SUMMARY
Unexpected events are part of everyday experience. They come in several varieties – action errors, unexpected action outcomes, and unexpected perceptual events – and they lead to motor slowing and cognitive distraction. While different varieties of unexpected events have been studied largely independently, and many different mechanisms are thought to explain their effects on action and cognition, we suggest a unifying theory. We propose that unexpected events recruit a fronto-basal-ganglia network for stopping. This network includes specific prefrontal cortical nodes and is posited to project to the subthalamic nucleus, with a putative global suppressive effect on basal-ganglia output. We argue that unexpected events interrupt action and impact cognition, partly at least, by recruiting this global suppressive network. This provides a common mechanistic basis for different types of unexpected events, links the literatures on motor inhibition, performance-monitoring, attention, and working memory, and is relevant for understanding clinical symptoms of distractibility and mental inflexibility.
Keywords: Errors, Motor inhibition, Unexpected events, Surprise, Attention, Novels, Distraction, Cognitive Control, Working Memory
A UNIFIED THEORY OF UNEXPECTED EVENTS
There are different varieties of unexpected events. For example, while driving a car, we sometimes accidentally activate the windscreen wipers when reaching for something else (FIGURE 1A). Here, an action error leads to an unexpected event, which necessitates an interruption and changing of the ongoing behavior: instead of placing our hand back on the wheel, we have to turn off the wipers. A second type of unexpected event is an unexpected action outcome – this can arise even when an action is performed without error, such as when aspects of the external environment behave unpredictably. For example, a failure in power steering can unexpectedly alter the planned outcome of turning the steering wheel (FIGURE 1B). As in the action error example, ongoing behavior needs to be interrupted and changed. Finally, some unexpected events are not directly related to our actions, but are instead unexpected perceptual events – for example, a deer jumping into the street in front of the car, which also necessitates an interruption and adaptation of ongoing actions (FIGURE 1C). Unexpected events occur in everyday life in many different situations and for many actions, such as speaking, typing, walking, or playing sports.
Figure 1.
Three kinds of unexpected events. Left: Action error (self-produced unexpected event). Middle: Unexpected action outcome (unexpected event produced by the interaction between self and environment). Right: Unexpected perceptual event (unexpected event produced in the environment).
Although all three types of events (action errors, unexpected action outcomes, unexpected perceptual events) are rooted in different aspects of the psychological environment (internally-vs. externally-generated, action-related or action-independent) they all share the common quality of being surprising. Moreover, they all may necessitate a change in behavior and cognitive processing to avoid immediate danger. Note that in most unexpected perceptual event tasks (e.g., Parmentier et al., 2008; Wessel and Aron, 2013) surprising events are not relevant to the action per se (i.e., they are not informative in regards to which response has to be made); but are informative in regards to the timing of the appearance of the imperative stimulus – on each trial, the tone carries information about the impending occurrence of the stimulus. In that sense, the unexpected perceptual events are task-relevant. In contrast to this, there is a fourth type of unexpected event in the literature – namely, unexpected perceptual events that are completely task-irrelevant. For example, instead of a deer jumping out in front of your car (task-relevant unexpected perceptual event), a deer could just unexpectedly stand outside the kitchen window (Summerfield and de Lange, 2014).
Much research has investigated the psychological and neural processes of these different types of unexpected events. However, these literatures are largely separate. Here, we attempt to provide a unified mechanistic theory of how behavior and cognition are interrupted by unexpected events. Specifically, we propose that all unexpected events recruit the same brain mechanism that has heretofore been characterized as responsible for the outright stopping of action (i.e., motor inhibition). We argue that the engagement of this mechanism leads to the interruption of behavior, and is also (at least partly) responsible for interruptions of cognition. This could help ‘refresh’ the current cognitive state, which could allow new information to be processed more efficiently.
