Abstract
High serum alkaline phosphatase concentrations are associated with elevated serum C-reactive protein (CRP) levels in the general population. To examine whether this association is independent of serum vitamin D levels or modified in chronic kidney disease (CKD), we determined if such associations exist using data from the National Health and Nutrition Examination Survey III of 14,420 adult participants in which 5.7% had CKD (defined as estimated glomerular filtration rate < 60 ml/min per 1.73m2). For each doubling of serum alkaline phosphatase, the odds of elevated serum CRP (over 3mg/l) were increased 2.73-fold in the non-chronic and 2.50-fold in the CKD sub-populations, respectively. Regression coefficients of each doubling of serum alkaline phosphatase with elevated CRP were not significantly different in between the sub-populations. Additional adjustment for the serum 25-hydroxy (OH) vitamin D level did not substantively change the results. Thus, associations of serum alkaline phosphatase with elevated CRP are independent of serum 25-OH vitamin D in the chronic and non-CKD populations. Hence, serum alkaline phosphatase might be a marker of the inflammatory milieu.
Keywords: alkaline phosphatase, C-reactive protein, chronic kidney disease, vitamin D
Alkaline phosphatase is primarily secreted by the liver and the bone,1 although a small amount is as well secreted by intestine, kidneys, and leukocytes. It is an indicator for bone turnover, particularly in patients with chronic kidney disease (CKD).1–2 Recent studies suggest that high serum alkaline phosphatase levels predict mortality independent of bone metabolism parameters and liver function tests in CKD3 and chronic hemodialysis patients.4–6
A possible explanation for the above associations of serum alkaline phosphatase with mortality in the CKD population is inflammation. Elevated markers of inflammation, such as serum C-reactive protein (CRP), are associated with increased risk of cardiovascular disease and mortality in the general and CKD populations.7–10 Studies in the general population suggest that higher serum alkaline phosphatase is associated with elevated serum CRP levels.11–12 However, this association might be confounded by serum vitamin D levels as lower vitamin D levels are associated with both elevated serum CRP levels13 and elevated serum alkaline phosphatase levels (resulting from increased osteoblastic activity).14 Therefore, we hypothesized that, in the CKD population, high serum alkaline phosphatase levels might be associated with elevated CRP and this association might in part explained by low serum vitamin D levels. Hence, we examined whether high serum alkaline phosphatase levels are indeed associated with elevated serum CRP levels and if this relationship can be attenuated or abolished by adjusting for serum vitamin D levels, specifically in the CKD population, using the National Health and Nutrition Examination Survey (NHANES) III database. Furthermore, we also examined whether the associations of serum alkaline phosphatase levels with serum CRP levels differed by the presence or absence of CKD.
RESULTS
Of the 15,837 adults (20 years or older) in NHANES III with valid data (serum creatinine, age, sex, and race) for glomerular filtration rate (GFR) estimation, 14,420 had non-missing data for serum alkaline phosphatase, vitamin D, CRP levels, and metabolic syndrome components. These participants were included in the current analysis. Of these, 5.7% had CKD.
Table 1 summarizes the clinical characteristics of the non-CKD and CKD populations categorized by the median value of serum alkaline phosphatase in the CKD sub-population. In general, higher serum alkaline phosphatase levels were associated with older age, greater prevalence of cardiovascular conditions, and physical inactivity in both non-CKD and CKD participants. Higher serum alkaline phosphatase levels were also associated with modest but statistically significant higher levels of serum aspartate amino transferase (AST) and alanine amino transferase (ALT). Higher serum alkaline phosphatase levels were significantly associated with diabetes, higher fasting plasma glucose, and greater waist circumference in the non-CKD population but not in CKD.
Table 1.
