development of the pH gradient across the thylakoid membranes is the same in mutant and wild-type cells, although the gradient was not measured directly (and should be).
One very important observation is that the “state transitions” are not changed in the mutant. Furthermore, the maximum photosynthetic rate, measured as Fv/Fm (maximum photochemical efficiency of PSII in the dark-adapted state), and the doubling time are the same for the mutant and the wild-type strains. Thus, under standard growth conditions, Lhcbm1 is not of crucial importance, although a more pronounced phenotype may be observed if the mutant is exposed to higher intensity light and/or other stressful conditions (e.g., desiccation and nutrient deprivation).
Future research also should focus on the role of PsbS (Li et al., 2000) and perhaps PsbZ (Swiatek et al., 2001) polypeptides in the use of excitation energy and their possible interactions with other poly-peptides of LHCII. Although no PsbS has been shown to exist in Chlamydomonas, its role in the NPQ of Arabidopsis has been established beyond any doubt.
CONCLUDING REMARKS
I end this essay with a quotation from Vauvenargues: “Those who cannot manage to look from many viewpoints sometimes attribute to one entire object what actually belongs only to the little they are aware of. The neatness of their ideas hinders them from being suspicious.” I would add “suspicious of their own ideas.” I admit that this may apply to me as it may apply to others involved in the debate regarding which antenna is more important in protecting against damage by excess light. I believe that we have an elephant before us, and different scientists are looking at different parts of this elephant. The key is to keep working hard but also to engage in rational discussions and focused collaborations with those who think differently than us.
I thank Nancy Eckardt, Arthur Grossman, Adam Gilmore, and Krishna Niyogi for their various suggestions. The arguments presented in published papers and even those presented here are not “watertight”; thus, this field of research has a tremendous future. The views expressed here are solely mine, and I take full responsibility for all errors.
References
- Allen, J.F., and Forsberg, J. (2001). Molecular recognition in thylakoid structure and function. Trends Plant Sci. 6, 317–326. [DOI] [PubMed] [Google Scholar]
- Andersson, J., Walters, R.G., Horton, P., and Jansson, S. (2001). Antisense inhibition of the photosynthetic antenna proteins CP29 and CP26: Implications for the mechanism of photoprotective energy dissipation. Plant Cell 13, 1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi, R., Pineau, B., Dainese, P., and Marquard, J. (1993). Carotenoid-binding proteins of photosystem II. Eur. J. Biochem. 212, 297–303. [DOI] [PubMed] [Google Scholar]
- Björkman, O., and Demmig-Adams, B. (1994). Regulation of photosynthetic light energy capture, conversion and dissipation in leaves of higher plants. In Ecological Studies, Vol. 100, E.-D. Schulze and M. Caldwell, eds (Berlin: Springer Verlag), pp. 17–47.
- Chow, W.S., Funk, C., Hope, A.B., and Govindjee (2000). Greening of intermittent-light-grown bean plants in continuous light: Thylakoid components in relation to photosynthetic performance and capacity for photoprotection. Indian J. Biochem. Biophys. 37, 395–404. [PubMed] [Google Scholar]
- Crimi, M., Dorra, D., Bösinger, C.S., Giuffra, E., Holzwarth, A.R., and Bassi, R. (2001). Time-resolved fluorescence analysis of the recombinant photosystem II antenna complex CP29: Effects of zeaxanthin, pH and phosphorylation. Eur. J. Biochem. 268, 260–267. [DOI] [PubMed] [Google Scholar]
- Crofts, A.R., and Yerkes, C.T. (1994). A molecular mechanism for QE quenching. FEBS Lett. 352, 265–270. [DOI] [PubMed] [Google Scholar]
- Demmig, B., Winter, K., Krüger, A., and Czygan, F.-C. (1988). Zeaxanthin and the heat dissipation of excess light energy in Nerium oleander exposed to a combination of high light and water stress. Plant Physiol. 87, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams, B., Gilmore, A.M., and Adams, W. (1996). Carotenoids. 3. In vivo functions of carotenoids in higher plants. FASEB J. 10, 403–412. [DOI] [PubMed] [Google Scholar]
