Abstract
Background
This study aimed to explore the correlation between FGFR1 and clinical features, including survival analysis and the promotion of angiogenesis by fibroblast growth factor receptor 1 (FGFR1) and vascular endothelial growth factor receptor 2 (VEGFR2). FGFR1 gene amplification has been found in non-small cell lung cancer (NSCLC). However, the prognostic value of FGFR1 and the correlation between FGFR1 and clinical features are still controversial.
Material/Methods
A total of 92 patients with NSCLC who underwent R0 resection between July 2006 and July 2008 were enrolled in the study. The expression of FGFR1, VEGFR2, and CD34 was detected by immunohistochemistry. The correlations between the aforementioned markers and the patients’ clinical features were analyzed by the chi-square test. The impact factors of prognosis were evaluated by Cox regression analyses.
Results
The expression ratios of FGFR1 and VEGFR2 were 26.1% and 43.4%, respectively. The intensity of FGFR1 expression was related to VEGFR2 and histopathology. To some extent, the average microvessel density (MVD) had correlation to the expression of FGFR1 and VGEFR2. The pathological stages III–IV and high expression of FGFR1 were found to be independent prognostic factors.
Conclusions
The expression intensity of FGFR1 and VEGFR2 was associated with MVD, and the expression of FGFR1 is one of the independent prognostic indicators for NSCLC.
MeSH Keywords: Carcinoma, Non-Small-Cell Lung; Microvessels; Prognosis; Receptor, Fibroblast Growth Factor, Type 1; Vascular Endothelial Growth Factor Receptor-2
Background
Lung cancer is the leading cause of cancer-related death in China [1], of which 85% are non-small cell lung cancer (NSCLC). During the last decades, the treatments for advanced NSCLC were confined to platinum-based chemotherapy, with an unsatisfactory response rate of 30% [2]. Intensive studies on tumor-related aberrant signaling pathways have led to rapid development in the treatment of lung cancer. Medicines targeted at epidermal growth factor receptor (EGFR) gene mutation or anaplastic lymphoma kinase fusion gene increase the response rate to 65–80% [3,4], whereas tumor progression is still unavoidable eventually. With a median progression-free survival of about 10 months and a poor overall survival (OS), patients with advanced NSCLC need new therapeutic targets.
Fibroblast growth factor receptor 1 (FGFR1), a transmembrane tyrosine kinase receptor of fibroblast growth factors (FGFs), is encoded by FGFR1 gene (8p11–12). After combining with FGFs, FGFR ligand-dependent dimerization activates tyrosine kinase domains, resulting in the phosphorylation of intracellular tyrosine residues [5]. Phosphorylated tyrosine residues work as docking sites for adaptor proteins, such as Grb2, SOS protein, recruiting Ras-guanosine diphosphate (Ras-GDP), activating mitogen-activated protein kinase, protein kinase C, phosphatidylinositol 3-kinase/AKT pathway, and signal transducer and activator of transcription signaling pathways [6]. FGFRs regulate cell proliferation, differentiation, antiapoptosis, and angiogenesis [7]. The overexpression of FGFR1 was found in NSCLC and recognized as a novel therapeutic target. Its expression status, however, is less studied in the Chinese population. Although several meta-analyses have been reported, the correlation between the expression status of FGFR1 and clinical pathological features remains controversial [8–10]. This study focused on these issues and also studied the promotion of angiogenesis with VEGFR2, which is the main receptor of VEGF-A that plays an important role in neoangiogenesis [11]. The expression of VRGFR2 can be detected in a variety of tumor cells, including colorectal cancer [12], breast cancer [13], and non-small cell lung cancer [14]. The overexpression of VEGFs and VEGFR2 is related to tumor invasion and metastasis, mainly because of their effect on angiogenesis [15,16]. Studies have shown interaction between FGF-FGFR and VEGF-VEGFR signaling pathways. FGF can upregulate the expression of VEGF, FGFR, and VEGFR in epithelial cells, and VEGF can upregulate the expression of FGF [17,18]. It is widely known that tumor development and metastasis depend on neoangiogenesis [19]. Prior studies indicated that neoangiogenesis is essential in developing lung cancer, and microvessel density (MVD) is increased even in premalignant lesions and early-stage lung cancer [20,21]. In this retrospective study, the correlation between FGFR1 and clinical features was explored, including survival analysis and promotion of angiogenesis by FGFR1 and VEGFR2.
Material and Methods
Patients and specimens
This was a retrospective study. Ninety-two patients pathologically diagnosed with NSCLC, who received radical resection (pneumonectomy + lymph node dissection) in West China Hospital of Sichuan University from July 2006 to July 2008 were enrolled in the study. The exclusion criteria were as follows: received neoadjuvant chemotherapy and/or radiotherapy; received EGFR tyrosine kinase inhibitors; had another kind of carcinoma; loss to follow-up; and histopathological specimens unavailable. The study was approved by the Hospital Ethics Committees, and all the patients enrolled gave informed consent. Follow-up data were obtained by telephone and/or outpatient department visits. The patients underwent chest computed tomography (CT) scan, abdomen CT scan, and brain magnetic resonance imaging, and also bone single-photon emission computed tomography if necessary, during periodic follow-up visit according to the National Comprehensive Cancer Network (NCCN) guideline. Staging was based on the NCCN guideline, and histological grading was evaluated according to the World Health Organization criteria. The clinical features included age, gender, stage, histological type, grade, lymph node status, smoking status, and postoperative adjuvant therapy. The primary endpoint was OS, and the secondary endpoint was recurrence-free survival (RFS).
