Abstract
Objective
This two-stage Phase II study assessed the activity of single agent alisertib in patients with recurrent/persistent uterine leiomyosarcoma (uLMS).
Methods
Eligibility criteria included histologically-confirmed, recurrent or persistent uLMS, age ≥18, 1-2 prior cytotoxic regimens, and RECIST version 1.1 measurable disease. The primary objective of the study was to evaluate the efficacy of alisertib through the frequency of patients with objective tumor responses and the frequency who survived event-free for at least 6 months (EFS6). The endpoints for EFS were RECIST progression, death, or beginning a subsequent therapy. The null hypothesis jointly specified the probability of a patient experiencing a tumor response to less than or equal to 5% and the probability of a patient surviving event-free for at least 6 months to less than or equal to 20%. A two-stage design was used with a target accrual of 23 patients for stage 1 and 47 pts cumulative for stage 2. Confidence intervals do not correct for multiplicity.
Results
Twenty-three patients were enrolled with two patients excluded on central histology review, yielding 21 eligible patients. Median age was 61 years. Prior treatment was either 1 cytotoxic regimen (71.4%) or 2 (28.6%). The most common treatment related AEs (grade 3 or worse) were anemia (4), leukopenia (5), neutropenia (7), thrombocytopenia (1), mucositis (4), diarrhea (1), and palmer-planter syndrome (2). There were no objective responses (0%; 90% CI: 0-10.4%). Best response was stable disease (38.1%); 12 patients had progressive disease (57.1%). EFS6 was 0% (90% CI: 0-10.4%). Median PFS and OS were 1.7 (90% CI: 1.4-3.2) and 14.5 months (90% CI: 7.6- NA), respectively.
Conclusion
Alisertib did not demonstrate clinically meaningful single agent activity in previously treated uLMS.
Keywords: Alisertib, Aurora Kinase, Uterine Leiomyosarcoma
Introduction
Uterine leiomyosarcoma (uLMS) is a rare and highly aggressive malignancy that accounts for approximately 60% of all uterine sarcomas.1 Although patients are often diagnosed with FIGO stage 1 (uterine confined) disease, recurrence rates are estimated to be between 50-70%.2,3 To date, the mainstay of treatment for patients who develop unresectable metastatic disease is cytotoxic chemotherapy with response rates to first-line multidrug chemotherapy ranging from 27-53%.4-9 The efficacy of second-line chemotherapy drops significantly with most regimens offering response rates of 10-15%.10-15 As a result, the overall survival of metastatic uLMS remains poor.
Developing treatment for advanced uLMS has been hampered by a limited understanding of the genomic and epigenetic mechanisms underlying the aggressive clinical phenotype of uLMS. Characterization of benign uterine leiomyomas by whole-genome sequencing has revealed that even these benign precursor lesions demonstrate remarkable genomic complexity consistent with chromosome shattering and reassembly resembling chromothripsis.16 These studies have also revealed frequent mutation of MED12 in benign leiomyomas but much lower rates in uLMS suggesting that many of these tumors may arise de novo rather than transforming from leiomyomas.17,18,19 To date there has been no large scale unbiased sequencing study reported in uLMS. As a result of the absence of clearly actionable genomic alterations in uLMS, the role of targeted therapy has remained limited. Pazopanib, a small molecule inhibitor of multiple tyrosine kinases including VEGF-1, -2, and -3, is currently the only approved targeted therapy for advanced soft tissue sarcoma (including LMS) although the overall degree of clinical benefit is modest and other VEGF targeted therapies have failed to demonstrate activity.20-23
Previous genome-wide transcriptional profiling of uLMS compared to benign myometrium and leiomyoma found that the majority of the most overexpressed gene products involve genes that regulate mitotic centrosome and spindle functions.24 Among the most highly overexpressed genes include Aurora A and B kinase. In aggregate, the aurora family of kinases play a central role in mitosis, specifically centrosome maturation and separation, bipolar spindle assembly, chromosome alignments, and cytokinesis.25 Overexpression of Aurora A has been shown to induce transformation in certain cell lines by disrupting the G2 checkpoint.26 Finally, aurora A kinase inhibition has demonstrated antiproliferative effects in multiple preclinical models of uLMS.27 Alisertib is a highly potent, Aurora kinase A selective inhibitor.28 Based on these data, as well as the ongoing and unmet need for effective therapies for this disease, a Phase II study of alisertib in advanced recurrent or persistent uLMS was undertaken. The primary objective was to evaluate the efficacy of the alisertib as defined by objective tumor responses and the frequency who survived event-free for at least 6 months.
