Abstract
Introduction
To evaluate contrast enhanced ultrasound (CEUS) as a modality to predict T stage of cancer of urinary bladder (CAUB) and to predict the grade of the tumor preoperatively.
Material and methods
110 patients with CAUB presenting to the Department of Urology at our institution between July 2014 and December 2015 underwent CEUS prior to endoscopic resection and the CEUS findings were compared with histopathology results.
Results
CEUS had a sensitivity of 75, 65 and 90% and specificity of 95, 85 and 92% in detecting Ta, T1 and muscle invasion respectively. CEUS had a sensitivity of 78% and specificity of 85% in detecting the grade of the lesion.
Conclusions
CEUS is a good alternative for T staging and grading of CAUB preoperatively. It is uniquely advantageous in detecting clots or necrosis and in patients with low eGFR where other imaging modalities are contraindicated.
Keywords: bladder cancer, contrast enhanced ultrasound, staging, grading, uroradiology, urooncology
INTRODUCTION
Urothelial carcinoma (UC) is the most common variant of carcinoma of the urinary bladder (CAUB) [1]. It constitutes 98% of all cancer of urinary bladder [1]. CAUB most commonly presents with gross painless hematuria. The management of CAUB is different for different stages and grades of the disease. Patients with non-muscle invasive bladder cancer (NMIBC) are managed with endoscopic resection and surveillance whereas patients with muscle invasive disease (MIBC) need radical extirpative surgery. The grade of the disease has been found to correlate with stage and is one of the primary drivers of the disease pathology. Thus, accurate staging and grading of UC is essential [2].
Conventional grey scale ultrasonography (USG) is a widely available, non-invasive examination and because of its low cost, it is often the first examination performed for patients presenting with hematuria, but it has limited utility in defining the disease. Contrast enhanced computed tomography (CECT) is the recommended investigation for staging of CAUB. However, its limitations include radiation exposure and contrast reactions. All patients will undergo cystoscopy and transurethral resection of bladder tumor (TURBT) for histopathological confirmation. TURBT can miss lesions or inadequate resection may be performed. One study reported that a repeat procedure within 6 weeks of the first TURBT found residual disease in 30% of patients [3].
Contrast enhanced ultrasound (CEUS) is a recent advancement in ultrasound technology. It utilizes ultrasonographic contrast agents (UCA), which are microbubbles composed of a lipid shell containing inert gases. These bubbles distribute in the body along vascular channels and their presence is detected by USG based on the reverberations they produce when they come in contact with sound waves. The uptake of UCA can be used to stage and grade CAUB in real time [4]. In the present study we evaluate CEUS as a method to predict invasive UC and determine the grade of CAUB.
MATERIAL AND METHODS
The study was conducted in the Departments of Urology, Radiology and Histopathology at our institute from July 2014 to December 2015. The study was approved by independent internal and an external ethics committees. A total of 278 consecutive patients of either sex presenting to the Department of Urology at our institute with suspected CAUB who met the inclusion and exclusion criteria (Table 1) were screened for CEUS.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
| 1. All patients with suspected carcinoma of the urinary bladder were included in the study. |
| Exclusion criteria |
| 1. Pregnancy |
| 2. Patients with prior lower abdominal surgery |
| 3. Patients who have undergone TURBT, TURP, any prior bladder surgery or have received intravesical chemotherapy or immunotherapy |
| 4. Patients with recent myocardial infarction, angina pectoris, cardiac insufficiency, severe cardiac arrhythmia or a right–left cardiac shunt |
| 5. Patients with severe pulmonary hypertension, uncontrolled systemic hypertension, acute respiratory distress syndrome or COPD |
| 6. Patients with a history of severe contrast allergy or any known drug allergy |
| 7. Morbidly obese patients (BMI >40) |
Inclusion criteria
All patients with suspected carcinoma of the urinary bladder were included in the study.
