Abstract
BACKGROUND
Cardiac autonomic perturbations frequently antecede onset of paroxysmal atrial fibrillation (AF). Interventions that influence autonomic inputs to myocardium may prevent AF. However, whether low heart rate or heart rate variability (HRV), which are noninvasive measures of cardiac autonomic dysfunction, are associated with AF incidence is unclear.
OBJECTIVES
We sought to study the association between HRV and risk of AF.
METHODS
We studied 11,715 middle-aged adults in the ARIC (Atherosclerosis Risk in Communities) cohort with heart rate and HRV measures obtained from 2-min electrocardiogram (ECG) recordings performed at baseline (1987 to 1989). These measures included standard deviation of RR intervals (SDNN), high frequency (HF) (0.15 to 0.40 Hz), low frequency (LF) (0.04 to 0.15 Hz), and the LF/HF ratio (denoting a higher sympathetic to parasympathetic dominance). Incident AF cases were ascertained by ECG at ARIC follow-up visits, hospital discharge diagnosis, or death certificates through 2011.
RESULTS
During an average follow-up of 19.4 years, 1,580 or 13.5% of participants developed AF. A baseline heart rate <60 beats/min was modestly associated with increased AF risk. Lower overall HRV as well as increased sympathetic/parasympathetic tone were independently associated with higher risk of AF; the hazard ratio for each 1 SD lower SDNN was 1.14 (95% confidence interval [CI]: 1.08 to 1.21), for HF was 1.12 (95% CI: 1.06 to 1.17), and for LF/HF was 1.08 (95% CI: 1.03 to 1.14).
CONCLUSIONS
Cardiac autonomic dysfunction denoted by low resting short-term HRV was associated with higher AF incidence. Also, a low heart rate may be associated with higher AF risk. Further studies are needed to determine whether interventions in the general population to restore autonomic balance may prevent AF.
Keywords: arrhythmia, frequency, heart rate variability, parasympathetic, sympathetic
Introduction
Atrial fibrillation (AF), a prevalent arrhythmia, is associated with increased risk of stroke, heart failure, mortality, and possibly dementia (1). Traditional cardiovascular disease (CVD) risk factors and CVD itself may explain only one-half of AF cases (2), prompting calls for improved understanding of its pathophysiological basis (3).
The role of cardiac autonomic dysfunction, an abnormality of the autonomic nervous system (ANS), has been long suspected in the initiation and maintenance of AF (4). While heart rate reflects an individual's baseline autonomic tone, heart rate variability (HRV) components provide some insight into the ANS-mediated modulation of heart rate (5). Variations in heart rate during breathing (high-frequency [HF] HRV, defined as 0.15 to 0.40 Hz, attributable mostly to parasympathetic modulation), and during the day and sleep (low-frequency [LF] HRV, specifically 0.04 to 0.15 Hz, mostly sympathetic modulation) require a well-functioning ANS. Both divisions of the ANS (sympathetic and parasympathetic) and their interactions with the underlying atrial substrate play a role in AF initiation or maintenance (6,7). For instance, ANS fluctuations are common prior to paroxysmal AF onset (8,9). Their role is becoming more evident with the success of multiple interventions that prevent AF recurrence via reductions in myocardial autonomic input (6,10).
Despite mounting evidence of a possible link between the ANS and AF, the only population-based studies examining such a relationship included a limited number of AF cases (fewer than 80 in each study) and did not find consistent independent associations between HRV and AF risk (11,12). Conversely, recent population-based studies have reported a consistent association between orthostatic hypotension, another proxy of autonomic dysregulation, and AF incidence (13). In this context, we studied the association between HRV measures and AF incidence among middle-aged men and women in the biracial, population-based ARIC (Atherosclerosis Risk in Communities) study.
Methods
ARIC enrolled 15,792 black and white men and women ages 45 to 64 years from 4 U.S. communities. The baseline examination (1987 to 1989) was followed by 3 triennial follow-up examinations, annual telephone interviews, and continuous active surveillance of hospitalizations and deaths in the communities (14).
For the main study analysis, we excluded those with missing HRV data on 2-min recordings (n = 804); prevalent AF determined by electrocardiogram (ECG) (n = 37); other heart rhythm abnormalities (atrial flutter, advanced atrioventricular block, pacemakers, supraventricular or ventricular tachycardia; n = 60); or self-identified race other than black or white (n = 78); or who were missing values of important covariates (n = 587), leaving 11,715 study participants. For sensitivity analyses, we used HRV measured from 12-lead, 10-second ECG instead of a 2-min rhythm strip; after similar exclusions, 14,019 participants were included.
The ARIC study was approved by institutional review boards at each participating center and written informed consent was given by all participants at every visit.
Assessment of HRV and AF
Cardiac autonomic function was measured using mean RR interval (inversely related to heart rate and linearly related to autonomic tone), and HRV measures. The HRV measures were derived from 2-min ECG recordings obtained from 3 electrocardiograph electrodes placed on the midline upper and midline lower epigastrium and left subcostal area and R-to-R interval recorded using standardized protocols while participants were in the supine position for at least 20 min (15,16). The 2-min, raw heart rate data were first subjected to a filter program to remove artifacts under visual control by a single, trained operator (17). A computer algorithm labeled data points (RR interval) outside the upper and lower limits generated by a 5-beat moving average ± 25% (or set manually by an operator using a mouse) with imputations. After smoothing, linear interpolation was applied to neighboring heart rate data points, and 256 heart rate data points were resampled with an equal distance of 0.4685 s (15). We excluded those participants with flagged HRV measurements (n = 2,511) if imputation of the beat-to-beat heart rate data throughout an artifact period was not possible while preserving the timing relationships of the adjacent, uncorrupted heart rate data (15). Most likely reasons for exclusion included presence of premature atrial or ventricular complexes or artifacts that might introduce appreciable errors when estimating RR intervals.
