Abstract
Objective: To investigate a possible association between common variations of the P2RY12 and the residual clopidogrel on-treatment platelet reactivity after adjusting for the influence of CYP2C19 tested by thromboelastography (TEG). Methods: One hundred and eighty patients with acute coronary syndrome (ACS) treated with clopidogrel and aspirin were included and platelet function was assessed by TEG. Five selected P2RY12 single nucleotide polymorphisms (SNPs; rs6798347, rs6787801, rs6801273, rs6785930, and rs2046934), which cover the common variations in the P2RY12 gene and its regulatory regions, and three CYP2C19 SNPs (*2,*3,*17) were genotyped and possible haplotypes were analyzed. Results: The high on-treatment platelet reactivity (HTPR) prevalence defined by a platelet inhibition rate <30% by TEG in adenosine diphosphate (ADP)-channel was 69 (38.33%). Six common haplotypes were inferred from four of the selected P2RY12 SNPs (denoted H0 to H5) according to the linkage disequilibrium R square (except for rs2046934). Haplotype H1 showed a significantly lower incidence of HTPR than the reference haplotype (H0) in the total study population while haplotypes H1 and H2 showed significantly lower incidences of HTPR than H0 in the nonsmoker subgroup after adjusting for CYP2C19 effects and demographic characteristics. rs2046934 (T744C) did not show any significant association with HTPR. Conclusions: The combination of common P2RY12 variations including regulatory regions rather than rs2046934 (T744C) that related to pharmacodynamics of clopidogrel in patients with ACS was independently associated with residual on-clopidogrel platelet reactivity. This is apart from the established association of the CYP2C19. This association seemed more important in the subgroup defined by smoking.
Keywords: P2RY12; CYP2C19; Haplotype; Single nucleotide polymorphism (SNP); Clopidogrel; Thromboelastography
1. Introduction
Dual antiplatelet therapy with aspirin and a platelet P2Y12 adenosine diphosphate (ADP) receptor antagonist reduces recurrent adverse cardiovascular events in acute coronary syndrome (ACS) and/or following percutaneous coronary intervention (PCI) (Yusuf et al., 2001; Beinart et al., 2005). It is recommended for patients with ACS and/or PCI in China (Emergency Medical Branch of Chinese Medical Doctor Association et al., 2016; Section of Interventional Cardiology of Chinese Society of Cardiology of Chinese Medical Association et al., 2016) and worldwide (Levine et al., 2016; SIGN, 2016). Clopidogrel is currently one of the most commonly used platelet P2Y12 receptor inhibitors in China, although new guidelines have recommended ticagrelor as the first-line medicine in patients with ACS or following PCI.
Clopidogrel is an orally administered thienopyridine prodrug, which needs to be biotransformed into its active metabolite. After oral administration, the absorption of clopidogrel is regulated by adenosine triphosphate (ATP)-binding cassette, sub-family B, member 1 (ABCB1) transporters (Mega et al., 2010a), and the hepatic biotransformation of clopidogrel prodrug to its active metabolite is mediated by CYP450 enzymes, especially CYP2C19. After activation, the active metabolite of clopidogrel selectively and irreversibly inhibits the P2Y12 receptor located on the surface of platelets and decreases platelet aggregation. Many studies have demonstrated the close relationship between CYP2C19 genotype and high on-treatment platelet reactivity (HTPR) (Mega et al., 2010b; Simon et al., 2011), including some studies focusing on Chinese subjects (Chen et al., 2014) and our previous study (Liu et al., 2015).
As well as CYP2C19, other genetic factors may contribute to the inter-individual variability of clopidogrel, among which P2RY12, which encodes the P2Y12 receptor on platelets, is of importance. Previous studies have implied that P2RY12 single nucleotide polymorphism (SNP) might play a role on the large variability in clopidogrel response, but the results were inconsistent and controversial (Angiolillo et al., 2007; Zee et al., 2008; Jana et al., 2014; Oestreich et al., 2014; Li et al., 2016; Zhang et al., 2016). Most of those studies focused on single haplotype-tagging SNPs (ht-SNPs), such as rs2046934 (i-T744C) or rs6785930, or two common haplotypes H1 and H2 (constituted by single nucleotide polymorphism database (dbSNP) rs10935838, rs2046934, rs5853517, and rs6809699), especially the previous studies in the Chinese population (Chen et al., 2014; Zhang et al., 2016). Several studies focused on rs10935838, rs5853517, and some other SNPs at the same time, but mostly in the Caucasian population (Zee et al., 2008; Rudež et al., 2009). rs10935838, rs2046934, rs5853517, and rs6809699 are in close linkage disequilibrium (LD) but not covering the regulatory regions of the P2RY12 gene. A comprehensive study of common variations in the P2RY12 gene that cover common haplotypes within the P2RY12 gene and its regulatory regions is lacking in the Chinese population with ACS. Moreover, most present studies focus on the P2RY12 gene separately, without adjusting for the influence of the main proven gene to affect clopidogrel on-treatment residual platelet activities—the CYP2C19 *2 and *3 alleles. The aim of this study was to investigate a possible association between the common genetic variations of the P2RY12 locus, including the promoter region after adjusting for the influence of CYP2C19 *2 and *3 alleles, and the residual on-clopidogrel platelet reactivity by thromboelastography (TEG) assay in a Chinese population with ACS.
2. Materials and methods
2.1. Study population
Consecutive patients aged from 18 to 80 years with established ACS at Peking University Second Hospital, Beijing, China, during May 2014 to April 2015 were included in the study. The diagnosis of ACS (unstable angina or myocardial infarction) was confirmed by ischemic symptoms and electrocardiograph (ECG) changes with or without increase in creatine kinase and troponin levels plus coronary angiography if needed. The exclusion criteria included contraindications to clopidogrel, aspirin, heparin, and contrast agents as well as quantitative coronary angiography drugs, and whether there had been a major operation within the last one month and the use of a glycoprotein IIb/IIIa antagonist during hospitalization.
All the eligible patients received 75 mg/d clopidogrel and 100 mg/d aspirin for at least 5 d with or without a loading dose of 300 or 600 mg clopidogrel according to institutional practice in addition to the other available medical therapy, including statins, β-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or antidiabetic agents.
2.2. Definition
Smokers were defined as current or recent smokers (stopped smoking for <1 year). Hypertension, diabetes mellitus, and dyslipidemia were defined according to the World Health Organization criteria. A family history of cardiovascular disease was defined as an early history of coronary artery disease in women younger than 65 years and in men younger than 55 years among the first-degree relatives.