Exclusion criteria
Pregnancy
Patients with prior lower abdominal surgery
Patients who had undergone TURBT, TURP, any prior bladder surgery or have received intravesical chemotherapy or immunotherapy
Patients with recent myocardial infarction, angina pectoris, cardiac insufficiency, severe cardiac arrhythmia or a right–left cardiac shunt
Patients with severe pulmonary hypertension, uncontrolled systemic hypertension, acute respiratory distress syndrome or COPD
Patients with a history of severe contrast allergy or any known drug allergy
Morbidly obese patients (BMI >40)
All patients provided written informed consent. CEUS was performed a day prior to cystoscopy and TURBT by a single radiologist with more than 10 years of experience in radiological evaluation of bladder cancer. CECT findings (if available) were not revealed to the operator. The same Philips IU 22 ultrasound with real time gray-scale and contrast harmonics was used to perform CEUS in all cases. A C5-1 pulse wave transducer with a frequency of 7.5 Hz and low mechanical index (0.047–0.115) was used. The patient was prepared for CEUS by confirming adequate bladder filling. Adequate bladder filling was confirmed by measuring the intravesical volume (250 to 300 ml) and ensuring that the bladder wall was not thinned out. Initially, a B mode grey scale ultrasound was done to identify all lesions and to survey the bladder for mobile clots, any diverticula, large median lobe or vesical calculi. All visualized lesions were recorded. The lesion of interest was focused and gain increased and the harmonic imaging mode was entered. With the probe held constant, the patient was asked to respire in a controlled manner to reduce respiratory artifacts.
Intravenous reconstituted contrast (Sonovue) was injected as a bolus. The timer was started from the time of flush and the contrast phase was recorded for 3 minutes post injection. After the procedure was done, the patient was allowed to void and observed for 24 hours for any adverse effects. SonoVue (Bracco, Italy), is a powder which is reconstituted in the solvent for injection. The active substance in SonoVue is sulphur hexafluoride in the form of microbubbles. Each vial contains 59 mg sulphur hexafluoride. The vial is reconstituted with 5 ml of normal saline and after vibration blending; 2.4 ml (5 mg/ml) was used for injection.
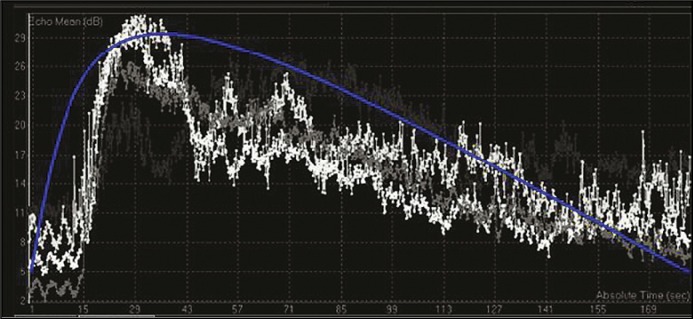
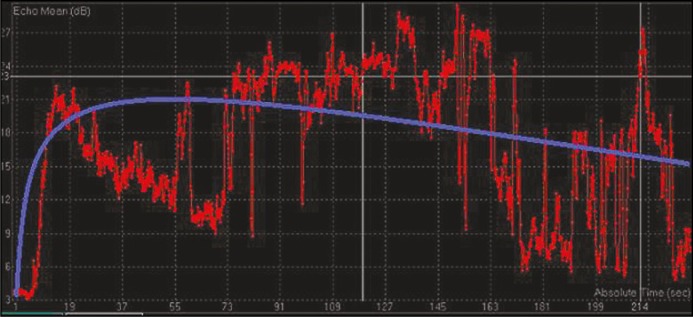
After the procedure, each recording was analyzed on the same day using a propriety software from Philips called Q Labs which came preinstalled on the machine. The region of interest (ROI) was marked as a polygon on the lesion to be studied. The computer software automatically generated contrast enhancement curves. Two types of curve shapes emerged. In curve type A there was rapid enhancement with high peak enhancement and disappearance of contrast within 3 minutes (Figure 1). For the the second curve, type B, there was an early enhancement peak but slow plateau and contrast disappearance did not take place until the end of study (Figure 2). Based on a review of literature, type A curves corresponded to high-grade lesions and type B as low-grade [4].
Figure 1.
Type A contrast enhancement curve (db – decibel, sec – second).