Immunohistochemical staining
Formalin-fixed and paraffin-embedded surgical specimens were immunostained according to streptavidin-peroxidase protocol. The paraffin-embedded tissues were sliced up into slices of 4 μm. Then, after deparaffinization and antigen retrieval, the tissue sections were incubated with FGFR1 antibodies (monoclonal rabbit anti-human, 1:500, Cell Signaling Technology Co., Ltd, MA, USA), VEGFR2 antibodies (polyclonal rabbit anti-human, 1:100, Golden Bridge Biotechnology Co., Ltd., Beijing, China), and CD34 antibodies (monoclonal mouse anti-human, 1:100, Golden Bridge Biotechnology Co., Ltd, Beijing, China) at 37°C for 1 h, and then at 4°C overnight. The next day the sections were rinsed 3 times with phosphate-buffered saline (PBS) before incubating with biotinylated goat anti-rabbit antibodies (1:200, Golden Bridge Biotechnology Co., Ltd, Beijing, China) and subsequently with horseradish-labeled streptavidin (1:200, Golden Bridge Biotechnology Co., Ltd., Beijing, China) at 37°C for 40 min. 3,3′-Diaminobenzidine was used as a chromogen and hematoxylin as a counterstain. Positive controls were provided by reagent companies, and PBS instead of primary antibodies was used as negative control.
Evaluation criteria
Brownish-black or brown particles displayed in certain places were defined as a specific positive reaction. The weighted score, evaluated in a semi-quantitative way, was calculated by multiplying the quantity score by staining intensity score, representing FGFR1 and VEGFR2 expression levels [22,23]. The quantity scores based on the percentage of stained cells were defined as follows: 0 for <5% cells stained; 1 for 5–25% cells stained; 2 for 26–50% cells stained; 3 for 51–75% cells stained; and 4 for ≥76% cells stained. The staining intensity was scored 0–3: 0 for no staining; 1 for pale yellow staining; 2 for brown staining; and 3 for brownish-black staining. Weighted score ≤1, 2–3, 4–6, or ≥7 was defined as negative, weak, moderate, or strong, respectively. For statistical analysis, the subgroups were finally dichotomized into high expression (moderate and strong) and low expression (negative and weak). CD34 was detected by immunostaining for MVD (24). The hot spots (intratumoral areas with a high density of CD34-positive vessels) were identified under low-power fields, while the CD34-positive vessels in hot spots were quantitated under high-power fields case by case. The average value of 5 fields was defined as the MVD for each sample. All the specimens were evaluated by 2 pathologists independently.
Statistical analysis
SPSS 16.0 software (SPSS Inc., IL, USA) was used for statistical analysis. The correlations between the expression of FGFR1and VEGFR2, MVD, and clinicopathological features were tested by chi-square test, t test, and Spearman’s rank correlation coefficient, respectively. Kaplan-Meier curve was used to analyze the correlation between the expression of FGFR1 and VEGFR2, MVD, and prognosis. Univariate and multivariate analyses were performed by Cox regression model. A P value less than 0.05 was considered to be statistically significant.
Results
Clinical characteristics
A total of 92 patients (70 males and 22 females) with a mean age of 58.5 (range 38–79) years were enrolled in the study. Classified by histological type, 49 patients had squamous lung cancer, 37 had adenocarcinoma, 4 had adenosquamous carcinoma, and 2 had large-cell lung cancer. Histologically, 54 samples were poorly differentiated, 36 moderately differentiated, and 2 well differentiated. According to the NCCN guideline of 2013, 25 patients were at stage I, 16 were at stage II, 42 were at stage III, and 9 were at stage IV. Moreover, 59 of all the patients were continuously smoking (defined as never quit or quit for less than 1 year), and 33 patients were nonsmokers or quit for more than 1 year.
Expression of FGFR1, VEGFR2, and CD34 in NSCLC
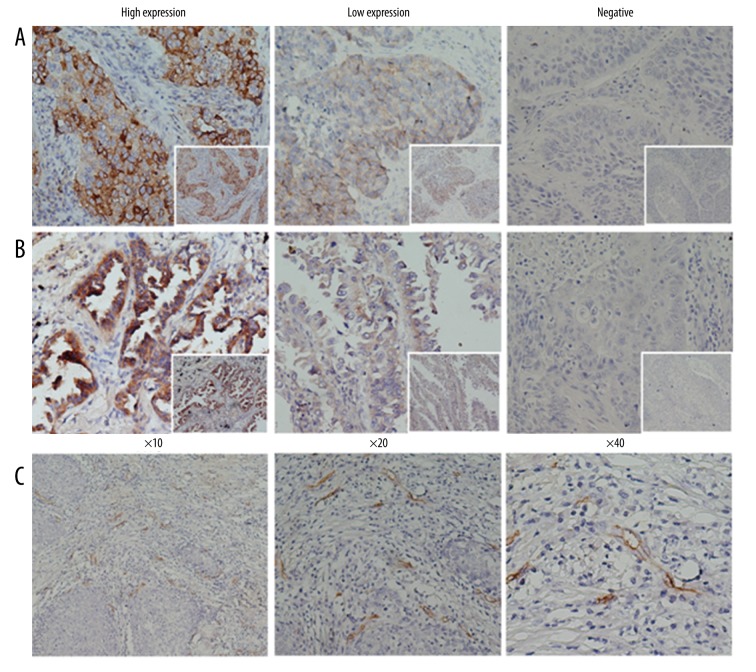
Both FGFR1 and VEGFR2 were mainly expressed in the cytoplasm (Figure 1A, 1B). In terms of FGFR1 expression, 25% (23/92) were negative, 48.9% (45/92) were weak, 18.5% (17/92) were moderate, and 7.6% (7/92) were strong. The high expression of FGFR1 was observed in 26.1% (24/92) of samples. Notably, the high expression rate of FGFR1 was significantly higher in squamous lung cancer than in adenocarcinoma (Table 1. 36.7% vs. 16.2%, P=0.036). The high expression of FGFR1 was not correlated with smoking (23.7% vs. 30.3%, P=0.491), while in statistical analysis, we found that the expression rate (patients that had been tested to be positive, including weak, moderate and strong) of FGFR1 was significantly higher in smoking patients (84.7% vs. 60.6%, P=0.009). Moreover, 5.4% (5/92) of samples were VEGFR2 negative, 52.2% (48/92) were weak, 29.3% (27/92) were moderate, and 13% (12/92) were strong. The high expression of VEGFR2 was detected in 43.4% (39/92) of samples. Patients with lymph node metastasis (P=0.022) and those with tumor size more than 4 cm (P=0.001) had higher expression of VEGFR2. The high expression of FGFR1 and VEGFR2 was not correlated with the patients’ age, gender, smoking status, differentiation, and stage. Microvascular endothelial cells could be stained yellow or brownish by CD34 antibody (Figure 1C). The number of stained endothelial cells in a certain area was defined as MVD. In this study, the average MVD was 34.6. Patients with lymph node metastasis (37.5±10.7 vs. 31.0±10.3, P=0.004) and stages III–IV (36.9±10.7 vs. 31.7±10.5, P=0.024) presented with a significantly higher MVD in tumor tissues, while no correlation was found between MVD and gender, age, smoking status, histological type, and differentiation.
