Abstract
Plant cells have an acute sense for pathogen-derived chemical stimuli, so-called elicitors, which induce the plant's defense response. To investigate the molecular basis of chemosensory transduction, elicitor-treated tomato cells were labeled with 1-min pulses of [32P] phosphate. This technique revealed drastic changes in protein phosphorylation in vivo within minutes of stimulation. The protein kinase inhibitors K-252a and staurosporine completely prevented these elicitor-induced changes in protein phosphorylation. They also blocked two early biochemical responses to elicitors, extracellular alkalinization and biosynthesis of ethylene. The ability of K-252a, staurosporine, and benzoylated staurosporine derivatives to inhibit elicitor responses in vivo correlated with their ability to inhibit tomato microsomal protein kinase in vitro. When K-252a was given to elicited cells 1 min after the[32] phosphate, the radioactivity in certain newly labeled phosphoprotein bands disappeared again within minutes. This correlated with an arrest of alkalinization within minutes when K-252a was applied in midcourse of elicitation. These data show that phosphorylation of protein substrates by K-252a-sensitive protein kinases is essential for transduction of elicitor signals in plant cells and that continuous phosphorylation of these proteins is required to maintain the elicited state.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biermann B., Johnson E. M., Feldman L. J. Characterization and distribution of a maize cDNA encoding a peptide similar to the catalytic region of second messenger dependent protein kinases. Plant Physiol. 1990;94:1609–1615. doi: 10.1104/pp.94.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A., Mayer J. E., Hahlbrock K. Fungal elicitor triggers rapid, transient, and specific protein phosphorylation in parsley cell suspension cultures. J Biol Chem. 1990 Apr 15;265(11):6360–6368. [PubMed] [Google Scholar]
- Farmer E. E., Pearce G., Ryan C. A. In vitro phosphorylation of plant plasma membrane proteins in response to the proteinase inhibitor inducing factor. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1539–1542. doi: 10.1073/pnas.86.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