Fast Fourier Transformation was performed to estimate Fourier transform of the heart rate residuals from which the power spectral density and the areas (power) under defined frequencies were computed.
The following HRV measures were estimated: 1) standard deviation of normal-to-normal RR intervals (SDNN); 2) root mean square of successive differences in normal-to-normal RR intervals (RMSSD); and, by partitioning of area under power spectral density curves, 3) HF and LF variations in heart rate, as defined earlier, as well as the ratio of LF/HF variation. Intradata operator reliability coefficient was 0.98 or more, and the interdata operator coefficient was 0.86 for HF (15). Given the low reliability of LF variations with short-term recordings, we particularly focused on the HF measures commonly reported as SDNN and HF, though associations with all measures are reported. Similarly, with the use of 10-s ECG, we only described the commonly reported HF time domain measure of SDNN. The correlation coefficient of SDNN measure in a study comparing 10-s with 2-min recordings was 0.72 (95% confidence interval [CI]: 0.63 to 0.80) (18).
Incident AF cases were identified from either ECG at ARIC follow-up visits, International Classification of Diseases, 9th Revision (ICD-9) diagnostic codes from hospital discharge records, or death certificate codes through 2011. At each ARIC examination, a supine 12-lead resting ECG was recorded at least 1 h after the use of any tobacco product or ingestion of caffeine, using MAC PC Personal Cardiographs (Marquette Electronics, Inc., Milwaukee, Wisconsin). ECG recordings were computer coded and reviewed by ARIC-certified cardiologists at a single reading center to confirm the AF diagnosis.
New hospitalizations or deaths were identified by annual follow-up telephone calls and by searching local hospitals records and the National Death Index. Presence of ICD-9 code 427.3 or ICD-10 code I48 at any position in the death certificate was also used to identify AF (19). An AF discharge code during a hospitalization with open heart surgery was not considered an event. A physician review of hospital discharge summaries in a subset of the ARIC cohort reported sensitivity and specificity of 84% and 98%, respectively, for the specified ICD codes (19).
Covariates
Race, education level, cigarette smoking status, pack-years of smoking, and alcohol drinking status were determined by self-report. Body mass index (BMI) was calculated as weight divided by height squared (kg/cm2). Diabetes was defined as fasting glucose >126 mg/dl (or nonfasting glucose >200 mg/dl), self-report of a physician diagnosis, or current use of medications for diabetes. Blood pressure was measured 3 times, with the last 2 measurements averaged. Hypertension was defined as an average blood pressure >140 mm Hg systolic or >90 mm Hg diastolic from visit measurements, or use of blood pressure medication in the past 2 weeks. Prevalent coronary heart disease (CHD) was defined as presence of a self-report of myocardial infarction (MI), coronary bypass, angioplasty, or MI suggested on the baseline ECG. Prevalent heart failure was identified using the Gothenburg criteria or self-report of heart failure medication use in the past 2 weeks. Participants were asked to bring all the medications they were currently taking to the field center visit. Given their potential influence on HRV, we stratified SDNN results based on beta-blocker use.
Statistical Methods
HRV measures were natural log transformed to normalize the distribution (20). Descriptive statistics of baseline characteristics were stratified by incident AF status. We used restricted cubic splines to examine the possibility that the association between HRV and incident AF was nonlinear. A partial likelihood ratio test compared models using only a linear term to models containing both the linear and cubic spline term. We found nonlinearity with threshold effects, thus study exposures were included in models described later as both a continuous term to compare with published studies and by creating custom categories such as RR interval between 600 ms and 1,000 ms as reference.
Cox proportional hazards models were used to estimate relative hazards of AF per SD decrease in log-transformed HRV measures in models with incremental adjustment for potential confounders. The primary results (model 3) were adjusted for age, race, sex, educational level, BMI, diabetes, hypertension, low-density and high-density lipoprotein cholesterol, smoking status, alcohol intake, prevalent CHD, and prevalent heart failure. Additional adjustment was done for incident CHD and incident heart failure (model 4) as a sensitivity analysis.
To test for differences in the HRV-AF relationship by age, sex, race, diabetes, hypertension, and CHD, we included an interaction term between these variables and the exposure variable (SDNN was used as it is an easily understood and commonly used metric for HF variations). We conducted several sensitivity analyses: 1) to assess selection bias due to excluded participants, we assessed the HRV-AF relationship using HRV measures from a 10-s ECG strip; 2) to reduce any reverse causality, we assessed the relationship after excluding those with incident AF events within 2 years of baseline; and 3) to account for confounding due to follow-up CVD events, we adjusted for incident CHD and incident heart failure events. Lastly, we were interested in the etiological relationship of HRV with AF and not risk prediction; thus, we explored the incremental value of ln (SDNN) to predict AF over CHA2DS2-VASc score and an AF-specific score such as CHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology)-AF score (21).
Statistical analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, North Carolina). A p value < 0.05 for a 2-sided null hypothesis was considered statistically significant, including for interaction terms.