2.3. Blood collection
On the 5th to 7th days after clopidogrel administration, blood samples were collected from peripheral veins with vacutainer tubes (BD Medical Systems) containing heparin or 32 g/L sodium citrate. TEG assay was conducted within 3 h after blood collection. The study protocol was approved by the Ethics Committee of Peking University, China (IRB 00001052-14087).
2.4. Platelet function assay
Citrated whole blood (2 ml) and heparin-anticoagulated whole blood (2 ml) were drawn at least 5 d after the administration of the first clopidogrel maintenance dose. These samples were sent to the hemostasis laboratory of the hospital and TEG was performed within 3 h after blood collection according to the manufacturer’s instruction (TEG 5000, Haemoscope Corp., IL, USA) by trained technicians at the hospital. Platelet reactivity was calculated using the TEG platelet mapping system. TEG utilizes four channels to detect the effects of antiplatelet therapy activity induced by arachidonic acid or ADP. The platelet inhibition rate (PIR) in the ADP channel was recorded. A TEG PIR of less than 30% was defined as clopidogrel HTPR, while patients with non-high on-treatment platelet reactivity (nHTPR) had a PIR of at least 30% according to the instruction and previous study (Bliden et al., 2007).
2.5. Selection of SNPs
Based on the latest LD data of the P2RY12 locus (P2RY12 gene with 2.5 kb flanking sequence) provided by SNP Annotation and Proxy Search (SNAP) for a population of Han Chinese in Beijing and Japanese in Tokyo (CHBJPT) (http://www.broadinstitute. org/mpg/snap), we chose the five most common P2RY12 SNPs which cover common haplotypes within the P2RY12 gene and its regulatory regions to be analyzed. LD data were calculated using Haploview 4.0, based on phased genotype data from the 1000 Genomes Project (1000 Genomes Pilot 1) (Table 1). The five selected SNPs that were genotyped were rs6798347 (c.−281−3614C>t), rs6787801 (c.−217+2739T>c), rs6801273 (c.−216−4445A>g), rs6785930 (c.18C>t, which was also identified as C34T), and rs2046934 (c.742T>c, which was also identified as T744C).
Table 1.
Linkage disequilibrium R-square of the selected P2RY12 SNPs
| P2RY12 SNP | Pairwise R
2
|
||||
| rs6798347 | rs6787801 | rs6801273 | rs2046934 | rs6785930 | |
| rs6798347 | rs6798347 | ||||
| rs6787801 | 0.354 | rs6787801 | |||
| rs6801273 | 0.347 | 0.144 | rs6801273 | ||
| rs2046934 | 0.147 | 0.114 | 0.169 | rs2046934 | |
| rs6785930 | 0.336 | 0.148 | 0.446 | 0.076 | rs6785930 |
Linkage disequilibrium data are calculated based on phased genotype data from the 1000 Genomes Project (1000 Genomes Pilot 1), and the pairwise R 2 values of >0.3 were selected for haplotype analysis (shown in bold numbers)
In addition to the five selected P2RY12 genes, we also genotyped the three most common reported CYP2C19 SNPs, CYP2C19 *2 (c.681G>A, rs4244285), CYP2C19 *3 (c.636G>A, rs4986893) and CYP2C19 *17 (c.806C>T, rs12248560), to adjust their effect on the platelet reactivity for the above selected SNPs.
2.6. Genetic and haplotype analysis
After TEG testing, the used heparin-anticoagulated whole blood was collected from the hemostasis laboratory. Blood sample genomic DNA was isolated using the DNA extractor AxyPrep-96 (AXYGEN Scientific Inc., California, USA). CYP2C19 *2 (rs4244285), CYP2C19 *3 (rs4986893), CYP2C19 *17 (rs12248560), and P2RY12 SNPs (rs6798347, rs6787801, rs6801273, rs2046934, and rs6785930) were identified by the ligase detection reaction (LDR)-real time polymerase chain reaction (PCR) analysis system (Perkin-Elmer Gene Amp PCR Systems 9600, Perkin-Elmer Shanghai Inc., Shanghai, China). GENESCAN™ 672 and Genemapper of the system were used to analyze the CYP2C19 alleles. In this study, 10% of the samples were randomly reanalyzed, and the results were confirmed in 98.92%. According to the LD R-square of the selected P2RY12 SNPs, SNPs with pairwise R 2 values >0.3 were selected for haplotype analysis, which turned out to be rs6798347, rs6787801, rs6801273, and rs6785930.
2.7. Statistical analysis
Statistical analysis was carried out using STATA for Windows, Version 12.0 (Stata Corp., Texas, USA). Demographic data were presented as means and standard deviations (SDs) for continuous variables and as counts and percentages for categorical variables. Hardy-Weinberg equilibrium analysis was conducted using a chi-square test. Multivariate logistic regression analysis was carried out to study the association between individual SNPs/haplotypes and HTPR. Homozygotes for the common allele of these SNPs were used as the reference for each comparison analysis. P2RY12 haplotype analysis was done separately in nonsmokers. Analyses were adjusted for age, sex, body mass index, smoker, diabetes, hypertension, hyperlipidemia, family history of cardiovascular disease, platelet count, clopidogrel dose regimen (with or without loading dose), prior vascular disease and diagnosis (ST-segment elevation myocardial infarction (STEMI) or non-ST-segment elevation acute coronary syndrome (NSTE-ACS)). Results were expressed as odds ratio (OR) with 95% confidence interval (95% CI). A 2-sided value of P<0.05 was considered statistically significant.
3. Results
3.1. Platelet reactivity detection and baseline characteristics
One hundred and eighty patients were included in this study. All were from the Chinese Han population, and 120 (66.67%) patients underwent PCI. We identified 69 (38.33%) patients with HTPR. Demographic characteristics according to different clopidogrel responses are shown in Table 2. There were no significant differences among age, gender, risk factors, medications, or other general information between the two groups (P>0.05) except for smoking (P=0.046).
Table 2.