Figure 2.
Type B contrast enhancement curve (db – decibel, sec – second).
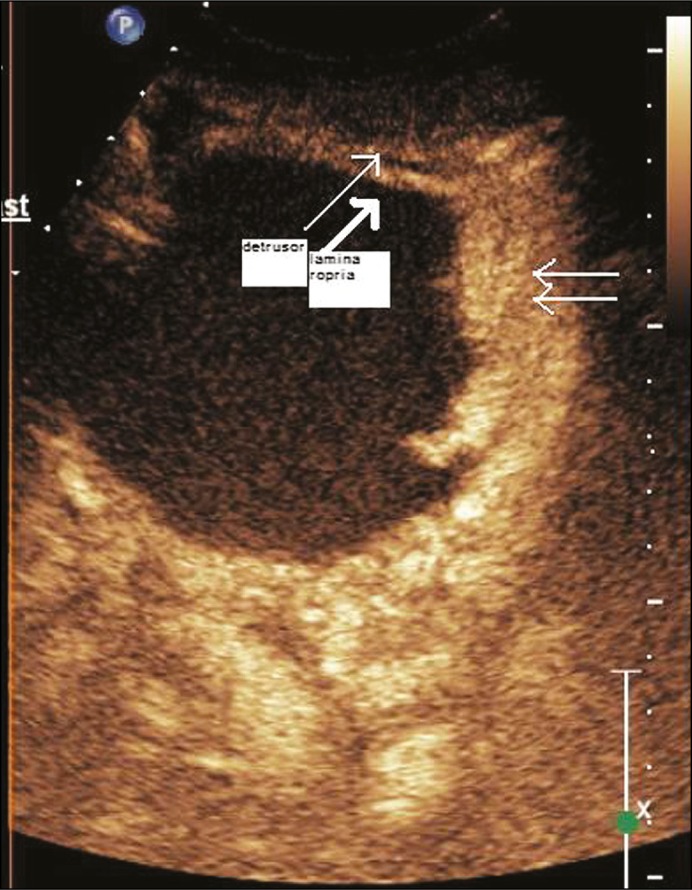
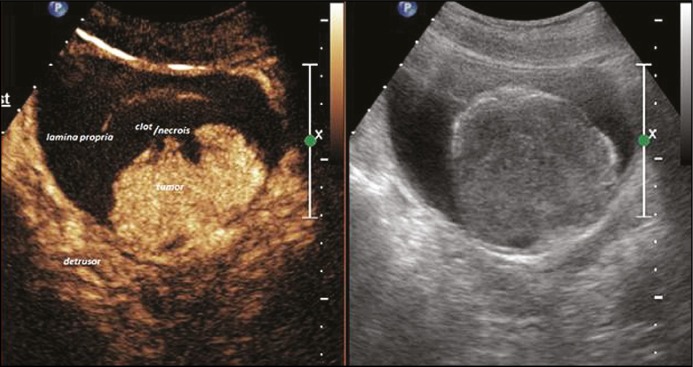
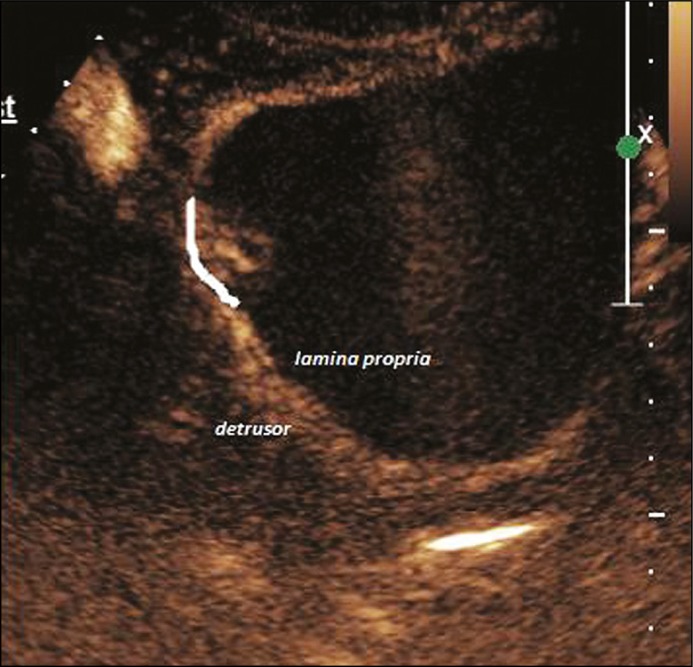
On contrast injection, the bladder wall separated into two distinct enhancing layers corresponding to the lamina propria and the muscularis propria. Depending on visual inspection and operator experience, the lesions were classified as muscle invasive if there was loss of planes between the lesion and the muscularis propria (Figure 3). If there was no muscle invasion, then the lesion was classified as NMIBC. It was further subdivided into Ta and T1 based on invasion of lamina propria (Figures 4 and 5). All patients underwent TURBT at the Department of Urology by a surgeon with at least 5 years of experience. The specimen was processed at the Department of Histopathology. If the HPR revealed absence of deep muscle in the specimen, a repeat TURBT was performed. All histopathology specimens were reevaluated individually by a single pathologist with 10 years experience, who was blinded to the CEUS and cystoscopy findings.