Results
At baseline, the mean age of cohort members was 54 ± 5.7 years (range: 44 to 66), with 57% females and 25% blacks. During an average follow-up of 19.4 years, 13.5% (1,580 of 11,715) had incident AF. Participants who developed AF were older and had a higher prevalence of CHD, heart failure, and traditional CVD risk factors (Table 1). Participants who developed AF had lower HRV across all measures, and a lower heart rate (higher mean RR interval) than those who did not (Table 1). Mean LF/HF ratio was not different by incident AF status.
Table 1.
Baseline Characteristics
| Characteristics | Incident Atrial Fibrillation |
p Value | |||
|---|---|---|---|---|---|
| Yes (n = 1,580) | No (n = 10,135) | ||||
| Mean* | SD | Mean* | SD | ||
| Age, yrs | 56.5 | 5.4 | 53.7 | 5.7 | <0.001 |
| Male | 0.53 | 0.42 | <0.001 | ||
| White | 0.82 | 0.74 | <0.001 | ||
| Education | <0.001 | ||||
| Less than high school | 0.28 | 0.22 | |||
| High school but less than college | 0.41 | 0.42 | |||
| College or professional | 0.31 | 0.36 | |||
| Hypertension | 0.45 | 0.32 | <0.001 | ||
| Diabetes | 0.16 | 0.10 | <0.001 | ||
| Prevalent CHD | 0.09 | 0.04 | <0.001 | ||
| Prevalent heart failure | 0.08 | 0.04 | <0.001 | ||
| Current smoker | 0.30 | 0.25 | <0.001 | ||
| Former smoker | 0.35 | 0.32 | <0.001 | ||
| Cigarette-years of smoking | 408.2 | 496.3 | 291.1 | 415.7 | <0.001 |
| Usual ethanol intake, g/week | 44.63 | 97.1 | 40.54 | 92.0 | 0.10 |
| LDL cholesterol, mg/dl | 139.0 | 39.1 | 137.0 | 39.1 | 0.05 |
| HDL cholesterol, mg/dl | 48.6 | 15.7 | 52.7 | 17.3 | <0.001 |
| BMI, kg/m2 | 28.7 | 5.6 | 27.4 | 5.2 | <0.001 |
| Cornell voltage, mm | 12.9 | 5.8 | 12.1 | 5.4 | <0.001 |
| Mean RR interval, ms | 916.2 | 144.2 | 904.4 | 135.3 | 0.001 |
| Log (SDNN) | 3.43 | 0.53 | 3.51 | 0.48 | <0.001 |
| Log (RMSSD) | 3.10 | 0.68 | 3.18 | 0.61 | <0.001 |
| Log (LF) | 2.40 | 1.48 | 2.71 | 1.34 | <0.001 |
| Log (HF) | 1.80 | 1.45 | 2.11 | 1.29 | <0.001 |
| Log (LF/HF) | 0.60 | 0.97 | 0.60 | 0.92 | 0.91 |
Mean or proportion
BMI = body mass index; CHD = coronary heart disease; HDL = high-density lipoprotein; HF = high frequency; LDL = low-density lipoprotein; LF = low frequency; RMSSD = root mean square of successive differences in normal-to-normal R-R intervals; SDNN = standard deviation of normal-to-normal R-R intervals.
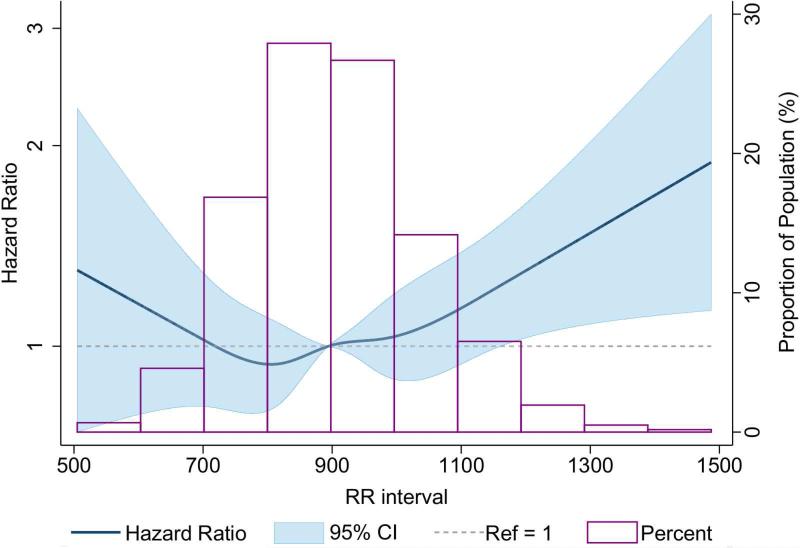
The multivariable-adjusted dose-response relationship of inverse of heart rate (mean RR) with incident AF (Figure 1) showed a threshold effect around 1,200 ms; above this threshold (heart rate around ≤50 beats/min) there was a linear relationship between mean RR interval and AF. A higher mean RR interval was associated with a higher incidence of AF that was nonsignificant after adjusting for confounders (Table 2). Further, mean RR interval >1,000 ms (heart rate <60 beats/min) was associated with higher incidence of AF (hazard ratio [HR]: 1.15; 95% CI: 0.99 to 1.32) compared to a mean RR interval between 600 and 1,000 ms); for mean RR >1,100 ms (heart rate <55 beats/min), the incidence of AF was 22% higher (HR: 1.22; 95% CI: 1.01 to 1.46) compared to those with mean RR interval between 600 and 1,100 ms.
Figure 1. Baseline RR Interval and Incident AF.