Demographic characteristics of the studied population according to residual platelet reactivity
| Characteristics | Overall (n=180) | HTPR (n=69) | nHTPR (n=111) | P-value |
| Demographics | ||||
| Age | 64.09±11.08 | 64.91±10.47 | 63.59±11.46 | 0.434 |
| Male | 137 (76.11%) | 50 (72.46%) | 87 (78.38%) | 0.367 |
| Risk factors | ||||
| BMI (kg/m2) | 25.81±3.49 | 25.78±3.69 | 25.83±3.38 | 0.921 |
| Smoker | 54 (30.00%) | 15 (21.74%) | 39 (35.14%) | 0.046 |
| DM | 70 (38.89%) | 28 (40.58%) | 42 (37.84%) | 0.714 |
| Hypertension | 122 (67.78%) | 48 (69.57%) | 74 (66.67%) | 0.686 |
| Dyslipideamia | 78 (43.33%) | 28 (40.58%) | 50 (45.05%) | 0.557 |
| FH of CVD | 63 (35.00%) | 25 (36.23%) | 38 (34.23%) | 0.785 |
| Medication | ||||
| Clopidogrel loading dose | 42 (23.33%) | 11 (15.94%) | 31 (27.93%) | 0.068 |
| β-Receptor antagonist | 132 (73.33%) | 46 (66.67%) | 86 (77.48%) | 0.113 |
| ACEIs or ARBs | 109 (60.56%) | 40 (57.97%) | 69 (62.16%) | 0.576 |
| CCBs | 48 (26.67%) | 23 (33.33%) | 25 (22.52%) | 0.113 |
| Statins | 165 (91.67%) | 64 (92.75%) | 101 (90.99%) | 0.678 |
| PPIs | 28 (15.56%) | 11 (15.94%) | 17 (15.32%) | 0.910 |
| NSTE-ACS | 124 (68.89%) | 44 (63.77%) | 80 (72.07%) | 0.243 |
| Prior MI | 29 (16.11%) | 12 (17.39%) | 17 (15.32%) | 0.713 |
| Prior PCI | 119 (66.11%) | 47 (68.12%) | 72 (64.86%) | 0.654 |
| Platelet count (109 L−1) | 189.11±61.19 | 193.06±64.50 | 186.70±59.25 | 0.502 |
| Haematocrit (%) | 38.35±6.37 | 37.74±5.16 | 38.73±7.01 | 0.320 |
| Hemoglobin (g/L) | 133.50±18.52 | 130.40±22.43 | 133.50±18.52 | 0.319 |
Values are expressed as mean±SD or n (%). P-values refer to comparisons between HTPR and nHTPR groups. HTPR: high on-treatment platelet reactivity; nHTPR: non-high on-treatment platelet reactivity; BMI: body mass index; DM: diabetes mellitus; FH of CVD: family history of cardiovascular disease; ACEIs/ARBs: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; CCBs: calcium channel blockers; PPIs: proton pump inhibitors; NSTE-ACS: non-ST-segment elevation acute coronary syndrome; MI: myocardial infarction; PCI: percutaneous coronary intervention
3.2. Genotyping and haplotype results
Allele frequencies and genotype distribution of the genetic variations studied are shown in Table 3. The distribution of all the CYP genetic variants did not deviate from Hardy-Weinberg equilibrium.
Table 3.
Genotype distribution and allele frequencies of investigated genetic variations
| Variation | Non-carriers,n (%) | Heterozygous carriers, n (%) | Homozygous carriers, n (%) | Carriers of at least one allele, n (%) | Allele frequency (%) |
| CYP2C19 | |||||
| *2 (rs4244285) | 91 (50.56%) | 67 (37.22%) | 22 (12.22%) | 89 (49.44%) | 30.83 |
| *3 (rs4986893) | 162 (90.00%) | 18 (10.00%) | 0 (0.00%) | 18 (10.00%) | 5.00 |
| *17 (rs12248560) | 179 (99.44%) | 1 (0.56%) | 0 (0.00%) | 1 (0.56%) | 0.28 |
| P2RY12 | |||||
| rs6798347 | 94 (52.22%) | 67 (37.22%) | 19 (10.56%) | 86 (47.78%) | 29.17 |
| rs6787801 | 60 (33.33%) | 81 (45.00%) | 39 (21.67%) | 120 (66.67%) | 44.17 |
| rs6801273 | 71 (39.44%) | 83 (46.11%) | 26 (14.44%) | 109 (60.56%) | 37.50 |
| rs6785930 | 116 (64.44%) | 54 (30.00%) | 10 (5.56%) | 64 (35.56%) | 20.56 |
| rs2046934 | 122 (67.78%) | 54 (30.00%) | 4 (2.22%) | 58 (32.33%) | 17.22 |
3.2.1 CYP2C19
Among the 180 patients studied, 89 (49.44%) were carriers of at least one CYP2C19 *2 loss-of-function (LOF) allele and 18 (10.00%) were carriers of at least one CYP2C19 *3 LOF allele. For CYP2C19 *17 gain of function (GOF) allele, only one heterozygous carrier was detected.
On the basis of the distribution of genotypes, the patients were divided into extensive metabolizers (EMs) without an LOF mutation allele (LOF non-carriers), which was also the CYP2C19 *1/*1 (681GG/636GG) wild type genotype, intermediate metabolizers (IMs) carrying only one LOF mutation allele (CYP2C19 *2 or CYP2C19 *3) with or without the GOF mutation allele, and poor metabolizers (PMs) carrying two LOF mutation alleles (CYP2C19 *2 and CYP2C19 *3), accounting for 42.78%, 42.78%, and 14.44% of all cases, respectively.
3.2.2 P2RY12
Genotype distribution and allele frequencies of the studied P2RY12 SNPs were given in Table 3. According to the LD R-square of the selected P2RY12 SNPs shown in Table 1, we combined the four P2RY12 ht-SNPs (rs6798347, rs6787801, rs6801273, and rs6785930) and 12 haplotype alleles were inferred, among which 6 had an allele frequency higher than 5%: (H0) CTAC (7.78%), (H1) tcgt (14.44%), (H2) tTgt (16.11%), (H3) CcgC (18.33%), (H4) CcAC (21.67%), and (H5) tTAC (7.22%), as shown in Table 4. Finally, the defined haplotypes cover 85.56% of the total common selected DNA sequence variations in the P2RY12 locus. The last P2RY12 SNP rs2046934 was not combined as the pairwise LD R 2 values with the other four SNPs were low (<0.3).
Table 4.