| Non-CKD participants | CKD participants | |||||
|---|---|---|---|---|---|---|
| ≤ 90 IU/l | > 90 IU/l | P-value | ≤ 90 IU/l | > 90 IU/l | P-value | |
| Alkaline phosphatase (IU/l) | 68±0.3 | 112±0.8 | 72±0.7 | 119±1.7 | ||
| Demographics | ||||||
| Age (years) | 42±0.4 | 47±0.5 | < 0.001 | 69±0.8 | 71±0.9 | 0.023 |
| Gender (male) (%) | 47 (46–48) | 54 (52–55) | < 0.001 | 39 (33–44) | 35 (29–41) | 0.214 |
| Race (African American) (%) | 9.9 (8.7–11.3) | 12.1 (10.7–13.5) | 0.019 | 7.9 (6.2–9.9) | 6.6 (4.8–8.9) | 0.232 |
| Clinical parameters | ||||||
| Myocardial infarction (%) | 2.1 (1.7–2.5) | 4.6 (3.8–5.7) | < 0.001 | 13.4 (10.0–17.8) | 19.0 (13.6–25.8) | 0.103 |
| Stroke (%) | 1.0 (0.7–1.2) | 2.8 (2.2–3.7) | < 0.001 | 7.2 (5.3–9.7) | 12.1 (9.1–15.9) | 0.002 |
| Congestive heart failure (%) | 1.0 (0.8–1.3) | 2.5 (1.8–3.3) | < 0.001 | 9.3 (6.6–13.0) | 16.2 (12.3–21.1) | 0.016 |
| Diabetes mellitus (%) | 4.6 (4.0–5.2) | 11.2 (10.0–12.5) | < 0.001 | 19.4 (16.4–22.8) | 24.2 (19.8–29.2) | 0.109 |
| Hypertension (%) | 25 (23–27) | 39 (36–42) | < 0.001 | 70 (65–75) | 77 (70–82) | 0.098 |
| Waist circumference (inches) | 35.1±0.1 | 37.4±0.1 | < 0.001 | 38.3±0.3 | 38.2±0.4 | 0.848 |
| Physically inactive (%) | 12 (10–14) | 17 (15–19) | < 0.001 | 21 (16–27) | 34 (29–40) | < 0.001 |
| Non-smoker (%) | 72 (70–74) | 68 (65–71) | 0.003 | 90 (86–93) | 84 (79–88) | 0.076 |
| Serum calcium (mg/dl) | 9.25±0.02 | 9.29±0.02 | 0.002 | 9.33±0.03 | 9.39±0.03 | 0.126 |
| Serum phosphorus (mg/dl) | 3.43±0.01 | 3.45±0.02 | 0.227 | 3.47±0.03 | 3.56±0.03 | 0.049 |
| Serum aspartate transaminase (IU/l) | 20.6±0.2 | 23.4±0.3 | < 0.001 | 20.8±0.5 | 21.6±0.4 | 0.261 |
| Serum alanine transaminase (IU/l) | 16.7±0.4 | 20.9±0.6 | < 0.001 | 13.3±0.6 | 15.0±0.6 | 0.040 |
| Serum bilirubin (mg/dl) | 0.63±0.01 | 0.60±0.01 | 0.027 | 0.58±0.02 | 0.55±0.01 | 0.165 |
| Estimated glomerular filtration rate (ml/min per 1.73 m2) | 94.1±0.5 | 93.2±0.5 | 0.124 | 50.3±0.4 | 47.9±0.6 | 0.001 |
| Serum vitamin D (ng/ml) | 30.3±0.4 | 28.2±0.4 | < 0.001 | 28.8±0.6 | 26.4±0.7 | 0.011 |
| Plasma fasting glucose (mg/dl) | 94.8±0.3 | 101.8±0.8 | < 0.001 | 106.1±1.6 | 106.2±2.1 | 0.988 |
| Hemoglobin (g/dl) | 14.1±0.03 | 14.4±0.04 | < 0.001 | 13.7±0.07 | 13.6±0.10 | 0.533 |
Abbreviation: CKD, chronic kidney disease.
Percentages shown as percent (95% confidence interval); continuous measures shown as mean±s.e.
Both CKD and non-CKD participants are divided into two groups by the median serum alkaline phosphatase level in the CKD group.