Figure 1.
Immunohistological staining of FGFR1 and VEGFR2, and MVD in NSCLC. (A) Expression of FGFR1. (B) Expression of VEGFR2; FGFR1 and VEGFR2 antibodies were mainly stained in the cytoplasm. (C) Microvascular endothelial cells were stained yellow or brownish by CD34 antibody.
Table 1.
The correlation between the expression of FGFR1, VEGFR2, MVD, and clinicopathological features.
| Clinical features | Total | FGFR1 high expression | VEGFR2 high expression | MVD | |||
|---|---|---|---|---|---|---|---|
| NO. (%) | P | NO. (%) | P | Value | P | ||
| Gender | |||||||
| Male | 70 | 18 (25.7) | 0.885 | 27 (38.6) | 0.186 | 33.6±10.3 | 0.139 |
| Female | 22 | 6 (27.3) | 12 (54.5) | 37.6±12.7 | |||
| Age (year) | |||||||
| <60 | 51 | 10 (19.6) | 0.114 | 18 (35.3) | 0.124 | 33.8±11.2 | 0.497 |
| ≥60 | 41 | 14 (34.1) | 21 (51.2) | 35.4±10.8 | |||
| Smoke | |||||||
| Yes | 59 | 14 (23.7) | 0.491 | 25 (42.4) | 0.996 | 38.1±11.9 | 0.058 |
| No | 33 | 10 (30.3) | 14 (42.4) | 33.1±10.6 | |||
| Tumor size | |||||||
| <4 cm | 47 | 11 (23.4) | 0.549 | 12 (25.5) | 0.001 | 35.9±10.5 | 0.261 |
| ≥4 cm | 45 | 13 (28.9) | 27 (60.0) | 33.4±11.3 | |||
| Histopathology | |||||||
| Squamous | 49 | 18 (36.7) | 0.036 | 21 (42.9) | 0.593 | 34.0±11.9 | 0.380 |
| Adenocarcinoma | 37 | 6 (16.2) | 18 (48.6) | 36.2±9.7 | |||
| Grade | |||||||
| Poor | 54 | 14 (25.9) | 0.967 | 21 (38.9) | 0.418 | 35.1±10.4 | 0.575 |
| Moderate/well | 38 | 10 (26.3) | 18 (47.4) | 33.8±11.9 | |||
| Lymph node metastasis | |||||||
| Yes | 51 | 12 (23.5) | 0.533 | 27 (52.9) | 0.022 | 37.5±10.7 | 0.004 |
| No | 41 | 12 (29.3) | 12 (29.3) | 31.0±10.3 | |||
| Stage | |||||||
| I–II | 41 | 11 (26.8) | 0.884 | 13 (31.7) | 0.063 | 31.7±10.5 | 0.024 |
| III–IV | 51 | 13 (25.5) | 26 (51.0) | 36.9±10.9 | |||
| Adjuvant therapy | |||||||
| Yes | 69 | 16 (23.2) | 0.273 | 28 (40.6) | 0.543 | 33.2±11.1 | 0.495 |
| No | 23 | 8 (34.8) | 11 (42.4) | 35.0±10.9 | |||
Correlations among the expression of MVD, FGFR1, and VEGFR2
MVD was significantly higher in patients with high expression of VEGFR2 than in patients with low expression of VEGFR2 (40.6±11.0 vs. 30.1±8.7, P<0.001), and a higher MVD was associated with the high expression of FGFR1 as well (43.1±10.1 vs. 31.5±9.6, P<0.001). The Spearman rank correlation analysis (Table 2) demonstrated that, to some extent, the expression intensity of VEGFR2 (r=0.224, P=0.032) and FGFR1 (r=0.265, P=0.011) was positively correlated with MVD. In addition, the expression levels of FGFR1 and VEGFR2 were significantly positively correlated (r=0.619, P<0.001).
Table 2.
