Abstract
This research was undertaken in order to characterize the chemical compositions and evaluate the antioxidant activities of essential oils obtained from different parts of the Origanum vulgare L. It is a medicinal plant used in traditional Chinese medicine for the treatment of heat stroke, fever, vomiting, acute gastroenteritis, and respiratory disorders. The chemical compositions of the three essential oils from different parts of the oregano (leaves-flowers, stems, and roots) were identified by gas chromatography-mass spectrometry (GC-MS). The antioxidant activity of each essential oil was assessed using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay and reducing the power test. Among the essential oils from different parts of the oregano, the leaf-flower oils have the best antioxidant activities, whereas the stem oils are the worst. The results of the DPPH free radical scavenging assay showed that the half maximal inhibitory concentration (IC50) values of the essential oils were (0.332±0.040) mg/ml (leaves-flowers), (0.357±0.031) mg/ml (roots), and (0.501±0.029) mg/ml (stems), respectively. Interestingly, the results of reducing the power test also revealed that when the concentration exceeded 1.25 mg/ml, the leaf-flower oils had the highest reducing power; however, the stem oils were the lowest.
This research was undertaken in order to characterize the chemical compositions and evaluate the antioxidant activities of essential oils obtained from different parts of the Origanum vulgare L. It is a medicinal plant used in traditional Chinese medicine for the treatment of heat stroke, fever, vomiting, acute gastroenteritis, and respiratory disorders. The chemical compositions of the three essential oils from different parts of the oregano (leaves-flowers, stems, and roots) were identified by gas chromatography-mass spectrometry (GC-MS). The antioxidant activity of each essential oil was assessed using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay and reducing the power test. Among the essential oils from different parts of the oregano, the leaf-flower oils have the best antioxidant activities, whereas the stem oils are the worst. The results of the DPPH free radical scavenging assay showed that the half maximal inhibitory concentration (IC50) values of the essential oils were (0.332±0.040) mg/ml (leaves-flowers), (0.357±0.031) mg/ml (roots), and (0.501±0.029) mg/ml (stems), respectively. Interestingly, the results of reducing the power test also revealed that when the concentration exceeded 1.25 mg/ml, the leaf-flower oils had the highest reducing power; however, the stem oils were the lowest.
O. vulgare L. is a Lamiaceae Origanum perennial herbaceous aromatic plant that primarily grows in Europe, North Africa, and Asia (Lagouri et al., 1993). It is grown at an altitude of 500–3600 m along roadsides, on hillsides, and in shrublands and grasslands. Additionally, it is widely distributed in many regions of China, including the Yunnan, Gansu, Sichuan, Hubei, Jiangxi, Anhui, and Fujian Provinces.
Oregano oil is an essential oil from the O. vulgare L. and is used worldwide as a raw material for medicinal and health products. Previous studies indicated more than 50% of oregano oil consists of phenolic compounds (primarily carvacrol and thymol). This oil also contains sesquiterpene, terpinene, terpineol alcohol, flavonoids, and other compounds (Arcila-Lozano et al., 2004; Ozkan et al., 2010).
In recent years, many reports have shown that oregano oil has strong antioxidant activities (Gilling et al., 2014; Bhargava et al., 2015; Fournomiti et al., 2015) and is a strong candidate to replace synthetic antioxidants used in the industry (Stuessy, 2009; Céspedes et al., 2013). Rosemary acid ester, which can be obtained from oregano oils, has strong antioxidant effects (Mechergui et al., 2010). Ruberto et al. (2002) analyzed the components of oregano essential oils from four different regions in Algeria and determined that the four types of essential oils exhibited strong antioxidant activities, even at very low concentrations (100 ppm (1 ppm=1 mg/L)).
However, few reports have investigated differences in the chemical compositions and antioxidant activities of the different parts of oregano. Indeed, previous works primarily concentrated on the aerial parts of oregano, which have been believed to possess various medicinal activities. However, little information is currently available on the underground parts (roots) (Ragi et al., 2011; Ok et al., 2015). Therefore, this research attempts to demonstrate the difference of chemical compositions and antioxidant activities of the essential oils from different parts of the oregano, and hopes to provide some experimental basis and references for screening the novel medical parts of the oregano.
