Abstract
Introduction
The aim of this article is to generally describe the roles of main surgical modalities in treatment of renal tumors, especially in the CT1a category in clinical practice. Surgical modalities include the following: laparoscopic or open resection (LR, OR) and laparoscopic or open nephrectomy (LN, ON). Representation of these methods has been changing over years due to improved operative skills and equipment and due to a shift of tumors to the lower T categories.
Material and methods
The sources of data were surgeries performed for renal tumors at the institution of the main author during the period 2002 to III/2016, reaching a total of 2204 cases (546 ONs, 647 LNs, 668 ORs and 343 LRs). Patients indicated for percutaneous ablative therapy or active surveillance were not included.
Results
During the whole period, the proportions of methods were: ONs 24.8%, LNs 29.4%, ORs 30.3%, LRs 15.6%. But during the years 2014 – III/2016, these changed to 12.6%:26.3%:31.6%:29.4% (in cT1a 1.7%:8.3%:37.8%:52.2%). Category cT1a constitutes in the years 2007 – III/2016 41.3%, in 2014 – III/2016 50.9%.
Conclusions
Resections and minimally invasive approaches are being performed more frequently and are the preferred methods in surgical treatment of kidney tumors. Resection is now indicated in about 60% of cases (open vs. laparoscopic resection are used nearly equally with a slight tendency for laparascopic predomination). In the cT1a category (amounting to approximately 50% of all surgically treated tumors), resection is possible in about 85–90% of cases.
Keywords: renal tumor, nephrectomy, laparoscopy, incidence, histology
INTRODUCTION
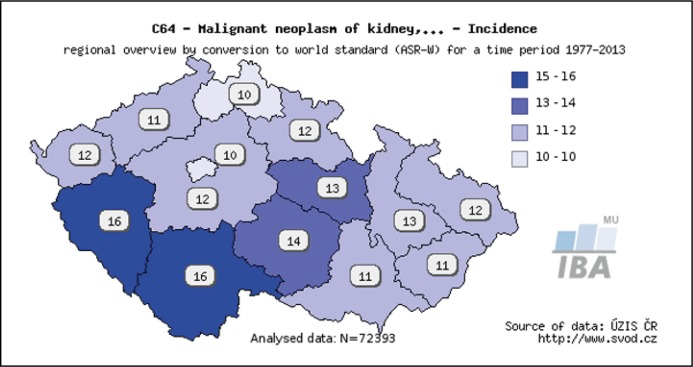
The diagnosis and management of renal cell carcinoma (RCC) have changed remarkably rapidly. Although the incidence of RCC has been increasing, survival has improved substantially. As incidental diagnosis of small indolent cancers has become more frequent, active surveillance, laparoscopic or robot-assisted nephron-sparing surgical techniques, and minimally invasive procedures, such as thermal ablation, have gained popularity. Despite progression in cancer control and survival, locally advanced disease and distant metastases are still diagnosed in a notable proportion of patients. An integrated management strategy that includes surgical debulking and systemic treatment with well-established targeted biological drugs has improved the care of patients [1–8]. The early diagnosis and a subsequent surgical treatment (or use of ablative modalities) of localised RCC remains the only method with a real chance at curing RCC. The modalities of surgery are the following: laparoscopic or open resection (LR, OR) and laparoscopic or open nephrectomy (LN, ON). Indications for surgery strictly follow the European Association of Urology (EAU) guidelines for treatment of renal tumors [2]. Resection is preferred before nephrectomy in all T1 category (≤7 cm) cases [9, 10, 11]. In resection, as well as in nephrectomy, preference is given to the less invasive approach (laparoscopic, even robotically assisted) before open surgery [12]. In metastatic disease, available targeted therapy has only a palliative and temporary effect even in less than 50% of treated cases [2]. Moreover, cytoreductive nephrectomy (or even cytoreductive resection) is part of the treatment of metastatic RCC (mRCC) as well. Surgery is not indicated only in: (a) smaller tumors (< about 3.5 cm) in elderly patients, unfit or refusing surgery – here are available active surveillance or percutaneous ablation (radiofrequency ablation or cryoablation) options; (b) in locally advanced disease and (c) mRCC not indicated for cytoreductive surgery [2, 4]. The aim of this article is to present the stratification of four basic methods of surgery performed at our department. Representation of these methods has been changing over the past years due to improved operative skills (in nephron sparing surgery and laparoscopy), enhancement of equipment (mainly for laparoscopy) and due to a shift of tumors to the lower T categories. Our department performs nearly all types of surgeries for kidney tumors in the Pilsen region which has 575 123 inhabitants. Thus, we have a representative spectrum of kidney tumors to assess the real needs of different surgical modalities. Additionallly, it is worth noting that the Czech Republic has the highest incidence of renal tumors worldwide and within the Czech Republic the ‘leader’ is the Pilsen region (Figures 1, 2).
