Abstract
BACKGROUND
The anti‐cancer mechanism of neo‐adjuvant hormonal therapy (NHT) is not well understood. Lymphangiogenesis plays an important role in cancer progression and is regulated by a complex mechanism that includes vascular endothelial growth factor (VEGF) signaling. However, there is little information regarding relationship between lymphangiogenesis and androgen deprivation. The aim of this study was to clarify changes in lymphangiogenesis and VEGF expression induced by androgen deprivation in prostate cancer in vivo and in vitro.
METHODS
Patients who had undergone a radical prostatectomy were enrolled in the study (NHT, n = 60 and non‐NHT, n = 64). Lymph vessels were identified by D2‐40 immunoreactivity and lymph vessel density and lymph vessel area (LVD and LVA, respectively) were measured from micrographs. The expression of VEGF‐A, ‐B, ‐C, and ‐D was evaluated by immunohistochemistry. The prognostic value of LVD and LVA for biochemical recurrence was also investigated.
RESULTS
Mean LVD ± SD was higher in the NHT than in the non‐NHT group (11.3 ± 3.0 vs. 7.1 ± 3.4 per high power field; P < 0.001). LVA was larger in the NHT than in the non‐NHT group (512.8 ± 174.9 vs. 202.7 ± 72.8 µm2; P < 0.001). VEGF‐A expression was lower whereas VEGF‐C and ‐D levels were higher in the NHT than in the non‐NHT group. VEGF‐B expression in specimens with NHT was lower than that in biopsy specimens at diagnosis. These results were confirmed by in vitro studies used androgen‐sensitive prostate cancer cell line. LVA was found to be an independent predictor of biochemical recurrence in patients who received NHT.
CONCLUSIONS
Our results demonstrate that NHT stimulates lymphangiogenesis via upregulation of VEGF‐C and ‐D, which may increase LVA and affect the outcome of prostate cancer patients. This findings were supported by in vitro data of prostate cancer cell. Prostate 77:255–262, 2017. © 2016 The Authors. The Prostate Published by Wiley Periodicals, Inc.
Keywords: lymphangiogenesis, neo‐adjuvant hormonal therapy, androgen deprivation, vascular endothelial growth factors, prostate cancer
INTRODUCTION
Lymphangiogenesis is associated with tumor progression and worse prognosis in many types of solid tumors, since it is a critical step for cancer cell dissemination into lymph nodes and distant organs 1, 2. However, several studies have reported that lymphangiogenesis is not required for lymph node metastasis in prostate cancer 3, 4. As such, the pathological and prognostic significance of this process in prostate cancer is unclear. In addition, mechanisms underlying lymphangiogenesis in human prostate cancer tissue are not well understood.
Vascular endothelial growth factors (VEGFs) strongly induce angiogenesis under many pathological conditions, including in prostate cancer 5. VEGFs are known to stimulate lymphangiogenesis in various malignancies, with VEGF‐C 6, VEGF‐D 7, 8, and VEGF‐A being the most widely studied; the expression of the latter is positively associated with metastasis 9, 10. Indeed, VEGF‐A, ‐C, and ‐D are associated with increased tumor aggressiveness in prostate cancer patients 11, 12. However, although several studies paid attention to the relationship between VEGF‐B and lymphangiogenesis in cancer 13, 14, there is little information in prostate cancer.
Androgen deprivation therapy (ADT) is a therapeutic strategy for treating patients with prostate cancer. Neo‐adjuvant hormonal therapy (NHT) by androgen deprivation is often used to improve the outcome of patients treated by radical prostatectomy (RP). However, there is no general agreement about its anti‐cancer effects or its clinical effectiveness. In addition, changes in lymphangiogenesis and its regulators by ADT in prostate cancer is not fully understood. Therefore, the main aim of this study is to clarify changes in lymph‐angiogenic status upon androgen deprivation in prostate cancer tissue by analyzing VEGF expression in vitro and in vivo. We also assessed the value of lymph angiogenesis‐related parameters for predicting the outcome of prostate cancer patients treated by radical prostatectomy after NHT.