Composition and frequency of P2RY12 haplotypes
| Haplotype | (1) (C>t) | (2) (T>c) | (3) (A>g) | (4) (C>t) | SNP allele composition | Frequency (%) |
| H0 | C | T | A | C | CTAC | 7.78 |
| H1 | t | c | g | t | tcgt | 14.44 |
| H2 | t | T | g | t | tTgt | 16.11 |
| H3 | C | c | g | C | CcgC | 18.33 |
| H4 | C | c | A | C | CcAC | 21.67 |
| H5 | t | T | A | C | tTAC | 7.22 |
Haplotype alleles were coded H1 to H5 in the descending order of their effect on platelet inhibition rate by TEG where H0 is the reference haplotype allele. (1)‒(4) represent rs6798347, rs6787801, rs6801273, and rs6785930, respectively, with the minor alleles in lower case
3.3. Relationship of genotypes and haplotypes with residual platelet reactivity
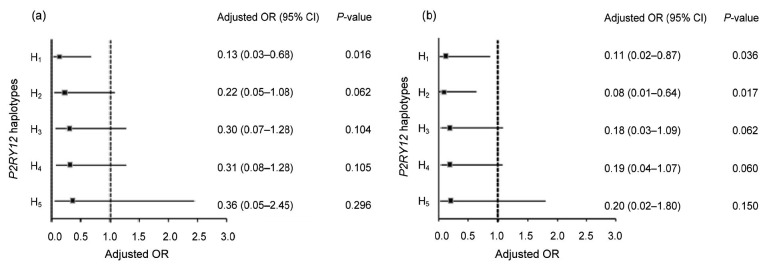
The results of multivariate logistic regression for HTPR in the entire study population are presented in Table 5. After adjusting for demographic characteristics, the presence of at least one LOF allele (PMs or IMs) or P2RY12 haplotype was independently associated with HTPR in the entire study population and in nonsmokers (Fig. 1).
Table 5.
Influence of all genetic variants/haplotypes on platelet reactivity
| SNP/haplotype | HTPR, n (%) | nHTPR, n (%) | Total, n (%) | P-value |
| CYP2C19 | ||||
| EM | 19 (24.68) | 58 (75.32) | 77 (42.78) | Reference |
| IM | 39 (50.65) | 38 (49.35) | 77 (42.78) | 0.001 |
| PM | 11 (42.31) | 15 (57.69) | 26 (14.44) | 0.091 |
| P2RY12 (rs2046934) | ||||
| Non-carriers | 44 (36.07) | 78 (63.93) | 122 (67.78) | Reference |
| Carriers | 25 (43.10) | 33 (56.90) | 58 (32.33) | 0.223 |
| P2RY12 haplotype | ||||
| H0 | 8 (57.14) | 6 (42.86) | 14 (7.78) | Reference |
| H1 | 9 (34.62) | 17 (65.38) | 26 (14.44) | 0.016 |
| H2 | 10 (34.48) | 19 (65.52) | 29 (16.11) | 0.062 |
| H3 | 13 (39.39) | 20 (60.61) | 33 (18.33) | 0.104 |
| H4 | 15 (38.46) | 24 (61.54) | 39 (21.67) | 0.105 |
| H5 | 7 (53.85) | 6 (46.15) | 13 (7.22) | 0.296 |
Values are expressed as n (%). P-values refer to comparisons between HTPR and nHTPR groups by multivariate logistic regression adjusted by CYP2C19 and covariate characteristics (including smoker). HTPR: high on-treatment platelet reactivity; nHTPR: non-high on-treatment platelet reactivity; EM: extensive metabolizer; IM: intermediate metabolizer; PM: poor metabolizer. Haplotypes H0 to H5 were coded by the compositions of rs6798347, rs6787801, rs6801273, and rs6785930
Fig. 1.
Effects of P2RY12 haplotypes on high on-clopidogrel platelet reactivity in all patients (a, n=154) and in nonsmokers (b, n=101) by multivariable logistic regression adjusted by CYP2C19 polymorphisms and covariate characteristics
In total group smoking was also adjusted compared to nonsmoker subgroup. The subgroup of smokers was excluded from the analysis due to small sample size (n=53)
3.3.1 CYP2C19
Carriers of at least one LOF allele (PMs and IMs) had a higher residual platelet reactivity than non-carriers (EMs), and IMs had a higher incidence of HTPR than EMs in the total study population and the nonsmoker subgroup (46 (46.46%) vs. 19 (24.68%), OR 6.00, P-value 0.000, 95% CI 2.27‒15.82; 32 (51.61%) vs. 20 (32.26%), OR 4.69, P-value 0.007, 95% CI 1.52‒14.46). PMs had 100% incidence of HTPR.
3.3.2 P2RY12
For the total study population, haplotype H1 showed a significantly lower incidence of HTPR than the reference haplotype (H0) after adjusting for demographic characteristics and smokers (9 (34.62%) vs. 8 (57.14%), OR 0.13, P-value 0.016, 95% CI 0.03–0.68; Fig. 1a). Among nonsmokers, after adjusting for demographic characteristics, haplotypes H1 and H2 showed a significantly lower incidence of HTPR than H0 (8 (47.06%) vs. 7 (63.64%), OR 0.11, P-value 0.036, 95% CI 0.02–0.87; 7 (38.89%) vs. 7 (63.64%), OR 0.08, P-value 0.017, 95% CI 0.01–0.64; Fig. 1b). P2RY12 allele rs2046934 (T744C) did not show any significant association with HTPR neither in the total population nor in nonsmokers.
4. Discussion
In our study, we found the possible association between the common genetic variations of the P2RY12 and residual on-clopidogrel platelet reactivity using TEG in a Chinese population with ACS after adjusting the influence of CYP2C19 *2 and *3 alleles. The P2RY12 SNPs we chose (rs6798347, rs6787801, rs6801273, rs6785930, and rs2046934) contained the promoter region of the P2RY12 gene, which may alter its transcriptional activity instead of changing the structure of the P2Y12 receptor as other mostly analyzed SNPs may do.
In our studied population, we identified by TEG 69 (38.33%) patients with HTPR. The incidence of HTPR was similar to those of other studies on a similar population (Liu et al., 2015; Tang et al., 2015) and higher than those on Caucasian and African-American populations with stable coronary heart disease (Bliden et al., 2007). The different incidences of HTPR might come from inadequate platelet inhibition in Chinese patients and because Chinese are more likely to carry the CYP2C19 LOF allele compared to Caucasian and Africans (Liu et al., 2015).
As well as the established association of the CYP2C19 *2 and *3 LOF alleles with HTPR, several SNPs of the P2RY12 gene have been studied. Most studies focused on rs2046934 (T744C) or rs6809699 (G52T) representing dbSNP rs10935838, rs2046934, rs5853517 and rs6809699, and rs6785930 (C34T). Some found that it can modulate platelet response to clopidogrel or affect patients’ outcomes (Fontana et al., 2003; Zee et al., 2008; Staritz et al., 2009; Shalia et al., 2013; Zoheir et al., 2013; Li et al., 2016), but most results were negative (Angiolillo et al., 2005; Smith et al., 2006; Cuisset et al., 2007; Lev et al., 2007; Bierend et al., 2008; Bonello et al., 2010; Jang et al., 2012; Namazi et al., 2012; Kar et al., 2013; Kim et al., 2013; Jana et al., 2014). Our results show that the P2RY12 allele rs2046934 (T744C) was not significantly associated with HTPR neither in the total study population nor in nonsmokers.