Figure 1.
Incidence of kidney tumor worldwide showing leading position of the Czech Republic. Source of data is www.svod.cz
Figure 2.
Incidence in different areas of the Czech Republic. The darkest region with overall incidence of 26 new cases per year per 100.000 inhabitants is the Pilsen region. Source of data is www.svod.cz
MATERIAL AND METHODS
All surgeries performed for treatment of kidney tumors at the Faculty Hospital Plzeň, CZ during the period from 2002 to March 2016 were included in the study. In 2003, laparoscopy was introduced as an option; 2003 LN and 2004 LR. The robotic system is not available at Pilsen. Patients with indications for percutaneous ablative therapy (mainly radiofrequency ablation – RFA) or active surveillance were not included.
RESULTS
During the period of 14.25 years (2002 – III/2016), 2204 men were surgically treated for kidney tumors by 4 basic surgical techniques. The total numbers are shown in Table 1. In the whole period, the proportion of methods was: ONs 24.8%, LNs 29.4%, ORs 30.3%, LRs 15.6%. But in years 2014 – III/2016, it changed to 12.6%:26.3%:31.6%:29.4% (in cT1a 1.7%:8.3%:37.8%:52.2%). During the years 2007 – III/2016, category cT1a constituted 41.3% of cases and in 2014 – III/2016 50.9%. Final histologies are shown in Table 2. Mostly renal cell carcinoma (RCC) – clear RCC 75.9%, papillary RCC 9.7% and chromophobe RCC 2.6%, but of course, some benign tumors (oncocytoma 4.2% and angiomyolipoma) and other unusual tumors were found. The stratification of four basic surgical techniques is shown in Table 1, and is graphically expressed in tab 3 as well. Figure 4 (source of data is in Table 1) demonstrates the growing representation of clinical category T1a up to about 50%. Figure 5 (sources of data are again found in Table 1) shows that nephron sparing surgery is feasible now in about 60% of all cases and in about 85% of cT1a category. Generally (resections and nephrectomies), over 60% of all tumors are treated with a laparoscopic approach (Figure 3).
Table 1.
Total numbers of surgeries performed in years 2002-III/2016 and stratified by different treatment modalities
| Total No or % of methods in topical year | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016-03-31 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All T categories | ONE | Total | 41 | 46 | 24 | 46 | 38 | 42 | 37 | 51 | 48 | 35 | 43 | 38 | 26 | 24 | 7 | 546 |
| % | 66.1% | 56.1% | 26.7% | 35.7% | 29.7% | 26.6% | 23.9% | 30.0% | 22.9% | 18.2% | 22.6% | 20.4% | 13.1% | 12.7% | 10.8% | 24.8% | ||
| LNE | Total | 0 | 14 | 35 | 40 | 47 | 57 | 56 | 46 | 58 | 56 | 60 | 59 | 53 | 45 | 21 | 647 | |
