Abstract
BACKGROUND
Maternal type 1 and 2 diabetes mellitus are strongly associated with high rates of severe structural birth defects, including congenital heart defects. Studies in type 1 diabetic embryopathy animal models have demonstrated that cellular stress-induced apoptosis mediates the teratogenicity of maternal diabetes leading to congenital heart defect formation. However, the mechanisms underlying maternal type 2 diabetes mellitus–induced congenital heart defects remain largely unknown.
OBJECTIVE
We aim to determine whether oxidative stress, endoplasmic reticulum stress, and excessive apoptosis are the intracellular molecular mechanisms underlying maternal type 2 diabetes mellitus–induced congenital heart defects.
STUDY DESIGN
A mouse model of maternal type 2 diabetes mellitus was established by feeding female mice a high-fat diet (60% fat). After 15 weeks on the high-fat diet, the mice showed characteristics of maternal type 2 diabetes mellitus. Control dams were either fed a normal diet (10% fat) or the high-fat diet during pregnancy only. Female mice from the high-fat diet group and the 2 control groups were mated with male mice that were fed a normal diet. At E12.5, embryonic hearts were harvested to determine the levels of lipid peroxides and superoxide, endoplasmic reticulum stress markers, cleaved caspase 3 and 8, and apoptosis. E17.5 embryonic hearts were harvested for the detection of congenital heart defect formation using India ink vessel patterning and histological examination.
RESULTS
Maternal type 2 diabetes mellitus significantly induced ventricular septal defects and persistent truncus arteriosus in the developing heart, along with increasing oxidative stress markers, including superoxide and lipid peroxidation; endoplasmic reticulum stress markers, including protein levels of phosphorylated-protein kinase RNA-like endoplasmic reticulum kinase, phosphorylated-IRE1α, phosphorylated-eIF2α, C/EBP homologous protein, and binding immunoglobulin protein; endoplasmic reticulum chaperone gene expression; and XBP1 messenger RNA splicing, as well as increased cleaved caspase 3 and 8 in embryonic hearts. Furthermore, maternal type 2 diabetes mellitus triggered excessive apoptosis in ventricular myocardium, endocardial cushion, and outflow tract of the embryonic heart.
CONCLUSION
Similar to those observations in type 1 diabetic embryopathy, maternal type 2 diabetes mellitus causes heart defects in the developing embryo manifested with oxidative stress, endoplasmic reticulum stress, and excessive apoptosis in heart cells.
Keywords: apoptosis, endoplasmic reticulum stress, heart defects, oxidative stress, type 2 diabetes mellitus
Introduction
Both maternal type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) increase the risk that offspring will have cardiovascular malformations 5-fold compared with the general population, even with modern preconception care.1–4 The most common maternal diabetes-associated congenital heart defects (CHDs) include cardiac septation defects such as ventricular septal defects (VSDs) and conotruncal cardiac anomalies such as persistent truncus arteriosus (PTA).4–6 However, the mechanisms underlying these anomalies remain largely unknown. Because the number of women of reproductive age with T2DM is increasing rapidly, understanding the molecular pathways involved in maternal T2DM-induced CHDs is essential for developing new strategies to prevent these types of birth defects.
Oxidative stress is triggered by an imbalance in intracellular reduction-oxidation (redox) homeostasis, and is sustained by the generation of reactive oxygen species.7 Our previous studies have revealed that both T1DM and T2DM significantly induce oxidative stress by increasing the levels of superoxide and lipid peroxidation in the developing neuroepithelium (T1DM and T2DM) and the embryonic heart (T1DM).8–26 Overexpression of the antioxidant enzyme, superoxide dismutase 1 (SOD1), diminishes maternal T1DM-induced oxidative stress in developing neuroepithelium and the embryonic heart, and thus ameliorates neural tube defects (NTDs) and CHDs in offspring of diabetic dams.20–22,24,26
Animal studies have shown that both maternal T1DM and T2DM trigger endoplasmic reticulum (ER) stress in neurulation-stage embryos (T1DM and T2DM) and in embryonic hearts (T1DM).13,18,20,24,26 ER stress occurs when misfolded proteins accumulate in the ER lumen and cause ER dysfunction. 27 Both maternal T1DMand T2DM disrupt ER luminal homeostasis by enhancing transcription of the proapoptotic C/EBP homologous protein (CHOP), and increasing other ER stress markers, such as binding immunoglobulin protein (BiP) and calnexin, in the developing neuroepithelium.28,29 In addition, we have shown in vitro that the ER stress inhibitor, 4-phenylbutyric acid, inhibits NTD formation in cultured embryos exposed to high glucose.30
Oxidative stress– and ER stress-induced cell apoptosis are causative events in maternal T1DM- and T2DM-induced NTDs.8,13,18,30–33 Apoptosis is a precisely controlled cellular event that is essential to many biological processes. 34 However, our previous studies have demonstrated that maternal T1DM and T2DM induces excessive apoptosis, leading to defective neurulation and failed neural tube closure.13,18,32 Furthermore, we have found that deletion of the gene for apoptosis signal-regulating kinase 1 significantly ameliorates diabetes-induced NTDs.24 Therefore, we hypothesize that oxidative stress, ER stress, and subsequent excessive apoptosis are contributing factors for maternal T2DM-induced CHDs.
Here, we use a high-fat diet (HFD)- induced T2DM mouse model to explore whether oxidative stress and ER stress-induced excessive apoptosis are present in T2DM-induced CHDs. A previous study demonstrated that this murine T2DM model exhibits modest hyperglycemia, glucose intolerance, insulin resistance, and hyperinsulinemia,18 all of which are characteristics of human T2DM. We showed that the levels of oxidative stress, ER stress, and apoptosis markers were increased in hearts of embryos from T2DM dams. We also found that maternal T2DM specifically induced VSDs and PTA. By investigating the role of abnormal cellular processes in embryonic heart development, we elucidated possible mechanisms in T2DM-induced CHDs.
Materials and Methods
Animal model of embryopathy
The procedures for animal use were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee. The mouse model of T2DM was established as previously described.18 Four-week-old female C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were maintained in a temperature-controlled room on a 12-hour light-dark cycle. After arrival, mice were divided into 2 groups and fed either a HFD (Research Diets Inc, New Brunswick, NJ) or a normal diet (Harlan Laboratories, Indianapolis, IN) for 15 weeks. The HFD contained 20% protein, 20% carbohydrate, and 60% fat. The normal diet contained 20% protein, 70% carbohydrate, and 10% fat. HFD mice and mice fed the normal diet were mated with lean male mice. During pregnancy, mice in the normal diet group were either maintained on the normal diet (control group 1) or subsequently fed HFD to serve as the high circulating free fatty acid control group (control group 2). Male and female mice were paired at 3:00 PM, and day 0.5 (E0.5) of pregnancy was established at noon of the day when a vaginal plug was present. Only CHDs were examined in these animals. NTDs were not the subject of the present study.