Methods
Patient selection
Histologic confirmation of the original primary tumor by the GOG Pathology Committee central review process was required. To be eligible, patients had to be ≥ 18 years of age and have had incurable recurrent or persistent uLMS. Patients were required to have had at least one prior chemotherapeutic regimen for management of leiomyosarcoma and were allowed to receive, but not required to receive, one additional cytotoxic regimen for recurrent or persistent disease. Biologic, small molecule and hormonal therapies were not counted towards prior therapy. GOG performance status of 0 to 2 was required; and had to be 1 or less if patients had received two prior cytotoxic regimens. All patients were required to have measurable disease by Response Criteria in Solid Tumors (RECIST 1.1). Patients must have had adequate hematologic parameters (absolute neutrophil count ≥ 1500/mcl, leukocytes ≥3000/mcl, and platelets ≥ 100,000/mcl), renal function (serum creatinine ≤ 1.5 × the institutional upper limit of normal [ULN] OR creatinine clearance ≥ 60 ml/min/1.73m2), and hepatic function (serum bilirubin ≤ 1.5 ULN, AST and ALT ≤ 3 × ULN, and alkaline phosphatase ≤ 2.5 × ULN). A signed approved informed consent in accordance with federal, state and local requirements and an authorization permitting release of personal health information were required for all patients. Participation in this trial required protocol approval by institutional review boards.
Patients were ineligible if they met any of the following criteria: prior therapy with an aurora kinase pathway inhibitor; prior malignancies (other than non-melanomatous skin cancer) evident within three years of prior cancer treatment; known CNS disease; clinically significant cardiovascular disease; history of hepatitis B, C or HIV; patients who were pregnant or nursing; patients unable to take oral medication and to maintain a fast for 2 hours before and 1 hour after alisertib administration; and patients unable to discontinue agents that effect gastric pH including proton pump inhibitors and histamine-2 antagonists.
Treatment
Enrolled patients received alisertib 50 mg twice daily by mouth on days 1-7 of each 21 day cycle. Treatment was continued until disease progression or adverse events prohibited further therapy. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Patients were required to have had an absolute neutrophil count ≥ 1000/mcl, platelets ≥ 100,000/mcl, AST and ALT ≤ 3 × ULN, and bilirubin ≤ ULN prior to initiating treatment on day 1 of each subsequent cycle. Dose reductions for neutropenia and thrombocytopenia occurring during a cycle were managed per protocol based on timing, depth and duration of the specific cytopenia. Unless otherwise specified, alisertib was held for grade 2 non-hematologic adverse events, resuming treatment at the same dose with resolution of toxicity to grade 1 or less, and held, then resumed with a one-level dose reduction for grade 3 non-hematologic adverse events after resolution of toxicity to grade 1 or less. A maximum of two dose reductions for management of adverse events were permitted. Treatment interruptions of up to 2 weeks are permitted for resolution of treatment-related toxicities. Failure of resolution of a treatment-related toxicity within 2 weeks required discontinuation of study treatment.
Evaluation Criteria
Activity of alisertib was assessed according to the RECIST version 1.1 guidelines by computed tomography or magnetic resonance imaging at baseline, every two cycles (or equivalent time frame for patients off treatment prior to disease progression) for the first 6 months, and then every 3 months thereafter until disease progression was documented. Responses (CR and PR) required confirmation at greater than or equal to 4 weeks from initial documentation of response. Event free survival (EFS) was defined in months from study entry until death, progression, or initiation of subsequent therapy with the minimum interval used. If none of these events had occurred, EFS was censored at the date of last contact.