| % | 0.0% | 17.1% | 38.9% | 31.0% | 36.7% | 36.1% | 36.1% | 27.1% | 27.6% | 29.2% | 31.6% | 31.7% | 26.8% | 23.8% | 32.3% | 29.4% | ||
| OR | Total | 21 | 22 | 27 | 29 | 26 | 51 | 44 | 54 | 67 | 78 | 58 | 48 | 71 | 57 | 15 | 668 | |
| % | 33.9% | 26.8% | 30.0% | 22.5% | 20.3% | 32.3% | 28.4% | 31.8% | 31.9% | 40.6% | 30.5% | 25.8% | 35.9% | 30.2% | 23.1% | 30.3% | ||
| LR | Total | 0 | 0 | 4 | 14 | 17 | 8 | 18 | 19 | 37 | 23 | 29 | 41 | 48 | 63 | 22 | 343 | |
| % | 0.0% | 0.0% | 4.4% | 10.9% | 13.3% | 5.1% | 11.6% | 11.2% | 17.6% | 12.0% | 15.3% | 22.0% | 24.2% | 33.3% | 33.8% | 15.6% | ||
| % of NSS in all | 33.9% | 26.8% | 34.4% | 33.3% | 33.6% | 37.3% | 40.0% | 42.9% | 49.5% | 52.6% | 45.8% | 47.8% | 60.1% | 63.5% | 56.9% | 45.9% | ||
| Total 2002-2016 | 62 | 82 | 90 | 129 | 128 | 158 | 155 | 170 | 210 | 192 | 190 | 186 | 198 | 189 | 65 | 2204 | ||
| cT1a | Total 2007-2016 | 158 | 155 | 170 | 210 | 192 | 190 | 186 | 198 | 189 | 65 | 1713 | ||||||
| cT1a | Total | 59 | 55 | 68 | 78 | 78 | 64 | 76 | 103 | 95 | 32 | 708 | ||||||
| % cT1a from all | 37.3% | 35.5% | 40.0% | 37.1% | 40.6% | 33.7% | 40.9% | 52.0% | 50.3% | 49.2% | 41.3% | |||||||
| ONE in cT1a | Total | 5 | 0 | 7 | 1 | 4 | 3 | 3 | 1 | 1 | 2 | 27 | ||||||
| % | 8.5% | 0.0% | 10.3% | 1.3% | 5.1% | 4.7% | 3.9% | 1.0% | 1.1% | 6.3% | 3.8% | |||||||
| LNE in cT1a | Total | 10 | 10 | 10 | 9 | 8 | 6 | 8 | 11 | 5 | 3 | 80 | ||||||
| % | 16.9% | 18.2% | 14.7% | 11.5% | 10.3% | 9.4% | 10.5% | 10.7% | 5.3% | 9.4% | 11.3% | |||||||
| OR in cT1a | Total | 38 | 28 | 36 | 38 | 44 | 34 | 26 | 46 | 34 | 7 | 331 | ||||||
| % | 64.4% | 50.9% | 52.9% | 48.7% | 56.4% | 53.1% | 34.2% | 44.7% | 35.8% | 21.9% | 46.8% | |||||||
| LR in cT1a | Total | 6 | 17 | 15 | 30 | 22 | 21 | 39 | 45 | 55 | 20 | 270 | ||||||
| % | 10.2% | 30.9% | 22.1% | 38.5% | 28.2% | 32.8% | 51.3% | 43.7% | 57.9% | 62.5% | 38.1% | |||||||
| % of NSS in cT1a | 74.6% | 81.8% | 75.0% | 87.2% | 84.6% | 85.9% | 85.5% | 88.3% | 93.7% | 84.4% | 84.9% | |||||||
Note: exact data for cT1a category from years 2002-2006 are not available
ONE – open nephrectomy, LNE – laparoscopic nephrectomy, OR – open resection, LR – laparoscopic resection
Table 2.
Result of histology in surgically treated tumors mentioned in Table 1:
| Histology in 2002-III/2016 | ||
|---|---|---|
| Histology | Total number | Percentage |
| CRCC | 1673 | 75.9% |
| PRCC | 213 | 9.7% |
| ChRCC | 58 | 2.6% |
| oncocytoma | 92 | 4.2% |
| angiomyolipoma | 48 | 2.2% |
| others | 120 | 5.4% |
| Together | 2204 | 100.0% |
RCC – renal cell carcinoma, CRCC – clear, PRCC – papillary, ChRCC – chromophobe. The 2016 WHO classification is used [13]
Figure 4.
Percentage of clinical T1a category (tumor ≤4 cm) in surgically treated tumors. The sources of data are found in Table 1.