METHODS
Patients
Prostate cancer specimens (n = 124) obtained by RP at our hospital were examined. Of these, 60 were from patients that had received NHT and 64 were from those that did not (NHT and non‐NHT groups, respectively). Exclusion criteria were short duration of NHT (<3 months); clinical or pathological invasion into the seminal vesicle or surrounding tissues or presence of metastasis; Gleason score (GS) of 10; or serum prostate antigen (PSA) levels >90 ng/ml. Clinicopathological features of NHT and non‐NHT groups were matched according to patient age, PSA level, GS, and pT stage. Clinicopathological features of both groups are shown in Table I.
Table I.
Clinicopathological Features
| Non‐NHT | NHT | P‐value | |
|---|---|---|---|
| Number of patients | 64 | 60 | |
| At diagnosis | |||
| Age, years; mean/SD | 63.3/5.5 | 64.8/4.4 | 0.101 |
| Serum PSA levels, ng/ml; mean/SD | 12.5/9.6 | 16.7/16.6 | 0.082 |
| Gleason score; N/% | 0.133 | ||
| Low (≤6) | 23/35.9 | 14/23.3 | |
| Middle (7) | 23/35.9 | 32/53.3 | |
| High (≥8) | 18/28.1 | 14/23.3 | |
| Clinical T stage; N/% | 0.082 | ||
| T1 | 20/31.3 | 10/16.7 | |
| T2 | 37/57.8 | 37/61.7 | |
| T3 | 7/10.9 | 13/21.7 | |
| In radical prostatectomy specimens | |||
| Pathological T stage; N/% | 0.934 | ||
| T2 | 39/60.9 | 37/61.7 | |
| T3 | 25/39.1 | 23/38.3 | |
NHT, neoadjuvant hormonal therapy; PSA, prostate‐specific antigen.
Pathological features were evaluated according to the 2002 tumor‐node‐metastasis staging system and GS. NHT consisted of anti‐androgen agent (n = 1, 1.7%), luteinizing hormone‐releasing hormone (LH‐RH) agonists (n = 28, 47.7%), or combination of the two (n = 31, 51.7%). The median/mean duration of NHT was 8/9.1 months (interquartile range: 5–11 months). Biochemical recurrence (BCR) was defined as serum PSA levels >0.2 ng/ml, as measured on two or more occasions. The study protocol was approved by the Human Ethics Review Committee of Nagasaki University Hospital, and written, informed consent form was obtained from each subject.
Immunohistochemistry
Prostate tissue sections (5 μm in thickness) were deparaffinized and rehydrated, and antigen retrieval was performed at 95°C for 40 min in 0.01 M sodium citrate buffer (pH 6.0). Sections were then immersed in 3% hydrogen peroxide for 30 min to block endogenous peroxidase activity. Antibodies against the following proteins were used: D2‐40 (DakoCytomation, Glostrup, Denmark); VEGF‐A and ‐B (Santa Cruz Biotechnology, Santa Cruz, CA); VEGF‐C (Zymed Laboratories, San Francisco, CA; and VEGF‐D (R&D Systems, Abingdon, UK). Sections were incubated with the primary antibodies at 4°C overnight, then treated with labeled polymer peroxidase from the EnVision+ Peroxidase kit (Dako, Carpinteria, CA) for 60 min. Immunoreactivity was visualized using a liquid diaminobenzidine substrate kit (Zymed Laboratories). Sections were counterstained with hematoxylin before mounting. Negative controls consisted of adjacent sections from each sample that were processed without the primary antibody. The positive control for all antibodies was kidney tissue with renal cell carcinoma. The immunohistochemical staining methods have been previously described 13, 15.
Evaluation of Immunoreactivity
VEGF expression was semi‐quantitatively analyzed as previously described 16. Briefly, specimens were assigned an immunoreactivity score (IRS), which was calculated by multiplying staining intensity (grade 0 = none, 1 = weak, 2 = moderate, and 3 = strong) by the percentage of positively stained cells (0, <1%; 1, 1–25%; 2, 26–50%; 3, 5175%; or 4, 76–100%). To determine lymph vessel density and area (LVD and LVA, respectively), sections labeled with anti‐D2‐40 antibody were examined. For each tumor section, three to five hot spots in the field of view (i.e., with the greatest density of positively stained vessels) were evaluated. LVD was defined as the number of positively stained vessels per high‐power field. Evaluation and measurements were performed by computer‐aided image analysis (WinROOF version 6.4; Mitani, Fukui, Japan). For statistical analyses, LVD and LVA were divided into two groups: low (median or less) and high (greater than the median).