In our research, a total of 37 compounds were identified in the leaf-flower oils, accounting for 98.78% of the total, and included carvacrol (30.73%), thymol (18.81%), P-cymene (10.88%), caryophyllene (7.73%), and 3-carene (4.06%). These components accounted for a total of 72.21%. Eleven compounds were identified in the stem oils, accounting for 99.51% of the total, and included large quantities of palmitic acid (60.18%), linoleic acid (14.25%), carvacrol (6.02%), thymol (3.46%), and oleic acid (5.65%). The above components accounted for a total of 89.56%. Finally, 29 compounds were identified in the root oils, accounting for 98.97% of the total, and also included large quantities of palmitic acid (58.23%), linoleic acid (12.11%), linolenic acid (3.66%), carvacrol (3.27%), and thymol (1.08%). These five components accounted for a total of 78.35% (Table S1). Fifty-nine compounds were identified in the essential oils from the three different parts, including three compounds (carvacrol, thymol, and caryophyllin) that were found in all parts (Table S1). The results also show that the chemical compositions of the leaf-flower oils are primarily composed of phenolic and terpenoid compounds (the total of the content was more than 60%). However, the chemical constituents of the root oils and stem oils are primarily composed of fatty acids (the total of the content was more than 70%).
The results of the DPPH free radical scavenging assay showed that the leaf-flower oils had the strongest antioxidant activities and that the stem oils had the weakest (Fig. 1, Table 1). Additionally, although the essential oils of each part showed strong antioxidant activities, their antioxidant capacities were much lower than those of the synthetic antioxidant butylated hydroxytoluene (BHT) (IC50=(0.161±0.009) mg/ml) (Table 1).
Fig. 1.
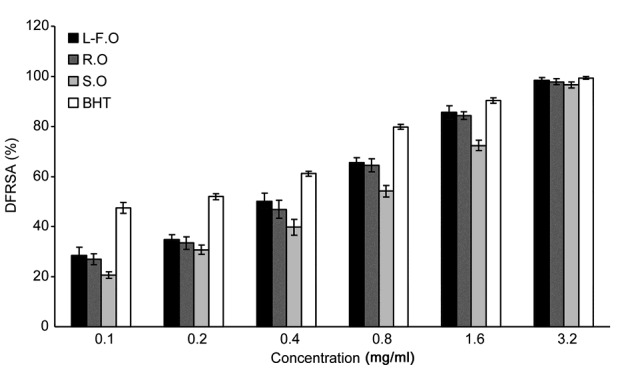
Comparison of the DFRSA of essential oils from different parts of O. vulgare L.
DFRSA: DPPH free radical scavenging activity; L-F.O: leaf-flower oil; R.O: root oil; S.O: stem oil; BHT: butylated hydroxytoluene. The DFRSAs of different concentrations of essential oils from different parts are expressed as inhibition percentage and as the mean±standard deviation (SD) (n=3)
Table 1.
IC50 values of essential oils from different parts of O. vulgare L.
| Sample | IC50 (mg/ml) |
| L-F.O | 0.332±0.040 |
| R.O | 0.357±0.031 |
| S.O | 0.501±0.029 |
| BHT | 0.161±0.009 |
L-F.O: leaf-flower oil; R.O: root oil; S.O: stem oil; BHT: butylated hydroxytoluene. The values are expressed as mean±standard deviation (SD) (n=3)
The ferric reducing power test indicates that the absorbance value and reducing power increased gradually with increasing concentration (Fig. 2). BHT had the best reducing abilities, surpassing those of all the concentrations of the three oils tested. When the concentration was less than 1.25 mg/ml, the absorbance value indicated no notable differences in the reducing abilities of the three types of essential oils. However, when the concentration exceeded 1.25 mg/ml, their reducing abilities were obviously different (Fig. 2). This may be attributable to the small contents of antioxidant constituents in each essential oil. As the concentration gradually increased, corresponding increases in the antioxidant constituent contents occurred in each essential oil. Additionally, when the concentration reached a certain level, the reducing powers of the essential oils became significantly different. The results revealed that when the concentration exceeded 1.25 mg/ml, the leaf-flower oils have the best reducing power, whereas the stem oils have the worst (Fig. 2).
Fig. 2.