Instead of any single polymorphisms of the analyzed genes, we found that the coexisting of the four SNPs (rs6798347, rs6787801, rs6801273, and rs6785930) was related to on-treatment platelet reactivity tested by TEG in the total population and in the nonsmoker subgroup. Haplotype H1, which carried four mutation alleles of the four selected SNPs at the same time, showed a significantly lower incidence of HTPR compared with the reference wild haplotype (H0). Similarly, Rudež et al. (2009) composited six P2Y12 SNPs (rs6798347, rs6787801, rs9859552, rs6801273, rs9848789, and rs2046934) and found that one haplotype was associated with significantly lower residual on-clopidogrel platelet reactivity compared with the reference haplotype tested by VerifyNow and light transmission aggregometry (LTA). Rudež et al. (2008) also composed five P2RY12 SNPs (rs6798347, rs10935842, rs6787801, rs6801273, and rs2046934) and found that they were associated with a higher risk of adverse cardiovascular events than the reference P2RY12 haplotype, which contains the common alleles of all the five P2RY12 SNPs.
Furthermore, we analyzed the co-contribution of P2RY12 and CYP2C19 together, after adjusting for the influence of CYP2C19 LOG alleles and GOF alleles, and we found the extra coexisting impact of P2RY12 on the residual platelet reactivities besides CYP2C19. This was partly examined in Malek et al. (2008)’s research with different alleles. Our study, in a similar way, found a new impact of P2RY12 genes on platelet reactivity but by a different combination of SNPs, which helps gain a better understanding of the effect of P2RY12 genes on clopidogrel on-treatment platelet reactivity.
In our studied population we found that smokers had a lower percentage of HTPR (OR 0.4945, P-value 0.046, 95% CI 0.25–0.99). This might be explained by Shanker et al. (2006)’s study stating that smokers might have higher platelet P2Y12 activity. Similarly, Cavallari et al. (2007)’s study stated that rs2046934 (T744C) was associated with a higher presence of coronary artery disease, particularly in nonsmokers, which in some way demonstrated that smoking affected P2Y12’s contribution to coronary artery disease although they showed the relationship with a different P2RY12 SNP. However, we are not the first to report the association of clopidogrel on-treatment platelet reactivity with smoking (Tantcheva-Poór et al., 1999; Bliden et al., 2008; Gremmel et al., 2009; Price et al., 2009; Jeong et al., 2010; Ueno et al., 2012; Gurbel et al., 2013; Peng et al., 2015). It was early reported that smoking increases the enzymatic activity of CYP1A2, one of the predominant pathways for the first oxidative step of the pro-drug clopidogrel, through polycyclic aromatic hydrocarbons and plasma nicotine, thereby enhancing the generation of the active metabolite of clopidogrel (Tantcheva-Poór et al., 1999). One hypothesis by Bliden et al. (2008)’s group was that the significant cigarette smoking might affect platelet inhibition in clopidogrel-treated patients via CYP1A2. However, a study conducted by Yousef et al. (2008) showed that the elimination half-life of clopidogrel was shorter in current smokers than in nonsmokers, which confused the established hypothesis. Meanwhile, there are several studies which reported a negative association with smokers in larger observation studies (Hochholzer et al., 2011; Sibbing et al., 2012; Kim et al., 2016). Kim et al. (2016) deduced that hemoglobin levels might be the key driver for the observed ex vivo phenomenon of lower on-treatment platelet reactivity levels and/or an enhanced response to ADP inhibitors like clopidogrel in active smokers in most of the studies published so far. More studies are needed. Recently, the study of Patti et al. (2016) found that cigarette smoking weakly influences antiplatelet effects of oral P2Y12 inhibition. The results on the association of smoking and platelet responsiveness to ADP-receptor antagonist are inconsistent and controversial within studies. There was one study of Cicko et al. (2010) reporting that smoke exposure led to an up-regulation of the P2Y2R subtypes in blood neutrophils and in bronchoalveolar lavage fluid neutrophils by in vitro mouse model. This might explain the observed relationship between HTPR and smoking on ACS patients in smokers. Further studies are required to compare the different on-treatment platelet activities between nonsmokers and smokers.
5. Conclusions
Our study showed that apart from the established association of the CYP2C19 *2 and *3 LOF alleles with HTPR, haplotypes of P2RY12 rs6798347, rs6787801, rs6801273, and rs6785930 rather than rs2046934 (T744C) that related to pharmacodynamics of clopidogrel in patients with ACS were independently associated with HTPR. This association seems to be more important in the subgroup defined by smoking. This finding suggests that P2RY12 genetic variation and common allele composition may be important in determining platelet reactivity for patients treated with clopidogrel, especially in nonsmokers.
6. Study limitations
The first limitation of our study is the small sample size, especially the small sample of the population smoking. Secondly we cannot completely eliminate the possible bias by clinical risk factors despite multivariate analysis. Thirdly, we did not observe the association between genetic variations and clinical outcome in this study. Finally, we did not perform pharmacokinetics analysis, which might be useful for a better explanation related to the ABCB1 and CYP1A2 polymorphisms.