Statistical methods
The primary objective of the study was to evaluate the efficacy of the study agent through the frequency of patients with objective tumor responses and the frequency who survived event-free for at least 6 months (6-month EFS). The endpoints for EFS were progression, death, or beginning a subsequent therapy. Activity on either scale is worthy of further investigation for phase II studies since they are expected to be useful surrogates for overall survival.
The null hypothesis (H0) relating to uninteresting levels of activity was determined from an analysis of a historical dataset based on a similar population of patients where the levels of activity of the study drugs were believed to be inactive to modestly active. The null hypothesis jointly specified the probability of a patient experiencing a tumor response to less than or equal to 5% and the probability of a patient surviving event-free for at least 6 months to less than or equal to 20%. The alternative hypothesis (Ha) is the complement of the parameter space under H0. Clinically significant differences were a 20% increase in the probability of being EFS at 6 months or a 20% increase in the probability of response.
The null hypothesis was evaluated with a flexible method provided by Sill et al.29, which is a two-stage design used to limit patient exposure to inactive regimens. If the observed numbers of patients with responses or 6-month EFS were both less than or equal to their respective critical values, then the study would close, and the regimen would be declared clinically uninteresting. Otherwise, with medical judgment indicating, the study would open to the second stage of accrual to further evaluate the regimen. If either the number of patients with responses or who were 6-month EFS exceeded their respective critical values, then the regimen would be considered worthy of further investigation in a phase III trial. The targeted accrual for the first stage was 23 patients but was allowed to deviate for administrative purposes. If 21 patients were accrued, the critical value for the number of patients with responses was 2 and the critical value for the number of patients who were 6-month EFS was 4. The cumulative targeted accrual for the second stage would have been 47 but was allowed to deviate. If 47 patients were accrued the critical value for the number of patients with responses would have been 6 and the critical value for the number of patients who were EFS at 6 months was thirteen, 13.
The goal of the design was to limit the expected probabilities of type I and II errors to approximately 10% under the assumed accrual ranges of 19 to 26 (stage 1) and 43 to 50 (cumulatively after stage 2). Using the method of Sill et al. (35: Sill et al.), the expected type I error at the end of stage two was about 7%, depending on the level of association between response and 6-month EFS. The expected probability of early termination when the agent is uninteresting was likely between 53% and 57%, depending on the level of association between the two variables.
The study had about 92 to 95% power of detecting a clinically significant effect, depending on the level of association between the primary endpoints.
Results
Patient characteristics
From August 6, 2012 to June 3, 2013, NRG/GOG member institutions enrolled 23 patients onto this trial. Two patients were subsequently deemed ineligible on GOG central pathology review; therefore 21 patients were evaluable for treatment efficacy. Baseline characteristics are shown in Table 1. The median age at study entry was 61 (range 46-83). Seventy one percent of patients received 1 prior chemotherapy regimen and 29% received two prior chemotherapy regimens. Prior radiotherapy was received by 19% of patients; prior immunotherapy by 10% of patients.
Table 1. Patient Characteristics.