India ink injection and hematoxylin-eosin staining
After euthanizing the pregnant dams at E17.5, fetuses were excised from uteri then the rib cage was removed and the hearts were exposed, morphologically examined, and imaged under a dissecting microscope (Leica, Wetzlar, Germany) while remained attaching to the spine. India ink was injected into the left ventricle of the heart using the μTIP (TIP10TW1-L, World Precision Instrument Inc, Sarasota, FL) tip, and images were taken for the examination of outflow tract defects. Subsequently, embryonic hearts were harvested and fixed in methacarn (methanol, 60%; chloroform, 30%; glacial acetic acid, 10%), embedded in paraffin, and cut into 8-μm sections. After deparaffinization and rehydration, all specimens then underwent hematoxylin-eosin staining in a standard procedure. All heart sections were photographed and examined for heart defects.
Dihydroethidium staining
Dihydroethidium (DHE) staining (Thermo Scientific, Rockford, IL) was used to detect the level of superoxide. DHE reacts with superoxide that is bound to cellular components including protein and DNA, and exhibits bright red fluorescence. E12.5 embryonic hearts were fixed in 4% paraformaldehyde for 30 minutes, washed 3 times with phosphate-buffered saline (PBS) (5 min/wash), and then embedded in optimum cutting temperature compound (Tissue-Tek; Sakura Finetek USA Inc, Torrance, CA). We incubated 10-μm frozen embryonic sections with 1.5 μmol/L DHE for 5 minutes at room temperature, then washed them 3 times with PBS for 5 min/wash. Sections were counterstained with 4′,6-diamidino-2-phenylindole (Sigma, St Louis, MO) and mounted with aqueous mounting medium (Sigma).
Lipid hydroperoxide quantification
As previously described,35 the degree of lipid peroxidation in E12.5 hearts was quantitatively assessed using the Calbiochem lipid hydroperoxide assay kit (Millipore, Bedford, MA) by following the manufacturer’s instructions. Lipid hydroperoxides of embryonic hearts were extracted by deoxygenated chloroform, and then measured by the absorbance of 500 nm after reaction with chromogen. The results were expressed as μmol/L lipid hydroperoxides/μg protein. Protein concentrations were determined by the BioRad detergent compatible protein assay kit (Hercules, CA).
Immunoblotting
Immunoblotting was performed as described by Yang et al.36,37 To extract protein, the lysis buffer (Cell Signaling Technology, Danvers, MA) containing a protease inhibitor cocktail (Sigma) was used. Equal amounts of protein and the Precision Plus Protein Standards (BioRad) were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto Immunobilon-Pmembranes (Millipore). Membranes were incubated in 5%nonfat milk for 45 minutes, and then were incubated for 18 hours at 4°C with the following primary antibodies at dilutions of 1:1000 in 5% nonfat milk: phosphorylated protein kinase RNA-like endoplasmic reticulum kinase; protein kinase RNA-like endoplasmic reticulum kinase; p-eIF2α; eIF2α; CHOP; BiP; IRE1α (cell signaling); p-IRE1α (Abcam, Cambridge, United Kingdom); caspase 8 (mouse specific) (Enzo Life Sciences, Farmingdale, NY); and caspase 3 (Millipore). Membranes were exposed to horseradish peroxidase–conjugated goat antirabbit, goat antimouse, or goat antirat (Millipore) secondary antibodies. Signals were detected using SuperSignal West Femto maximum sensitivity substrate kit (Thermo Scientific), and chemiluminescence emitted from the bands was directly captured using a Bioimage EC3 system (UVP Inc, Upland, CA). Densitometric analysis of chemiluminescence signals was performed using software (VisionWorks LS; UVP Inc). To ensure that equivalent amounts of protein were loaded among samples, membranes were stripped and incubated with a β-actin antibody (Abcam). All experiments were repeated in triplicate with the use of independently prepared tissue lysates.
Detection of XBP1 messenger RNA splicing
Messenger RNA (mRNA) was extracted from E12.5 embryonic hearts and reverse-transcribed using the QuantiTect reverse transcription kit (Qiagen, Venlo, The Netherlands). The polymerase chain reaction (PCR) primers for X-box binding protein 1 were as follows: forward, 5′-GAACCAGGAGTTAAGAACACG-3′ and reverse, 5′-AGGCAACAG TGTCAGAGTCC-3′. If no XBP1 mRNA splicing occurred, a 205-base pair (bp) band was produced. When XBP1 splicing occurred, a 205-bp band and a 179-bp main band were produced.
Real-time quantitative PCR
Using the Rneasy mini kit (Qiagen), mRNA was isolated from E12.5 embryonic hearts, and then reverse-transcribed using the high-capacity complimentary DNA archive kit (Applied Biosystems, Grand Island, NY). Real-time PCR for BiP, CHOP, calnexin, IRE1α, protein disulphide isomerase A, 94 kDa glucose-regulated protein, and β-actin were performed using the Maxima SYBR green/ROX quantitative PCR Master Mix assay (Thermo Scientific) in the StepOnePlus system (Applied Biosystems). Primer sequences for real-time PCR are listed in Table 1.
TABLE 1.
Sequences of primers used in real-time quantitative polymerase chain reaction
| Primers name | Primer sequences |
|---|---|
| BiP | Forward primer 5′-ACTTGGGGACCACCTATTCCT-3′ |
| Reverse primer 5′-ATCGCCAATCAGACGCTCC-3′ | |
| CHOP | Forward primer 5′-CGGAACCTGAGGAGAGAGTG-3′ |
| Reverse primer 5′-CTGTCAGCCAAGCTAGGGAC-3′ | |
| Calnexin | Forward primer 5′-ATGGAAGGGAAGTGGTTACTGT-3′ |
| Reverse primer 5′-GCTTTGTAGGTGACCTTTGGAG-3′ | |
| IRE1α | Forward primer 5′-ACACCGACCACCGTATCTCA-3′ |
| Reverse primer 5′-CTCAGGATAATGGTAGCCATGTC-3′ | |
| PDIA | Forward primer 5′-CGCCTCCGATGTGTTGGA-3′ |
| Reverse primer 5′-CAGTGCAATCCACCTTTGCTAA-3′ | |
| GRP94 | Forward primer 5′-TCGTCAGAGCTGATGATGAAGT-3′ |
| Reverse primer 5′-GCGTTTAACCCATCCAACTGAAT-3′ | |
| β-Actin | Forward primer 5′-GAACCAGGAGTTAAGAACACG-3′ |
| Reverse primer 5′-AGGCAACAGTGTCAGAGTCC-3′ |
BiP, binding immunoglobulin protein; CHOP, C/EBP homologous protein.