- Elrad, D., Niyogi, K.K., and Grossman, A. (2002). A major light harvesting polypeptide of photosystem II functions in thermal dissipation. Plant Cell 14, 1801–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, H.A., Das, S.K., Bautista, J.A., Bruce, D., Vasil'ev, S., Crimi, M., Croce, R., and Bassi, R. (2001). Photochemical behavior of xanthophylls in the recombinant photosystem II antenna complex, CP26. Biochemistry 40, 1220–1225. [DOI] [PubMed] [Google Scholar]
- Gilmore, A.M., Hazlett, T.L., Debrunner, P.G., and Govindjee (1996). Photosystem II chlorophyll a fluorescence lifetimes and intensity are independent of the antenna size differences between barley wild type and chlorina mutants: Photochemical quenching and xanthophyll cycle-dependent non-photochemical quenching of fluorescence. Photosynth. Res. 48, 171–187. [DOI] [PubMed] [Google Scholar]
- Gilmore, A.M., Hazlett, T.L., and Govindjee (1995). Xanthophyll cycle dependent quenching of photosystem II chlorophyll a fluorescence: Formation of a quenching complex with a short lifetime. Proc. Natl. Acad. Sci. USA 92, 2273–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore, A.M., Shinkarev, V.P., Hazlett, T.L., and Govindjee (1998). Quantitative analysis of the effects of intrathylakoid pH and xanthophyll cycle pigments on chlorophyll a lifetime distributions and intensity in thylakoids. Biochemistry 37, 13582–13593. [DOI] [PubMed] [Google Scholar]
- Govindjee (1995). Sixty-three years since Kautsky: Chlorophyll a fluorescence. Aust. J. Plant Physiol. 22, 131–160. [Google Scholar]
- Govindjee, and Seufferheld, M. (2002). Non-photochemical quenching of chlorophyll a fluorescence: Early history and characterization of two xanthophyll cycle mutants of Chlamydomonas reinhardtii. Funct. Plant Biol., in press. [DOI] [PubMed]
- Govindjee, and Spilotro, P. (2002). An Arabidopsis thaliana mutant, altered in the γ subunit of ATP synthase, has a different pattern of intensity-dependent changes in non-photochemical quenching and kinetics of the P-to-S fluorescence decay. Funct. Plant Biol. 29, 425–434. [DOI] [PubMed] [Google Scholar]
- Grossman, A.R. (2000). Chlamydomonas reinhardtii and photosynthesis: Genetics to genomics. Curr. Opin. Plant Biol. 3, 132–137. [DOI] [PubMed] [Google Scholar]
- Holub, O., Seufferheld, M.J., Gohlke, C., Govindjee, and Clegg, R.M. (2000). Fluorescence lifetime imaging (FLI) in real time: A new technique in photosynthesis research. Photosynthetica 38, 581–599. [Google Scholar]
- Horton, P., Ruban, A.V., and Young, A.J. (1999). Regulation of the structure and function of the light harvesting complexes of photosystem II by the xanthophyll cycle. In The Photochemistry of Carotenoids, H.A. Frank, A.J. Young, G. Britton, and R.J. Cogdell, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 271–291.
- Li, X.I., Björkman, O., Shih, C., Grossman, A.R., Rosenquist, M., Jansson, S., and Niyogi, K.K. (2000). A pigment-binding protein essential for regulation of pho-tosynthetic light harvesting. Nature 403, 391–395. [DOI] [PubMed] [Google Scholar]
- Niyogi, K.K. (1999). Photoprotection revisited: Genetic and molecular approaches. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 333–359. [DOI] [PubMed] [Google Scholar]
- Niyogi, K.K., Björkman, O., and Grossman, A.R. (1997). Chlamydomonas xanthophyll cycle mutants identified by video imaging of chlorophyll fluorescence quenching. Plant Cell 9, 1369–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi, K.K., Grossman, A.R., and Björkman, O. (1998). Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10, 1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort, D., and Baker, N.R. (2002). A photoprotective role for O2 as an electron sink in photosynthesis? Curr. Opin. Plant Biol. 5, 193–198. [DOI] [PubMed] [Google Scholar]
- Rochaix, J.-D., Goldschmidt-Clermont, M., and Merchant, S., eds (1998). The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. (Dordrecht, The Netherlands: Kluwer Academic Publishers).
- Swiatek, M., Kuras, R., Sokolenko, A., Higgs, D., Olive, J., Cinque, G., Müller, B., Eichacker, L.A., Stern, D.B., Bassi, R., Herrman, R.G., and Wollman, F.-A. (2001). The chloroplast gene ycf9 encodes a photosystem II (PSII) core subunit, PsbZ, that participates in PSII supramolecular architecture. Plant Cell 13, 1347–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, H.Y., Bugos, R.C., and Hieber, A.D. (1999). Biochemistry and molecular biology of the xanthophyll cycle. In The Photochemistry of Carotenoids, H.A. Frank, A.J. Young, G. Britton, and R.J. Cogdell, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 293–303.