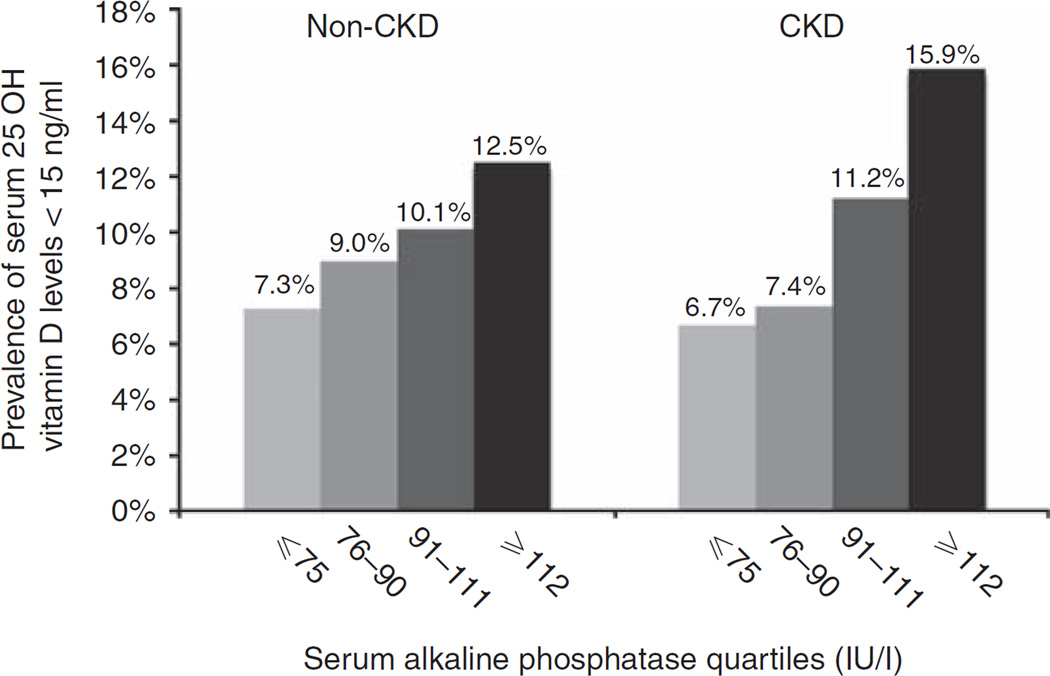
A higher prevalence of 25-hydroxy (OH) vitamin D deficiency (< 15 ng/ml) was observed with increasing alkaline phosphatase levels in the non-CKD and CKD sub-populations (Figure 1). In separate logistic regression models, for each doubling of serum alkaline phosphatase, the unadjusted odds of low 25-OH vitamin D were increased by a factor 1.66 (95% confidence interval (CI) 1.38–2.01) in the non-CKD and by a factor of 2.09 (95% CI 1.31–3.34) in the CKD subpopulations, respectively. When adjusted for demographics, comorbidities, waist circumference, physical inactivity, smoking, serum calcium and phosphorous, AST, ALT, bilirubin, estimated GFR, hemoglobin, and fasting glucose, these associations were significant in the non-CKD population (odds ratio 1.30, 95% CI 1.04–1.62) but not in the CKD population (odds ratio 1.44, 95% CI 0.72–2.86 in CKD).
Figure 1.
Prevalence of low serum 25-hydroxy (OH) vitamin D levels by serum alkaline phosphatase groups in non-chronic kidney disease (CKD) and CKD populations.
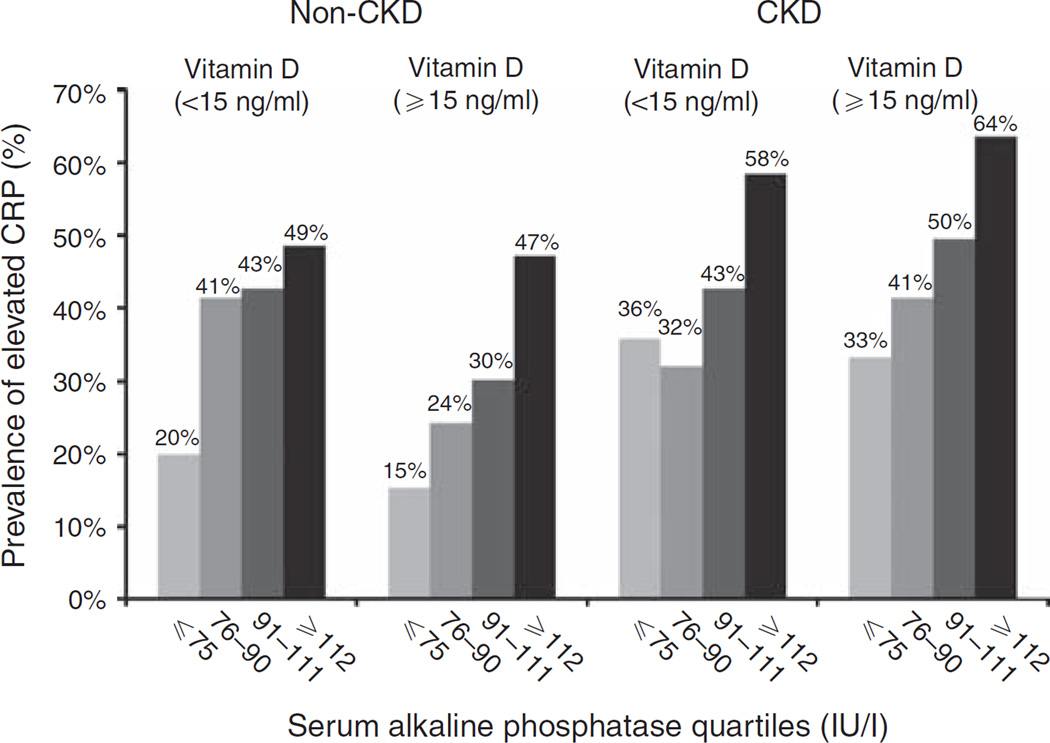
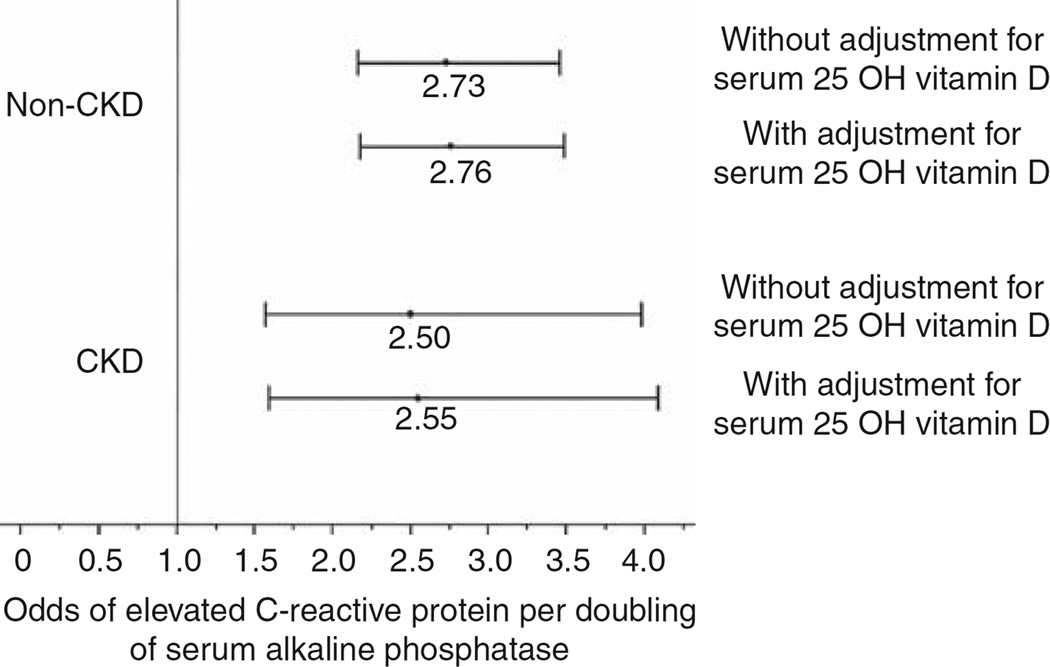
The detection threshold lower limit of the assay used to measure CRP in NHANES III was 2.1 mg/l. The median (interquartile range) for CRP distribution in the entire cohort, non-CKD sub-population, and CKD sub-population was 2.10 (2.10, 2.78), 2.10 (2.10, 2.58), and 2.10 (2.10, 6.92) mg/l, respectively. Using the 25th, 50th, and 75th percentiles of serum alkaline phosphatase in the CKD sub-population, both CKD and non-CKD sub-populations were divided into four groups. Figure 2 displays the prevalence of elevated CRP, defined as serum CRP level > 3.0 mg/l, by serum alkaline phosphatase and serum vitamin D levels in the non-CKD and CKD sub-populations. In separate multivariable models without adjustment for serum vitamin D levels, each