Figure 5.
Percentage of nephron sparing surgery (NSS) in all cT categories and in category cT1a. The sources of data are found in Table 1.
Figure 3.
Stratification of surgeries (in percentages) by basic surgical procedures in years 2002-III/2016. The sources of data are found in Table 1.
DISCUSSION
The surgical management of renal masses has undergone rapid evolution in clinical practice during the past two decades [14] and this is visible on our series as well. In 2002, 2/3 of all cases were treated with open radical nephrectomy and 1/3 with open resection. However, in 2015-2016, the ratio of resections increased from 1/3 to up to 60% of cases (Figure 3). This is due to (I) the expansion of general EAU guideline indications from category T1a only (tumor ≤4 cm) to T1b (>4 cm and ≤7 cm) and even T2a (>7 cm and ≤10 cm) as well [2]; (II) improved technique of laparoscopic resection [15] and technical equipment; and (III) a shift of diagnosis to less advanced cases (increasing of clinical T1a category between years 2007–2016 from 37% to about 50%) (Figure 4). For comparison, in the Netherlands in 2014, 62% of cT1a underwent nephron sparing surgery (NSS) and a further 5% underwent thermal ablation therapy. Partial nephrectomy (PN) was more common in the under 70 age group, in patients with non-centrally located tumors and in patients treated in high-volume hospitals. In the same study, 70% of cT1b patients underwent radical nephrectomy (RN), which were mainly performed laparoscopically. The rate of open RN vs. laparoscopic NE has increased in patients with larger tumor sizes and tumors located in the right kidney [16]. Elderly patients with T1a are treated with RN more frequently than with PN [16]. A higher rate of indication of PN by urologists at academic hospitals and a lower rate of PN in elderly patients are confirmed by several studies [10, 16, 17, 18]. Data from the USA (from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample from 2009 to 2012) supports a higher rate of PN in teaching hospitals (cumulative rates of PN were 48% vs. 33% in teaching vs. nonteaching hospitals respectively (p <0.0001) [10]. In the UK, the British Association of Urological Surgeons (BAUS) performed a survey in 2012 and found that the indications for NSS were: elective with a tumor of ≤4.5 cm in 59%, elective with a tumor of >4.5 cm in 10% (relative in 7%, imperative in 12%). The median (range) tumor size was 3.4 (0.8–30) cm. The technique used was minimally invasive surgery in 42%, open in 58%, with conversions in 4% [9]. The situation in the whole Czech Republic is documented in a survey performed by the Czech Urological Society and by the National Health Information System (NHIS). Selected urological surgical procedures were quantified in the a six-year period (2009–2014). A total of 20 634 (4 809 – 23.3% resections; 6 772 – 32.8% laparascopies) urgical procedures for kidney tumors (without any stratification by cT category) were performed in the mentioned period [19]. Nephron sparing surgery is generally underutilised [10, 20, 21, 22], but our presented study shows it can be performed in over 85% of cT1a cases (Figure 5). When technically feasible, RN is no longer an acceptable option when a PN is indicated, and might even be considered as malpractice [14]. The indications for nephrectomy in cT1a remain mainly centrally located tumors and tumor combined with other pathologies (e.g. end-stage kidney disease). What is a role of laparoscopy? In at least half of all cases (not only nephrectomies but also resections), laparoscopy can be applied. Laparoscopic surgery is technically more difficult and more expensive than open surgery, but is a more comfortable option for patients [15, 23]. Laparoscopic/robotic surgery is more commonly recommended by academic urologists and for patients under 65 year of age [24]. Robotic technology is associated with increased use of PN [25]. However, the open approach remains an important part of kidney tumor surgery. Open resections are indicated mainly in more complex tumors with higher nephrometry scores [26]. Open nephrectomy remains the standard for treating patients with the following findings: large advanced-stage tumors and/or perinephric extension, lymphadenopathy, thrombus in the renal vein and vena cava inferior – frequently with need of cooperation of urologist with cardiovascular surgeons [12, 27–30].