The correlation between FGFR1, VEGFR2, and MVD.
| VEGFR2 | MVD | FGFR1 | ||
|---|---|---|---|---|
| VEGFR2 | Correlation coefficient | 1.000 | 0.224* | 0.619** |
| Sig. (2-tailed) | . | 0.032 | <0.001 | |
| N | 92 | 92 | 92 | |
| MVD | Correlation coefficient | 0.224* | 1.000 | 0.265* |
| Sig. (2-tailed) | 0.032 | . | 0.011 | |
| N | 92 | 92 | 92 | |
| FGFR1 | Correlation coefficient | 0.619** | 0.265* | 1.000 |
| Sig. (2-tailed) | <0.001 | 0.011 | . | |
| N | 92 | 92 | 92 |
Impact of FGFR1, VEGFR2, and MVD on prognosis
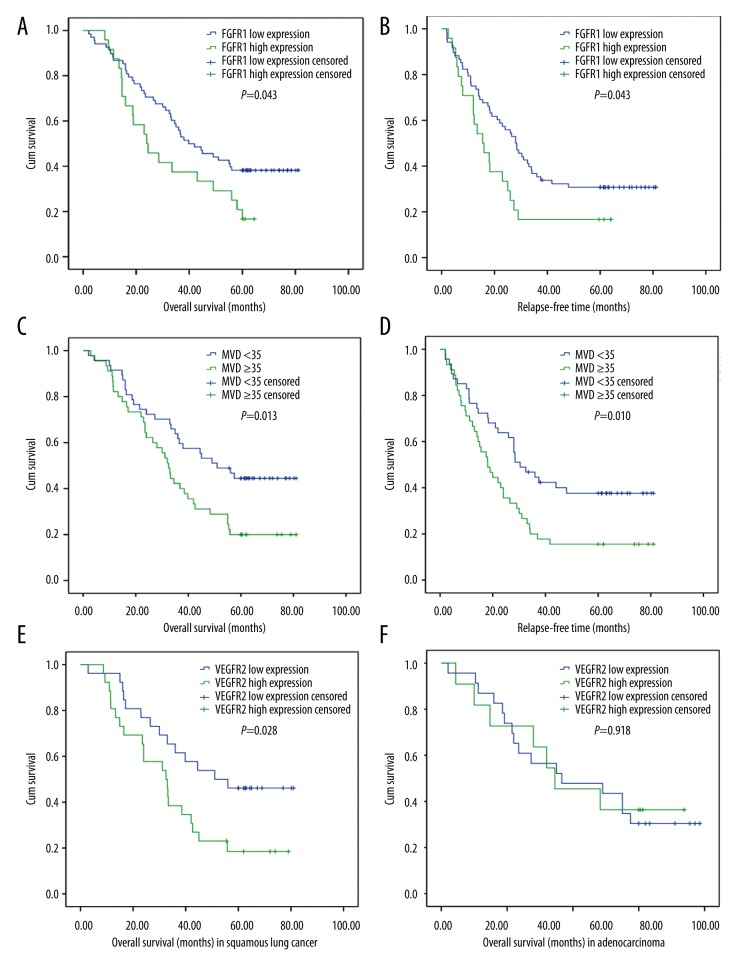
With a total follow-up of 80 months, the patients with the high expression of FGFR1 presented with a significantly shorter OS (23.5 months vs. 39.8 months, P=0.043) and RFS (15.0 months vs. 28.0 months, P=0.043) than those with the low expression of FGFR1 (Figure 2A, 2B). The expression level of VEGFR2 showed no significant impact on the survival of patients with NSCLC. Notably, in squamous lung cancer, the OS in patients with the high expression of VEGFR2 was 32.5 months, which was shorter than that in patients with the low expression (51.0 months, P=0.028) (Figure 2E). However, no significant difference was observed in the survival of patients with adenocarcinoma between high-expression and low-expression groups of VEGFR2 (P = 0.918) (Figure 2F). The analysis of the impact of MVD on OS and RFS is shown in Figure 2C and 2D. The RFS of patients with a high and a low value of MVD was 18.1 and 30.4 months (P=0.010), respectively, while the OS in the low-MVD group was significantly longer than the OS in the high-MVD group (51.0 months vs. 32.5 months, P=0.013).
Figure 2.
Overall survival (OS) and recurrence-free survival (RFS) curves for patients with NSCLC. (A) The OS in FGFR1 low- and high-expression groups; a significant difference can be seen (P=0.043). (B) The RFS was significantly different between FGFR1 low- and high-expression groups (P=0.043). (C) The OS was significantly longer in the group with MVD <35 than in the group with MVD ≥35 (P=0.013). (D) The RFS was significantly longer in the group with MVD <35 than in the group with MVD ≥35 (P=0.010). (E) In squamous lung cancer, the OS was shorter in patients with the high expression of VEGFR2 than in the low-expression group (51.0 months, P=0.028). (F) No significant difference was observed in the survival of patients with adenocarcinoma between the high- and low-expression groups of VEGFR2 (P=0.918).
Univariate and multivariate analyses
All clinical and pathological features, including gender, age, smoking status, tumor size, differentiation, histological type, lymph node status, stage, adjuvant therapy, FGFR1 expression, VEGFR2 expression, and MVD value, were considered in the Cox regression model. The univariate analysis showed that tumor stage (P<0.001), lymph node metastasis (P=0.003), high expression of FGFR1 (P=0.043), and high value of MVD (p=0.013) in tumor tissue suggested shorter OS, while only stage (stage III: hazard ratio [HR] 3.451, 95% confidence interval [CI]: 1.621–7.345, P=0.001; stage IV: HR 39.207, 95% CI: 12.201–125.986, P<0.001) and high expression of FGFR1 (HR 2.194,95% CI: 1.232–3.908,P=0.008) remained independent risk factors of OS in multivariate analysis. Adjuvant chemotherapy was the protection factor for OS (HR 0.492, 95% CI: 0.251–0.964, P=0.039) (Table 3).
Table 3.