| Characteristic | Category | n | % |
|---|---|---|---|
| Age Group | |||
| 40-49 | 3 | 14.3 | |
| 50-59 | 6 | 28.6 | |
| 60-69 | 8 | 38.1 | |
| 70-79 | 3 | 14.3 | |
| >=80 | 1 | 4.8 | |
| Ethnicity | |||
| Hispanic | 1 | 4.8 | |
| Non-Hispanic | 19 | 90.5 | |
| Unknown | 1 | 4.8 | |
| Race | |||
| White | 15 | 71.4 | |
| Black/African | 1 | 4.8 | |
| Asian | 2 | 9.5 | |
| Native Hawaiian/Pacific Islander | 1 | 4.8 | |
| Other | 1 | 4.8 | |
| Unknown | 1 | 4.8 | |
| Performance Status | |||
| 0 | 18 | 85.7 | |
| 1 | 3 | 14.3 | |
| Prior Chemotherapy | |||
| 1 Prior Regimen | 15 | 71.4 | |
| 2 Prior Regimens | 6 | 28.6 | |
| Prior Radiation | |||
| No | 17 | 81.0 | |
| Yes | 4 | 19.0 | |
| Prior Immunotherapy | |||
| No | 19 | 90.5 | |
| Yes | 2 | 9.5 | |
| Prior Surgery | |||
| Yes | 21 | 100.0 |
Adverse events
As shown in Table 2, safety of alisertib was analyzed descriptively. There were no treatment related deaths. No new safety signals of alisertib were identified. The most commonly reported grade 1-2 adverse events included: thrombocytopenia (n=9), anemia (n=12), nausea (n=6), GI (n=13), General and administration site (n=12), nervous system (n=9), and skin (n=12). The most commonly reported grade ≥3 adverse events included: anemia (n=4), leukopenia (n=5), neutropenia (n=7), thrombocytopenia (n=1), mucositis (n=4), diarrhea (n=1), and palmar-planter syndrome (n=2). Six out of 21 (28.6%) patients required a dose reduction and 1 patient (5%) permanently discontinued alisertib due to adverse events, respectively.
Table 2.
| Grade | |||||||
|---|---|---|---|---|---|---|---|
| AE Category | 0 | 1 | 2 | 3 | 4 | 5 | Total |
| Leukopenia | 9 | 3 | 4 | 5 | 0 | 0 | 21 |
| Thrombocytopenia | 11 | 9 | 0 | 1 | 0 | 0 | 21 |
| Neutropenia | 11 | 0 | 3 | 5 | 2 | 0 | 21 |
| Anemia | 5 | 3 | 9 | 3 | 1 | 0 | 21 |
| Other Investigations | 18 | 3 | 0 | 0 | 0 | 0 | 21 |
| Other Blood/Lymphatics | 19 | 0 | 0 | 1 | 1 | 0 | 21 |
| Cardiac | 20 | 0 | 0 | 1 | 0 | 0 | 21 |
| Nausea | 15 | 3 | 3 | 0 | 0 | 0 | 21 |
| Vomiting | 18 | 3 | 0 | 0 | 0 | 0 | 21 |
| Other Gastrointestinal | 4 | 7 | 6 | 4 | 0 | 0 | 21 |
| General and administration site | 9 | 5 | 7 | 0 | 0 | 0 | 21 |
| Infections/infestations | 19 | 0 | 2 | 0 | 0 | 0 | 21 |
| Metabolism/nutrition | 14 | 1 | 6 | 0 | 0 | 0 | 21 |
| Musculoskeletal/connective tissue | 16 | 3 | 1 | 1 | 0 | 0 | 21 |
| Peripheral sensory neuropathy | 20 | 1 | 0 | 0 | 0 | 0 | 21 |
| Nervous system | 12 | 5 | 4 | 0 | 0 | 0 | 21 |
| Psychiatric | 19 | 2 | 0 | 0 | 0 | 0 | 21 |
| Renal/urinary | 19 | 2 | 0 | 0 | 0 | 0 | 21 |
| Reproductive/breast | 19 | 1 | 0 | 1 | 0 | 0 | 21 |
| Respiratory/thoracic/mediastinal | 16 | 4 | 0 | 1 | 0 | 0 | 21 |
| Skin/subcutaneous | 7 | 5 | 7 | 2 | 0 | 0 | 21 |
| Vascular disorders | 19 | 0 | 1 | 1 | 0 | 0 | 21 |
Activity of alisertib
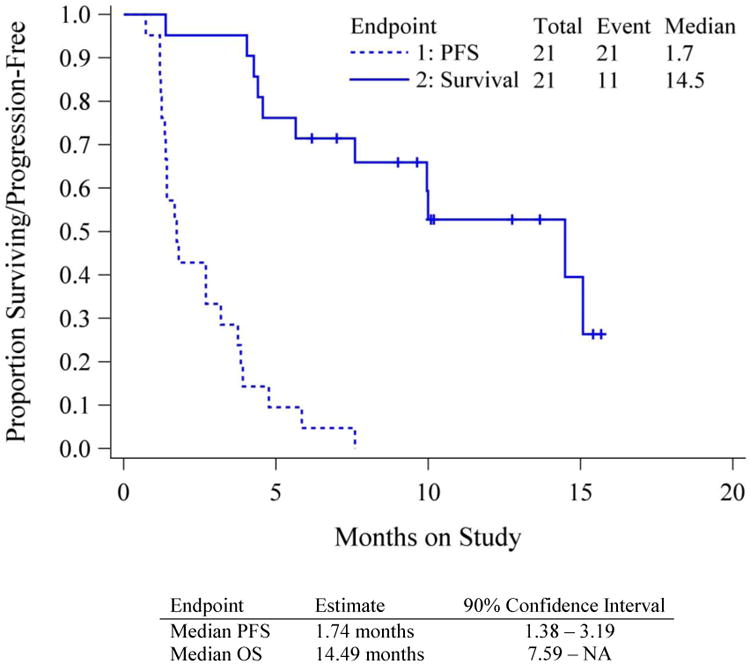