CONCLUSIONS
Resections and minimally invasive approaches are being performed more frequently and are the preferred methods in surgical treatment of kidney tumors. Clinical sequences are the following: resection (laparoscopic > open) > nephrectomy (laparoscopic > open). Resection is now (2016) indicated in about 60% (open vs. laparoscopic resection are used nearly equally with a slight tendency for laparascopic predomination). In the cT1a category (tumors up to 4 cm, making up approximately 50% of all surgically treated tumors), resection is possible in about 85–90% of cases. Over 60% of all tumors are treated with a laparoscopic approach. The open approach is indicated mainly in resections of more complex tumors and in nephrectomy for locally advanced tumors.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Capitanio U, Montorsi F. Renal cancer. Lancet. 2016;387:894–906. doi: 10.1016/S0140-6736(15)00046-X. [DOI] [PubMed] [Google Scholar]
- 2.Ljungberg B, Bensalah K, Canfield S, et al. EAU Guidelines on Renal Cell Carcinoma: 2014 Update. Eur Urol. 2015;67:913–924. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 3.MacLennan S, Imamura M, Lapitan MC, et al. Systematic review of oncological outcomes following surgical management of localised renal cancer. Eur Urol. 2012;61:972–993. doi: 10.1016/j.eururo.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 4.Harris KT, Ball MW, Gorin MA, Allaf ME, Pierorazio PM. Outcomes of partial nephrectomy in patients who meet percutaneous ablation criteria. Cent European J Urol. 2015;68:132–136. doi: 10.5173/ceju.2015.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ljungberg B, Hedin O, Lundstam S, et al. Nephron Sparing Surgery Associated With Better Survival Than Radical Nephrectomy in Patients Treated for Unforeseen Benign Renal Tumors. Urology. 2016;93:117–123. doi: 10.1016/j.urology.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 6.Pierorazio PM, Johnson MH, Patel HD, et al. Management of Renal Masses and Localized Renal Cancer: Systematic Review and Meta-Analysis. J Urol. 2016 doi: 10.1016/j.juro.2016.04.081. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Poppel H, Becker F, Cadeddu JA, et al. Treatment of localised renal cell carcinoma. Eur Urol. 2011;60:662–672. doi: 10.1016/j.eururo.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 8.Volpe A, Cadeddu JA, Cestari A, et al. Contemporary management of small renal masses. Eur Urol. 2011;60:501–515. doi: 10.1016/j.eururo.2011.05.044. [DOI] [PubMed] [Google Scholar]
- 9.Fernando A, Fowler S, O'Brien T. Nephron-sparing surgery across a nation-outcomes from the British Association of Urological Surgeons 2012 national partial nephrectomy audit. BJU Int. 2016;117:874–882. doi: 10.1111/bju.13353. [DOI] [PubMed] [Google Scholar]
- 10.Vigneswaran HT, Lec P, Brito J, Turini G, Pareek G, Golijanin D. Partial Nephrectomy for Small Renal Masses: Do Teaching and Nonteaching Institutions Adhere to Guidelines Equally? J Endurol. 2016;30:714–721. doi: 10.1089/end.2016.0112. [DOI] [PubMed] [Google Scholar]
- 11.Aben KKH, Osanto S, Hulsbergen-van de Kaa CA, Soetekouw PM, Stemkens D, Bex A. Adherence to guideline recommendations for management of clinical T1 renal cancers in the Netherlands: a population-based study. World J Urol. 2016;34:1053–1068. doi: 10.1007/s00345-016-1841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amer T, Biju RD, Hutton R, et al. Laparoscopic nephrectomy-Pfannenstiel or expanded port site specimen extraction: a systematic review and meta-analysis. Cent European J Urol. 2015;68:322–329. doi: 10.5173/ceju.2015.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016;70:93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 14.Bianchi M, Becker A, Abdollah F, et al. Rates of open versus laparoscopic and partial versus radical nephrectomy for T1a renal cell carcinoma: a population-based evaluation. Int J Urol. 2013;20:1064–1071. doi: 10.1111/iju.12110. [DOI] [PubMed] [Google Scholar]