Univariate and multivariate analyses with regard to RFS and OS (n=92).
| Variable | Total No. | Univariate analysis | Multivariate Cox regression | |
|---|---|---|---|---|
| P | HR (95%CI) | P | ||
| Gender | ||||
| Male | 70 | 0.830 | ||
| Female | 22 | |||
| Age (year) | ||||
| <60 | 51 | 0.662 | ||
| ≥60 | 41 | |||
| Smoke | ||||
| Yes | 59 | 0.742 | ||
| No | 33 | |||
| Tumor size | ||||
| <4 cm | 47 | 0.095 | ||
| ≥4 cm | 45 | |||
| Grade | ||||
| Poor | 54 | 0.536 | ||
| Moderate or well | 38 | |||
| Histopathology | ||||
| Squamous | 49 | 0.593 | ||
| Adenocarcinoma | 37 | |||
| Lymph node metastasis | ||||
| Yes | 51 | 0.003 | ||
| No | 41 | |||
| Stage | ||||
| I | 25 | <0.001 | ||
| II | 16 | |||
| III | 42 | 3.451 (1.621,7.345) | 0.001 | |
| IV | 9 | 39.207 (12.201,125.986) | <0.001 | |
| Adjuvant therapy | ||||
| Yes | 69 | 0.708 | 0.492 (0.251,0.96) | 0.039 |
| No | 23 | |||
| FGFR1 expression | ||||
| Low | 68 | 0.043 | 2.194 (1.232,3.908) | 0.008 |
| High | 24 | |||
| VEGFR2 expression | ||||
| Low | 53 | 0.174 | ||
| High | 39 | |||
| MVD | ||||
| <35 | 47 | 0.013 | ||
| ≥35 | 45 | |||
HR – hazard risk; CI – confidence interval.
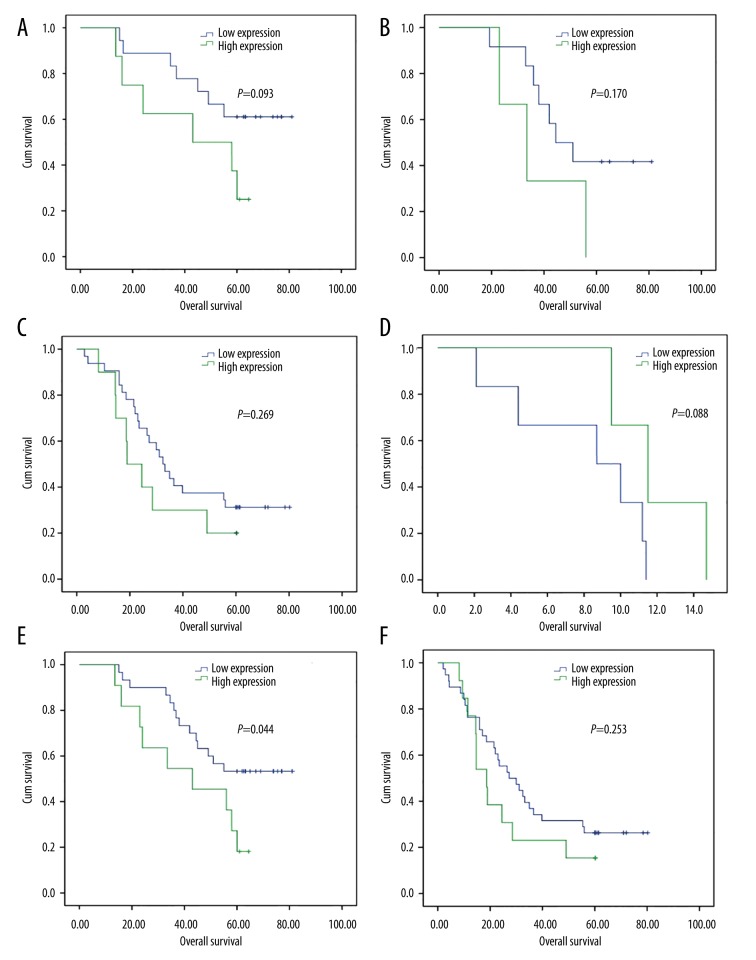
Considering that stage is universally thought to be the most important prognostic factor, stratified analysis based on stages was done (Figure 3). The OS was not significantly different between low- and high-expression of FGFR1 in stage I, II, III and IV, respectively, while when the patients with stage I and II were all taken into account, the OS was significantly longer in those with low FGFR1 expression than high FGFR1 expression (P=0.044). No significant difference was observed in the survival of patients with low and high FGFR1 expression in stage III–IV (P=0.253).
Figure 3.
Overall survival (OS) curves for patients with NSCLC in different stages. (A) The OS in stage I; no significant difference can be seen (P=0.093). (B) The OS was not significantly different between FGFR1 low- and high-expression groups in stage II (P=0.170). (C) The OS was not significantly longer in the group with low FGFR1 expression in stage III (P=0.269). (D) The OS was not significantly longer in the group with low FGFR1 expression in stage IV (P=0.088). (E) The OS was significantly longer in the group with low FGFR1 expression in stage I–II (P=0.044). (F) No significant difference was observed in the survival of patients with low and high FGFR1 expression in stage III–IV (P=0.253).
Discussion
Understanding the molecular alterations in lung cancer might provide better choices for targeted therapy. The malfunction of FGFR signaling pathway, mainly caused by the overexpression and/or mutation of receptor genes, was found in a variety of epithelial tumors [25–28], such as breast cancer, prostatic cancer, oral carcinoma, and gastric cancer. Both in vitro and in vivo experiments showed that the FGFR signal pathway played an important role in growth, survival, and migration of lung cancer cells, which could be blocked by FGFR tyrosine kinase inhibitor [29,30]. Previous studies compared the results between fluorescence in situ hybridization and immunohistochemistry (IHC) in detecting the overexpression of FGFR1, where no significant difference was detected between the 2 methods [31]. In this study, the expression of FGFR1 by IHC, and its correlation with VEGFR2 and MVD, was analyzed to evaluate its correlation with clinical features and the impact on prognosis.