Figure 3.
Contrast enhanced ultrasound image of a T2 lesion (TNM Tumor stage T2, double thin arrow showing site of invasion).
Figure 4.
Composite image of contrast enhanced ultrasound and grey scale image of a T1 lesion (TNM tumor stage T1).
Figure 5.
Contrast enhanced ultrasound image of a Ta lesion (TNM Tumor stage Ta).
Statistical methods and data analysis
Statistical analyses were performed using a computer software package (SPSS Inc. Chicago, IL). Student T tests and the Chi Square test were applied. For CEUS the sensitivity and specificity was calculated using histopathology as a gold standard.
RESULTS
Two hundred and seventy-eight (278) patients were found eligible for participation in the study. One hundred and eight patients did not give thir consent. Forty-six patients could not undergo CEUS before TURBT. One hundred and twenty-four patients were enrolled in the study and underwent CEUS. Nine patients did not undergo restage TURBT after CEUS and were thus excluded. Three patients were excluded by the pathologist due to inability to determine grade and two patients had squamous cell variant of bladder cancer. In summary, for final analysis, 110 patients who had both CEUS and final histopathology were included for analysis.
The characteristics of the 110 patients and the final histopathology of the patients are shown in Table 2.
Table 2.
Characteristics of 110 patients
| Parameter | Variable |
|---|---|
| Age | 19–85 (median 60) |
| Sex | Male: 96 Female: 14 |
| Hematuria | Yes: 102 No: 8 |
| Histopathological T Stage | Ta: 21 T1:32 MIBC 53 Clot: 4 |
| Histopathological Grade | Low grade: 36 High Grade: 70 |
| CEUS Grade | Ta:21 T1:31 MIBC: 54 Clot: 4 |
| CEUS Grade | Low Grade: 32 High Grade: 74 |
CEUS – Contrast enhanced ultrasound; T – tumor; NMIBC – non muscle invasive bladder cancer; MIBC – muscle invasive bladder cancer
The Ta lesions detected in CEUS are not the same as defined by pathology
The sensitivity, specificity and predictive value of CEUS in detecting T stage are shown in Table 3. CEUS can predict NMIBC and MIBC with a sensitivity of 90 and 90.74% respectively and specificity of 75.71 and 92.76% respectively. CEUS can predict Ta and T1 cancer with a sensitivity of 75 and 65.62% respectively and a specificity of 93.33 and 85.9% respectively.
Table 3.