Footnotes
Project supported by the Beijing Higher Education Young Elite Teacher Project (No. YETP0064), China
Compliance with ethics guidelines: Xiao-yan NIE, Jun-lei LI, Yong ZHANG, Yang XU, Xue-li YANG, Yu FU, Guang-kai LIANG, Yun LU, Jian LIU, and Lu-wen SHI declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
References
- 1.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Lack of association between the P2Y12 receptor gene polymorphism and platelet response to clopidogrel in patients with coronary artery disease. Thromb Res. 2005;116(6):491–497. doi: 10.1016/j.thromres.2005.03.001. (Available from: http://dx.doi.org/10.1016/j.thromres.2005.03.001) [DOI] [PubMed] [Google Scholar]
- 2.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007;49(14):1505–1516. doi: 10.1016/j.jacc.2006.11.044. (Available from: http://dx.doi.org/10.1016/j.jacc.2006.11.044) [DOI] [PubMed] [Google Scholar]
- 3.Beinart SC, Kolm P, Veledar E, et al. Long-term cost effectiveness of early and sustained dual oral antiplatelet therapy with clopidogrel given for up to one year after percutaneous coronary intervention: results from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. J Am Coll Cardiol. 2005;46(5):761–769. doi: 10.1016/j.jacc.2005.03.073. (Available from: http://dx.doi.org/10.1016/j.jacc.2005.03.073) [DOI] [PubMed] [Google Scholar]
- 4.Bierend A, Rau T, Maas R, et al. P2Y12 polymorphisms and antiplatelet effects of aspirin in patients with coronary artery disease. Br J Clin Pharmacol. 2008;65(4):540–547. doi: 10.1111/j.1365-2125.2007.03044.x. (Available from: http://dx.doi.org/10.1111/j.1365-2125.2007.03044.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bliden KP, DiChiara J, Tantry US, et al. Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention: is the current antiplatelet therapy adequate? J Am Coll Cardiol. 2007;49(6):657–666. doi: 10.1016/j.jacc.2006.10.050. (Available from: http://dx.doi.org/10.1016/j.jacc.2006.10.050) [DOI] [PubMed] [Google Scholar]
- 6.Bliden KP, Dichiara J, Lawal L, et al. The association of cigarette smoking with enhanced platelet inhibition by clopidogrel. J Am Coll Cardiol. 2008;52(7):531–533. doi: 10.1016/j.jacc.2008.04.045. (Available from: http://dx.doi.org/10.1016/j.jacc.2008.04.045) [DOI] [PubMed] [Google Scholar]
- 7.Bonello L, Bonello-Palot N, Armero S, et al. Impact of P2Y12-ADP receptor polymorphism on the efficacy of clopidogrel dose-adjustment according to platelet reactivity monitoring in coronary artery disease patients. Thromb Res. 2010;125(4):e167–e170. doi: 10.1016/j.thromres.2009.10.014. (Available from: http://dx.doi.org/10.1016/j.thromres.2009.10.014) [DOI] [PubMed] [Google Scholar]
- 8.Cavallari U, Trabetti E, Malerba G, et al. Gene sequence variations of the platelet P2Y12 receptor are associated with coronary artery disease. BMC Med Genet. 2007;8:59. doi: 10.1186/1471-2350-8-59. (Available from: http://dx.doi.org/10.1186/1471-2350-8-59) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Huang X, Tang Y, et al. GW25-e3303 Both PON1 Q192R and CYP2C19*2 influence platelet response to clopidogrel and ischemic events in Chinese patients undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2014;64(16):C203. (Available from: http://dx.doi.org/10.1016/j.jacc.2014.06.943) [PMC free article] [PubMed] [Google Scholar]
- 10.Cicko S, Lucattelli M, Müller T, et al. Purinergic receptor inhibition prevents the development of smoke-induced lung injury and emphysema. J Immunol. 2010;185(1):688–697. doi: 10.4049/jimmunol.0904042. (Available from: http://dx.doi.org/10.4049/jimmunol.0904042) [DOI] [PubMed] [Google Scholar]
- 11.Cuisset T, Frere C, Quilici J, et al. Role of the T744C polymorphism of the P2Y12 gene on platelet response to a 600-mg loading dose of clopidogrel in 597 patients with non-ST-segment elevation acute coronary syndrome. Thromb Res. 2007;120(6):893–899. doi: 10.1016/j.thromres.2007.01.012. (Available from: http://dx.doi.org/10.1016/j.thromres.2007.01.012) [DOI] [PubMed] [Google Scholar]
- 12.Emergency Medical Branch of Chinese Medical Doctor Association, Cardiovascular Epidemiology Branch of Chinese Medical Association, Laboratory Medicine Brance of Chinese Medical Association. Emergency rapid diagnosis and treatment of guidelines acute coronary syndrome. Chin J Emerg Med. 2016;25(4):397-404(in Chinese):397–404 (in Chinese). (Available from: http://dx.doi.org/10.3760/cma.j.issn.1671-0282.2016.04.002) [Google Scholar]
- 13.Fontana P, Gaussem P, Aiach M, et al. P2Y12 H2 haplotype is associated with peripheral arterial disease: a case-control study. Circulation. 2003;108(24):2971–2973. doi: 10.1161/01.CIR.0000106904.80795.35. (Available from: http://dx.doi.org/10.1161/01.cir.0000106904.80795.35) [DOI] [PubMed] [Google Scholar]
- 14.Gremmel T, Steiner S, Seidinger D, et al. Smoking promotes clopidogrel-mediated platelet inhibition in patients receiving dual antiplatelet therapy. Thromb Res. 2009;124(5):588–591. doi: 10.1016/j.thromres.2009.06.012. (Available from: http://dx.doi.org/10.1016/j.thromres.2009.06.012) [DOI] [PubMed] [Google Scholar]
- 15.Gurbel PA, Bliden KP, Logan DK, et al. The influence of smoking status on the pharmacokinetics and pharmacodynamics of clopidogrel and prasugrel: the PARADOX study. J Am Coll Cardiol. 2013;62(6):505–512. doi: 10.1016/j.jacc.2013.03.037. (Available from: http://dx.doi.org/10.1016/j.jacc.2013.03.037) [DOI] [PubMed] [Google Scholar]
- 16.Hochholzer W, Trenk D, Mega JL, et al. Impact of smoking on antiplatelet effect of clopidogrel and prasugrel after loading dose and on maintenance therapy. Am Heart J. 2011;162(3):518–526e5. doi: 10.1016/j.ahj.2011.06.005. (Available from: http://dx.doi.org/10.1016/j.ahj.2011.06.005) [DOI] [PubMed] [Google Scholar]