The activity of alisertib was analyzed in 21 patients and is presented in Table 3. At the time of this report all patients are off study treatment with 19 (90%) discontinuing for disease progression. Best overall response was stable disease in 8 (38%). There were no confirmed partial or complete responses. There was only one patient (5%) progression free at ≥6 months. The median progression free survival was 1.7 months (90% CI: CI 1.4-3.2), and median overall survival 14.5 months (90% CI 7.6-NE) as shown in Figure 1. Neither endpoint met its respective criteria for proceeding to the second stage (>2 responses or >6 patients with EFS > 6 months).
Table 3. Response, 6-month PFS, cycle of treatment and status.
| Endpoint | Category | n | % |
|---|---|---|---|
| Off Study Therapy | Yes | 21 | 100 |
| Cycles of Treatment | |||
| 1 | 2 | 9.5 | |
| 2 | 12 | 57.1 | |
| 3 | 4 | 19.0 | |
| 4 | 1 | 4.8 | |
| 5 | 1 | 4.8 | |
| 6 | 1 | 4.8 | |
| Reason Off Therapy | |||
| Disease Progression | 19 | 90.5 | |
| Adverse Events | 1 | 4.8 | |
| Other Reason | 1 | 4.8 | |
| Stable disease | 8 | 38.1 | |
| RECIST Response | |||
| Stable disease | 8 | 38.1 | |
| Increasing disease | 12 | 57.1 | |
| Indeterminate | 1 | 4.8 | |
| PFS > 6 Months | |||
| No | 20 | 95.2 | |
| Yes | 1 | 4.8 | |
| EFS > 6 Months | |||
| No | 21 | 100 | |
| Survival Status | |||
| Alive | 10 | 47.6 | |
| Dead - Disease-related | 9 | 42.9 | |
| Dead - Neither Rx/Dis | 1 | 4.8 | |
| Dead - Reason Pending | 1 | 4.8 |
Figure 1. Progression Free and Overall Survival.
Discussion
The use of alisertib in advanced recurrent/persistent uLMS failed to reach the primary endpoint thresholds for further accrual in this study and has insufficient activity to warrant further investigation as a single agent. Moreover, the use of alisertib was associated with significant, although typically not treatment limiting, toxicity. The potential role for drug combinations incorporating aurora kinase inhibitors into uLMS treatment remains unknown although further investigation may be difficult to justify in the absence of meaningful single agent activity with this class of agents.
The activity of alisertib in soft tissue sarcoma was also explored in a Cancer Therapy Evaluation Program (CTEP)-sponsored phase II study.30 This study included multiple histologically-defined sarcoma subtypes including liposarcoma, leiomyosarcoma (primarily non-uterine), undifferentiated sarcoma, malignant peripheral nerve sheath tumor, and finally all other sarcomas not otherwise specified. A total of 72 patients were enrolled with none of the five cohorts meeting prespecified response criteria necessary to continue to a second stage of accrual. Two partial responses were observed, both in patients with angiosarcoma. The overall safety profile was consistent with our findings. While the study failed to meet its primary response rate endpoint, the twelve-week progression free survival was considered favorable compared to historic controls for some cohorts. However, when combined with our experience reported here, these data suggest that alisertib has limited activity in sarcomas. Despite these disappointing data, other studies in breast cancer, small cell lung cancer, and peripheral T-cell lymphoma have reported promising activity with the use of alisertib.28,31 These findings provide initial validation of the aurora A kinase as a potentially important drug target in certain cancer types.