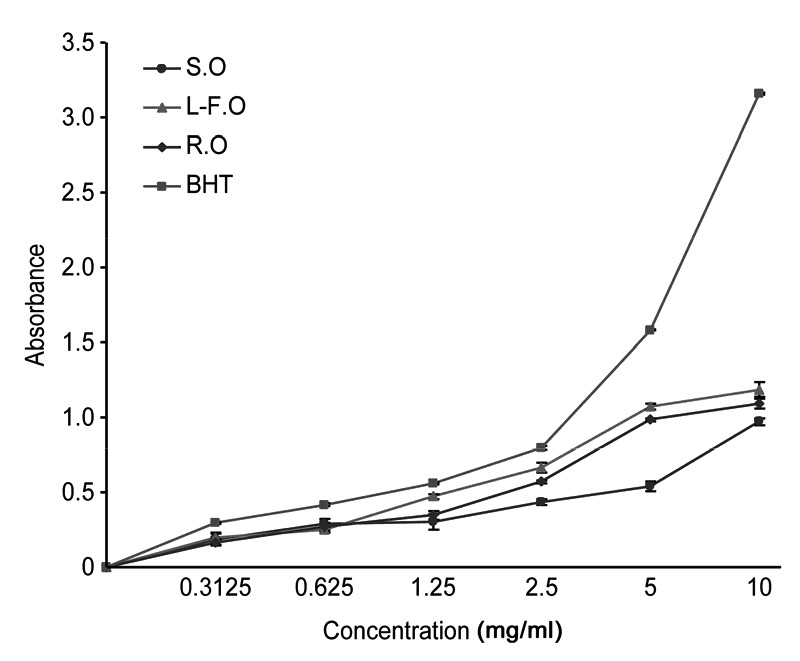
Comparison of the reducing powers of essential oils from different parts of O. vulgare L.
L-F.O: leaf-flower oil; R.O: root oil; S.O: stem oil; BHT: butylated hydroxytoluene. The reducing powers of essential oils from different parts are expressed as absorbance values and as the mean±standard deviation (SD) (n=3)
In our study, O. vulgare L. was collected from wild-growing plants in Huanggang City, Tuanfeng County, Hubei Province, China during flowering time, when the plants contain higher carvacrol and thymol contents. The results show that the contents of carvacrol and thymol in essential oil from the leaves-flowers were close to 50%, and meet the experimental requirements (support information). Our antioxidant activity results are similar to those reported previously (Castilho et al., 2012; Teixeira et al., 2013). However, in our study, the IC50 values of the oregano essential oils were slightly different from those identified in other works (Teixeira et al., 2013; Grondona et al., 2014), possibly because of the various origins of the medicinal materials or differences between oregano extracts and essential oils. Therefore, the overall results are reasonable.
According to previous reports (Mechergui et al., 2010), the antioxidant activities of oregano oil primarily depend on the contents of carvacrol and thymol in the essential oils. From Table S1, the data showed that carvacrol and thymol in the essential oils were rarely from the underground parts (roots), where the total percentage of two components just was only 4.35%, far below the total percentage from the aerial parts (leaves-flowers), which was 49.54%. However, the results of the antioxidant activity tests showed that the essential oils from the underground parts were similar to those from the aerial parts (Fig. 2, Table 1). This suggests that the antioxidant capacities of the root oils are not just dependent on the content of the phenolic compounds. It shows that the antioxidant capacity is the result of the comprehensive effect of the compound components in the essential oils.
The data in Table S1 also showed that the root oils contained large amounts of fatty acids, such as palmitic acid, linoleic acid, and linolenic acid, and their total content was more than 60%. These compounds having strong antioxidant activities may have some important significance for the antioxidant effects of the roots of the oregano.
Our research reveals that essential oils from the different parts of O. vulgare L. have excellent antioxidant activities as traditional Chinese medicines. Additionally, the roots of O. vulgare L. have significant medicinal values and should not be discarded when oregano is used for its antioxidant properties. These findings are highly significant for improving the overall antioxidant activity of the oregano essential oils for novel medical applications. However, the antioxidant activities of this research are only for in vitro experimentation. The antioxidant capacity in vivo of the essential oils from the different parts of O. vulgare L. also needs to be further investigated.
Materials and methods
Chemicals and reagents
Potassium ferricyanide, DPPH, BHT, and ferric chloride (FeCl3) were purchased from the China National Pharmaceutical Group (Chengdu, China). Acetate buffer, anhydrous ethanol, trichloroacetic acid, and other chemicals were of analytical grade.