- 17.Jana U, Ludek S, Jana K, et al. Genetic polymorphisms of platelet receptors in patients with acute myocardial infarction and resistance to antiplatelet therapy. Genet Test Mol Biomarkers. 2014;18(9):599–604. doi: 10.1089/gtmb.2014.0077. (Available from: http://dx.doi.org/10.1089/gtmb.2014.0077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang MJ, Jeon YJ, Min KT, et al. Polymorphisms of platelet ADP receptor P2RY12 in the risk of venous thromboembolism in the Korean population. Clin Appl Thromb Hemost. 2012;18(4):416–420. doi: 10.1177/1076029611426283. (Available from: http://dx.doi.org/10.1177/1076029611426283) [DOI] [PubMed] [Google Scholar]
- 19.Jeong YH, Cho JH, Kang MK, et al. Smoking at least 10 cigarettes per day increases platelet inhibition by clopidogrel in patients with ST-segment-elevation myocardial infarction. Thromb Res. 2010;126(4):e334–e338. doi: 10.1016/j.thromres.2010.03.020. (Available from: http://dx.doi.org/10.1016/j.thromres.2010.03.020) [DOI] [PubMed] [Google Scholar]
- 20.Kar R, Meena A, Yadav BK, et al. Clopidogrel resistance in North Indian patients of coronary artery disease and lack of its association with platelet ADP receptors P2Y1 and P2Y12 gene polymorphisms. Platelets. 2013;24(4):297–302. doi: 10.3109/09537104.2012.693992. (Available from: http://dx.doi.org/10.3109/09537104.2012.693992) [DOI] [PubMed] [Google Scholar]
- 21.Kim KA, Song WG, Lee HM, et al. Effect of P2Y1 and P2Y12 genetic polymorphisms on the ADP-induced platelet aggregation in a Korean population. Thromb Res. 2013;132(2):221–226. doi: 10.1016/j.thromres.2013.06.020. (Available from: http://dx.doi.org/10.1016/j.thromres.2013.06.020) [DOI] [PubMed] [Google Scholar]
- 22.Kim YG, Suh JW, Kang SH, et al. Cigarette smoking does not enhance clopidogrel responsiveness after adjusting VerifyNow P2Y12 reaction unit for the influence of hemoglobin level. JACC Cardiovasc Interv. 2016;9(16):1680–1690. doi: 10.1016/j.jcin.2016.05.036. (Available from: http://dx.doi.org/10.1016/j.jcin.2016.05.036) [DOI] [PubMed] [Google Scholar]
- 23.Lev EI, Patel RT, Guthikonda S, et al. Genetic polymorphisms of the platelet receptors P2Y12, P2Y1 and GP IIIa and response to aspirin and clopidogrel. Thromb Res. 2007;119(3):355–360. doi: 10.1016/j.thromres.2006.02.006. (Available from: http://dx.doi.org/10.1016/j.thromres.2006.02.006) [DOI] [PubMed] [Google Scholar]
- 24.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134(10):e123–e155. doi: 10.1161/CIR.0000000000000404. (Available from: http://dx.doi.org/10.1161/cir.0000000000000404) [DOI] [PubMed] [Google Scholar]
- 25.Li XQ, Ma N, Li XG, et al. Association of PON1, P2Y12 and COX1 with recurrent ischemic events in patients with extracranial or intracranial stenting. PLoS ONE. 2016;11(2):e0148891. doi: 10.1371/journal.pone.0148891. (Available from: http://dx.doi.org/10.1371/journal.pone.0148891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Nie XY, Zhang Y, et al. CYP2C19*2 and other allelic variants affecting platelet response to clopidogrel tested by thrombelastography in patients with acute coronary syndrome. Chin Med J (Engl) 2015;128(16):2183–2188. doi: 10.4103/0366-6999.162515. (Available from: http://dx.doi.org/10.4103/0366-6999.162515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malek LA, Kisiel B, Spiewak M, et al. Coexisting polymorphisms of P2Y12 and CYP2C19 genes as a risk factor for persistent platelet activation with clopidogrel. Circ J. 2008;72(7):1165–1169. doi: 10.1253/circj.72.1165. (Available from: http://dx.doi.org/10.1253/circj.72.1165) [DOI] [PubMed] [Google Scholar]
- 28.Mega JL, Close SL, Wiviott SD, et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010;376(9749):1312–1319. doi: 10.1016/S0140-6736(10)61273-1. (Available from: http://dx.doi.org/10.1016/s0140-6736(10)61273-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mega JL, Simon T, Collet JP, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304(16):1821–1830. doi: 10.1001/jama.2010.1543. (Available from: http://dx.doi.org/10.1001/jama.2010.1543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Namazi S, Kojuri J, Khalili A, et al. The impact of genetic polymorphisms of P2Y12, CYP3A5 and CYP2C19 on clopidogrel response variability in Iranian patients. Biochem Pharmacol. 2012;83(7):903–908. doi: 10.1016/j.bcp.2012.01.003. (Available from: http://dx.doi.org/10.1016/j.bcp.2012.01.003) [DOI] [PubMed] [Google Scholar]
- 31.Oestreich JH, Steinhubl SR, Ferraris SP, et al. Effect of genetic variation in P2Y12 on TRAP-stimulated platelet response in healthy subjects. J Thromb Thrombolysis. 2014;38(3):372–379. doi: 10.1007/s11239-014-1058-5. (Available from: http://dx.doi.org/10.1007/s11239-014-1058-5) [DOI] [PubMed] [Google Scholar]
- 32.Patti G, Polacco M, Taurino E, et al. Effects of cigarette smoking on platelet reactivity during P2Y12 inhibition in patients with myocardial infarction undergoing drug-eluting stent implantation: results from the prospective cigarette smoking on platelet reactivity (COPTER) study. J Thromb Thrombolysis. 2016;41(4):648–653. doi: 10.1007/s11239-016-1341-8. (Available from: http://dx.doi.org/10.1007/s11239-016-1341-8) [DOI] [PubMed] [Google Scholar]
- 33.Peng L, Zhang L, Yang J, et al. Joint effects of CYP2C19*2 and smoking status on clopidogrel responsiveness in patients with acute coronary syndrome. Int J Cardiol. 2015;180(1):196–198. doi: 10.1016/j.ijcard.2014.11.210. (Available from: http://dx.doi.org/10.1016/j.ijcard.2014.11.210) [DOI] [PubMed] [Google Scholar]
- 34.Price MJ, Nayak KR, Barker CM, et al. Predictors of heightened platelet reactivity despite dual-antiplatelet therapy in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2009;103(10):1339–1343. doi: 10.1016/j.amjcard.2009.01.341. (Available from: http://dx.doi.org/10.1016/j.amjcard.2009.01.341) [DOI] [PubMed] [Google Scholar]