doubling of serum alkaline phosphatase was associated with 2.73-fold (95% CI 2.16–3.46) higher odds of elevated CRP in the non-CKD group and 2.50-fold (95% CI 1.57–3.98) higher odds of elevated CRP in the CKD group, respectively (Figure 3). Additional adjustment for serum 25-OH vitamin D level did not significantly change the results (Figure 3), suggesting that serum vitamin D level has minimal effects on the association between serum alkaline phosphatase level and elevated serum CRP in the general population or those with CKD.
Figure 2.
Prevalence of elevated serum C-reactive protein (CRP) (> 3 mg/l) by serum alkaline phosphatase groups and serum 25-hydroxy vitamin D levels in non-chronic kidney disease (CKD) and CKD populations.
Figure 3. Associations of serum alkaline phosphatase with elevated serum C-reactive protein (> 3 mg/l) in non-chronic kidney disease (CKD) and CKD populations.
All models were adjusted for age, gender, race, myocardial infarction, stroke, congestive heart failure, diabetes, hypertension, waist circumference, exercise level, smoking, serum calcium, serum phosphorus, aspartate amino transferase, alanine amino transferase, bilirubin, glomerular filtration rate, serum hemoglobin, and serum fasting glucose. 25-OH vitamin D, 25-hydroxy vitamin D.
In order to examine whether the associations of serum alkaline phosphatase with elevated CRP varies by the presence or absence of CKD, two approaches were adopted. In the first approach, an interaction term of serum alkaline phosphatase with CKD was entered into the full model of the entire cohort. This interaction term was nonsignificant (P = 0.741). In the second approach, the regression coefficients of each doubling of serum alkaline phosphatase with elevated CRP in the non-CKD and CKD sub-populations were compared and these were also not significantly different (P = 0.734).