Plant materials
O. vulgare L. samples were collected at altitudes between 800 and 1500 m in Fanggaoping Town, Huanggang City, Tuanfeng County, Hubei Province, of China in July 2015 and grown in dry and ventilated places away from the sun. Three different lots of voucher specimens (Batch Nos. 20150421, 20150523, and 20150629) were identified as the dry whole herb by Prof. Fei GE, Director of the Department of Pharmacognosy, College of Pharmacy, Jiangxi University of Traditional Chinese Medicine (Nanchang, China).
Essential oil extraction
The essential oils were extracted from dried plant materials using a Clevenger-type apparatus for steam distillation, and the contents of the essential oils were then determined (Kayode and Afolayan, 2015).
The oregano was air-dried in a shady place at room temperature for 14 d. The dry whole herbs were used for the isolation of the essential oils after drying. The oregano leaves-flowers, stems, and roots were crushed into powders (particle size less than 250 μm); subsequently, 600 g of each powder and 6 L of water were placed into a 10-L round-bottom flask. Herbal powders were extracted for 8 h via steam distillation, and the essential oils were collected, dewatered, dried over anhydrous sodium sulfate, and stored at −20 °C.
Essential oil chemical composition determination
GC-MS analysis of the essential oils was performed using an Agilent 6890N Network GC system equipped with an Agilent-Technologies 5975 inert XL mass selective detector and Agilent-Technologies 7683B series auto injector (Agilent Technologies, Little Falls, CA, USA). A fused-silica HP-20 M polyethylene glycol column (50 m×0.2 mm, 0.2 µm thickness, Hewlett Packard, Vienna, Austria) was directly coupled to the MS. The chromatographic conditions were as follows: the injection and detector temperatures were 250 and 230 °C, respectively. The column temperature was programmed to increase from 60 to 250 °C at a rate of 10 °C/min. The lower and upper temperatures were held for 2 and 20 min, respectively. Helium was used as the carrier gas at a flow rate of 1 ml/min. A 10-µl sample was injected using the split mode (split ratio: 1:20). For MS detection, electron ionization was used with an ionization energy of 70 eV, and the m/z scanning range spanned from 35 to 450. The compound quantities were calculated by integrating the peak areas of the spectrograms. Each sample was analyzed three times. The average peak areas of all GC signals were determined, and the percentage of each component peak was calculated by comparing its average area to the total area.
The components of the essential oils were identified by comparing their retention times with those reported in the literature (Berrehal et al., 2010; Teixeira et al., 2013) and their mass spectra with those from the Wiley 275 and NIST/NBS libraries and through the co-injection of standards for the main components. The quantification of each peak was performed using the mass reported by the mass detector. The results were expressed as percentages (Table S1).
DPPH free radical scavenging assay and determination of IC50
The DPPH free radical scavenging activity (DFRSA) is one of the most popular methods for determining antioxidant activity (Kandaswami and Middleton, 1994). The radical scavenging activity (RSA) was determined using the stable radical DPPH according to the method reported by Parejo et al. (2000) and de Gaulejac et al. (1999). This experiment established a scavenging rate-concentration probability model equation by investigating different concentrations (0.10, 0.20, 0.40, 0.80, 1.60, and 3.20 mg/ml). The RSA was calculated as the percentage of DPPH discoloration using the following equation: RSA=(1−A sample/A blank)×100%, where A sample and A blank are the absorbances of the sample and blank, respectively.
DFRSA was evaluated in terms of the IC50 value (the sample concentration required to scavenge 50% of the free radicals). The IC50 value was obtained by extrapolating a nonlinear regression analysis. The data were analyzed using Microsoft Excel 2013 and SPSS 19.0 software, and all experiments were performed in triplicate. The DFRSA of the sample solution was detected by the same method using BHT as a control.
Reducing power assay
Reducing power is commonly used to evaluate a substance’s antioxidant activity (Benzie and Strain, 1996). According to the reported methods (Benzie and Szeto, 1999; Dlugosz et al., 2006), essential oils from different parts were diluted to 0.3125, 0.625, 1.25, 2.5, 5.0, and 10.0 mg/ml, respectively, using anhydrous ethanol. Then, 1 ml of the sample solution, 2.5 ml of phosphate butter (0.2 mol/L, pH 6.6) and 2.5 ml of 1% (0.01 g/ml) potassium ferricyanide solution were mixed. The mixtures were kept at 50 °C for 20 min, added 2.5 ml of 10% (0.1 g/ml) trichloroacetic acid solution, and centrifuged at 10 000 r/min for 10 min. Next, 2.5 ml of the supernatant was combined with 2.5 ml of distilled water and 0.5 ml of 0.1% (1 g/L) FeCl3 solution. The absorbance values of the mixtures were determined in parallel and in triplicate at 700 nm, and the mean value was calculated.