Wu et al. Type 2 diabetes mellitus induces congenital heart defects. Am J Obstet Gynecol 2016.
TUNEL assay
The TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) assay was performed using the ApopTag fluorescein in situ apoptosis detection kit (Millipore) as previously described.30–32 Briefly, 10-μm frozen heart sections were fixed with 4% paraformaldehyde in PBS and incubated with TUNEL reaction agents. Three embryonic hearts from 3 different dams (n = 3) per group were used, and 2 sections per heart were examined.
Statistical analyses
Data were presented as means ± SE. Statistical differences were determined by 1-way analysis of variance using software (SigmaStat 3.5, Systat Software Inc, San Jose, CA). In 1-way analysis of variance analysis, Tukey test was used to estimate the significance of the results (P <.05). The χ2 test was used to estimate the significance of difference in VSDs and PTA rates among experimental and control groups.
Results
Maternal T2DM induces heart defects in the offspring
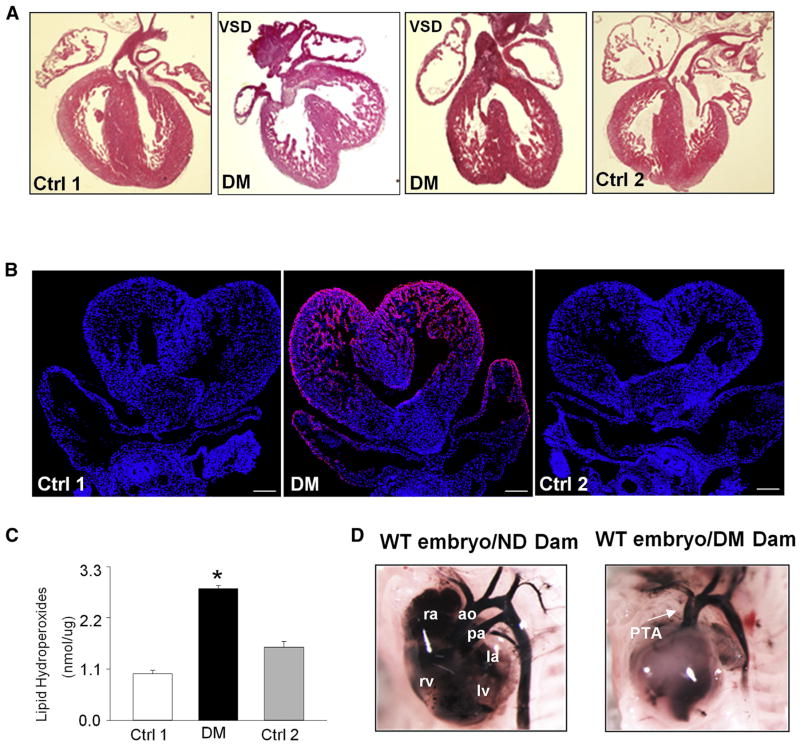
Previous studies using a model of T1DM demonstrated that maternal hyperglycemia significantly induces heart defects in the developing embryo.24,26 Here, we investigated whether T2DM could induce embryonic heart defects. After feeding on a HFD (60% fat) for 15 weeks, mice in the experimental group had higher blood glucose levels than mice in the 2 control groups that were fed a normal diet (10% fat) or a HFD during pregnancy only (Table 2). Using India ink injections, 3.7% embryos (2 of 46) from the HFD group exhibited PTA, and there was no PTA in embryos of the 2 nondiabetic control groups (Figure 1, D, and Table 2). Hematoxylin-eosin staining of E17.5 embryonic heart sections showed that 6 of 46 (13%) embryos in the HFD group had VSDs, whereas embryos from the 2 nondiabetic control groups had no VSDs (Table 2). The VSDs were located at the cardiac outlet and associated with an overriding aorta (Figure 1, A). These data suggest that maternal T2DM significantly induced heart defects.
TABLE 2.
Maternal type 2 diabetes mellitus induces heart defects in developing embryos
| Group | Dams, n | Glucose level, mmol/L | Date | Hearts, n | VSD rate (%) | PTA rate (%) |
|---|---|---|---|---|---|---|
| Ctrl 1 | 5 | 5.8 ± 0.46 | E17.5 | 47 | 0 (0.0) | 0 (0.0) |
| DM | 7 | 9.3 ± 0.31a | E17.5 | 46 | 6 (13.0)a | 2 (3.7)a |
| Ctrl 2 | 5 | 6.9 ± 0.35 | E17.5 | 44 | 0 (0.0) | 0 (0.0) |
Ctrl 1, control group fed normal diet; Ctrl 2, control group fed 60% high-fat diet during pregnancy; DM, group fed high-fat diet; PTA, persistent truncus arteriosus; VSD, ventricular septal defect.
Significant differences compared with other 2 groups as analyzed by 1-way analysis of variance followed by Tukey (blood glucose levels) or χ2 (VSD and PTA rates) test.
Wu et al. Type 2 diabetes mellitus induces congenital heart defects. Am J Obstet Gynecol 2016.
FIGURE 1. Maternal type 2 diabetes mellitus induces heart defects and oxidative stress in developing heart.
A, Morphological structures of E17.5 embryonic hearts. B, Representative images of dihydroethidium staining of E12.5 embryonic hearts. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (blue). Scale bar = 30 μm. C, Lipid peroxidation levels in E12.5 embryonic hearts. Experiments were performed using 3 embryonic hearts from 3 different dams per group. D, E17.5 heart and blood vessels imaged in whole mount after injection of India ink.
*Significant differences (P < .05) compared with other groups.
ao, aorta; Ctrl 1, control group fed normal diet; Ctrl 2, control group fed 60% high-fat diet during pregnancy; DM, group fed high-fat diet; la, left atrium; lv, left ventricle; ND, nondiabetic; pa, pulmonary artery; PTA, persistent truncus arterious; ra, right atrium; rv, right ventricle; VSD, ventricular septum defect; WT, wild type.