Diagnostic parameters of CEUS for T stage
| T stage | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Ta | 75 | 93.33 | 71.43 | 94.38 |
| T1 | 65.62 | 85.9 | 65.62 | 85.9 |
| NMIBC (T1 +Ta) | 90 | 75.71 | 67.92 | 92.98 |
| MIBC | 90.74 | 92.76 | 92.45 | 91.23 |
*NMIBC – non muscle invasive bladder cancer; MIBC – muscle invasive bladder cancer; PPV – positive predictive value; NPV – negative predictive value
The sensitivity, specificity and predictive value of CEUS enhancement curve in predicting the grade are shown in Table 4. CEUS predicted low grade CAUB with a sensitivity, specificity, positive predictive valve and negative predictive value of 78.12, 85.14, 69.44 and 90% respectively. CEUS predicted high grade CAUB with a sensitivity, specificity, positive predictive value and negative predictive value of 85.14, 78.12, 90 and 69.44% respectively.
Table 4.
Type of enhancement curve in predicting grade
| Grade | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Low | 78.12 | 85.14 | 69.44 | 90 |
| High | 85.14 | 78.12 | 90 | 69.44 |
PPV – positive predictive value; NPV – negative predictive value
DISCUSSION
Urothelial carcinoma of the urinary bladder is a common malignant neoplasm among smokers, with a rising incidence in India [2].
Macroscopic hematuria is an indication for complete evaluation of the genitourinary system for malignancy. Active hematuria can interfere with certain screening and diagnostic tests, such as malignant cytology. In our study, 4 patients were found to have organized clots in the bladder. The presence of clots in the bladder can hamper adequate evaluation of the bladder with CECT. CEUS has a practical advantage in this scenario as it can detect tumor vascularity and clot mobility in real time. A unique practical advantage of CEUS in evaluation of bladder tumor is that large papillary tumors with necrosis or adherent clots can be identified preoperatively and may help the surgeon in planning the resection. Patients with active hematuria on bladder irrigation pose a problem for CEUS, as stopping the irrigation can lead to clot formation and blockage of the return, causing the patient discomfort. Additionally, in situ large foley bulbs can hide small sessile lesions.
Serum creatinine may be raised in bladder cancer secondary to obstructive uropathy or due to CKD due to diabetes and hypertension. Serum creatinine has an effect on the choice of urinary diversion after cystectomy. Raised serum creatinine is a relative contraindication for CECT and MRI as it predisposes to nephropathy and nephrogenic systemic fibrosis respectively. CEUS has a significant advantage in this patient subgroup. UCA are metabolized and excreted by the lungs. In our study, 11 patients presented with a serum creatinine above 1.5 and all underwent CEUS without any toxicity or side effect.
In our study, pathology of TURBT specimens was used as the reference standard. This is a potential weakness of our study. Endoscopic resection was performed by different surgeons in the department depending on the OR schedule. TURBT can understage and overstage the disease. A study in 2014 showed that radical cystectomy post TURBT can change the T stage in more than half of the cases. All TURBT specimens were evaluated by the same pathologist with more than 10 years of experience in evaluating uro-oncology specimens. However, there can be significant inter reader variability among different pathologists. Tritschlr et al. reported that just the knowledge of endoscopic findings could change the pathologist’s interpretation. Studies have shown that there is significant inter observer variability and poor reproducibility in interpreting the grades of bladder cancer. Even with these drawbacks, our unique situation as a center in a resource poor setting prevented the use of a better methodology. Histopathology from radical cystectomy is ideal. The waiting period at our center can be long due to very high volumes and limited resources. Disease may progress during this period. Secondly, many patients received neo-adjuvant chemotherapy prior to radical cystectomy. This demonstrates the variability in management of CAUB. Several factors including the resource setting can potentially modify the final treatment plan that the patient receives [5–8].
CEUS was able to delineate the T stage with specificity (85–100%) and sensitivity (75–92%) for tumor staging. CEUS was not able to accurately determine lamina propria invasion. If Ta and T1 lesions were combined as NMIBC, then the sensitivity of the test improved but specificity declined. Our results are in concordance with previously published studies [9, 10, 11].
Differentiating lamina propria on CEUS is difficult. This wispy thin layer usually disappears with bladder overdistension. It is difficult to visualize in females and patients with thin bladders. Only in small solitary pedunculated tumors, can a comment on lamina propria be attempted. Differentiating Ta and T1 lesions is important for management decisions, as solitary Ta lesions require lesser follow up. However, from the surgeon’s point of view, where most small solitary tumors will undergo en bloc resection with underlying muscle biopsy, the knowledge of Ta preoperatively does not make a difference in the surgical approach [7].