The associations of serum alkaline phosphatase as a categorical variable with elevated CRP are summarized in Tables 2 and 3.
Table 2.
Associations of serum alkaline phosphatase levels as a categorical variablea with elevated serum CRP levels (> 3 mg/l) in non-CKD population in logistic regression models without and with adjustment for serum vitamin D levels
| Model 1b | Model 2c | |||
|---|---|---|---|---|
| Serum alkaline phosphatase group | Odds | 95% CI | Odds | 95% CI |
| ≤ 75 IU/l | 1.00 | 1.00 | ||
| 76–90 IU/l | 1.49 | 1.26–1.74 | 1.50 | 1.28–1.75 |
| 91–111 IU/l | 1.99 | 1.69–2.36 | 2.01 | 1.70–2.37 |
| ≥ 112 IU/l | 3.53 | 2.76–4.50 | 3.56 | 2.80–4.52 |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; CRP, C-reactive protein.
Serum alkaline phosphatase groups defined by serum alkaline phosphatase quartiles in the CKD participants.
Model 1. Adjusted for age, gender, race, myocardial infarction, stroke, congestive heart failure, diabetes, hypertension, waist circumference, physical inactivity, smoking, estimated glomerular filtration rate, hemoglobin, serum calcium, phosphorus, aspartate transaminase, alanine transaminase, bilirubin, and fasting glucose.
Model 2. Adjusted for Model 1 variables and serum 25-hydroxy vitamin D level.
Table 3.
Associations of serum alkaline phosphatase levels as a categorical variablea with elevated serum CRP levels (> 3 mg/l) in CKD population in logistic regression models without and with adjustment for serum vitamin D levels
| Model 1b | Model 2c | |||
|---|---|---|---|---|
| Serum alkaline phosphatase group | Odds | 95% CI | Odds | 95% CI |
| ≤ 75 IU/l | 1.00 | 1.00 | ||
| 76–90 IU/l | 1.24 | 0.71–2.16 | 1.24 | 0.71–2.18 |
| 91–111 IU/l | 1.93 | 1.26–2.94 | 1.97 | 1.28–3.01 |
| ≥ 112 IU/l | 2.52 | 1.44–4.43 | 2.59 | 1.47–4.57 |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; CRP, C-reactive protein.
Serum alkaline phosphatase groups defined by serum alkaline phosphatase quartiles in the CKD participants.
Model 1. Adjusted for age, gender, race, myocardial infarction, stroke, congestive heart failure, diabetes, hypertension, waist circumference, physical inactivity, smoking, estimated glomerular filtration rate, hemoglobin, serum calcium, phosphorus, aspartate transaminase, alanine transaminase, bilirubin, and fasting glucose.
Model 2. Adjusted for Model 1 variables and serum 25-hydroxy vitamin D level.
In sensitivity analyses, we performed an additional analysis using data only from participants who had γ-glutamyl transpeptidase (GGT) measured to evaluate further the hepatobiliary component of the association between alkaline phosphatase and CRP. In separate multivariable models, the odds ratio of elevated CRP with each doubling of serum alkaline phosphatase without adjusting for GGT (but adjusted for all the covariates listed in Table 2) in the non-CKD sub-population was 2.78 (95% CI 2.14–3.59) and in the CKD sub-population was 2.64 (95% CI 1.47–4.74). With further adjustment for GGT, the odds ratios for each doubling of serum alkaline phosphatase with elevated CRP remained statistically significant (in the non-CKD sub-population 2.64 (95% CI 2.05–3.40) and CKD subpopulation 2.53 (95% CI 1.40–4.58).
DISCUSSION
Serum alkaline phosphatase levels are commonly elevated in CKD and dialysis patients. The osteoblast is a prominent source of alkaline phosphatase. As hyperparathyroidism and high-turnover bone disease are common in dialysis patients, an elevated serum alkaline phosphatase level is usually considered as a marker of bone disease. However, the potential role of alkaline phosphatase in the pathogenesis of diseases has been increasingly recognized.