Wu et al. Type 2 diabetes mellitus induces congenital heart defects. Am J Obstet Gynecol 2016.
Maternal T2DM leads to oxidative stress in the embryonic heart
To test whether oxidative stress participates in maternal T2DM-induced heart defects, we determined the levels of superoxide and lipid peroxidation in E12.5 hearts from diabetic and nondiabetic dams. The level of superoxide was assessed by DHE staining. There were robust DHE-positive signals in the embryonic hearts from diabetic dams, whereas almost no DHE signals were observed in control groups (Figure 1, B). Furthermore, maternal T2DM significantly increased the levels of lipid peroxidation in the developing heart (Figure 1, C). These findings suggest that maternal T2DM induces oxidative stress in developing heart.
Maternal T2DM triggers ER stress in the developing heart
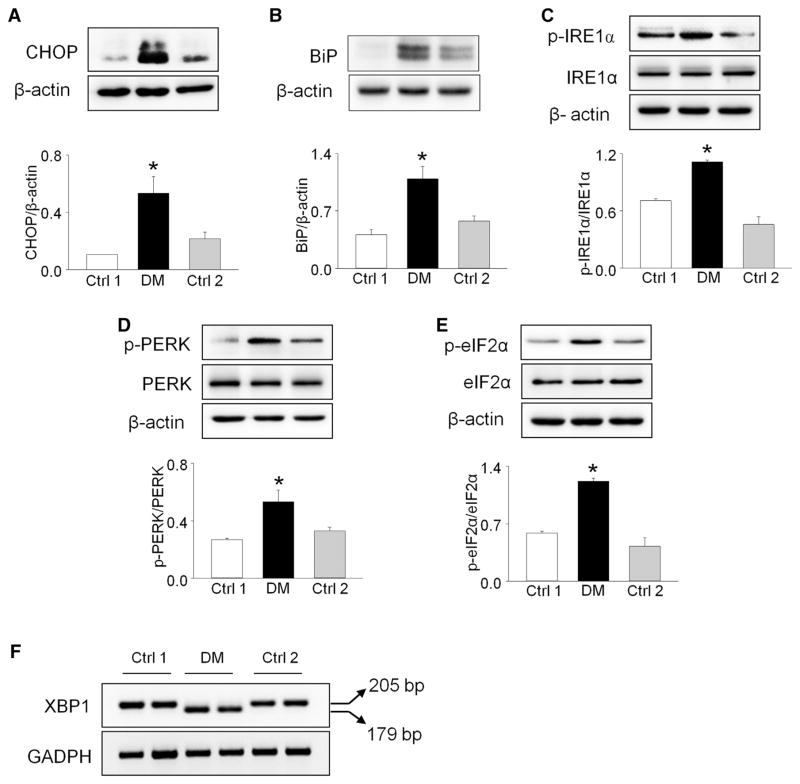
ER stress is a primary molecular mechanism underlying maternal T1DM and T2DM-induced NTDs.18,20,26,30 We examined the levels of ER stress makers to evaluate whether ER stress is also associated with maternal T2DMinduced heart defects. Protein levels of CHOP, BiP, phosphorylated protein kinase RNA-like endoplasmic reticulum kinase, p-eIF2α, and p-IRE1α in the embryonic hearts from diabetic dams were significantly increased, compared with those in embryonic hearts from the 2 nondiabetic control groups (Figure 2, A to E).
FIGURE 2. Maternal type 2 diabetes mellitus triggers endoplasmic reticulum stress in developing heart.
A to E, Protein levels of phosphorylated protein kinase RNA-like endoplasmic reticulum kinase, protein kinase RNA-like endoplasmic reticulum kinase, p-IRE1α, IRE1α, p-eIF2α, eIF2α, C/EBP homologous protein (CHOP), and binding immunoglobulin protein (BiP) were determined in E12.5 embryonic hearts. F, XBP1 messenger RNA splicing in E12.5 embryonic hearts. Arrows point to actual size of bands. Experiments were performed using 3 embryonic hearts from 3 different dams per group. *Significant differences (P < .05) compared with other groups.
Ctrl 1, control group fed normal diet; Ctrl 2, control group fed 60% high-fat diet during pregnancy; DM, group fed high-fat diet.
Wu et al. Type 2 diabetes mellitus induces congenital heart defects. Am J Obstet Gynecol 2016.
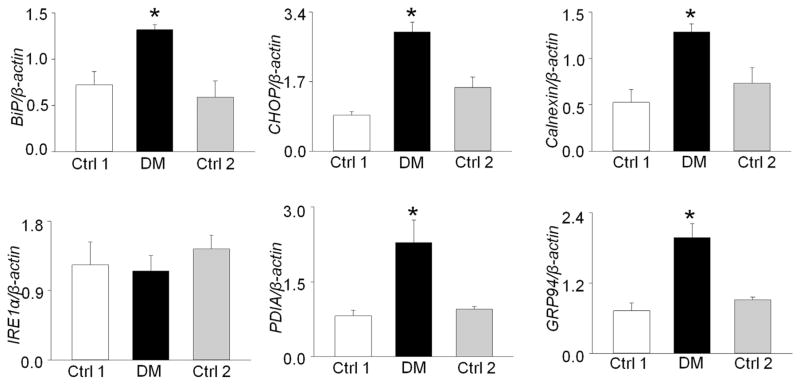
Additionally, we used reverse-transcription PCR to detect the level of XBP1 mRNA splicing, another indicator of ER stress, in embryonic hearts from diabetic vs nondiabetic control dams. Robust XBP1 mRNA splicing was observed in the embryonic hearts exposed to T2DM, with the PCR products showing 2 bands at 205 bp and 179 bp (Figure 2, F). In contrast, there was no spliced XBP1 mRNA in hearts from the 2 nondiabetic control groups (Figure 2, F). Furthermore, maternal T2DM significantly up-regulated the mRNA levels of the ER chaperone genes for BiP, CHOP, calnexin, PDIA, and GRP94 in the embryonic hearts, except IRE1α (Figure 3). These data indicate that ER stress is involved in maternal T2DM-induced heart defects.
FIGURE 3. Maternal type 2 diabetes mellitus up-regulates messenger RNA (mRNA) levels of endoplasmic reticulum chaperone genes in developing heart.
mRNA levels of binding immunoglobulin protein (BiP), C/EBP homologous protein (CHOP), calnexin, IRE1α, PDIA, and GRP94 were determined in E12.5 embryonic hearts. Experiments were performed using 3 embryonic hearts from 3 different dams per group. *Significant differences (P < .05) compared with other groups.