A total of 46 paired biopsy specimens were obtained from 60 NHT patients at the time of diagnosis and radical operation and were used to compare expression of VEGF‐A to ‐D before and after NHT. Analyses of LVD and LVA were not carried out because they could not be accurately determined in biopsy specimens.
Cell Culture and Androgen Depletion
The LNCaP androgen‐dependent human prostate cancer cell line was purchased from the American Type Culture Collection (Manassas, VA). Cells were cultured in Roswell Park Memorial Institute 1640 medium (Gibco/Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) and 50 μg/ml gentamicin (Gibco/Life Technologies) at 37°C in a humidified atmosphere of 5% CO2 and 95% air. To prepare androgen‐depleted FBS by charcoal stripping, 100 ml FBS were incubated with 1,000 mg of activated charcoal for 3 hr with constant stripping, followed by filtration 17.
Western Blot Analysis
Western blotting was carried out as previously described 18. Aliquots of total cellular lysate (40–50 µg/lane) were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane, which was blocked with 5% nonfat milk in Tris‐buffered saline containing 0.1% Tween 20 (TBS‐T) for 1 hr at room temperature and then incubated with primary antibody overnight at 4°C. After washing three times with TBS‐T, the membrane was incubated with the appropriate secondary antibody for 1 hr at room temperature. Protein bands were detected with the ECL Prime kit (GE Healthcare, Little Chalfont, UK).
Statistical Analysis
Results are expressed as mean ± SD. The Student's t‐test was applied to continuous variables and the Mann–Whitney U‐test was used for other data. Pearson's correlation and the correlation coefficient (r) were used to evaluate the relationship between continuous variables, and the corresponding p values are reported. The Kaplan–Meier survival curve and log‐rank test along with multivariate analysis using the Cox proportional hazards model were used to assess patient survival. All statistical analyses were performed using the StatView v.5.0 for Windows software (Abacus Concepts, San Francisco, CA).
RESULTS
D2‐40‐Positive Lymph Vessels and VEGF Expression
Nearly all D2‐40‐positive vessels were relapsed in the non‐NHT group and the intra‐luminal space was narrow (Fig. 1A). In particular, there were few lymph vessels with a lumen in the intra‐tumoral area. In contrast, D2‐40‐positive lymph vessels in the NHT group had a wider inner cavity (Fig. 1B and C) and contained some cells (Fig. 1D). It was not possible to determine the intra‐tumoral area in some samples from the NHT group; we, therefore, evaluated LVD and LVA in the peri‐tumoral area. VEGF immunoreactivity was mostly detected in the cytoplasm of cancer cells. There were no differences in the expression patterns of the four VEGFs; however, the staining intensity and percentage of positively stained cells was higher for VEGF‐A and ‐C than for VEGF‐B and ‐D.
Figure 1.
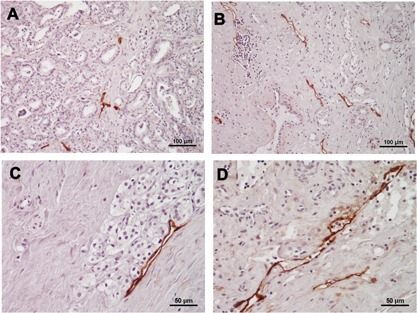
Representative micrographs of D2‐40‐positive lymph vessels in prostate cancer tissue samples with/without NHT. Most lymph vessels were relapsed and the intra‐luminal space was narrow in non‐NHT specimens (A: magnification ×200). Lymph vessels had a relatively wide inner cavity in NHT specimens (B: magnification ×200, C: ×400). Some cells were detected within D2‐40‐positive lymph vessels in some NHT specimens (D: magnification ×400).
Lymphangiogenesis‐Related Parameters and VEGF Expression
LVD was higher (Fig. 2A) and LVA was greater (Fig. 2B) in the NHT group than in the non‐NHT group (P < 0.001 for both). The mean IRS score ± SD of VEGF‐A was lower in the NHT than in the non‐NHT group (P < 0.001) (Fig. 2C). A similar trend was observed for VEGF‐B, although the difference did not reach statistical significance (P = 0.253; Fig. 2D). In contrast to VEGF‐A, the IRS of VEGF‐C and ‐D was higher in the NHT than in the non‐NHT group (P < 0.001 for both; Fig. 2E and F).
Figure 2.