In CKD3 and chronic hemodialysis patients,4–6 high serum alkaline phosphatase level was associated with increased mortality. A potential mechanism for this increased mortality is vascular calcification. Pyrophosphate in the arterial wall is a potent inhibitor of vascular calcification. By hydrolyzing pyrophosphate, alkaline phosphatase can promote vascular calcification.15 Experimental support of a pathogenic role of alkaline phosphatase in vascular calcification is as follows. In calcified diabetic arteries, the expression of alkaline phosphatase is upregulated.15 The hydrolysis rate of pyrophosphate was increased in the aorta of uremic rats,16 which was reduced by levamisole, a nonspecific inhibitor of alkaline phosphatase. Although these studies support a role of alkaline phosphatase in the arterial wall in the pathogenesis of vascular calcification, the role of circulating alkaline phosphatase is less clear. Nonetheless, higher levels of serum alkaline phosphatase have been associated with progressive arterial calcification in stage IV and V CKD patients.17
In addition to vascular calcification, there are other potential mechanisms that may mediate the associations of serum alkaline phosphatase with increased mortality. One of the potential explanations is that higher serum alkaline phosphatase might be associated with inflammation. In this study, we examined whether serum alkaline phosphatase level was associated with elevated serum CRP level.
As shown in Table 1, higher serum alkaline phosphatase levels were associated with greater baseline prevalence of cardiovascular disease and physical inactivity in both non-CKD and CKD populations. In the non-CKD population, higher serum alkaline phosphatase levels were also associated with higher waist circumference and diabetes. These data suggest that elevated serum alkaline phosphatase levels might reflect not only altered bone mineral metabolism but also an atherogenic milieu.
Atherosclerosis has been well established to be an inflammatory process. In an earlier study of 1740 middleaged adults, compared with the normal alkaline phosphatase group, serum alkaline phosphatase in the upper quartile was associated with higher levels of serum CRP (2.58 vs 1.66 mg/l, P < 0.001).12 In another study of Chinese adults, higher serum alkaline phosphatase levels were also associated with elevated CRP levels.11 In an earlier analysis of the Insulin Resistance Atherosclerosis Study cohort, compared with the lowest quartile of serum alkaline phosphatase, those in the highest quartile had a 2.3-fold higher odds of developing incident metabolic syndrome subsequently.18 To our knowledge, whether elevated serum alkaline phosphatase is associated with elevated CRP has not been examined in the CKD population. As shown in Figure 2, higher serum levels of alkaline phosphatase were indeed associated with greater prevalence of elevated serum CRP in the CKD and non-CKD populations. As shown in Figure 3, even after adjustment for other factors, in both CKD and non-CKD populations, there was about a 2.5-fold increase in the odds of elevated CRP with each doubling of serum alkaline phosphatase. There was no evidence of effect modification of this association by the presence or absence of CKD.
Vitamin D deficiency, as reflected by low serum level of 25-OH vitamin D, is very prevalent. Vitamin D deficiency is particularly prominent in CKD population. Lower levels of serum vitamin D could be associated with both inflammatory markers13 and elevated serum alkaline phosphatase14 levels. Therefore, the associations of alkaline phosphatase with inflammation could be confounded by serum 25-OH vitamin D levels. We tested this hypothesis by examining the associations of serum alkaline phosphatase with elevated CRP, without and with adjusting for serum 25-OH vitamin D levels. As shown in Tables 2 and 3 and Figure 3, adjustment for serum 25-OH vitamin D levels had little effect on the associations of serum alkaline phosphatase with elevated serum CRP in both the non-CKD and CKD groups, suggesting a 25-OH vitamin D independent association of serum alkaline phosphatase with elevated CRP.
Finally, the associations of elevated serum alkaline phosphatase with elevated serum CRP could reflect underlying liver disorder, such as non-alcoholic fatty liver disease.19 Although non-alcoholic fatty liver disease is the most common cause for mild-to-moderate elevation in serum AST and ALT, in about 70% of those with fatty liver, serum AST, and ALT levels are normal.20 To attempt to characterize this further, we conducted separate analyses in those NHANES III participants who had GGT measured. Adjusting for GGT had no significant effect on the odds of elevated CRP. However, the presence of occult liver disease cannot be definitively ruled out.
A major strength of this study is the careful data collection in NHANES III, including the uniform measurements of serum levels of alkaline phosphatase, serum 25-OH vitamin D, and CRP. The major limitations of this study include those that are usually present in all observational studies that use existing databases. The observational nature of the study limits inference beyond associations. Furthermore, as this study is cross-sectional, the ability to deduce causality is further limited. An additional limitation is that data on serum parathyroid hormone levels and bone-specific alkaline phosphatase are not available in NHANES III.
In summary, this study shows that elevated serum alkaline phosphatase levels in CKD and non-CKD are associated with elevated serum CRP levels and this association is independent of serum 25-OH vitamin D levels. Furthermore, elevated serum alkaline phosphatase levels were also associated with diabetes, hypertension, and cardiovascular disease. Thus, serum alkaline phosphatase in CKD could reflect an inflammatory and atherogenic milieu and not merely a biomarker of bone mineral metabolism. These might be potential mechanisms for the observed increased mortality associated with elevated serum alkaline phosphatase seen in CKD and hemodialysis patients.