Ctrl 1, control group fed normal diet; Ctrl 2, control group fed 60% high-fat diet during pregnancy; DM, group fed high-fat diet.
Wu et al. Type 2 diabetes mellitus induces congenital heart defects. Am J Obstet Gynecol 2016.
Maternal T2DM activates caspase and induces apoptosis in the developing heart
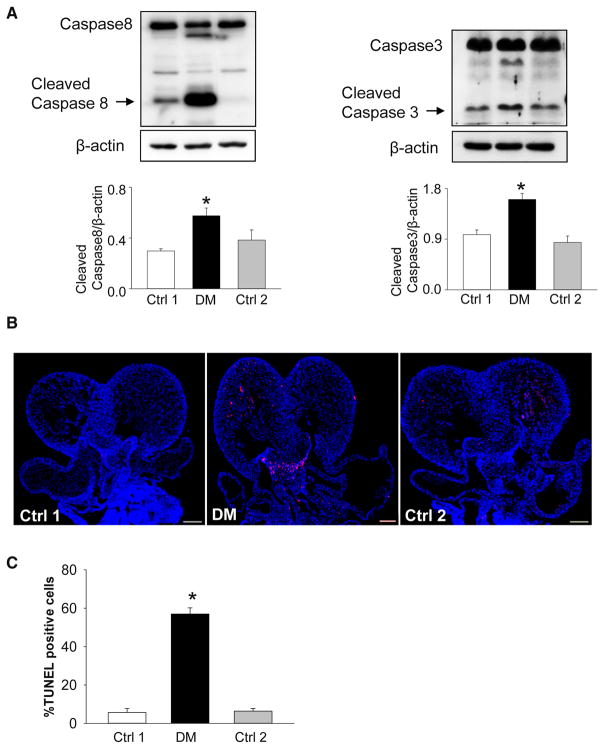
We have previously shown that maternal T1DM significantly increases aberrant apoptosis in the developing neuroepithelium by cleaving caspase 8 (an initiator of apoptosis) and caspase 3 (an executor of apoptosis).31,32 To evaluate whether maternal T2DM increases apoptosis in the embryonic heart, we assessed protein levels of cleaved caspase 8 and 3. There was an abundance of cleaved caspase 3 and 8 in developing hearts from the diabetic group, compared with hearts from the 2 nondiabetic control groups (Figure 4, A). In addition, we observed significantly higher numbers of apoptotic cells in the endocardial cushion, the ventricular myocardium (Figure 4, B), and the outflow tract (Figure 4, C) of embryonic hearts compared with those in embryonic hearts from nondiabetic control dams.
FIGURE 4. Maternal type 2 diabetes mellitus activates caspase cleavages and induces excessive apoptosis in developing heart.
A, Protein levels of caspase 8 and 3 in E12.5 embryonic hearts. B, Representative images of TUNEL assay showing apoptotic cells (red signal). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (blue). Scale bar = 30 μm. Experiments were performed using 3 embryonic hearts from 3 different dams per group. C, Quantification of TUNEL-positive cells per section. *Significant differences (P < .05) compared with other groups. Ctrl 1, control group fed normal diet; Ctrl 2, control group fed 60% high-fat diet during pregnancy; DM, group fed high-fat diet.
Comment
Previous research to delineate mechanisms underlying maternal diabetes-induced heart defects has primarily been performed using mouse models of T1DM.24,26 In the present study, we investigated the possible mechanisms underlying maternal T2DM-induced heart defects. In models of T1DM, the types of CHDs observed are VSDs and PTA.24,26 We observed the same types of heart defects in our model of T2DM. However, the heart defect incidence (16.7%) in the T2DM model is much lower than that (approximately 26%) of the T1DM model.24,26 The discrepancy may be due to different levels of blood glucose found in T1DMvs T2DM. Blood glucose levels in animals with T2DMare typically 9.3 ± 0.31 mmol/L, which is significantly lower than that in animals with T1DM, which averages 24.3 ± 1.12 mmol/L.24,26 In our previous study,18 the T2DM model as well as control group 2 (lean mice fed HFD during pregnancy) had significantly higher fatty acid levels than those in the nondiabetic control group. Our previous results showed that high fatty acid did not contribute to the teratogenicity of T2DM.18 In the present study, to eliminate the effect of high fatty acid on heart development, group 2 was used as a control group, which did not affect heart development. Our T2DM resembles some key features of human T2DM.18 First, it recapitulates insulin resistance and glucose intolerance associated with human T2DM,18 and these factors contribute to the degree of hyperglycemia in T2DM. Second, obesity is associated with 60–90% of human T2DM patients.38
Our previous work and those of others have demonstrated that oxidative stress plays a causal role in T1DM- and T2DM-induced NTDs.8,13,14,20–22,31 We have shown that overexpression of the antioxidant enzyme, SOD1, ameliorates T1DM- and T2DM-induced NTDs, and T1DM-induced heart defects, by reducing oxidative stress.20–22,24 In our current study, we observed the presence of elevated levels of superoxide and lipid peroxidation in embryonic hearts exposed to maternal T2DM. These results suggest that oxidative stress also plays a vital role in T2DM-induced heart defects. However, further studies are needed to assess the involvement of oxidative stress in T2DM-induced heart defects, and further elucidate the downstream molecular intermediates of oxidative stress.
ER stress is a primary intracellular regulatory mechanism of maternal T1DM-induced NTDs.13,20,30,31 Our previous work has demonstrated that SOD1 overexpression blocks maternal T1DM-triggered ER stress and consequently reduces NTD formation.20,30,31 In addition, a naturally occurring antioxidant, curcumin, blunts high glucose–induced ER stress, which leads to amelioration of NTD formation.13 Mechanistic studies performed in T2DM models have revealed that ER stress also is a causal event for NTD formation.18 The present study shows that maternal T2DM induces ER stress in the developing heart, suggesting that ER stress may play an important role in the induction of heart defects in maternal T2DM. Our recent studies demonstrated in the T1DM that impaired Wnt signaling and activation of the proapoptotic kinase apoptosis signal-regulating kinase 1 are involved in the induction of heart defects.24,39 Future studies will reveal whether alterations of these signaling pathways contribute to the etiology of T2DM-induced heart defects.