We begin by describing foundational issues in the behavioral and neural consequences of action errors, unexpected action outcomes, and unexpected perceptual events. Based on results from computerized tasks in the lab, we argue that there is a strong overlap between all three. We then describe a fronto-basal-ganglia brain network for outright stopping of action. We highlight recent studies showing that this same network is active following all three types of unexpected events, and that its activity relates to the resultant motor slowing and cognitive interruption. This motivates our novel theory of how behavior and cognition are interrupted by unexpected events. We discuss the neurobiology of the putative underlying frontal-basal-ganglia system, the relation to attention, alternative interpretations of the data that might challenge the theory, and clinical implications.
MOTOR SLOWING FOLLOWING UNEXPECTED EVENTS
Recent research points to common behavioral and cognitive effects for action errors, unexpected action outcomes, and unexpected perceptual events. These include a reactive slowing of reaction times (RT), and an impairment of ongoing cognitive processing. In the first section of this review we address the motor slowing. In a later section entitled Unexpected Events May Affect Cognition Via the Stopping System, we address the interruption of cognition.
Motor slowing after action errors
Motor slowing after errors was first reported by Rabbitt (1966) and Laming (1968). Participants performed speeded forced-choice manual RT tasks (FIGURE 2A). Compared to the mean RTs of all correct responses, the RTs of correct responses following trials with an erroneous response were significantly increased. This basic behavioral pattern is known as post-error slowing (PES). Beyond forced-choice reaction time tasks, PES occurs for mental rotation (Band and Kok, 2000), for perceptual decision making (Danielmeier et al., 2011), lexical decision making (Dutilh et al., 2012), typewriting (Logan and Crump, 2010), and many other tasks. In addition to manual responses, PES occurs after erroneous saccades (Endrass et al., 2007), and after responses made with the feet. PES is also observed across age-groups (Brooker and Buss, 2014; Friedman et al., 2009) and species (Jedema et al., 2011; Narayanan and Laubach, 2008). Theories of PES can be coarsely grouped into ‘adaptive’ theories, proposing that PES is aimed at improving ongoing behavior, and ‘maladaptive’ theories, proposing that PES signifies behavioral impairment caused by an error (for detailed reviews, see Danielmeier and Ullsperger, 2011; Ullsperger et al., 2014).
Figure 2.
Behavioral tasks, and EEG and fMRI activity associated with three kinds of unexpected event. Top row shows typical tasks used to study each type of event and typical behavioral results (slowing of reaction time after the unexpected event). Middle row shows typical scalp EEG activity, highlighting the presence of a negativity-positivity complex waveform with fronto-central distribution after each type of event. Bottom shows typical brain networks activated (BOLD response) after all three types of events, including areas strongly implicated in motor stopping such as the right inferior frontal cortex (rIFC), the presupplementary motor area (preSMA) and the region in the vicinity of the subthalamic nucleus (STN). Figures reproduced based on data from Danielmeier et al., 2009; Wessel et al., 2012, Wessel & Aron, 2013, Kiehl et al., 2005.
Adaptive Theories
Adaptive theories can be grouped by whether their proposed mechanism applies to the perceptual level, the motor level, or their interaction.
Laming (1968) proposed that the processing of perceptual information after errors is delayed to avoid unnecessary, potentially interfering information that could lead to further errors. This was based on the assumption that people start sampling information even in the absence of upcoming task-relevant stimuli. If this information-accumulation starts before the task-relevant stimulus it could lead to premature, potentially erroneous responses. Hence, a delay of processing (i.e., PES) is adaptive.
A different, currently influential, theory suggests that after an error, a speed-accuracy trade-off is enacted. This is done by increasing the motor threshold – i.e., the amount of sensory evidence required to initiate a motor response (Botvinick et al., 2001). For example, drift-diffusion modeling shows that a behavioral parameter reflecting response caution is increased following errors (Dutilh et al., 2012). While the mechanism proposed in Laming’s perceptual delay theory operates purely on the perceptual level, the speed-accuracy theory concerns the interaction between perceptual and motor systems.
A third theory of PES pertains entirely to the motor level (Ridderinkhof, 2002; Ridderinkhof et al., 2002). It proposes that errors increase the amount of motor inhibition on the next trial. This is evidenced by the fact that slower responses after errors exhibit a stronger reduction of interference from task-irrelevant stimuli. The authors interpret this as a selective suppression of the inappropriate response tendency after an error, specifically on trials with greater PES.