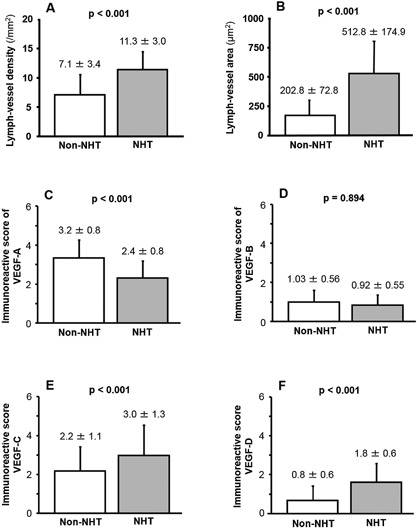
(A) LVD was higher and (B) LVA is greater in the NHT than in the non‐NHT group. (C and D) The IRS for VEGF‐A (C) and VEGF‐B (D) was lower in the NHT than in the non‐NHT group, although the difference was statistically significant only for VEGF‐A. (E and F) The IRS of VEGF‐C (E) and VEGF‐D (F) was higher in the NHT than in the non‐NHT group.
A comparative analysis with the paired Student's t‐test of VEGF expression in biopsy specimens obtained at the time of diagnosis and RP specimens obtained after NHT from the same patient yielded similar findings. Briefly, the IRS of VEGF‐A and ‐B was lower in RP specimen treated with NHT than in biopsy specimens at diagnosis (VEGF‐A: 2.4 ± 0.8 vs. 2.8 ± 1.3, P < 0.001 and VEGF‐B: 0.92 ± 0.55 vs. 0.96 ± 0.67, P = 0.030). In contrast, the IRS of VEGF‐C and ‐D was higher in RP than in biopsy specimens (2.9 ± 1.5 and 1.8 ± 0.7, respectively, vs. 1.9 ± 0.8 and 0.7 ± 0.6, respectively) (P < 0.001).
LVD was positively correlated with expression of VEGF‐A (r = 0.49, P < 0.001) and VEGF‐C (r = 0.47, P < 0.001) in the non‐NHT group. LVD was weakly but significantly correlated with VEGF‐D (r = 0.27, P = 0.034) but not with VEGF‐B (r = 0.01, P = 0.953) expression. On the other hand, LVA was correlated with expression of VEGF‐A (r = 0.25, P = 0.044) and VEGF‐C (r = 0.52, P < 0.001) but not of VEGF‐B (r = 0.07, P = 0.580) or VEGF‐D (r = 0.14, P = 0.273).
In vitro study showed that VEGF‐A expression in androgen‐dependent LNCaP human prostate cancer cells cultured in medium lacking androgen was lower compared to those cultured in standard medium (Fig. 3). Similar trend was also found in VEGF‐B expression whereas VEGF‐C and ‐D expression was increased by androgen deprivation (Fig. 3).
Figure 3.
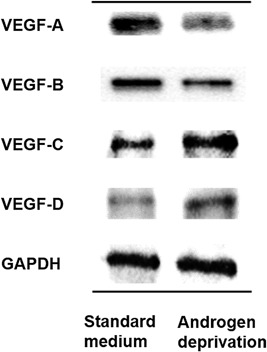
Expressions of VEGF‐A and ‐B were reduced by androgen depletion, as determined by western blotting. In contrast, expressions of VEGF‐C and VEGF‐D were increased in cancer cells cultured in androgen‐deficient as compared to standard medium.
Predictive Value for Biochemical Recurrence
Kaplan–Meier curves for BCR in the NHT group revealed that high values for LVD (Fig. 4A) and LVA (Fig. 4B) were associated with shorter time to BCR (P = 0.037 and 0.004, respectively; log‐rank test). The multivariate analysis of pathological features (pT stage and GS) showed that high LVA was an independent predictor of BCR (hazard ratio [HR] = 3.33, 95% confidence interval (CI): 1.24–8.97, P = 0.017), whereas LVD was not (HR = 2.37, 95%CI: 0.87–6.47, P = 0.092).
Figure 4.
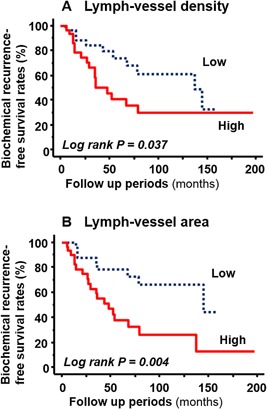
Kaplan–Meier curves of BCR in patients treated with NHT. (A) Higher LVD was associated with shorter time to BCR (P = 0.037, log‐rank test). (B) Larger LVA predicted BCR (P = 0.004, log‐rank test).