The dose-response relationship of inverse heart rate and incident atrial fibrillation (AF) showed a threshold effect around 1,200 ms. The model was adjusted for baseline age, race, sex, diabetes, hypertension, high- and low-density lipoprotein cholesterol, prevalent coronary heart disease, prevalent heart failure, current smoking status, alcohol drinking, and body mass index. Although, the shape of the relationship remained unchanged after additional adjustment for beta-blockers, the confidence interval (CI) widened.
Table 2.
Relative Hazards of AF with HRV Decrease
| Measure | Model 1* | Model 2† | Model 3‡ | Model 4§ | ||||
|---|---|---|---|---|---|---|---|---|
| HR∥ | 95% CI | HR∥ | 95% CI | HR∥ | 95% CI | HR∥ | 95% CI | |
| Mean RR interval | 1.01 | 0.96-1.06 | 0.96 | 0.92-1.01 | 0.91 | 0.83-1.00 | 0.91 | 0.83-1.01 |
| Log (SDNN) | 1.18 | 1.13-1.25 | 1.10 | 1.05-1.16 | 1.14 | 1.08-1.21 | 1.12 | 1.06-1.19 |
| Log (RMSSD) | 1.07 | 1.02-1.13 | 1.04 | 0.99-1.10 | 1.07 | 1.01-1.14 | 1.06 | 1.00-1.12 |
| Log (HF) | 1.14 | 1.09-1.20 | 1.11 | 1.06-1.17 | 1.12 | 1.06-1.17 | 1.10 | 1.05-1.16 |
| Log (LF) | 1.24 | 1.18-1.30 | 1.16 | 1.11-1.22 | 1.17 | 1.11-1.23 | 1.16 | 1.10-1.22 |
| Log (LF/HF) | 1.15 | 1.09-1.21 | 1.08 | 1.03-1.14 | 1.08 | 1.03-1.14 | 1.08 | 1.03-1.14 |
Model 1: adjusted for age, race, and sex.
Model 2: adjusted for adjusted for model 1 covariates, and prevalent CHD, diabetes, hypertension, prevalent heart failure, smoking status, alcohol intake, education level, LDL cholesterol, HDL cholesterol, study center, and BMI.
Model 3: adjusted for model 2 covariates, and heart rate (no change with additional adjustment of Cornell voltage).
Model 4: adjusted for model 3 covariates, and incident CHD, and incident heart failure.
Hazard ratios (HR) are expressed per SD lower heart rate variability (HRV) measures.
AF = atrial fibrillation; CI = confidence interval; other abbreviations as in Table 1.
HRV, AF Incidence, and Related Variables
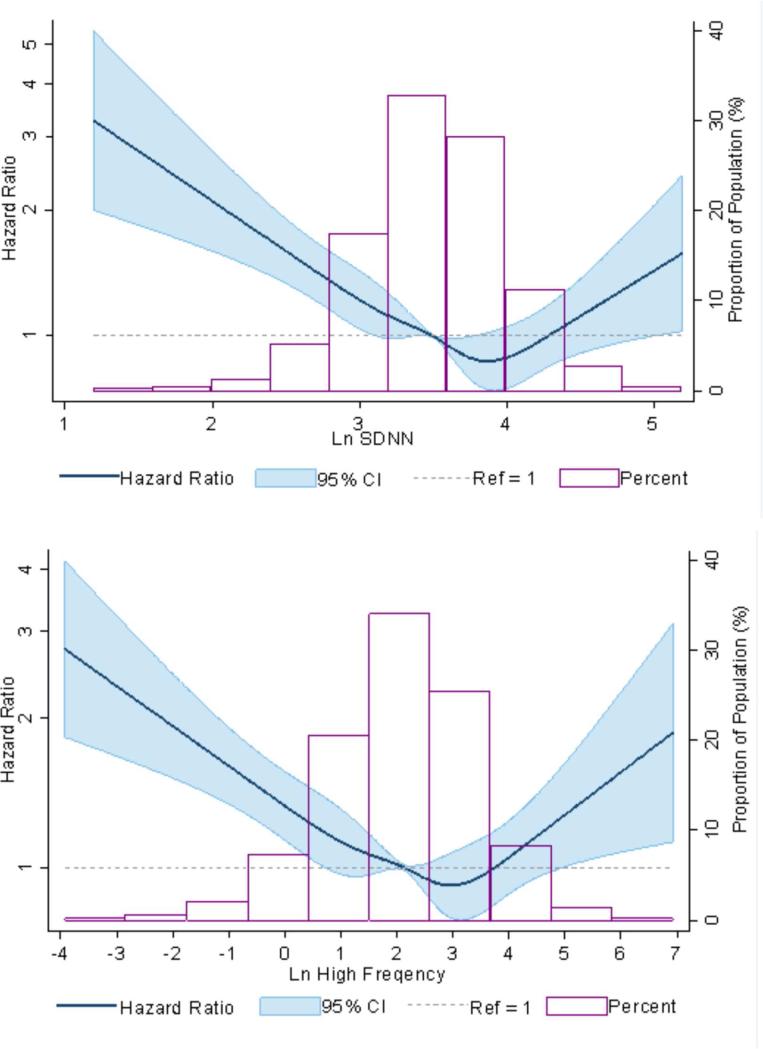
The multivariable-adjusted dose-response relationship between HRV measures (SDNN and high frequency) with incident AF suggested an almost log linear relationship with threshold effect (Figure 2). A lower HRV was associated with a significantly higher risk of incident AF for all measures of HRV (Table 2). In fully-adjusted Cox proportional hazard models (model 3 of Table 2), for every 1 SD lower measure, the HR of incident AF was 1.14 for log(SDNN), 1.07 for log(RMSSD), 1.12 for log(HF), and 1.17 for log(LF). Also, a higher sympathetic tone compared to parasympathetic tone as suggested by lower log(LF/HF) was associated with higher risk of AF (HR: 1.08; 95% CI: 1.03 to 1.14 per SD). Additional adjustment for incident CHD and incident heart failure did not influence the estimates much (Table 2).