Significantly improving the outcomes for patients with uLMS will ultimately require an improved understanding of the key drivers of this disease. The genomic complexity associated with this cancer, as well as the apparent absence of recurrent and potentially targetable kinase mutations, suggests that the selective targeting of cell signaling pathways is unlikely to result in the dramatic efficacy observed in some oncogene addicted cancers. With a number of studies demonstrating an association between increased mutational burdens with response to immune checkpoint blockade, it is possible that the genomic complexity of uLMS may make these tumors good candidates for immunotherapy.32-34 However, it remains unclear whether the class of genomic alterations induced by chromothripsis characteristic of these tumors will lead to the same neoadjuvant formation believed to underlie this association. Ultimately determining the utility of immune checkpoint blockade and other novel agents such as those targeting tumor epigenetics will require further clinical studies.
This study continues to demonstrate the importance of the National Institutes of Health (NIH) National Clinical Trials Network (NCTN) for conducting therapeutic studies in very rare tumors that might otherwise go largely uninvestigated.35 This NCTN study accrued 23 patients with advanced and previously treated uLMS over a period of only 10 months, or approximately 2.3 patients per month. Given that fewer then 3,000 cases of uLMS are diagnosed annually in the United States, this enrollment represents a significant accomplishment and demonstrates the unique ability of the NCTN mechanism to rapidly evaluate the efficacy of new drugs for uLMS and other very rare cancers.36,37 This study also demonstrates the continued importance of incorporating central pathology review for studies evaluated treatment in rare cancers. As a result of this central review, 2/23 (8.7%) patients enrolled on the basis of a local pathology diagnosis of uLMS were excluded, thereby avoiding potential confounding of the study.
In summary, this study failed to demonstrate single agent activity of the Aurora kinase inhibitor alisertib in uLMS and does not support further studies of this class of agents in this disease.
Research Highlights.
Uterine leiomyosarcomas are poor prognosis tumors with few effective treatments
Aurora-A kinase deregulations are common in uterine leiomyosarcoma
Alisertib monotherapy was not active in advanced/recurrent uterine leiomyosarcoma
Acknowledgments
This study was supported by National Cancer Institute grants NRG Oncology SDMC grant U10 CA180822 and the NRG Oncology Operations grant U10CA 180868. DH was supported by National Institutes of Healthy grant P30 CA008748. The authors would like to thank Millenium Pharmaceuticals for their support of this research.
The following NRG Oncology/Gynecologic Oncology Group member institutions participated in the primary treatment studies: Memorial Sloan Kettering Cancer Center, University of Hawaii, Abington Memorial Hospital, University of Mississippi Medical Center, University of North Carolina at Chapel Hill, Riverside Methodist Hospital, University of Chicago, Women and Infants Hospital and the Hospital of Central Connecticut.
Footnotes
Parts of this study were presented in abstract form at the ASCO annual meeting held in Chicago, Illinois on 5/29 to 6/2/2015.