DISCUSSION
Our study demonstrated that LVA was closely associated with prognosis in prostate cancer patients in NHT group. This result is supported by previous report that LVA has also been linked to malignant potential and patient prognosis in prostate cancer 19. Accordingly, we observed that D2‐40‐positive lymph vessels in the non‐NHT group were small and collapsed and that the lumen of vessels in the intra‐tumoral area were almost non‐existent. Several previous studies have reported similar findings in prostate cancer tissue 11, 19, 20. Pressure from interstitial fluid and mechanical compression in the intra‐tumoral area have been proposed to explain these observations 3, 20. It is speculated that collapsed and occluded lymph vessels contribute little to the pathology of prostate cancer given the narrow space of the lumen 3. On the other hand, our results showed that LVA was higher in the NHT than in the non‐NHT group; this may be explained by the decrease in pressure and mechanical compression within and around the tumor mass by ADT. Thus, the anti‐cancer effects of ADT create a favorable microenvironment for lymphatic vessel expansion. Indeed, our multivariate analysis showed that LVA in the NHT group was an independent predictor for BCR.
VEGF‐C and ‐D are recognized as the most important regulators of lymph‐angiogenesis in various types of malignancies including prostate cancer 4, 21. We therefore hypothesized that VEGF‐C and ‐D expression would decrease upon NHT. Unexpectedly, the levels of both factors were higher in the NHT than in the non‐NHT group. In addition, our in vitro studies demonstrated that VEGF‐C and ‐D expression were upregulated by androgen deprivation. This study is the first to report a change in VEGF‐D expression by androgen deprivation in human prostate cancer, although it was previously shown that serum VEGF‐D level was increased by ADT 22 as well as by castration in an animal model 23. Our result that VEGF‐C expression was increased by NHT in human prostate cancer tissue was confirmed in an androgen‐dependent prostate cancer cell line, in accordance with a previous report that VEGF‐C is upregulated by androgen depletion in LNCaP cells 24, 25. However, in human prostate cancer tissue, VEGF‐C expression was found to be lower in RP as compared to biopsy specimens 26. We speculate that the discrepancy between these results and ours is due to differences in methodology, including the antibody used and incubation time as well as the analytical method that was applied. In addition, the duration of NHT differed between the two studies; the mean duration of NHT was approximately 3 months in the earlier study, since mean interval between diagnosis and prostatectomy was reportedly 119 days 26. In contrast, the mean duration in our study population was 9.1 months. This may have influenced VEGF‐C expression level in human prostate cancer tissue.
Another interesting finding of this study is that androgen deprivation decreased VEGF‐B expression in a prostate cancer cell line and human tissue. A similar finding was reported in an animal model 23. These results suggest that VEGF‐B expression is decreased by ADT in prostate cancer. However, our results also showed that VEGF‐B was unaffected by lymphangiogenesis in human prostate cancer tissue, implying that it plays a negligible role in lymphangiogenesis in prostate cancer.
VEGF‐A immunoreactivity was reportedly decreased by androgen deprivation in 20 prostate cancer patients 27. This is in agreement with the results of the present study in a larger population. Another study reported that serum VEGF‐A level was reduced by ADT in prostate cancer patients 22, and VEGF‐A expression in androgen‐responsive prostate cancer cells was suppressed by castration in an animal model 23, 28. These observations indicate that ADT inhibits VEGF‐A expression in prostate cancer. Our in vitro study supports these findings. On the other hand, we also found that VEGF‐A expression was positively associated with lymphangiogenesis in prostate cancer patients. Given that NHT suppressed lymphangiogenesis, we speculate that the decrease in VEGF‐A expression may be insufficient to counter the pro‐lymphangiogenic activity of VEGF‐C and ‐D.
CONCLUSIONS
We showed that lymphangiogenesis as well as VEGF‐C and ‐D expression in prostate cancer was stimulated by NHT whereas expressions of VEGF‐A and ‐B were suppressed by NHT. We also found that LVA was associated with VEGF‐A and ‐C, and it was identified as an independent predictor of BCR after RP in patients who had undergone NHT. Based on these findings, we conclude that NHT‐induced upregulation of VEGF‐C expression affects prostate cancer patient outcome after RP by increasing LVA.