All analyses were conducted in triplicate, and the results are shown as mean±standard deviation (SD). Statistical analysis was performed using Microsoft Excel 2013.
Acknowledgments
We would like to thank Prof. Fei GE from Jiangxi University of Traditional Chinese Medicine (Nanchang, China) for identifying Origanum vulgare L.
List of electronic supplementary materials
Chemical compositions of essential oils from different parts of O. vulgare L.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 81560657) and the Jiangxi Provincial Department of Science and Technology (No. 20142BAB205083), China
Electronic supplementary materials: The online version of this article (http://dx.doi.org/10.1631/jzus.B1600377) contains supplementary materials, which are available to authorized users
Compliance with ethics guidelines: Fei HAN, Guang-qiang MA, Ming YANG, Li YAN, Wei XIONG, Ji-cheng SHU, Zhi-dong ZHAO, and Han-lin XU declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Arcila-Lozano CC, Loarca-Pina G, Lecona-Uribe S, et al. Oregano: properties, composition and biological activity. Arch Latinoam Nutr. 2004;54(1):100–111. [PubMed] [Google Scholar]
- 2.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. (Available from: http://dx.doi.org/10.1006/abio.1996.0292) [DOI] [PubMed] [Google Scholar]
- 3.Benzie IF, Szeto YT. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J Agric Food Chem. 1999;47(2):633–636. doi: 10.1021/jf9807768. (Available from: http://dx.doi.org/10.1021/jf9807768) [DOI] [PubMed] [Google Scholar]
- 4.Berrehal D, Boudiar T, Hichem L, et al. Comparative composition of four essential oils of oregano used in Algerian and Jordanian folk medicine. Nat Prod Commun. 2010;5(6):957–960. [PubMed] [Google Scholar]
- 5.Bhargava K, Conti DS, da Rocha SR, et al. Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food Microbiol. 2015;47:69–73. doi: 10.1016/j.fm.2014.11.007. (Available from: http://dx.doi.org/10.1016/j.fm.2014.11.007) [DOI] [PubMed] [Google Scholar]
- 6.Castilho PC, Savluchinske-Feio S, Weinhold TS, et al. Evaluation of the antimicrobial and antioxidant activities of essential oils, extracts and their main components from oregano from Madeira Island, Portugal. Food Control. 2012;23(2):552–558. (Available from: http://dx.doi.org/10.1016/j.foodcont.2011.08.031) [Google Scholar]
- 7.Céspedes CL, Sampietro DA, Seigler DS, et al. Natural Antioxidants and Biocides from Wild Medicinal Plants. Cambridge: CABI; 2013. [Google Scholar]
- 8.de Gaulejac NS, Provost C, Vivas N. Comparative study of polyphenol scavenging activities assessed by different methods. J Agric Food Chem. 1999;47(2):425–431. doi: 10.1021/jf980700b. (Available from: http://dx.doi.org/10.1021/jf980700b) [DOI] [PubMed] [Google Scholar]
- 9.Dlugosz A, Lembas-Bogaczyk J, Lamer-Zarawska E. Antoxid increases ferric reducing antioxidant power (FRAP) even stronger than vitamin C. Acta Pol Pharm. 2006;63(5):446–448. [PubMed] [Google Scholar]
- 10.Fournomiti M, Kimbaris A, Mantzourani I, et al. Antimicrobial activity of essential oils of cultivated oregano (Origanum vulgare), sage (Salvia officinalis), and thyme (Thymus vulgaris) against clinical isolates of Escherichia coli, Klebsiella oxytoca, and Klebsiella pneumoniae . Microb Ecol Health Dis. 2015;26:23289. doi: 10.3402/mehd.v26.23289. (Available from: http://dx.doi.org/10.3402/mehd.v26.23289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilling DH, Kitajima M, Torrey JR, et al. Antiviral efficacy and mechanisms of action of oregano essential oil and its primary component carvacrol against murine norovirus. J Appl Microbiol. 2014;116(5):1149–1163. doi: 10.1111/jam.12453. (Available from: http://dx.doi.org/10.1111/jam.12453) [DOI] [PubMed] [Google Scholar]