According to previous reports, gene amplification of FGFR1 was about 16–20% in squamous lung cancer and 5% in adenocarcinoma. Inconsistently, researches from Weiss [32] and Schildhaus [33] showed no FGFR1 gene amplification in adenocarcinoma. Related researches were less conducted China. Several common histological types, such as squamous lung cancer, adenocarcinoma, large-cell lung cancer, and adenosquamous carcinoma, were included in this study. The present study showed that 2 patients with large-cell lung cancer and 4 with adenosquamous carcinoma had FGFR1 negative or weak expression. FGFR1 expression was significantly higher in squamous lung cancer than in adenocarcinoma (36.7% vs. 16.2%, P=0.036), which was consistent with most previous reports [31,34]. The similarity between these results implicated no significant ethnic diversity in the FGFR1 status while large-scale researches are still in need. The expression rate of FGFR1 was slightly higher in this study than in previous reports, which might be due to different detection methods. For example, a newly published research using reverse transcription-polymerase chain reaction showed an extremely higher amplification rate of FGFR1 (41.5% in squamous lung cancer and 14.3% in nonsquamous lung cancer) than in any other researches. IHC is a widely used method in detecting the expression of specific proteins, which could test the expression of FGFR1. The standard method has not been identified yet. Zhang’s research showed that FGFR1 inhibitor induced tumor stasis or regression in the FGFR1-amplified NSCLC model [29]. This study demonstrated that FGFR1 was overexpressed in some NSCLCs, especially in squamous lung cancer in the Chinese population, suggesting that FGFR1 might be a potential target for targeted therapy and its inhibitor might be applied for the treatment of squamous lung cancer and a small part of adenocarcinoma harboring the high expression of FGFR1.
It is widely known that EGFR mutation frequently emerges in Asian female nonsmokers. Some studies suggested that smoking was also positively correlated with FGFR1 amplification [31], while some did not [34]. This study showed some differences, as the high expression of FGFR1 was not correlated with smoking (23.7% vs. 30.3%, P=0.491), while the expression rate of FGFR1 was significantly higher in smoking patients (84.7% vs. 60.6%, P=0.009). In conclusion, no correlation was found between high expression of FGFR1 and clinical characteristics such as age, gender, stage, grade, smoking status, and lymph node metastasis, suggesting no special predictive potential of these clinical characteristics for FGFR1 status in patients with NSCLC. Weiss [32] demonstrated that gene amplification of FGFR1 led to poor prognosis. Conversely, Trans [35] demonstrated that FGFR1 amplification suggested a longer survival. Inconsistent pathological types and different EGFR-TKI treatment status in analysis might explain the differing results. In this study, patients who had taken EGFR-TKI after relapse were excluded, and multivariate analysis was used to identify the correlation between FGFR1 expression and clinical features. It showed that the high expression of FGFR1 was related to poor prognosis, which was consistent with the findings of Weiss’s research. However, in Weiss’s study, clinical pathological characteristics (e.g., stage, smoking status, and adjuvant chemotherapy) were not taken into consideration. The present study, using multivariate analysis (including gender, age, smoking status, tumor size, degree of differentiation, lymph node metastasis, stage, and adjuvant chemotherapy), demonstrated that patients with high expression of FGFR1 had shorter RFS and OS, suggesting that the high expression of FGFR1 was the independent prognostic indicator of NSCLC. We also took stage, the most important prognostic factor, into account. Although we found no significant difference in survival between low and high FGFR1 expression in stage I, II, III, and IV, when we combined the patients with stage I and II, the OS was significantly longer in those with low FGFR1 expression than high FGFR1 expression (P=0.044). No significant difference was observed in the survival of patients with low- and high-FGFR1 expression in stage III–IV (P=0.253), indicating that the prognostic value could be more important in early stage of lung cancer. The small sample size may be why we did not get statistical significance in stage I and II separately.
VEGFR2 expression, mainly located in the cytoplasm, was found in most tumor tissues (94.6%) in this study, which was consistent with Decaussin’s report [36]. The expression of VEGFR2 was not influenced by age, gender, smoking status, stage, or grade, but it was significantly higher in patients with lymph node metastasis (P=0.022) and/or tumor size more than 4 cm (P=0.001). Some studies suggested that the expression status of VEGFR2 was correlated with prognosis [37]. Seto’s study [38], involving 60 postsurgery patients with stage I lung cancer, indicated that patients with VEGFR2 expression in tumor cells presented with poorer survival, while some other studies showed no correlation between VEGFR2 expression and prognosis [39]. A recent meta-analysis found no statistically significant effect of VEGFR2 expression on survival, but VEGFA/VEGFR2 co-expression had an influence on survival (40). The differences might be caused by the diversity in stage, histological type, period of follow-up, and sample size between different studies. The present study showed no correlation between the expression statuses of VEGFR2 and RFS and OS in patients with NSCLC after surgery. The subgroup analysis, however, showed that in a subgroup of squamous lung cancer, patients with the high expression of VEGFR2 had shorter RFS and OS compared with the low-expression group (P=0.028; P=0.033), while this correlation was not found in adenocarcinoma. According to the literature, similar results were found in the Holzer’s study [41]. In other words, the expression status of VEGFR2 might play different roles in the prognosis of different histological types. In the meantime, it reflected indirectly that carcinomas with different histological types present with different biological characteristics, so that personalized treatment should be emphasized during treatment.