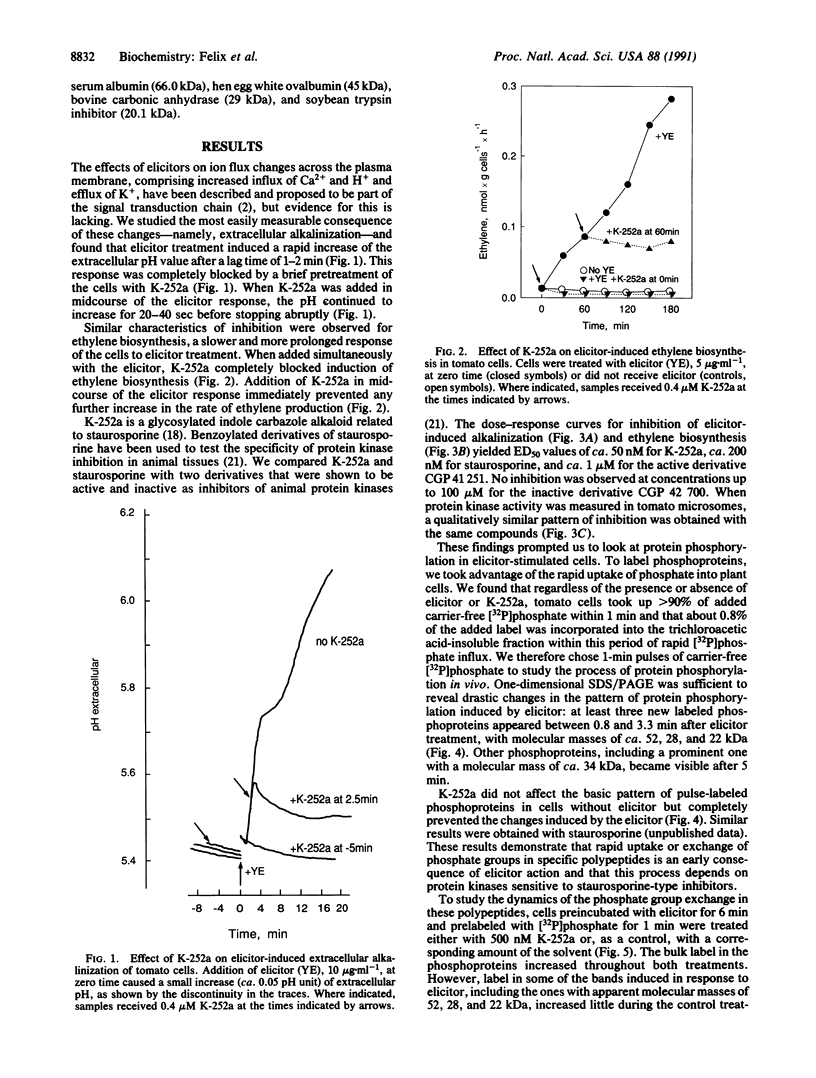
- Grosskopf D. G., Felix G., Boller T. K-252a inhibits the response of tomato cells to fungal elicitors in vivo and their microsomal protein kinase in vitro. FEBS Lett. 1990 Nov 26;275(1-2):177–180. doi: 10.1016/0014-5793(90)81466-2. [DOI] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawton M. A., Yamamoto R. T., Hanks S. K., Lamb C. J. Molecular cloning of plant transcripts encoding protein kinase homologs. Proc Natl Acad Sci U S A. 1989 May;86(9):3140–3144. doi: 10.1073/pnas.86.9.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh C., Cohen P. Identification of high levels of type 1 and type 2A protein phosphatases in higher plants. Biochem J. 1989 Aug 15;262(1):335–339. doi: 10.1042/bj2620335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiny-Baron G., Scherer G. F. Phospholipid-stimulated protein kinase in plants. J Biol Chem. 1989 Oct 25;264(30):18052–18059. [PubMed] [Google Scholar]
- Meyer T., Regenass U., Fabbro D., Alteri E., Rösel J., Müller M., Caravatti G., Matter A. A derivative of staurosporine (CGP 41 251) shows selectivity for protein kinase C inhibition and in vitro anti-proliferative as well as in vivo anti-tumor activity. Int J Cancer. 1989 May 15;43(5):851–856. doi: 10.1002/ijc.2910430519. [DOI] [PubMed] [Google Scholar]
- Rüegg U. T., Burgess G. M. Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci. 1989 Jun;10(6):218–220. doi: 10.1016/0165-6147(89)90263-0. [DOI] [PubMed] [Google Scholar]
- Schmidt W. E., Ebel J. Specific binding of a fungal glucan phytoalexin elicitor to membrane fractions from soybean Glycine max. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4117–4121. doi: 10.1073/pnas.84.12.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
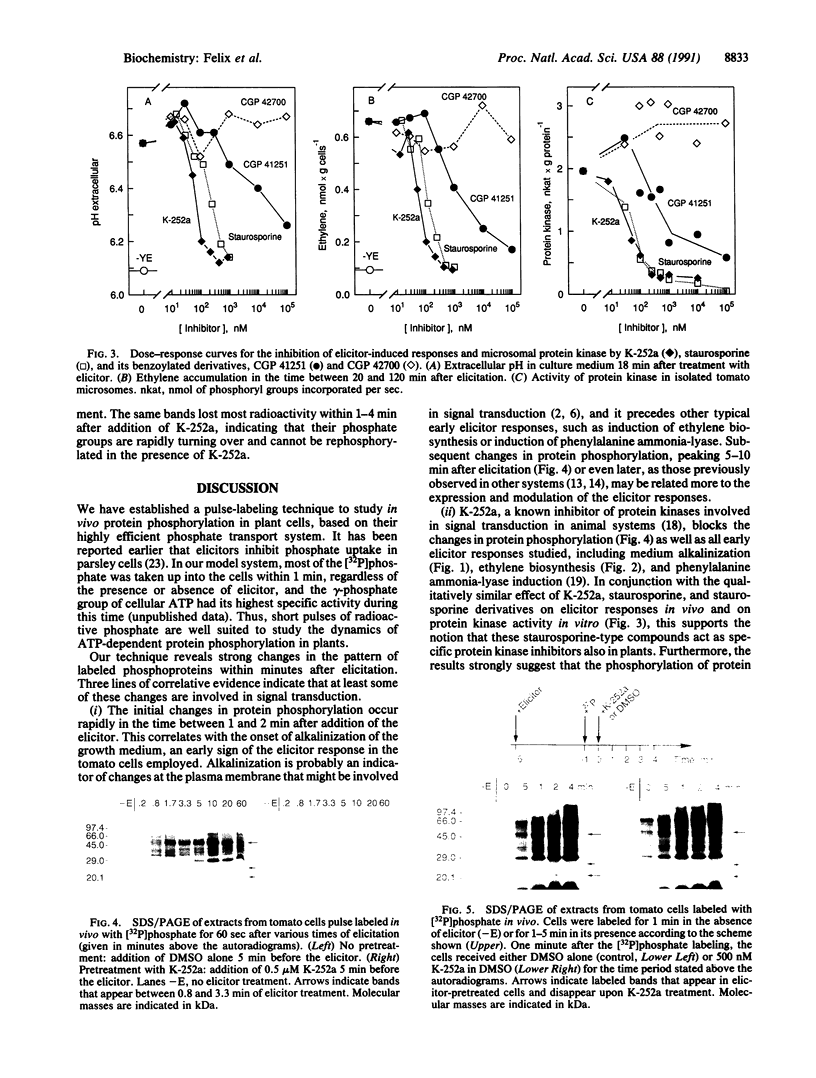
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. C., Zhang R. Relationship of a putative receptor protein kinase from maize to the S-locus glycoproteins of Brassica. Nature. 1990 Jun 21;345(6277):743–746. doi: 10.1038/345743a0. [DOI] [PubMed] [Google Scholar]