ACKNOWLEDGMENTS
This study was supported in part by a Grant‐in‐Aid from Japan Society for the Promotion of Science (to YM). However, it was not supported financially by any company and other funding agency.
Conflicts of interest: There are no conflicts of interest to declare.
REFERENCES
- 1. Arya M, Bott SR, Shergill IS, Ahmed HU, Williamson M, Patel HR. The metastatic cascade in prostate cancer. Surg Oncol 2006; 15:117–128. [DOI] [PubMed] [Google Scholar]
- 2. Miyata Y, Kanda S, Mitsunari K, Asai A, Sakai H. Heme oxygenase‐1 expression is associated with tumor aggressiveness and outcomes in patients with bladder cancer: A correlation with smoking intensity. Transl Res 2014; 164:468–476. [DOI] [PubMed] [Google Scholar]
- 3. Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, Munn LL, Jain RK. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 2002; 296:1883–1886. [DOI] [PubMed] [Google Scholar]
- 4. Wong SY, Haack H, Crowley D, Barry M, Bronson RT, Hynes RO. Tumor‐secreted vascular endothelial growth factor‐C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis. Cancer Res 2005; 65:9789–9798. [DOI] [PubMed] [Google Scholar]
- 5. de Brot S, Ntekim A, Cardenas R, James V, Allegrucci C, Heery DM, Bates DO, Ødum N, Persson JL, Mongan NP. Regulation of vascular endothelial growth factor in prostate cancer. Endocr Relat Cancer 2015; 22:107–123. [DOI] [PubMed] [Google Scholar]
- 6. Achen MG, Stacker SA. Molecular control of lymphatic metastasis. Ann NY Acad Sci 2008; 1131:225–234. [DOI] [PubMed] [Google Scholar]
- 7. Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF‐D promotes the metastasis spread of tumor cells via the lymphatics. Nat Med 2011; 7:186–191. [DOI] [PubMed] [Google Scholar]
- 8. Miyata Y, Kanda S, Ohba K, Nomata K, Hayashida Y, Eguchi J, Hayashi T, Kanetake H. Lymphangiogenesis and angiogenesis in bladder cancer: Prognostic implications and regulation by vascular endothelial growth factors ‐A, ‐C, and ‐D. Clin Cancer Res 2006; 12:800–806. [DOI] [PubMed] [Google Scholar]
- 9. Crusiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF‐A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest 2004; 113:1040–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miyata Y, Watanabe S, Sagara Y, Mitsunari K, Matsuo T, Ohba K, Sakai H. High expression of HuR in cytoplasm, but not nuclei, is associated with malignant aggressiveness and prognosis in bladder cancer. PLoS ONE 2013; 8:e59095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeng Y, Opeskin K, Baldwin ME, Horvath LG, Achen MG, Stacker SA, Sutherland RL, Williams ED. Expression of vascular endothelial growth factor receptor‐3 by lymphatic endothelial cell is associated with lymph node metastasis in prostate cancer. Clin Cancer Res 2004; 10:5137–5144. [DOI] [PubMed] [Google Scholar]
- 12. Stearns ME, Wang M, Hu Y, Kim G, Garcia FU. Expression of a flt‐4 (VEGFR3) splicing variant in primary human prostate tumors. VEGF D and flt‐4t(Delta773‐1081) overexpression is diagnostic for sentinel lymph node metastasis. Lab Invest 2004; 84:785–795. [DOI] [PubMed] [Google Scholar]
- 13. Iwata T, Miyata Y, Kanda S, Nishikido M, Hayashi T, Sakai H, Kanetake H. Lymphangiogenesis and angiogenesis in conventional renal cell carcinoma: Association with vascular endothelial growth factors A to D immunohistochemistry. Urology 2008; 71:749–754. [DOI] [PubMed] [Google Scholar]
- 14. Yang X, Zhang Y, Hosaka K, Andersson P, Wang J, Tholander F, Cao Z, Morikawa H, Tegnér J, Yang Y, Iwamoto H, Lim S, Cao Y. VEGF‐B promotes cancer metastasis through a VEGF‐A‐independent mechanism and serves as a marker of poor prognosis for cancer patients. Proc Natl Acad Sci USA 2015; 112:2900–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miyata Y, Kanda S, Ohba K, Nomata K, Eguchi J, Hayashida Y, Hayashi T, Kanetake H. Tumor lymphangiogenesis in transitional cell carcinoma of the upper urinary tract: Association with clinicopathological features and prognosis. J Urol 2006; 176:348–353. [DOI] [PubMed] [Google Scholar]