Figure 2. HRV and AF Incidence.
The multivariable-adjusted dose-response relationship between HRV measures (SDNN and high frequency) with incident AF suggested an almost log linear relationship with threshold effect between incident AF and (A) standard deviation normal to normal (SDNN) and (B) high frequency, both measures of HRV. The curves were adjusted for prevalent coronary heart disease, diabetes, hypertension, prevalent heart failure, smoking status, alcohol intake, education level, high- and low-density lipoprotein cholesterol, study center, body mass index, heart rate, and Cornell voltage. Abbreviations as in Figure 1.
On examining differences in HRV-AF relationship by several characteristics (Table 3), there was no difference in the relationship by race, sex, CHD, heart failure, or beta-blocker therapy. There was a difference in association by diabetes status and age group; specifically, a much stronger association was seen among those with diabetes than those without (interaction p < 0.01), and no association was found among younger cohort participants.
Table 3.
Demographic Differences in Relative Hazards of AF with HRV Decrease
| HR* | 95% CI | p for interaction | ||
|---|---|---|---|---|
| Male | 1.14 | 1.05 | 1.23 | 0.75 |
| Female | 1.14 | 1.05 | 1.24 | |
| White | 1.15 | 1.08 | 1.22 | 0.87 |
| Black | 1.09 | 0.97 | 1.24 | |
| Age <54 yrs | 1.02 | 0.91 | 1.15 | 0.05 |
| Age ≥54 yrs | 1.22 | 1.14 | 1.30 | |
| No diabetes | 1.09 | 1.03 | 1.16 | <0.01 |
| Diabetes | 1.33 | 1.16 | 1.52 | |
The estimates did not differ appreciably after excluding AF events that occurred within 2 years of follow-up. Also, additional adjustment for beta-blockers did not change the estimates. Further, there was an association of smaller magnitude seen when using 10-s ECG recordings (Online Table 1).
On addition of ln (SDNN) to a model predicting 10-year AF risk with CHA2DS2-VASc score as an independent variable (base model), c-statistic improved from 0.576 (95% CI: 0.547 to 0.605) to 0.611 (95% CI: 0.582 to 0.640). However, the addition of ln (SDNN) to a similar model with CHA2DS2-VASc score components as individual variables (age, sex, diabetes, hypertension, stroke, etc.), c-statistic did not change appreciably (0.746 [95% CI: 0.724 to 0.768] to 0.750 [95% CI: 0.728 to 0.773]). The addition of ln (SDNN) to CHARGE-AF risk score did not improve prediction.
Discussion
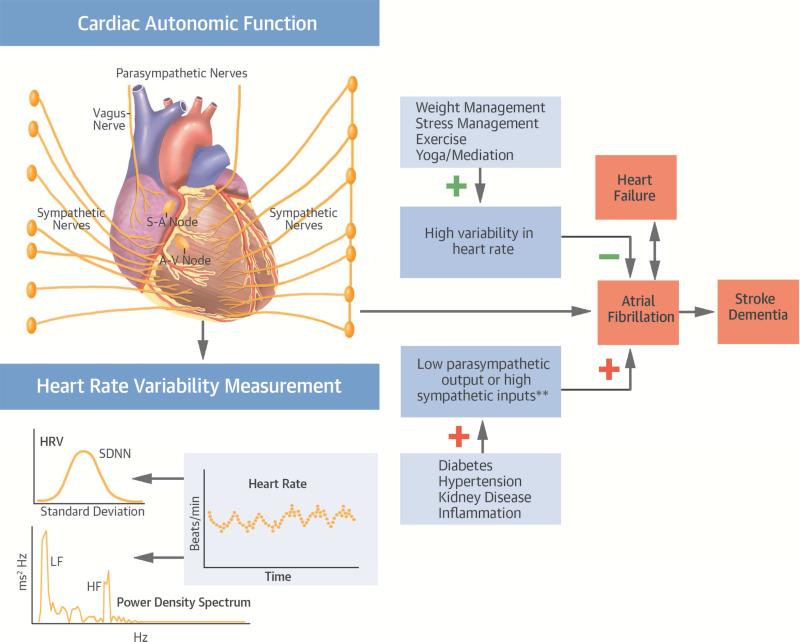
In this large middle-aged, biracial, population-based study, cardiac autonomic dysfunction as marked by low HRV and low heart rate was associated with a higher incidence of AF (Central Illustration). We found a modest relationship between higher mean RR interval, a marker of autonomic tone, and AF incidence. We also observed a log-linear relationship with threshold effect for both time and frequency domain measures of HRV and AF incidence. Our results suggested that presence of either a high basal parasympathetic tone (as seen by a higher mean RR) or poor modulation of heart rate (as assessed by HRV measures on a 2-min ECG rhythm strip, relating more to poor parasympathetic modulation and independent of heart rate) are associated with a higher AF incidence. Also, a higher sympathetic-to-parasympathetic modulation as denoted by an elevated LF/HF ratio was associated with higher incidence of AF.
Central Illustration. Cardiac Autonomic Function and Atrial Fibrillation: Potential Interplay.