Conflicts of Interest: The authors report that there are no conflicts of interest to disclose other than Dr. Hyman who reports grants and non-financial support from PUMA Biotechnology, AstraZeneca and LOXO Oncology. He has also received personal fees from both CytomX and Atara Biotherapeutics and non –financial support from Roche/Genentech outside the submitted work. Additionally, Dr. Carol Aghajanian reports that she has received compensation from Oxigene for a one time Steering Committee meeting in 2016 as well as compensation for serving on a one time Advisory Board in 2014 for Astra Zeneca. Dr. Aghajanian was reimbursed for travel expenses by AbbVie in 2014.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huang CY, Chen CA, Chen YL, et al. Nationwide surveillance in uterine cancer: survival analysis and the importance of birth cohort: 30-year population-based registry in Taiwan. PLoS One. 2012;7:e51372. doi: 10.1371/journal.pone.0051372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zivanovic O, Jacks LM, Iasonos A, et al. A nomogram to predict postresection 5-year overall survival for patients with uterine leiomyosarcoma. Cancer. 2012;118:660–9. doi: 10.1002/cncr.26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iasonos A, Keung EZ, Zivanovic O, et al. External validation of a prognostic nomogram for overall survival in women with uterine leiomyosarcoma. Cancer. 2013;119:1816–22. doi: 10.1002/cncr.27971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hensley ML, Blessing JA, Mannel R, et al. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II trial. Gynecol Oncol. 2008;109:329–34. doi: 10.1016/j.ygyno.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hensley ML, Blessing JA, Degeest K, et al. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II study. Gynecol Oncol. 2008;109:323–8. doi: 10.1016/j.ygyno.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol. 2002;20:2824–31. doi: 10.1200/JCO.2002.11.050. [DOI] [PubMed] [Google Scholar]
- 7.Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected] J Clin Oncol. 2007;25:2755–63. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 8.Sutton G, Blessing JA, Malfetano JH. Ifosfamide and doxorubicin in the treatment of advanced leiomyosarcomas of the uterus: A Gynecologic Oncology Group study. Gynecologic Oncology. 1996;62:226–229. doi: 10.1006/gyno.1996.0220. [DOI] [PubMed] [Google Scholar]
- 9.Omura GA, Major FJ, Blessing JA, et al. A Randomized Study of Adriamycin with and Without Dimethyl Triazenoimidazole Carboxamide in Advanced Uterine Sarcomas. Cancer. 1983;52:626–632. doi: 10.1002/1097-0142(19830815)52:4<626::aid-cncr2820520409>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Muss HB, Bundy B, Disaia PJ, et al. Treatment of Recurrent or Advanced Uterine Sarcoma - A Randomized Trial of Doxorubicin Versus Doxorubicin and Cyclophosphamide (A Phase-Iii Trial of the Gynecologic Oncology Group) Cancer. 1985;55:1648–1653. doi: 10.1002/1097-0142(19850415)55:8<1648::aid-cncr2820550806>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Sutton G, Blessing J, Hanjani P, et al. Phase II evaluation of liposomal doxorubicin (Doxil) in recurrent or advanced leiomyosarcoma of the uterus: a Gynecologic oncology Group study. Gynecologic Oncology. 2005;96:749–752. doi: 10.1016/j.ygyno.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 12.Sutton GP, Blessing JA, Barrett RJ, et al. Phase-II Trial of Ifosfamide and Mesna in Leiomyosarcoma of the Uterus - A Gynecologic Oncology Group-Study. American Journal of Obstetrics and Gynecology. 1992;166:556–559. doi: 10.1016/0002-9378(92)91671-v. [DOI] [PubMed] [Google Scholar]
- 13.Le Cesne A, Blay JY, Judson I, et al. Phase II study of ET-743 in advanced soft tissue sarcomas: a European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin Oncol. 2005;23:576–84. doi: 10.1200/JCO.2005.01.180. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Carbonero R, Supko JG, Manola J, et al. Phase II and pharmacokinetic study of ecteinascidin 743 in patients with progressive sarcomas of soft tissues refractory to chemotherapy. Journal of Clinical Oncology. 2004;22:1480–1490. doi: 10.1200/JCO.2004.02.098. [DOI] [PubMed] [Google Scholar]
- 15.Demetri GD, Chawla SP, von Mehren M, et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedules. J Clin Oncol. 2009;27:4188–96. doi: 10.1200/JCO.2008.21.0088. [DOI] [PubMed] [Google Scholar]