The balance between cell proliferation and apoptosis is essential for normal embryonic development. The developing neuroepithelium and heart are especially vulnerable to abnormal apoptosis.40–43 Multiple studies have demonstrated that maternal T1DM increases apoptosis in developing embryo.11,13,20,26,31,32 We have shown that abnormal apoptosis occurs in neuroepithelial cells of the developing neuroepithelium leading to NTD formation under T1DM conditions.32 Studies in models of T1DM have revealed that enhanced apoptosis and reduced cell proliferation are involved in heart defect formation.24,44–47 Consistent with findings from the T1DM model, maternal T2DM induces excessive apoptosis in the endocardial cushion, the ventricular myocardium, and the outflow tract of the developing heart. Thus, excessive apoptosis is likely the cause of heart defects under maternal T2DM conditions. Future studies will assess whether abnormal cell proliferation level is also a potential regulatory mechanism of maternal T2DM-induced heart defects.
Because T2DMis on rise in women of reproductive age,48 the clinical significance of our findings is high. Hyperglycemia caused by T2DM is associated with a variety of adverse pregnancy outcomes, including structural birth defects, preterm birth, small-for-gestational- age fetuses, fetal hypertrophic cardiomyopathy, preeclampsia, stillbirth, and perinatal deaths.48–54 Modern preconception care, which aims to control hyperglycemia, has failed to prevent the higher incidence of adverse pregnancy outcomes in diabetic pregnancies, compared with the general population.55,56 Therefore, therapeutics that can prevent diabetes-induced birth defects are needed. Antioxidants are effective in preventing adverse pregnancy outcomes associated with maternal diabetes in animal models8–10,12–15,17,20–22,36,37; however, their effects on human pregnancies are controversial.57,58 Our previous studies revealed that a group of natural compounds with antioxidant and signaling modulating properties reduces birth defects in animal diabetic pregnancies. 9,13,14,20–22,24 Dietary supplementation of these natural compounds may be effective in treating diabetes-associated pregnancy outcomes in human beings but this remains to be determined.59 New preventive measures by targeting cellular organelle stress may be also effective to ameliorate birth defects caused by factors other than diabetes.60–66
In summary, our present work demonstrates that maternal T2DM has adverse effects on the embryonic heart and leads to heart defects, including VSDs and PTA. Maternal T2DM triggers oxidative stress and persistent ER stress, leading to excessive apoptosis in the endocardial cushion, the ventricular myocardium, and the outflow tract of the developing heart. Because oxidative stress, ER stress, and apoptosis are causal events in T1DM embryopathy, our results suggest that T1DM and T2DM may share similar molecular mechanisms to cause birth defects. Based on our work to identify possible therapeutic inhibitors of the signaling pathways involved inT1DM embryopathy,8–10,12–15,17,20–22,33,36,37 it is possible that the same interventions could successfully reduce birth defects induced by T2DM.
Acknowledgments
This research is supported by NIH R01DK083243; R01DK101972; R01DK103024; and Basic Science Award, American Diabetes Association (1-13-BS-220) (all to Dr Yang).
The authors are grateful to Dr Julie Wu at the University of Maryland School of Medicine for critical reading and editing.
Footnotes
The authors report no conflict of interest.
References
- 1.Correa A, Gilboa SM, Besser LM, et al. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199:237.e1–9. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J, Cummings EA, O’Connell C, Jangaard K. Fetal and neonatal outcomes of diabetic pregnancies. Obstet Gynecol. 2006;108:644–50. doi: 10.1097/01.AOG.0000231688.08263.47. [DOI] [PubMed] [Google Scholar]
- 3.Loffredo CA, Wilson PD, Ferencz C. Maternal diabetes: an independent risk factor for major cardiovascular malformations with increased mortality of affected infants. Teratology. 2001;64:98–106. doi: 10.1002/tera.1051. [DOI] [PubMed] [Google Scholar]
- 4.Wren C, Birrell G, Hawthorne G. Cardiovascular malformations in infants of diabetic mothers. Heart. 2003;89:1217–20. doi: 10.1136/heart.89.10.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enciso JM, Gratzinger D, Camenisch TD, Canosa S, Pinter E, Madri JA. Elevated glucose inhibits VEGF-A-mediated endocardial cushion formation: modulation by PECAM-1 and MMP-2. J Cell Biol. 2003;160:605–15. doi: 10.1083/jcb.200209014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu-Sulaiman RM, Subaih B. Congenital heart disease in infants of diabetic mothers: echocardiographic study. Pediatr Cardiol. 2004;25:137–40. doi: 10.1007/s00246-003-0538-8. [DOI] [PubMed] [Google Scholar]
- 7.Ueda S, Masutani H, Nakamura H, Tanaka T, Ueno M, Yodoi J. Redox control of cell death. Antioxid Redox Signal. 2002;4:405–14. doi: 10.1089/15230860260196209. [DOI] [PubMed] [Google Scholar]
- 8.Yang P, Zhao Z, Reece EA. Activation of oxidative stress signaling that is implicated in apoptosis with a mouse model of diabetic embryopathy. Am J Obstet Gynecol. 2008;198:130.e1–7. doi: 10.1016/j.ajog.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 9.Yang P, Li H. Epigallocatechin-3-gallate ameliorates hyperglycemia-induced embryonic vasculopathy and malformation by inhibition of Foxo3a activation. Am J Obstet Gynecol. 2010;203:75.e1–6. doi: 10.1016/j.ajog.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang P, Cao Y, Li H. Hyperglycemia induces inducible nitric oxide synthase gene expression and consequent nitrosative stress via c-Jun N-terminal kinase activation. Am J Obstet Gynecol. 2010;203:185.e5–11. doi: 10.1016/j.ajog.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu H, Yu J, Dong D, Zhou Q, Wang JY, Yang P. The miR-322-TRAF3 circuit mediates the pro-apoptotic effect of high glucose on neural stem cells. Toxicol Sci. 2015;144:186–96. doi: 10.1093/toxsci/kfu271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang P, Reece EA, Wang F, Gabbay-Benziv R. Decoding the oxidative stress hypothesis in diabetic embryopathy through proapoptotic kinase signaling. Am J Obstet Gynecol. 2015;212:569–79. doi: 10.1016/j.ajog.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Wang F, Reece EA, Yang P. Curcumin ameliorates high glucose-induced neural tube defects by suppressing cellular stress and apoptosis. Am J Obstet Gynecol. 2015;212:802.e1–8. doi: 10.1016/j.ajog.