Abnormalities in cardiac autonomic function, as denoted by a lower heart rate variability (HRV) or unbalanced sympathetic to parasympathetic tone, were associated with a higher risk of developing atrial fibrillation (AF) in a population-based cohort over 20 years of follow-up. Future studies should investigate whether modulation of the autonomic nervous system, through strategies such as weight management, exercise, and yoga, may be effective in preventing AF. **One SDNN lower HRV measure was associated with 21% higher risk of AF development over 20 years of follow up in the ARIC study cohort. Abbreviations: ARIC = Atherosclerosis Risk in Communities; A-V = atrioventricular; HF = high frequency; LF = low frequency; S-A = sino-atrial; SDNN = standard deviation of normal-to-normal R-R intervals.
A high HRV indicates that both parasympathetic and sympathetic ANSs are at physiological level, while its reduction is complex and difficult to interpret (5). Our findings persisted after adjustment for well-measured confounders and indicated that low HRV measures might reflect the possibility of AF initiation in a susceptible patient with sympathetic surge due to poor vagal modulation. The LF measure or its ratio with HF is unreliable for the sympathetic component when using short-term recordings measured in the resting state, and it might reflect not cardiac sympathetic innervation but baroreflex responses (22). The LF component has major contributions from the parasympathetic system (Pearson correlation coefficient = 0.82 was seen between HF and LF HRV measures in our study) when measured for a short duration. LF/HF ratio was also associated with a higher AF incidence in our study. The associations were stronger among those age ≥54 years and among patients with diabetes, likely reflecting a higher variation in degree of ANS dysfunction in these subgroups as well as a higher risk of AF. An earlier report from the Framingham cohort with limited power suggested a positive association between SDNN and LF/HF derived from ambulatory ECG and incident AF (sex- and age-adjusted), which attenuated to an HR of 1.13 (95% CI: 0.94 to 1.38) per unit decrease in log (SDNN) after adjustment for CVD risk factors (11). In another cohort of patients with hypertension and their controls, only the LF component of HRV was associated with incident AF (12).
In our study, we observed a higher AF risk among those with a higher mean RR interval. In previous studies, both a higher resting heart rate (23), as well as a low heart rate after moderate exercise, have been associated with higher AF risk (24). A sympathetic overtone is often seen with organic heart disease as well as acute states like sepsis and post-cardiac surgeries, which are all associated with higher AF risk/burden (25-27), whereas a vagal dominance has been implicated for AF especially in young individuals and women (28). Also, nonautonomic system-related pathologies such as a sick conduction system in a small fraction of participants may present with a low heart rate at baseline, which may also predispose them to a higher risk of AF.
In terms of mechanisms, a low HRV is associated with a poor cardiovascular profile as well as a higher risk of incident coronary artery disease (29). Though HRV has been reported as a marker of general well-being, its association with CVD deaths was higher than a small association observed with cancer deaths in the ARIC cohort (30). Experimental evidence exists in both animals and humans about a strong role of the ANS in AF initiation and maintenance. Vagal stimulation could induce and maintain AF, whereas such induction was abolished following ablation of vagal inputs to the atria (31,32). Lowering vagal inputs by pulmonary vein isolation or use of class I antiarrhythmic drugs may explain their success in reducing AF recurrence (33-36) Also, a drop in blood pressure after 2 min of standing from a supine position reflects vascular sympathetic input and response, and has been associated with higher AF incidence (13,37).
Risk factors such as obesity, obstructive sleep apnea, lack of physical activity, and physiological or emotional stress acts may adversely influence the autonomic nervous system and be implicated in the causation of AF (38). Attempts at weight reduction with lifestyle changes, moderate physical activity, use of continuous positive airway pressure in those with obstructive sleep apnea, and use of mindful meditation and breathing exercises in those with high sympathetic tone, such as heart failure patients, may lead to improved autonomic tone and modulation, thus preventing atrial fibrillation. Modifying the ANS to reduce AF incidence or recurrence such as during AF ablation through wide circumferential ablation, ablation of ganglionated plexi, and dissection of fat pad, or use of drugs/devices is an area of active debate and research (39-42). It is worth noting that for a given patient, a lower heart rate might not always be a marker of high parasympathetic tone but could be a marker of conduction disease. Our findings supported interventions attempting to improve autonomic tone as a potential way to reduce AF incidence or recurrence.
Study Limitations
The strengths of our study included its inclusion of a large number of men and women in 2 race groups, HRV measured from a 2-min rhythm strip as compared to a 10-s ECG strip, well-measured confounders, an extended follow-up, and a large number of AF events. However, use of 2-min ECG data could also be a study limitation. A resting, supine, ECG rhythm strip of 2 min is appropriate to estimate HRV or the parasympathetic component but not the sympathetic component, which is better estimated with long records (43). Thus, our analysis was primarily focused on high frequency or time domain measures. While use of HRV data from the 2-min rhythm strip led to the exclusion of a large proportion of our cohort, their characteristics were similar to those included (Online Table 2), and the results were robust on using 10-s ECG data with fewer exclusions. Lastly, we did not see attenuation of effect estimates after excluding AF events during the initial 2 years of follow-up; thus, reverse causality is unlikely. Case ascertainment in the absence of continuous ambulatory ECG monitoring for paroxysmal AF and outpatient surveillance has lower sensitivity especially after 1998 (last visit with field center ECG recordings) and remains another limitation, but specificity and positive predictive value of hospital diagnosis is high.
Conclusions
Our study found that a decrease in modulation of heart rate was associated with increased risk of new-onset AF. Modulation of ANS inputs have proven beneficial among patients undergoing wide circumferential AF ablation. Whether interventions ranging from exercise and yoga to direct modulation of ANS can prevent AF in high- and moderate-risk individuals remains to be examined.