VEGF/VEGFR signal and FGF/FGFR signal play crucial roles in angiogenesis. The proangiogenic effect of VEGF and bFGF is mediated through the VEGF receptor 2 and FGF receptor 1, respectively. Previous researches also showed crosstalk between them in vitro and in vivo [42]. In this study, MVD, which reflected the number of vessels, was significantly higher in tumors with the high expression of VEGFR2 than in tumors with the low expression of VEGFR2. The same situation was found in the case of FGFR1. The present study, using human tumor tissues, verified in terms of histopathology that the expression of FGFR1 and VEGFR2 was significantly correlated (r=0.619), and both of them had more or less positive correlation with MVD (r=0.224; r=0.265). Although the sample size was small, this study found and verified some important findings in China. The role of FGFR1 in squamous lung cancer and its crosstalk with VEGFR2 in tumor development need to be explored further. It is hoped that FGFR1 would serve as one of the treatment targets in NSCLC, especially in squamous lung cancer.
Conclusions
The high expression of FGFR1 existed in part of NSCLC (including squamous lung cancer and adenocarcinoma) and was the independent prognostic factor associated with poor prognosis, especially in early stages. The expression of FGFR1 and VEGFR2 was positively correlated, and both of them were associated with angiogenesis.
Acknowledgment
The authors would like to thank all their colleagues who participated in this study.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Source of support: Departmental sources
References
- 1.Chen W, Zheng R, Zeng H, et al. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27:2–12. doi: 10.3978/j.issn.1000-9604.2015.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smit EF, van Meerbeeck JP, Lianes P, et al. Three-arm randomized study of two cisplatin-based regimens and paclitaxel plus gemcitabine in advanced non-small-cell lung cancer: A phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group – EORTC 08975. J Clin Oncol. 2003;21:3909–17. doi: 10.1200/JCO.2003.03.195. [DOI] [PubMed] [Google Scholar]
- 3.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 4.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 5.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–49. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Raju R, Palapetta SM, Sandhya VK, et al. A Network Map of FGF-1/FGFR signaling system. J Signal Transduct. 2014;2014:962962. doi: 10.1155/2014/962962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–37. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Yang W, Yao YW, Zeng JL, et al. Prognostic value of FGFR1 gene copy number in patients with non-small cell lung cancer: A meta-analysis. J Thorac Dis. 2014;6:803–9. doi: 10.3978/j.issn.2072-1439.2014.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang T, Gao G, Fan G, et al. FGFR1 amplification in lung squamous cell carcinoma: A systematic review with meta-analysis. Lung Cancer. 2015;87:1–7. doi: 10.1016/j.lungcan.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Chang J, Liu X, Wang S, et al. Prognostic value of FGFR gene amplification in patients with different types of cancer: A systematic review and meta-analysis. PLoS One. 2014;9:e105524. doi: 10.1371/journal.pone.0105524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cascone T, Troiani T, Morelli MP, et al. Antiangiogenic drugs in non-small cell lung cancer treatment. Curr Opin Oncol. 2006;18:151–55. doi: 10.1097/01.cco.0000208788.99570.0e. [DOI] [PubMed] [Google Scholar]
- 12.Duff SE, Jeziorska M, Rosa DD, et al. Vascular endothelial growth factors and receptors in colorectal cancer: Implications for anti-angiogenic therapy. Eur J Cancer. 2006;42:112–17. doi: 10.1016/j.ejca.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Dhakal HP, Naume B, Synnestvedt M, et al. Expression of vascular endothelial growth factor and vascular endothelial growth factor receptors 1 and 2 in invasive breast carcinoma: Prognostic significance and relationship with markers for aggressiveness. Histopathology. 2012;61:350–64. doi: 10.1111/j.1365-2559.2012.04223.x. [DOI] [PubMed] [Google Scholar]
- 14.Pomme G, Augustin F, Fiegl M, et al. Detailed assessment of microvasculature markers in non-small cell lung cancer reveals potentially clinically relevant characteristics. Virchows Arch. 2015;467:55–66. doi: 10.1007/s00428-015-1767-y. [DOI] [PubMed] [Google Scholar]
- 15.Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273:114–27. doi: 10.1111/joim.12019. [DOI] [PubMed] [Google Scholar]
- 16.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–78. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 17.Tsunoda S, Nakamura T, Sakurai H, Saiki I. Fibroblast growth factor-2-induced host stroma reaction during initial tumor growth promotes progression of mouse melanoma via vascular endothelial growth factor A-dependent neovascularization. Cancer Sci. 2007;98:541–48. doi: 10.1111/j.1349-7006.2007.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seghezzi G, Patel S, Ren CJ, et al. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: An autocrine mechanism contributing to angiogenesis. J Cell Biol. 1998;141:1659–73. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–86. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 20.Fontanini G, Calcinai A, Boldrini L, et al. Modulation of neoangiogenesis in bronchial preneoplastic lesions. Oncol Rep. 1999;6:813–17. doi: 10.3892/or.6.4.813. [DOI] [PubMed] [Google Scholar]
- 21.Keith RL, Miller YE, Gemmill RM, et al. Angiogenic squamous dysplasia in bronchi of individuals at high risk for lung cancer. Clin Cancer Res. 2000;6:1616–25. [PubMed] [Google Scholar]