CEUS is truly unique in its ability to define the tumor grade preoperatively. We defined two types of TIC. When the type of curve was used to define the grade, we found that type A curve can define high-grade lesions with a sensitivity and specificity of 85 and 78% respectively (p <0.05). Similarly, a type B curve correlates well with a low-grade lesion. This is similar to results reported previously in literature. Drudi et al. reported a sensitivity and specificity of 90 and 85% respectively [4]. Their higher accuracy may be due to consensus analysis by two radiologists and availability of supplementary information in the form of color Doppler.
The software that is used for the quantitative analysis a CEUS recording is different with different manufacturers [10, 11]. There are many types of propriety software available. The TIC values recorded will vary with the machine, probe, signal gain settings and the software being used [10, 11]. We also noticed that the amount of contrast used and the dilution used also had an effect on the TIC values. Thus, we feel that each center should develop their own TIC curves and cutoffs during preliminary examinations using these parameters as a rough guide until more robust data is available and there is homogenous reporting of these values.
CONCLUSIONS
CEUS is a reliable investigation modality for preoperative characterization of urothelial carcinoma. It is an easily available, safe and non-invasive examination that is advantageous in patients with low GFR as it is not nephrotoxic.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27:289–293. doi: 10.1007/s00345-009-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan P, Bogillot O, Cordier S, et al. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 casecontrol studies. Int J Cancer. 2000;86:289–294. doi: 10.1002/(sici)1097-0215(20000415)86:2<289::aid-ijc21>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.Jimenez RE, Gheiler E, Oskanian P, et al. Grading the invasive component of urothelial carcinoma of the bladder and its relationship with progressionfree survival. Am J Surg Pathol. 2000;24:980–987. doi: 10.1097/00000478-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Drudi F, Di Leo N, Malpassini F, Antonini F, Corongiu E, Iori F. CEUS in the differentiation between low and high-grade bladder carcinoma. J Ultrasound. 2012;15:247–251. doi: 10.1016/j.jus.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schips L, Augustin h, Zigeuner RE, et al. Is repeated transurethral resection justified in patients with newly diagnosed superficial bladder cancer? Urology. 2002;59:220–223. doi: 10.1016/s0090-4295(01)01522-9. [DOI] [PubMed] [Google Scholar]
- 6.Tekes A, Kamel I, Imam K, et al. Dynamic MRI of bladder cancer: evaluation of staging accuracy. AJR Am J Roentgenol. 2005;184:121–127. doi: 10.2214/ajr.184.1.01840121. [DOI] [PubMed] [Google Scholar]
- 7.Tritschler S, Karl A, Sommer M, et al. Influence of clinical information on the interpretation of urinary cytology in bladder cancer: how suggestible is a cytologist? BJU Int. 2010;106:1165–1168. doi: 10.1111/j.1464-410X.2010.09285.x. [DOI] [PubMed] [Google Scholar]
- 8.Poletajew S, Fus Ł, Walędziak M, et al. Comparison of pathological staging and grading of urothelial bladder carcinoma in post-transurethral resection and post-radical cystectomy specimens. Pol J Pathol. 2014;65:305–312. doi: 10.5114/pjp.2014.48192. [DOI] [PubMed] [Google Scholar]
- 9.Caruso G, Salvaggio G, Campisi A, et al. Bladder tumor staging: comparison of contrast-enhanced and gray-scale ultrasound. AJR Am J Roentgenol. 2010;194:151–156. doi: 10.2214/AJR.09.2741. [DOI] [PubMed] [Google Scholar]
- 10.Drudi F, Di Leo N, Maghella F, et al. CEUS in the study of bladder, method, administration and evaluation, a technical note. J Ultrasound. 2013;17:57–63. doi: 10.1007/s40477-013-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shariat SF, Karam JA, Lerner SP. Molecular markers in bladder cancer. Curr Opin Urol. 2008;18:1–8. doi: 10.1097/MOU.0b013e3282f1c5c1. [DOI] [PubMed] [Google Scholar]