Supplementary Material
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In a large, biracial, population-based cohort of middle-aged men and women, impaired cardiac autonomic responsiveness, as reflected in low heart rate variability, was associated with an increased risk of AF independent of several traditional risk factors.
TRANSLATIONAL OUTLOOK: Future studies should investigate whether modulation of the autonomic nervous system is effective in preventing AF. Candidate approaches include weight management, exercise, yoga, prevention or treatment of sleep apnea, or administration of adrenergic inhibitor drugs.
Acknowledgements
The authors thank the ARIC participants, staff, and investigators for their long-term contributions to ARIC.
Funding: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions. Additional funding for this study was provided by grants 16EIA26410001 from the American Heart Association and 1RC1HL099452-01 from NHLBI. SKA was supported by a National Institutes of Health/National Heart, Lung, and Blood Institute T32HL007024 (PI: Prof. Coresh) Cardiovascular Epidemiology Training Grant.
ABBREVIATIONS AND ACRONYMS
- AF
atrial fibrillation
- ANS
autonomic nervous system
- CHD
coronary heart disease
- CVD
cardiovascular disease
- ECG
electrocardiogram
- HF
heart failure
- HRV
heart rate variability
- MI
myocardial infarction
- RMSSD
root mean square of successive differences in normal-to-normal R-R intervals
- SDNN
standard deviation of normal-to-normal R-R intervals
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics—2016 Update: A Report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–8. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Chen PS, Bild DE, et al. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119:606–18. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coumel P. Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur Heart J. 1994;15(Suppl A):9–16. doi: 10.1093/eurheartj/15.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- 5.Malik M, Camm AJ. Components of heart rate variability--what they really mean and what we really measure. Am J Cardiol. 1993;72:821–2. doi: 10.1016/0002-9149(93)91070-x. [DOI] [PubMed] [Google Scholar]
- 6.Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114:1500–15. doi: 10.1161/CIRCRESAHA.114.303772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu Z, Scherlag BJ, Lin J, et al. Atrial fibrillation begets atrial fibrillation: autonomic mechanism for atrial electrical remodeling induced by short-term rapid atrial pacing. Circ Arrhythm Electrophysiol. 2008;1:184–92. doi: 10.1161/CIRCEP.108.784272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002;105:2753–9. doi: 10.1161/01.cir.0000018443.44005.d8. [DOI] [PubMed] [Google Scholar]
- 9.Amar D, Zhang H, Miodownik S, Kadish AH. Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. J Am Coll Cardiol. 2003;42:1262–8. doi: 10.1016/s0735-1097(03)00955-0. [DOI] [PubMed] [Google Scholar]
- 10.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm. 2012;9:632–96. e21. [Google Scholar]
- 11.Singh JP, Larson MG, Levy D, Evans JC, Tsuji H, Benjamin EJ. Is baseline autonomic tone associated with new onset atrial fibrillation? Insights from the Framingham Heart Study. Ann Noninvasive Electrocardiol. 2004;9:215–20. doi: 10.1111/j.1542-474X.2004.93550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkiomaki J, Ukkola O, Kiviniemi A, et al. Heart Rate Variability Findings as a Predictor of Atrial Fibrillation in Middle-Aged Population. J Cardiovasc Electrophysiol. 2014;25:719–24. doi: 10.1111/jce.12402. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal SK, Alonso A, Whelton SP, et al. Orthostatic change in blood pressure and incidence of atrial fibrillation: results from a bi-ethnic population based study. PloS One. 2013;8:e79030. doi: 10.1371/journal.pone.0079030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 15.Liao D, Barnes RW, Chambless LE, Heiss G. A computer algorithm to impute interrupted heart rate data for the spectral analysis of heart rate variability--the ARIC study. Comput Biomed Res. 1996;29:140–51. doi: 10.1006/cbmr.1996.0012. [DOI] [PubMed] [Google Scholar]
- 16.Liao D, Barnes RW, Chambless LE, Simpson RJ, Jr., Sorlie P, Heiss G. Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability--the ARIC study. Atherosclerosis Risk in Communities. Am J Cardiol. 1995;76:906–12. doi: 10.1016/s0002-9149(99)80260-4. [DOI] [PubMed] [Google Scholar]
- 17.National Heart, Lung, and Blood Institute . The ARIC Manuals of Operation: no. 11. Sitting blood pressure and postural changes in blood pressure and heart rate. ARIC Coordinating Center, School of Public Health, University of North Carolina; Chapel Hill, NC: 1987. [Google Scholar]
- 18.Schroeder EB, Whitsel EA, Evans GW, Prineas RJ, Chambless LE, Heiss G. Repeatability of heart rate variability measures. J Electrocardiol. 2004;37:163–72. doi: 10.1016/j.jelectrocard.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–7. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354–81. [No authors listed] [PubMed] [Google Scholar]