- 16. Miyata Y, Mitsunari K, Asai A, Takehara K, Mochizuki Y, Sakai H. Pathological significance and prognostic role of microvessel density, evaluated using CD31, CD34, and CD105 in prostate cancer patients after radical prostatectomy with neoadjuvant therapy. Prostate 2015; 75:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mizutani N, Inoue M, Omori Y, Ito H, Tamiya‐Koizumi K, Takagi A, Kojima T, Nakamura M, Iwaki S, Nakatochi M, Suzuki M, Nozawa Y, Murate T. Increased acid ceramidase expression depends on upregulation of androgen‐dependent deubiquitinases, USP2, in a human prostate cancer cell line, LNCaP. J Biochem 2015; 158:309–319. [DOI] [PubMed] [Google Scholar]
- 18. Mitsunari K, Miyata Y, Asai A, Matsuo T, Shida Y, Hakariya T, Sakai H. Human antigen R is positively associated with malignant aggressiveness via upregulation of cell proliferation, migration, and vascular endothelial growth factors and cyclooxygenase‐2 in prostate cancer. Transl Res 2016. pii: S1931‐5244(16)30020‐2. DOI: 10.1016/j.trsl.2016.04.002 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 19. Ambrosio MR, Rocca BJ, Barone A, Ginori A, Crivelli F, Pirtoli L, Del Vecchio MT. Lymphatic vascularization in prostate adenocarcinoma: Correlation with tumor grade, androgen withdrawal and prognosis. Anticancer Res 2105; 35:5595–5600. [PubMed] [Google Scholar]
- 20. Kim H‐S, Sung W, Lee S, Chang SG, Park YK. Lymphatic vessel densities of lymph node‐negative prostate adenocarcinoma in Korea. Pathol Res Pract 2009; 205:249–254. [DOI] [PubMed] [Google Scholar]
- 21. Woollard DJ, Opeskin K, Coso S, Wu D, Baldwin ME, Williams ED. Differential expression of VEGF ligands and receptors in prostate cancer. Prostate 2013; 73:563–572. [DOI] [PubMed] [Google Scholar]
- 22. Verdroorn BP, Feng C, Ricke WA, Sahasrabudhe DM, Kilari D, Kohli M. An observational study of plasma vascular endothelial growth factor (VEGF) A and D expression in non‐localized prostate cancer. J Mens Health 2012; 9:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang GM, Kovalenko B, Huang Y, Moscatelli D. Vascular endothelial growth factor and angiopoietin are required for prostate regeneration. Prostate 2007; 67:485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jinping Li, Wang E, Rinaldo F, Datta K. Upregulation of VEGF‐C by androgen depletion: The involvement of IGF‐1R‐FOXO pathway. Oncogene 2005; 24:5510–5520. [DOI] [PubMed] [Google Scholar]
- 25. Rinaldo F, Li J, Wang E, Muders M, Datta K. RalA regulates vascular endothelial growth factor‐C (VEGF‐C) synthesis in prostate cancer cells during androgen ablation. Oncogene 2007; 26:1731–1738. [DOI] [PubMed] [Google Scholar]
- 26. Kozakowski N, Hartmann C, Klingler HC, Susani M, Mazal PR, Scharrer A, Haitel A. Immunohistochemical expression of PDGFR, VEGF‐C, and proteins of the mToR pathway before and after androgen deprivation therapy in prostate carcinoma: Significant decrease after treatment. Target Oncol 2014; 9:359–366. [DOI] [PubMed] [Google Scholar]
- 27. Aslan G, Cimen S, Yorukoglu K, Tuna B, Sonmez D, Mungan U, Celebi I. Vascular endothelial growth factor expression in untreated and androgen‐deprived patients with prostate cancer. Pathol Res Pract 2005; 201:593–598. [DOI] [PubMed] [Google Scholar]
- 28. Joseph IBJK, Isaacs JT. Potential of the antiangiogenic ability of linomide by androgen ablation involves down‐regulation of vascular endothelial growth factor in human androgen‐responsive prostate cancers. Cancer Res 1997; 57:1054–1057. [PubMed] [Google Scholar]