- 16.Mehine M, Kaasinen E, Mäkinen N, et al. Characterization of Uterine Leiomyomas by Whole-Genome Sequencing. New England Journal of Medicine. 2013;369:43–53. doi: 10.1056/NEJMoa1302736. [DOI] [PubMed] [Google Scholar]
- 17.Makinen N, Mehine M, Tolvanen J, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011;334:252–5. doi: 10.1126/science.1208930. [DOI] [PubMed] [Google Scholar]
- 18.Mittal P, Shin YH, Yatsenko SA, et al. Med12 gain-of-function mutation causes leiomyomas and genomic instability. The Journal of Clinical Investigation. 2015;125:3280–3284. doi: 10.1172/JCI81534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perot G, Croce S, Ribeiro A, et al. MED12 alterations in both human benign and malignant uterine soft tissue tumors. PLoS One. 2012;7:e40015. doi: 10.1371/journal.pone.0040015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–86. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 21.Hensley ML, Sill MW, Scribner DR, Jr, et al. Sunitinib malate in the treatment of recurrent or persistent uterine leiomyosarcoma: A Gynecologic Oncology Group phase II study. Gynecologic Oncology. 2009;115:460–465. doi: 10.1016/j.ygyno.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maki RG, D'Adamo DR, Keohan ML, et al. Phase II Study of Sorafenib in Patients With Metastatic or Recurrent Sarcomas. Journal of Clinical Oncology. 2009;27:3133–3140. doi: 10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hensley ML, Miller A, O'Malley DM, et al. Randomized phase III trial of gemcitabine plus docetaxel plus bevacizumab or placebo as first-line treatment for metastatic uterine leiomyosarcoma: an NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol. 2015;33:1180–5. doi: 10.1200/JCO.2014.58.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shan W, Akinfenwa PY, Savannah KB, et al. A small-molecule inhibitor targeting the mitotic spindle checkpoint impairs the growth of uterine leiomyosarcoma. Clin Cancer Res. 2012;18:3352–65. doi: 10.1158/1078-0432.CCR-11-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marumoto T, Zhang D, Saya H. Aurora-A - a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 26.Vader G, Lens SM. The Aurora kinase family in cell division and cancer. Biochim Biophys Acta. 2008;1786:60–72. doi: 10.1016/j.bbcan.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Brewer Savannah KJ, Demicco EG, Lusby K, et al. Dual targeting of mTOR and aurora-A kinase for the treatment of uterine Leiomyosarcoma. Clin Cancer Res. 2012;18:4633–45. doi: 10.1158/1078-0432.CCR-12-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melichar B, Adenis A, Lockhart AC, et al. Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: a five-arm phase 2 study. Lancet Oncol. 2015;16:395–405. doi: 10.1016/S1470-2045(15)70051-3. [DOI] [PubMed] [Google Scholar]
- 29.Sill MW, Rubinstein L, Litwin S, et al. A method for utilizing co-primary efficacy outcome measures to screen regimens for activity in two-stage Phase II clinical trials. Clin Trials. 2012;9:385–95. doi: 10.1177/1740774512450101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickson MA, Mahoney MR, Tap WD, et al. Phase II study of MLN8237 (Alisertib) in advanced/metastatic sarcoma. Ann Oncol. 2016;27:1855–60. doi: 10.1093/annonc/mdw281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barr PM, Li H, Spier C, et al. Phase II Intergroup Trial of Alisertib in Relapsed and Refractory Peripheral T-Cell Lymphoma and Transformed Mycosis Fungoides: SWOG 1108. J Clin Oncol. 2015;33:2399–404. doi: 10.1200/JCO.2014.60.6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–11. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schott AF, Welch JJ, Verschraegen CF, et al. The National Clinical Trials Network: Conducting Successful Clinical Trials of New Therapies for Rare Cancers. Semin Oncol. 2015;42:731–9. doi: 10.1053/j.seminoncol.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueda SM, Kapp DS, Cheung MK, et al. Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. Am J Obstet Gynecol. 2008;198:218 e1–6. doi: 10.1016/j.ajog.2007.08.075. [DOI] [PubMed] [Google Scholar]
- 37.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: A Cancer Journal for Clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]


