Abstract
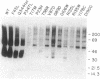
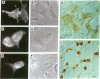
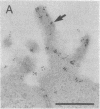
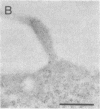
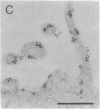
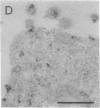
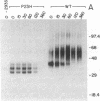
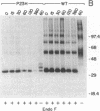
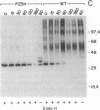
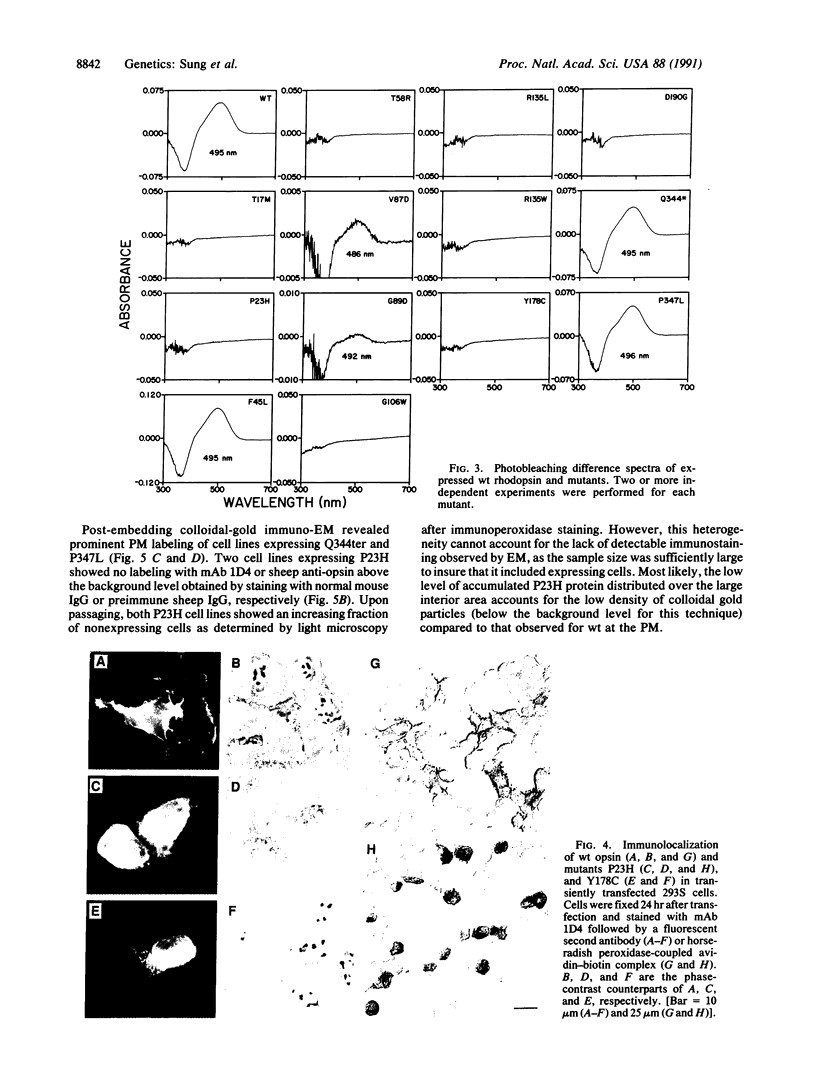
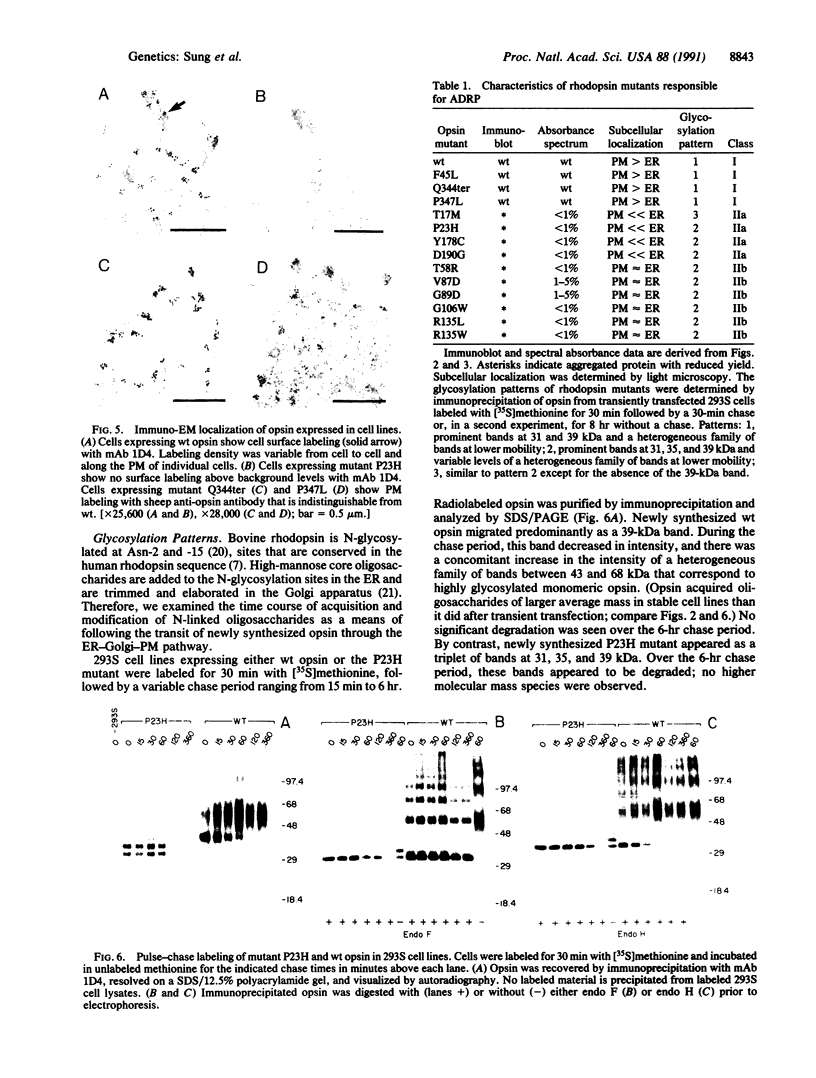
Thirteen mutant rhodopsins responsible for autosomal dominant retinitis pigmentosa (ADRP) have been produced by transfection of cloned cDNA into tissue culture cells. Three mutants [class I: Phe-45----Leu, Gln-344----termination (deletion of C-terminal positions 344-348), and Pro-347----Leu] resemble wild-type rhodopsin in yield, regenerability with 11-cis-retinal, and plasma membrane localization. Ten mutants [class II: Thr-17----Met, Pro-23----His, Thr-58----Arg, Val-87----Asp, Gly-89----Asp, Gly-106----Trp, Arg-135----Leu, Arg-135----Trp, Tyr-178----Cys, and Asp-190----Gly] accumulate to significantly lower levels, regenerate with 11-cis-retinal variably or not at all, and are transported inefficiently to the plasma membrane, remaining primarily in the endoplasmic reticulum. These data suggest that there are at least two distinct biochemical defects associated with different rhodopsin mutants in ADRP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamus G., Arendt A., Zam Z. S., McDowell J. H., Hargrave P. A. Use of peptides to select for anti-rhodopsin antibodies with desired amino acid sequence specificities. Pept Res. 1988 Sep-Oct;1(1):42–47. [PubMed] [Google Scholar]
- Altman L. G., Schneider B. G., Papermaster D. S. Rapid embedding of tissues in Lowicryl K4M for immunoelectron microscopy. J Histochem Cytochem. 1984 Nov;32(11):1217–1223. doi: 10.1177/32.11.6436366. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990 Nov 16;63(4):827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- Doi T., Molday R. S., Khorana H. G. Role of the intradiscal domain in rhodopsin assembly and function. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4991–4995. doi: 10.1073/pnas.87.13.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., McGee T. L., Hahn L. B., Cowley G. S., Olsson J. E., Reichel E., Sandberg M. A., Berson E. L. Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N Engl J Med. 1990 Nov 8;323(19):1302–1307. doi: 10.1056/NEJM199011083231903. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., McGee T. L., Reichel E., Hahn L. B., Cowley G. S., Yandell D. W., Sandberg M. A., Berson E. L. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990 Jan 25;343(6256):364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- Goldman B. M., Blobel G. In vitro biosynthesis, core glycosylation, and membrane integration of opsin. J Cell Biol. 1981 Jul;90(1):236–242. doi: 10.1083/jcb.90.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs H. H., Russell D. W., Brown M. S., Goldstein J. L. The LDL receptor locus in familial hypercholesterolemia: mutational analysis of a membrane protein. Annu Rev Genet. 1990;24:133–170. doi: 10.1146/annurev.ge.24.120190.001025. [DOI] [PubMed] [Google Scholar]
- Hodges R. S., Heaton R. J., Parker J. M., Molday L., Molday R. S. Antigen-antibody interaction. Synthetic peptides define linear antigenic determinants recognized by monoclonal antibodies directed to the cytoplasmic carboxyl terminus of rhodopsin. J Biol Chem. 1988 Aug 25;263(24):11768–11775. [PubMed] [Google Scholar]
- Inglehearn C. F., Bashir R., Lester D. H., Jay M., Bird A. C., Bhattacharya S. S. A 3-bp deletion in the rhodopsin gene in a family with autosomal dominant retinitis pigmentosa. Am J Hum Genet. 1991 Jan;48(1):26–30. [PMC free article] [PubMed] [Google Scholar]
- Karnik S. S., Khorana H. G. Assembly of functional rhodopsin requires a disulfide bond between cysteine residues 110 and 187. J Biol Chem. 1990 Oct 15;265(29):17520–17524. [PubMed] [Google Scholar]