MATERIALS AND METHODS
From 1988 to 1994, the National Center for Health Statistics conducted NHANES III, a cross-sectional survey of the US population. A complex, multistage sampling design was used to allow results to be extrapolated to the entire non-institutionalized civilian US population as of the early 1990s.21 Data on age, sex, race, current or past cigarette smoking, and history of comorbid conditions, such as myocardial infarction, stroke, and congestive heart failure, were collected in a structured home interview conducted by trained personnel. Diabetes was defined as the history of diabetes or the use of insulin or oral diabetes medications or fasting blood glucose ≥ 125 mg/dl. A detailed questionnaire on leisure time physical activity was also administered during the home interview. The home interview was followed by an examination by a physician at a mobile examination center, which included blood pressure measurement as well as extensive anthropometric, physiological, and laboratory testing.
Serum specimens from collection sites were transported on dry ice to the central laboratories and stored at −70 °C until analysis. Serum biochemistry panel including serum alkaline phosphatase was measured by a Hitachi 737 automated analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN, USA). Serum 25-OH vitamin D level was measured by a radioimmunoassay using the 25-OH D 125I radioimmunoassay kit (INCSTAR, Stillwater, MN, USA). Serum CRP was measured by latex-enhanced nephelometry using a Behring Nephelometer Analyzer System and reagents from Behring Diagnostics, Somerville, NJ, USA.
Serum creatinine was measured using a kinetic-rate Jaffe method in NHANES III. These serum creatinine measurements were recalibrated to the creatinine standard provided by the Cleveland Clinic Research Laboratory (Cleveland, OH, USA) using the equation (−0.184 + 0.960 × NHANES III measured serum creatinine in mg/dl).22 GFR was estimated as 175 × (standardized serum creatinine)−1.154 × (age)−0.203 × 0.742 (if the individual is woman) × 1.212 (if the individual is African American) ml/min per 1.73m2 (ref. 23). CKD was defined as GFR < 60 ml/min per 1.73m2.
A consensus statement of the Centers for Disease Control and the American Heart Association categorized serum CRP level > 3 mg/l as high risk.24 Therefore, serum CRP > 3 mg/l is defined as elevated CRP in this study.
Data analysis
Several aspects of the NHANES design must be taken into account in data analysis, including the sampling weights and the complex survey design. We used the svy suite of commands in Stata 10 (Stata 10, College station, TX, USA) and followed the analytical guidelines for NHANES data proposed by the Centers for Disease Control.21 It should be noted that the svy suite of commands in Stata use the complex survey design of NHANES to calculate the expected means and proportions of the entire US non-institutionalized civilian population, and hence means and proportions are presented with the estimated value and 95% CIs.
Association of serum alkaline phosphatase with serum vitamin D level
As serum alkaline phosphatase levels were positively skewed, these values were log transformed before statistical analyses. The occurrence of a low serum 25 OH vitamin D level (defined as < 15 ng/ml) was related to serum alkaline phosphatase in separate multivariable logistic regression analyses in the CKD and non-CKD sub-populations. These analyses included covariates to adjust for age, gender, race, history of myocardial infarction, stroke, congestive heart failure, diabetes and hypertension, waist circumference, physical inactivity, smoking, estimated GFR, hemoglobin, fasting serum glucose, serum calcium, phosphorus, AST, ALT and bilirubin.
Association of serum alkaline phosphatase with serum CRP level
The presence of inflammation, defined as serum CRP > 3 mg/l, was related to serum alkaline phosphatase by fitting separate multivariate logistic regression models in the non-CKD and CKD sub-populations. The models included the following factors as covariates: age, gender, race, history of myocardial infarction, stroke, congestive heart failure, diabetes and hypertension, waist circumference, physical inactivity, smoking, estimated GFR, hemoglobin, fasting serum glucose, serum calcium, phosphorus, AST, ALT, and bilirubin. Serum vitamin 25-OH vitamin D level was subsequently added to each model as an additional covariate to examine whether the association of serum alkaline phosphatase with elevated CRP is independent of serum 25 OH vitamin D.
Two approaches were adopted to examine whether presence or absence of CKD modified the association between CRP and serum alkaline phosphatase. In the first approach, the logistic regression coefficients relating elevated CRP to serum alkaline phosphatase were first estimated in separate models for the CKD and non-CKD populations. The coefficients for the two populations were compared by computing the ratio of difference between the coefficients and the square root of the sum of their squared standard errors, and then relating this quantity to the standard normal distribution. In the second approach, above logistic regression models were constructed for the entire cohort (including CKD and non-CKD participants) with the above covariates, and an interaction term of serum alklaline phosphatase with CKD was entered into the model to test whether this interaction term was significant.