- 15.Hora M, Eret V, Ürge T, et al. Results of laparoscopic resection of kidney tumor in everyday clinical practice. Cent European J Urol. 2009;62:160–166. [Google Scholar]
- 16.Kates M, Badalato G, Pitman M, McKiernan J. Persistent overuse of radical nephrectomy in the elderly. Urology. 2011;78:555–559. doi: 10.1016/j.urology.2011.02.066. [DOI] [PubMed] [Google Scholar]
- 17.Breau RH, Crispen PL, Jenkins SM, Blute ML, Leibovich BC. Treatment of patients with small renal masses: a survey of the American Urological Association. J Urol. 2011;185:407–413. doi: 10.1016/j.juro.2010.09.092. [DOI] [PubMed] [Google Scholar]
- 18.Kim SP, Shah ND, Weight CJ, et al. Contemporary trends in nephrectomy for renal cell carcinoma in the United States: results from a population based cohort. J Urol. 2011;186:1779–1785. doi: 10.1016/j.juro.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 19.Hora M, Babjuk M, Brodák M, Tomáš H, Ladislav J, Krhut J, et al. Key urological surgical procedures in the Czech Republic in period 2009-2014. Czes Urol. 2016;20:135–140. [Google Scholar]
- 20.Ljungberg B, Gudmundsson E, Christensen S, Lundstam S. Practice patterns for the surgical treatment of T1 renal cell carcinoma: a nationwide population-based register study. Scandin J Urol. 2014;48:445–452. doi: 10.3109/21681805.2014.898686. [DOI] [PubMed] [Google Scholar]
- 21.Smaldone MC, Kutikov A, Egleston B, et al. Assessing performance trends in laparoscopic nephrectomy and nephron-sparing surgery for localized renal tumors. Urology. 2012;80:286–291. doi: 10.1016/j.urology.2012.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooperberg MR, Mallin K, Kane CJ, Carroll PR. Treatment trends for stage I renal cell carcinoma. J Urol. 2011;186:394–399. doi: 10.1016/j.juro.2011.03.130. [DOI] [PubMed] [Google Scholar]
- 23.Hora M, Klecka J, Hes O, Ferda J, Urge T. [Miniinvasive laparoscopic or retroperitoneoscopic radical nephrectomy for the parenchymal tumor] Rozhl Chir. 2005;84:246–252. [PubMed] [Google Scholar]
- 24.Millman AL, Pace KT, Ordon M, Lee JY. Surgeon-specific factors affecting treatment decisions among Canadian urologists in the management of pT1a renal tumors. Can Urol Assoc. 2014;8:183–189. doi: 10.5489/cuaj.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel HD, Mullins JK, Pierorazio PM, et al. Trends in renal surgery: robotic technology is associated with increased use of partial nephrectomy. J Urol. 2013;189:1229–1235. doi: 10.1016/j.juro.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Klatte T, Ficarra V, Gratzke C, et al. A Literature Review of Renal Surgical Anatomy and Surgical Strategies for Partial Nephrectomy. Eur Urol. 2015;68:980–992. doi: 10.1016/j.eururo.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sobczyński R, Golabek T, Przydacz M, et al. Modified technique of cavoatrial tumor thrombectomy without cardiopulmonary by-pass and hypothermic circulatory arrest: a preliminary report. Cent European J Urol. 2015;68:311–317. doi: 10.5173/ceju.2015.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verhoest G, Couapel JP, Oger E, et al. Safety and Feasibility of Laparoscopic Nephrectomy for Big Tumors (≥10 cm): A Retrospective Multicentric Study. Clin Genitourin Cancer. 2016;14:e335–340. doi: 10.1016/j.clgc.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Gill IS, Metcalfe C, Abreu A, et al. Robotic Level III Inferior Vena Cava Tumor Thrombectomy: Initial Series. J Urol. 2015;194:929–938. doi: 10.1016/j.juro.2015.03.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang M, Zhang J, Niu Y, Xing N. Feasibility of Pure Conventional Retroperitoneal Laparoscopic Radical Nephrectomy With Level II Vena Caval Tumor Thrombectomy. Urology. 2016;90:101–105. doi: 10.1016/j.urology.2015.10.037. [DOI] [PubMed] [Google Scholar]