- 22.Bonnesen B, Pappot H, Holmstav J, Skov BG. Vascular endothelial growth factor A and vascular endothelial growth factor receptor 2 expression in non-small cell lung cancer patients: Relation to prognosis. Lung Cancer. 2009;66:314–18. doi: 10.1016/j.lungcan.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Behrens C, Lin HY, Lee JJ, et al. Immunohistochemical expression of basic fibroblast growth factor and fibroblast growth factor receptors 1 and 2 in the pathogenesis of lung cancer. Clin Cancer Res. 2008;14:6014–22. doi: 10.1158/1078-0432.CCR-08-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bing Z, Jian-ru Y, Yao-quan J, Shi-feng C. Evaluation of angiogenesis in non-small cell lung carcinoma by CD34 immunohistochemistry. Cell Biochem Biophys. 2014;70:327–31. doi: 10.1007/s12013-014-9916-5. [DOI] [PubMed] [Google Scholar]
- 25.Turner N, Pearson A, Sharpe R, et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010;70:2085–94. doi: 10.1158/0008-5472.CAN-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwabi-Addo B, Ozen M, Ittmann M. The role of fibroblast growth factors and their receptors in prostate cancer. Endocr Relat Cancer. 2004;11:709–24. doi: 10.1677/erc.1.00535. [DOI] [PubMed] [Google Scholar]
- 27.Freier K, Schwaenen C, Sticht C, et al. Recurrent FGFR1 amplification and high FGFR1 protein expression in oral squamous cell carcinoma (OSCC) Oral Oncol. 2007;43:60–66. doi: 10.1016/j.oraloncology.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Nakazawa K, Yashiro M, Hirakawa K. Keratinocyte growth factor produced by gastric fibroblasts specifically stimulates proliferation of cancer cells from scirrhous gastric carcinoma. Cancer Res. 2003;63:8848–52. [PubMed] [Google Scholar]
- 29.Zhang J, Zhang L, Su X, et al. Translating the therapeutic potential of AZD4547 in FGFR1-amplified non-small cell lung cancer through the use of patient-derived tumor xenograft models. Clin Cancer Res. 2012;18:6658–67. doi: 10.1158/1078-0432.CCR-12-2694. [DOI] [PubMed] [Google Scholar]
- 30.Marek L, Ware KE, Fritzsche A, et al. Fibroblast growth factor (FGF) and FGF receptor-mediated autocrine signaling in non-small cell lung cancer cells. Mol Pharmacol. 2009;75:196–207. doi: 10.1124/mol.108.049544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HR, Kim DJ, Kang DR, et al. Fibroblast growth factor receptor 1 gene amplification is associated with poor survival and cigarette smoking dosage in patients with resected squamous cell lung cancer. J Clin Oncol. 2013;31:731–37. doi: 10.1200/JCO.2012.43.8622. [DOI] [PubMed] [Google Scholar]
- 32.Weiss J, Sos ML, Seidel D, et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med. 2010;2:62ra93. doi: 10.1126/scitranslmed.3001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schildhaus HU, Heukamp LC, Merkelbach-Bruse S, et al. Definition of a fluorescence in-situ hybridization score identifies high- and low-level FGFR1 amplification types in squamous cell lung cancer. Mod Pathol. 2012;25:1473–80. doi: 10.1038/modpathol.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heist RS, Mino-Kenudson M, Sequist LV, et al. FGFR1 amplification in squamous cell carcinoma of the lung. J Thorac Oncol. 2012;7:1775–80. doi: 10.1097/JTO.0b013e31826aed28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tran TN, Selinger CI, Kohonen-Corish MR, et al. Fibroblast growth factor receptor 1 (FGFR1) copy number is an independent prognostic factor in non-small cell lung cancer. Lung Cancer. 2013;81:462–67. doi: 10.1016/j.lungcan.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 36.Decaussin M, Sartelet H, Robert C, et al. Expression of vascular endothelial growth factor (VEGF) and its two receptors (VEGF-R1-Flt1 and VEGF-R2-Flk1/KDR) in non-small cell lung carcinomas (NSCLCs): Correlation with angiogenesis and survival. J Pathol. 1999;188:369–77. doi: 10.1002/(SICI)1096-9896(199908)188:4<369::AID-PATH381>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 37.Carrillo de Santa Pau E, Arias FC, Caso Pelaez E, et al. Prognostic significance of the expression of vascular endothelial growth factors A, B, C, and D and their receptors R1, R2, and R3 in patients with nonsmall cell lung cancer. Cancer. 2009;115:1701–12. doi: 10.1002/cncr.24193. [DOI] [PubMed] [Google Scholar]
- 38.Seto T, Higashiyama M, Funai H, et al. Prognostic value of expression of vascular endothelial growth factor and its flt-1 and KDR receptors in stage I non-small-cell lung cancer. Lung Cancer. 2006;53:91–96. doi: 10.1016/j.lungcan.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Dziadziuszko R, Chyczewski L, Jassem E, Jassem J. Expression of vascular endothelial growth factor (VEGF) and its receptor FLK-1 in non-small cell lung cancer (NSCLC) – a preliminary report. Folia Histochem Cytobiol. 2001;39(Suppl 2):100–1. [PubMed] [Google Scholar]
- 40.Zheng CL, Qiu C, Shen MX, et al. Prognostic impact of elevation of vascular endothelial growth factor family expression in patients with non-small cell lung cancer: an updated meta-analysis. Asian Pac J Cancer Prev. 2015;16:1881–95. doi: 10.7314/apjcp.2015.16.5.1881. [DOI] [PubMed] [Google Scholar]
- 41.Holzer TR, Fulford AD, Nedderman DM, et al. Tumor cell expression of vascular endothelial growth factor receptor 2 is an adverse prognostic factor in patients with squamous cell carcinoma of the lung. PLoS One. 2013;8:e80292. doi: 10.1371/journal.pone.0080292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao R, Ji H, Feng N, et al. Collaborative interplay between FGF-2 and VEGF-C promotes lymphangiogenesis and metastasis. Proc Natl Acad Sci USA. 2012;109:15894–99. doi: 10.1073/pnas.1208324109. [DOI] [PMC free article] [PubMed] [Google Scholar]