To examine the association of serum alkaline phosphatase as a categorical variable with serum CRP, both CKD and non-CKD subpopulations were divided into four groups using the 25th, 50th, and 75th percentiles of serum alkaline phosphatase in the CKD sub-population. These groups were used in multivariable logistic regression models as described above with the lowest alkaline phosphatase as the reference.
Sensitivity analyses
Serum GGT was added to the above covariates in the multivariable logistic regression models for the subgroup of participants with serum GGT measurements.
Acknowledgments
This work is supported by a grant from the Dialysis Research Foundation of Utah. SB is the recipient of grants RO1-DK077298 and RO1-DK078112.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.Magnusson P, Sharp CA, Magnusson M, et al. Effect of chronic renal failure on bone turnover and bone alkaline phosphatase isoforms. Kidney Int. 2001;60:257–265. doi: 10.1046/j.1523-1755.2001.00794.x. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher S, Jones RG, Rayner HC, et al. Assessment of renal osteodystrophy in dialysis patients: use of bone alkaline phosphatase, bone mineral density and parathyroid ultrasound in comparison with bone histology. Nephron. 1997;75:412–419. doi: 10.1159/000189578. [DOI] [PubMed] [Google Scholar]
- 3.Beddhu S, Ma X, Baird B, et al. Serum alkaline phosphatase and mortality in African Americans with chronic kidney disease. Clin J Am Soc Nephrol. 2009 doi: 10.2215/CJN.01560309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blayney MJ, Pisoni RL, Bragg-Gresham JL, et al. High alkaline phosphatase levels in hemodialysis patients are associated with higher risk of hospitalization and death. Kidney Int. 2008;74:655–663. doi: 10.1038/ki.2008.248. [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 6.Regidor DL, Kovesdy CP, Mehrotra R, et al. Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. J Am Soc Nephrol. 2008;19:2193–2203. doi: 10.1681/ASN.2008010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalantar-Zadeh K, Brennan ML, Hazen SL. Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2006;48:59–68. doi: 10.1053/j.ajkd.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 8.Menon V, Wang X, Greene T, et al. Relationship between C-reactive protein, albumin, and cardiovascular disease in patients with chronic kidney disease. Am J Kidney Dis. 2003;42:44–52. doi: 10.1016/s0272-6386(03)00407-4. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 10.Yeun JY, Levine RA, Mantadilok V, et al. C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000;35:469–476. doi: 10.1016/s0272-6386(00)70200-9. [DOI] [PubMed] [Google Scholar]
- 11.Cheung BM, Ong KL, Cheung RV, et al. Association between plasma alkaline phosphatase and C-reactive protein in Hong Kong Chinese. Clin Chem Lab Med. 2008;46:523–527. doi: 10.1515/CCLM.2008.111. [DOI] [PubMed] [Google Scholar]
- 12.Kerner A, Avizohar O, Sella R, et al. Association between elevated liver enzymes and C-reactive protein: possible hepatic contribution to systemic inflammation in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2005;25:193–197. doi: 10.1161/01.ATV.0000148324.63685.6a. [DOI] [PubMed] [Google Scholar]
- 13.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 14.Jean G, Terrat JC, Vanel T, et al. Daily oral 25-hydroxycholecalciferol supplementation for vitamin D deficiency in haemodialysis patients: effects on mineral metabolism and bone markers. Nephrol Dial Transplant. 2008;23:3670–3676. doi: 10.1093/ndt/gfn339. [DOI] [PubMed] [Google Scholar]
- 15.Fadini GP, Pauletto P, Avogaro A, et al. The good and the bad in the link between insulin resistance and vascular calcification. Atherosclerosis. 2007;193:241–244. doi: 10.1016/j.atherosclerosis.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Lomashvili KA, Garg P, Narisawa S, et al. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int. 2008;73:1024–1030. doi: 10.1038/ki.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigrist MK, Taal MW, Bungay P, et al. Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:1241–1248. doi: 10.2215/CJN.02190507. [DOI] [PubMed] [Google Scholar]
- 18.Hanley AJ, Williams K, Festa A, et al. Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes. 2005;54:3140–3147. doi: 10.2337/diabetes.54.11.3140. [DOI] [PubMed] [Google Scholar]
- 19.Kotronen A, Yki-Jarvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27–38. doi: 10.1161/ATVBAHA.107.147538. [DOI] [PubMed] [Google Scholar]
- 20.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 21.National Center for Health Statistics. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Hyattsville: MD: 1995. [Google Scholar]
- 22.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 24.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]