- Kingsley R. E., Cole N. L. Preparation of cultured mammalian cells for transmission and scanning electron microscopy using Aclar film. J Electron Microsc Tech. 1988 Sep;10(1):77–85. doi: 10.1002/jemt.1060100110. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990 Aug 24;62(4):611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Nathans J. Determinants of visual pigment absorbance: role of charged amino acids in the putative transmembrane segments. Biochemistry. 1990 Jan 30;29(4):937–942. doi: 10.1021/bi00456a013. [DOI] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation and nucleotide sequence of the gene encoding human rhodopsin. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4851–4855. doi: 10.1073/pnas.81.15.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J., Thomas D., Hogness D. S. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986 Apr 11;232(4747):193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Nathans J., Weitz C. J., Agarwal N., Nir I., Papermaster D. S. Production of bovine rhodopsin by mammalian cell lines expressing cloned cDNA: spectrophotometry and subcellular localization. Vision Res. 1989;29(8):907–914. doi: 10.1016/0042-6989(89)90105-3. [DOI] [PubMed] [Google Scholar]
- Papermaster D. S., Schneider B. G., Zorn M. A., Kraehenbuhl J. P. Immunocytochemical localization of opsin in outer segments and Golgi zones of frog photoreceptor cells. An electron microscope analysis of cross-linked albumin-embedded retinas. J Cell Biol. 1978 Apr;77(1):196–210. doi: 10.1083/jcb.77.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider B. G., Papermaster D. S. Immunocytochemistry of retinal membrane protein biosynthesis at the electron microscopic level by the albumin embedding technique. Methods Enzymol. 1983;96:485–495. doi: 10.1016/s0076-6879(83)96042-1. [DOI] [PubMed] [Google Scholar]
- Sung C. H., Davenport C. M., Hennessey J. C., Maumenee I. H., Jacobson S. G., Heckenlively J. R., Nowakowski R., Fishman G., Gouras P., Nathans J. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6481–6485. doi: 10.1073/pnas.88.15.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALD G., BROWN P. K. Human rhodopsin. Science. 1958 Jan 31;127(3292):222–226. doi: 10.1126/science.127.3292.222. [DOI] [PubMed] [Google Scholar]