- 12.Grondona E, Gatti G, Lopez AG, et al. Bio-efficacy of the essential oil of oregano (Origanum vulgare Lamiaceae. ssp. Hirtum) Plant Foods Hum Nutr. 2014;69(4):351–357. doi: 10.1007/s11130-014-0441-x. (Available from: http://dx.doi.org/10.1007/s11130-014-0441-x) [DOI] [PubMed] [Google Scholar]
- 13.Kandaswami C, Middleton EJr. Armstrong, D. (Ed.), Free Radicals in Diagnostic Medicine. US: Springer; 1994. Free radical scavenging and antioxidant activity of plant flavonoids; pp. 351–376. (Available from: http://dx.doi.org/10.1007/978-1-4615-1833-4_25) [DOI] [PubMed] [Google Scholar]
- 14.Kayode RMO, Afolayan AJ. Cytotoxicity and effect of extraction methods on the chemical composition of essential oils of Moringa oleifera seeds. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(4):680–689. doi: 10.1631/jzus.B1400303. (Available from: http://dx.doi.org/10.1631/jzus.B1400303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagouri V, Blekas G, Tsimidou M, et al. Composition and antioxidant activity of essential oils from oregano plants grown wild in Greece. Z Lebensm Unters Forsch. 1993;197(1):20–23. (Available from: http://dx.doi.org/10.1007/BF01202694) [Google Scholar]
- 16.Mechergui K, Coelho JA, Serra MC, et al. Essential oils of Origanum vulgare L. subsp. glandulosum (Desf.) Ietswaart from Tunisia: chemical composition and antioxidant activity. J Sci Food Agric. 2010;90(10):1745–1749. doi: 10.1002/jsfa.4011. (Available from: http://dx.doi.org/10.1002/jsfa.4011) [DOI] [PubMed] [Google Scholar]
- 17.Ok E, Adanir N, Ozturk T. Antibacterial and smear layer removal capability of oregano extract solution. Eur J Dent. 2015;9(1):20–24. doi: 10.4103/1305-7456.149633. (Available from: http://dx.doi.org/10.4103/1305-7456.149633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozkan G, Baydar H, Erbas S. The influence of harvest time on essential oil composition, phenolic constituents and antioxidant properties of Turkish oregano (Origanum onites L.) J Sci Food Agric. 2010;90(2):205–209. doi: 10.1002/jsfa.3788. (Available from: http://dx.doi.org/10.1002/jsfa.3788) [DOI] [PubMed] [Google Scholar]
- 19.Parejo I, Codina C, Petrakis C, et al. Evaluation of scavenging activity assessed by Co(II)/EDTA-induced luminol chemiluminescence and DPPH∙ (2,2-diphenyl-1-picrylhydrazyl) free radical assay. J Pharmacol Toxicol Methods. 2000;44(3):507–512. doi: 10.1016/s1056-8719(01)00110-1. (Available from: http://dx.doi.org/10.1016/S1056-8719(01)00110-1) [DOI] [PubMed] [Google Scholar]
- 20.Ragi J, Pappert A, Rao B, et al. Oregano extract ointment for wound healing: a randomized, double-blind, petrolatum-controlled study evaluating efficacy. J Drugs Dermatol. 2011;10(10):1168–1172. [PubMed] [Google Scholar]
- 21.Ruberto G, Baratta MT, Sari M, et al. Chemical composition and antioxidant activity of essential oils from Algerian Origanum glandulosum Desf. Flavour Frag J. 2002;17(4):251–254. (Available from: http://dx.doi.org/10.1002/ffj.1101) [Google Scholar]
- 22.Stuessy TF. Plant Taxonomy: the Systematic Evaluation of Comparative Data. New York: Columbia University Press; 2009. [Google Scholar]
- 23.Teixeira B, Marques A, Ramos C, et al. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J Sci Food Agric. 2013;93(11):2707–2714. doi: 10.1002/jsfa.6089. (Available from: http://dx.doi.org/10.1002/jsfa.6089) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chemical compositions of essential oils from different parts of O. vulgare L.