- 35.Rudež G, Pons D, Leebeek F, et al. Platelet receptor P2RY12 haplotypes predict restenosis after percutaneous coronary interventions. Hum Mutat. 2008;29(3):375–380. doi: 10.1002/humu.20641. (Available from: http://dx.doi.org/10.1002/humu.20641) [DOI] [PubMed] [Google Scholar]
- 36.Rudež G, Bouman HJ, van Werkum JW, et al. Common variation in the platelet receptor P2RY12 gene is associated with residual on-clopidogrel platelet reactivity in patients undergoing elective percutaneous coronary interventions. Circ Cardiovasc Genet. 2009;2(5):515–521. doi: 10.1161/CIRCGENETICS.109.861799. (Available from: http://dx.doi.org/10.1161/circgenetics.109.861799) [DOI] [PubMed] [Google Scholar]
- 37.SIGN . SIGN 148 Acute Coronary Syndrome. Edinburgh: Scottish Intercollegiate Guidelines Network (SIGN); 2016. [Google Scholar]
- 38.Section of Interventional Cardiology of Chinese Society of Cardiology of Chinese Medical Association, Specialty Committee on Prevention and Treatment of Thrombosis of Chinese College of Cardiovascular Physicians, Editorial Board of Chinese Journal of Cardiology. Chinese guideline for percutaneous coronary intervention (2016) Chin J Cardiol. 2016;44(5):382-400(in Chinese):382–400 (in Chinese). doi: 10.3760/cma.j.issn.0253-3758.2016.05.006. (Available from: http://dx.doi.org/10.3760/cma.j.issn.0253-3758.2016.05.006) [DOI] [PubMed] [Google Scholar]
- 39.Shalia KK, Shah VK, Pawar P, et al. Polymorphisms of MDR1, CYP2C19 and P2Y12 genes in Indian population: effects on clopidogrel response. Indian Heart J. 2013;65(2):158–167. doi: 10.1016/j.ihj.2013.02.012. (Available from: http://dx.doi.org/10.1016/j.ihj.2013.02.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shanker G, Kontos JL, Eckman DM, et al. Nicotine upregulates the expression of P2Y12 on vascular cells and megakaryoblasts. J Thromb Thrombolysis. 2006;22(3):213–220. doi: 10.1007/s11239-006-9033-4. (Available from: http://dx.doi.org/10.1007/s11239-006-9033-4) [DOI] [PubMed] [Google Scholar]
- 41.Sibbing D, Bernlochner I, Schulz S, et al. The impact of smoking on the antiplatelet action of clopidogrel in non-ST-elevation myocardial infarction patients: results from the ISAR-REACT 4 platelet substudy. J Thromb Haemost. 2012;10(10):2199–2202. doi: 10.1111/j.1538-7836.2012.04867.x. (Available from: http://dx.doi.org/10.1111/j.1538-7836.2012.04867.x) [DOI] [PubMed] [Google Scholar]
- 42.Simon T, Bhatt DL, Bergougnan L, et al. Genetic polymorphisms and the impact of a higher clopidogrel dose regimen on active metabolite exposure and antiplatelet response in healthy subjects. Clin Pharmacol Ther. 2011;90(2):287–295. doi: 10.1038/clpt.2011.127. (Available from: http://dx.doi.org/10.1038/clpt.2011.127) [DOI] [PubMed] [Google Scholar]
- 43.Smith SM, Judge HM, Peters G, et al. Common sequence variations in the P2Y12 and CYP3A5 genes do not explain the variability in the inhibitory effects of clopidogrel therapy. Platelets. 2006;17(4):250–258. doi: 10.1080/09537100500475844. (Available from: http://dx.doi.org/10.1080/09537100500475844) [DOI] [PubMed] [Google Scholar]
- 44.Staritz P, Kurz K, Stoll M, et al. Platelet reactivity and clopidogrel resistance are associated with the H2 haplotype of the P2Y12-ADP receptor gene. Int J Cardiol. 2009;133(3):341–345. doi: 10.1016/j.ijcard.2007.12.118. (Available from: http://dx.doi.org/10.1016/j.ijcard.2007.12.118) [DOI] [PubMed] [Google Scholar]
- 45.Tang XF, Han YL, Zhang JH, et al. Comparing of light transmittance aggregometry and modified thrombelastograph in predicting clinical outcomes in Chinese patients undergoing coronary stenting with clopidogrel. Chin Med J (Engl) 2015;128(6):774–779. doi: 10.4103/0366-6999.152611. (Available from: http://dx.doi.org/10.4103/0366-6999.152611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tantcheva-Poór I, Zaigler M, Rietbrock S, et al. Estimation of cytochrome P-450 CYP1A2 activity in 863 healthy Caucasians using a saliva-based caffeine test. Pharmacogenetics. 1999;9(2):131–144. [PubMed] [Google Scholar]
- 47.Ueno M, Ferreiro JL, Desai B, et al. Cigarette smoking is associated with a dose-response effect in clopidogrel-treated patients with diabetes mellitus and coronary artery disease: results of a pharmacodynamic study. JACC Cardiovasc Interv. 2012;5(3):293–300. doi: 10.1016/j.jcin.2011.09.027. (Available from: http://dx.doi.org/10.1016/j.jcin.2011.09.027) [DOI] [PubMed] [Google Scholar]
- 48.Yousef AM, Arafat T, Bulatova NR, et al. Smoking behaviour modulates pharmacokinetics of orally administered clopidogrel. J Clin Pharm Ther. 2008;33(4):439–449. doi: 10.1111/j.1365-2710.2008.00936.x. (Available from: http://dx.doi.org/10.1111/j.1365-2710.2008.00936.x) [DOI] [PubMed] [Google Scholar]
- 49.Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494–502. doi: 10.1056/NEJMoa010746. (Available from: http://dx.doi.org/10.1056/NEJMoa010746) [DOI] [PubMed] [Google Scholar]
- 50.Zee RY, Michaud SE, Diehl KA, et al. Purinergic receptor P2Y, G-protein coupled, 12 gene variants and risk of incident ischemic stroke, myocardial infarction, and venous thromboembolism. Atherosclerosis. 2008;197(2):694–699. doi: 10.1016/j.atherosclerosis.2007.07.001. (Available from: http://dx.doi.org/10.1016/j.atherosclerosis.2007.07.001) [DOI] [PubMed] [Google Scholar]
- 51.Zhang JH, Wang J, Tang XF, et al. Effect of platelet receptor gene polymorphisms on outcomes in ST-elevation myocardial infarction patients after percutaneous coronary intervention. Platelets. 2016;27(1):75–79. doi: 10.3109/09537104.2015.1034096. (Available from: http://dx.doi.org/10.3109/09537104.2015.1034096) [DOI] [PubMed] [Google Scholar]
- 52.Zoheir N, Abd Elhamid S, Abulata N, et al. P2Y12 receptor gene polymorphism and antiplatelet effect of clopidogrel in patients with coronary artery disease after coronary stenting. Blood Coagul Fibrinolysis. 2013;24(5):525–531. doi: 10.1097/MBC.0b013e32835e98bf. (Available from: http://dx.doi.org/10.1097/MBC.0b013e32835e98bf) [DOI] [PubMed] [Google Scholar]