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F, Reece EA, Yang P. Oxidative stress is responsible for maternal diabetes-impaired transforming growth factor beta signaling in the developing mouse heart. Am J Obstet Gynecol. 2015;212:650.e1–11. doi: 10.1016/j.ajog.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F, Reece EA, Yang P. Advances in revealing the molecular targets downstream of oxidative stress-induced proapoptotic kinase signaling in diabetic embryopathy. Am J Obstet Gynecol. 2015;213:125–34. doi: 10.1016/j.ajog.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabbay-Benziv R, Reece EA, Wang F, Yang P. Birth defects in pregestational diabetes: defect range, glycemic threshold and pathogenesis. World J Diabetes. 2015;6:481–8. doi: 10.4239/wjd.v6.i3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong D, Reece EA, Lin X, Wu Y, Arias Villela N, Yang P. New development of the yolk sac theory in diabetic embryopathy: molecular mechanism and link to structural birth defects. Am J Obstet Gynecol. 2016;214:192–202. doi: 10.1016/j.ajog.2015.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y, Wang F, Fu M, Wang C, Quon MJ, Yang P. Cellular stress, excessive apoptosis, and the effect of metformin in a mouse model of type 2 diabetic embryopathy. Diabetes. 2015;64:2526–36. doi: 10.2337/db14-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong D, Yu J, Wu Y, Fu N, Villela NA, Yang P. Maternal diabetes triggers DNA damage and DNA damage response in neurulation stage embryos through oxidative stress. Biochem Biophys Res Commun. 2015;467:407–12. doi: 10.1016/j.bbrc.2015.09.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F, Reece EA, Yang P. Superoxide dismutase 1 overexpression in mice abolishes maternal diabetes-induced endoplasmic reticulum stress in diabetic embryopathy. Am J Obstet Gynecol. 2013;209:345.e1–7. doi: 10.1016/j.ajog.2013.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weng H, Li X, Reece EA, Yang P. SOD1 suppresses maternal hyperglycemia-increased iNOS expression and consequent nitrosative stress in diabetic embryopathy. Am J Obstet Gynecol. 2012;206:448.e1–7. doi: 10.1016/j.ajog.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Weng H, Reece EA, Yang P. SOD1 overexpression in vivo blocks hyperglycemia-induced specific PKC isoforms: substrate activation and consequent lipid peroxidation in diabetic embryopathy. Am J Obstet Gynecol. 2011;205:84.e1–6. doi: 10.1016/j.ajog.2011.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu H, Yu J, Dong D, et al. High glucose-repressed CITED2 expression through miR-200b triggers the unfolded protein response and endoplasmic reticulum stress. Diabetes. 2016;65:149–63. doi: 10.2337/db15-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang F, Wu Y, Quon MJ, Li X, Yang P. ASK1 mediates the teratogenicity of diabetes in the developing heart by inducing ER stress and inhibiting critical factors essential for cardiac development. Am J Physiol Endocrinol Metab. 2015;309:E487–99. doi: 10.1152/ajpendo.00121.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong D, Reece EA, Yang P. The Nrf2 activator vinylsulfone reduces high glucose-induced neural tube defects by suppressing cellular stress and apoptosis. Reprod Sci. 2016 Jan 21; doi: 10.1177/1933719115625846. pii: 1933719115625846. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang F, Wu Y, Gu H, et al. Ask1 gene deletion blocks maternal diabetes-induced endoplasmic reticulum stress in the developing embryo by disrupting the unfolded protein response signalosome. Diabetes. 2015;64:973–88. doi: 10.2337/db14-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Ann Rev Biochem. 2005;74:739–89. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 28.Oyadomari S, Koizumi A, Takeda K, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–32. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laybutt DR, Preston AM, Akerfeldt MC, et al. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–63. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Xu C, Yang P. c-Jun NH2-terminal kinase 1/2 and endoplasmic reticulum stress as interdependent and reciprocal causation in diabetic embryopathy. Diabetes. 2013;62:599–608. doi: 10.2337/db12-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Weng H, Xu C, Reece EA, Yang P. Oxidative stress-induced JNK1/2 activation triggers proapoptotic signaling and apoptosis that leads to diabetic embryopathy. Diabetes. 2012;61:2084–92. doi: 10.2337/db11-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang P, Li X, Xu C, et al. Maternal hyperglycemia activates an ASK1-FoxO3a-caspase 8 pathway that leads to embryonic neural tube defects. Sci Signal. 2013;6:ra74. doi: 10.1126/scisignal.2004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong D, Fu N, Yang P. MiR-17 down-regulation by high glucose stabilizes thioredoxin-interacting protein and removes thioredoxin inhibition on ASK1 leading to apoptosis. Toxicol Sci. 2016;150:84–96. doi: 10.1093/toxsci/kfv313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buja LM, Eigenbrodt ML, Eigenbrodt EH. Apoptosis and necrosis. Basic types and mechanisms of cell death. Arch Pathol Lab Med. 1993;117:1208–14. [PubMed] [Google Scholar]
- 35.Smyth JW, Hong TT, Gao D, et al. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J Clin Invest. 2010;120:266–79. doi: 10.1172/JCI39740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang P, Zhao Z, Reece EA. Blockade of c-Jun N-terminal kinase activation abrogates hyperglycemia-induced yolk sac vasculopathy in vitro. Am J Obstet Gynecol. 2008;198:321.e1–7. doi: 10.1016/j.ajog.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Yang P, Reece EA. Role of HIF-1alpha in maternal hyperglycemia-induced embryonic vasculopathy. Am J Obstet Gynecol. 2011;204:332.e1–7. doi: 10.1016/j.ajog.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golay A, Ybarra J. Link between obesity and type 2 diabetes. Best Pract Res Clin Endocrinol Metab. 2005;19:649–63. doi: 10.1016/j.beem.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Wang F, Fisher SA, Zhong J, Wu Y, Yang P. Superoxide dismutase 1 in vivo ameliorates maternal diabetes-induced apoptosis and heart defects through restoration of impaired Wnt signaling. Circ Cardiovasc Genet. 2015;8:665–76. doi: 10.1161/CIRCGENETICS.115.001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun F, Kawasaki E, Akazawa S, et al. Apoptosis and its pathway in early post-implantation embryos of diabetic rats. Diabetes Res Clin Pract. 2005;67:110–8. doi: 10.1016/j.diabres.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Forsberg H, Eriksson UJ, Welsh N. Apoptosis in embryos of diabetic rats. Pharmacol Toxicol. 1998;83:104–11. doi: 10.1111/j.1600-0773.1998.tb01452.x. [DOI] [PubMed] [Google Scholar]