- 21.Alonso A, Roetker NS, Soliman EZ, et al. Prediction of Atrial Fibrillation in a Racially Diverse Cohort: The Multi-Ethnic Study of Atherosclerosis (MESA). J Am Heart Assoc. 2016;5(2):e003077. doi: 10.1161/JAHA.115.003077. doi: 10.1161/JAHA.115.003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moak JP, Goldstein DS, Eldadah BA, et al. Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm. 2007;4:1523–9. doi: 10.1016/j.hrthm.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okin PM, Wachtell K, Kjeldsen SE, et al. Incidence of atrial fibrillation in relation to changing heart rate over time in hypertensive patients: the LIFE study. Circ Arrhythm Electrophysiol. 2008;1:337–43. doi: 10.1161/CIRCEP.108.795351. [DOI] [PubMed] [Google Scholar]
- 24.Grundvold I, Skretteberg PT, Liestol K, et al. Low heart rates predict incident atrial fibrillation in healthy middle-aged men. Circ Arrhythm Electrophysiol. 2013;6:726–31. doi: 10.1161/CIRCEP.113.000267. [DOI] [PubMed] [Google Scholar]
- 25.Kalman JM, Munawar M, Howes LG, et al. Atrial fibrillation after coronary artery bypass grafting is associated with sympathetic activation. Ann Thorac Surg. 1995;60:1709–15. doi: 10.1016/0003-4975(95)00718-0. [DOI] [PubMed] [Google Scholar]
- 26.Bauernschmitt R, Malberg H, Wessel N, et al. Autonomic control in patients experiencing atrial fibrillation after cardiac surgery. Pacing Clin Electrophysiol. 2007;30:77–84. doi: 10.1111/j.1540-8159.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- 27.Kalisnik JM, Hrovat E, Hrastovec A, Avbelj V, Zibert J, Gersak B. Severe Cardiac Autonomic Derangement and Altered Ventricular Repolarization Pave the Way to Postoperative Atrial Fibrillation. Innovations (Phila) 2015;10:398–405. doi: 10.1097/IMI.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 28.Coumel P. Autonomic Influences of Atrial Fibrillation. In: Malik M, Camm AJ, editors. Dynamic Electrocardiography. Futura; Elmsford, NY: 2004. pp. 530–36. [Google Scholar]
- 29.Hillebrand S, Gast KB, de Mutsert R, et al. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose-response meta-regression. Europace. 2013;15:742–9. doi: 10.1093/europace/eus341. [DOI] [PubMed] [Google Scholar]
- 30.Dekker JM, Crow RS, Folsom AR, et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk In Communities. Circulation. 2000;102:1239–44. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 31.Schauerte P, Scherlag BJ, Pitha J, et al. Catheter ablation of cardiac autonomic nerves for prevention of vagal atrial fibrillation. Circulation. 2000;102:2774–80. doi: 10.1161/01.cir.102.22.2774. [DOI] [PubMed] [Google Scholar]
- 32.Aistrup GL, Villuendas R, Ng J, et al. Targeted G-protein inhibition as a novel approach to decrease vagal atrial fibrillation by selective parasympathetic attenuation. Cardiovasc Res. 2009;83:481–92. doi: 10.1093/cvr/cvp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyanaga S, Yamane T, Date T, et al. Impact of pulmonary vein isolation on the autonomic modulation in patients with paroxysmal atrial fibrillation and prolonged sinus pauses. Europace. 2009;11:576–81. doi: 10.1093/europace/eup082. [DOI] [PubMed] [Google Scholar]
- 34.Pappone C, Santinelli V, Manguso F, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–34. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 35.Miyakoshi M, Ikeda T, Miwa Y, et al. Quantitative assessment of cibenzoline administration for vagally mediated paroxysmal atrial fibrillation using frequency-domain heart rate variability analysis. J Cardiol. 2009;54:86–92. doi: 10.1016/j.jjcc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 36.White CM, Sander S, Coleman CI, et al. Impact of epicardial anterior fat pad retention on postcardiothoracic surgery atrial fibrillation incidence: the AFIST-III Study. J Am Coll Cardiol. 2007;49:298–303. doi: 10.1016/j.jacc.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 37.Fedorowski A, Hedblad B, Engstrom G, Gustav Smith J, Melander O. Orthostatic hypotension and long-term incidence of atrial fibrillation: the Malmo Preventive Project. J Intern Med. 2010;268:383–9. doi: 10.1111/j.1365-2796.2010.02261.x. [DOI] [PubMed] [Google Scholar]
- 38.Miller JD, Aronis KN, Chrispin J, et al. Obesity, Exercise, Obstructive Sleep Apnea, and Modifiable Atherosclerotic Cardiovascular Disease Risk Factors in Atrial Fibrillation. J Am Coll Cardiol. 2015;66:2899–906. doi: 10.1016/j.jacc.2015.10.047. [DOI] [PubMed] [Google Scholar]
- 39.Rajendran PS, Buch E, Shivkumar K. Marshaling the autonomic nervous system for treatment of atrial fibrillation. J Am Coll Cardiol. 2014;63:1902–3. doi: 10.1016/j.jacc.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S, Jing Y, Zhang J, Bian C, Zhang YU, Zhang X. Does Anterior Fat Pad Removal Reduce the Incidence of Atrial Fibrillation after CABG? A Meta-Analysis of Randomized Controlled Trials. Pacing Clin Electrophysiol. 2015;38:1363–8. doi: 10.1111/pace.12740. [DOI] [PubMed] [Google Scholar]
- 41.Giannopoulos G, Kossyvakis C, Efremidis M, et al. Central sympathetic inhibition to reduce postablation atrial fibrillation recurrences in hypertensive patients: a randomized, controlled study. Circulation. 2014;130:1346–52. doi: 10.1161/CIRCULATIONAHA.114.010999. [DOI] [PubMed] [Google Scholar]
- 42.Krul SPJ, Berger WR, Veldkamp MW, et al. Treatment of Atrial and Ventricular Arrhythmias Through Autonomic Modulation. JACC: Clin Electrophysiol. 2015;1:496–508. doi: 10.1016/j.jacep.2015.09.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.