- 42.Reece EA, Ma XD, Zhao Z, Wu YK, Dhanasekaran D. Aberrant patterns of cellular communication in diabetes-induced embryopathy in rats: II, apoptotic pathways. Am J Obstet Gynecol. 2005;192:967–72. doi: 10.1016/j.ajog.2004.10.592. [DOI] [PubMed] [Google Scholar]
- 43.Phelan SA, Ito M, Loeken MR. Neural tube defects in embryos of diabetic mice: role of the Pax-3 gene and apoptosis. Diabetes. 1997;46:1189–97. doi: 10.2337/diab.46.7.1189. [DOI] [PubMed] [Google Scholar]
- 44.Molin DG, Roest PA, Nordstrand H, et al. Disturbed morphogenesis of cardiac outflow tract and increased rate of aortic arch anomalies in the offspring of diabetic rats. Birth Defects Res A Clin Mol Teratol. 2004;70:927–38. doi: 10.1002/bdra.20101. [DOI] [PubMed] [Google Scholar]
- 45.Morgan SC, Relaix F, Sandell LL, Loeken MR. Oxidative stress during diabetic pregnancy disrupts cardiac neural crest migration and causes outflow tract defects. Birth Defects Res A Clin Mol Teratol. 2008;82:453–63. doi: 10.1002/bdra.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wentzel P, Gareskog M, Eriksson UJ. Decreased cardiac glutathione peroxidase levels and enhanced mandibular apoptosis in malformed embryos of diabetic rats. Diabetes. 2008;57:3344–52. doi: 10.2337/db08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bohuslavova R, Skvorova L, Sedmera D, Semenza GL, Pavlinkova G. Increased susceptibility of HIF-1alpha heterozygous-null mice to cardiovascular malformations associated with maternal diabetes. J Mol Cell Cardiol. 2013;60:129–41. doi: 10.1016/j.yjmcc.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 48.Yee LM, Cheng YW, Inturrisi M, Caughey AB. Effect of gestational weight gain on perinatal outcomes in women with type 2 diabetes mellitus using the 2009 Institute of Medicine guidelines. Am J Obstet Gynecol. 2011;205:257.e1–6. doi: 10.1016/j.ajog.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boghossian NS, Yeung E, Albert PS, et al. Changes in diabetes status between pregnancies and impact on subsequent newborn outcomes. Am J Obstet Gynecol. 2014;210:431.e1–14. doi: 10.1016/j.ajog.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peterson C, Grosse SD, Li R, et al. Preventable health and cost burden of adverse birth outcomes associated with pregestational diabetes in the United States. Am J Obstet Gynecol. 2015;212:74.e1–9. doi: 10.1016/j.ajog.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yanit KE, Snowden JM, Cheng YW, Caughey AB. The impact of chronic hypertension and pregestational diabetes on pregnancy outcomes. Am J Obstet Gynecol. 2012;207:333.e1–6. doi: 10.1016/j.ajog.2012.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzalez AB, Young L, Doll JA, Morgan GM, Crawford SE, Plunkett BA. Elevated neonatal insulin-like growth factor I is associated with fetal hypertrophic cardiomyopathy in diabetic women. Am J Obstet Gynecol. 2014;211:290.e1–7. doi: 10.1016/j.ajog.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 53.Desai N, Roman A, Rochelson B, et al. Maternal metformin treatment decreases fetal inflammation in a rat model of obesity and metabolic syndrome. Am J Obstet Gynecol. 2013;209:136.e1–9. doi: 10.1016/j.ajog.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Hauth JC, Clifton RG, Roberts JM, et al. Maternal insulin resistance and preeclampsia. Am J Obstet Gynecol. 2011;204:327.e1–6. doi: 10.1016/j.ajog.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bacon S, Schmid J, McCarthy A, et al. The clinical management of hyperglycemia in pregnancy complicated by maturity-onset diabetes of the young. Am J Obstet Gynecol. 2015;213:236.e1–7. doi: 10.1016/j.ajog.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 56.Herrera KM, Rosenn BM, Foroutan J, et al. Randomized controlled trial of insulin detemir versus NPH for the treatment of pregnant women with diabetes. Am J Obstet Gynecol. 2015;213:426.e1–7. doi: 10.1016/j.ajog.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 57.Parker SE, Yazdy MM, Tinker SC, Mitchell AA, Werler MM. The impact of folic acid intake on the association among diabetes mellitus, obesity, and spina bifida. Am J Obstet Gynecol. 2013;209:239.e1–8. doi: 10.1016/j.ajog.2013.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Correa A, Gilboa SM, Botto LD, et al. Lack of periconceptional vitamins or supplements that contain folic acid and diabetes mellitus-associated birth defects. Am J Obstet Gynecol. 2012;206:218.e1–13. doi: 10.1016/j.ajog.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Renault KM, Norgaard K, Nilas L, et al. The Treatment of Obese Pregnant Women (TOP) study: a randomized controlled trial of the effect of physical activity intervention assessed by pedometer with or without dietary intervention in obese pregnant women. Am J Obstet Gynecol. 2014;210:134.e1–9. doi: 10.1016/j.ajog.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 60.Bateman BT, Huybrechts KF, Fischer MA, et al. Chronic hypertension in pregnancy and the risk of congenital malformations: a cohort study. Am J Obstet Gynecol. 2015;212:337.e1–14. doi: 10.1016/j.ajog.2014.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agopian AJ, Lupo PJ, Canfield MA, Mitchell LE. National Birth Defects Prevention Study. Swimming pool use and birth defect risk. Am J Obstet Gynecol. 2013;209:219.e1–9. doi: 10.1016/j.ajog.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 62.Dolan SM. Interpregnancy interval and congenital anomalies. Am J Obstet Gynecol. 2014;210:498–9. doi: 10.1016/j.ajog.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Chen I, Jhangri GS, Chandra S. Relationship between interpregnancy interval and congenital anomalies. Am J Obstet Gynecol. 2014;210:564.e1–8. doi: 10.1016/j.ajog.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Hernandez RK, Werler MM, Romitti P, Sun L, Anderka M National Birth Defects Prevention Study. Nonsteroidal antiinflammatory drug use among women and the risk of birth defects. Am J Obstet Gynecol. 2012;206:228.e1–8. doi: 10.1016/j.ajog.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Broussard CS, Rasmussen SA, Reefhuis J, et al. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1, 11. doi: 10.1016/j.ajog.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 66.Garabedian C, Sfeir R, Langlois C, et al. Does prenatal diagnosis modify neonatal treatment and early outcome of children with esophageal atresia? Am J Obstet Gynecol. 2015;212:340.e1–7. doi: 10.1016/j.ajog.2014.09.030. [DOI] [PubMed] [Google Scholar]