Maladaptive Theories
Maladaptive theories postulate that PES does not index a directed cognitive control effort aimed at improving behavior after errors, but instead reflects impaired processing after errors. A prominent theory proposes that errors are followed by an orienting response (Notebaert et al., 2009) – i.e., a cascade of autonomic and central nervous system activity in response to an unexpected change in the environment (Sokolov, 1963). According to this theory, PES is an index of this orienting response, which occurs after errors, but also after less frequent trial types in general. Indeed, in experiments in which correct responses were less frequent than errors, the slowing effect was reversed, now occurring after correct responses (Notebaert et al., 2009).
A second maladaptive theory suggests that PES occurs because error processing is a resource-intensive process (Jentzsch and Dudschig, 2009). It thus interferes with the processing of the subsequent stimulus, thereby slowing RT. Evidence for this ‘bottleneck’ account comes from the fact that PES is significantly larger when the inter-trial interval is short, whereas when more time is given to process an error (longer inter-trial intervals), PES is reduced.
Bridging adaptive and maladaptive theories
The key difference between adaptive and maladaptive theories is the prediction they make regarding post-trial accuracy. According to maladaptive theories, errors should impair post-trial accuracy, whereas for adaptive theories PES signifies a mechanism that improves post-trial accuracy. While several studies have tried to adjudicate between these competing theories, the evidence is inconclusive. Some studies show that accuracy increases after errors, while others show the reverse (this is reviewed in detail elsewhere, e.g., Danielmeier and Ullsperger, 2011, and we will return to this issue later).
To presage our theory, we propose – like Ridderinkhof et al. – that errors trigger motor inhibition. However, whereas that theory suggested that motor inhibition is targeted specifically at the inappropriate response on the next trial, we argue that the inhibition that is triggered by errors (and other unexpected events) is global at the time it occurs (i.e., broadly impacts the motor system and also the cognitive system), and that it is likely short-lived. Consequently, according to our theory, the effects on the next trial do not relate to lingering motor suppression, but rather to momentary disruptions of the task set. As we will argue, this theory can potentially bridge the perceived chasm between maladaptive and adaptive theories of PES.
Motor slowing after unexpected outcomes of correct actions
Motor slowing also follows a second type of unexpected event, namely, unexpected action outcomes. In studies of unexpected action outcomes, participants usually receive action feedback after each response, which is then altered in an unpredictable fashion following some correct responses (FIGURE 2B). For example, in a flanker task, visual action feedback was provided immediately after the pressing of a response button, regardless of response accuracy (Gentsch et al., 2009). On a minority of correct trials, however, this feedback was unexpectedly absent (the explanation to participants was that responses would not always register due to a hardware malfunction). Importantly, such trials were followed by slower responses compared to correct trials with regular feedback.
Similar post-trial slowing was also found when the feedback was unexpectedly altered (instead of unexpectedly absent). In a series of studies, participants performed a hybrid flanker-novelty/oddball task, in which they responded to flanker stimuli and were asked to actively monitor the action feedback. While this was usually an upward triangle displayed immediately after response emission, the participants were monitoring for rare target events (a downward triangle) (Wessel et al., 2012; Wessel et al., 2014a). On some correct trials, however, the visual response feedback was instead a completely unexpected ‘novel’ stimulus (pictograms of everyday objects). Across all three studies, participants exhibited significantly increased RTs on trials following these unexpected action effects.
Together, these studies show that unexpected action outcomes of otherwise correct responses can lead to post-trial slowing akin to PES. Furthermore, they show that both the unexpected absence and the unexpected presence of action outcomes lead to post-trial slowing.
Motor slowing following unexpected, action-independent perceptual events
A largely separate literature has described motor slowing after a third type of unexpected event, namely, unexpected perceptual events that are not related to (or produced by) any specific action. This has been studied with simple tasks such as dichotic listening (Schroger, 1996) or cross-modal oddball/novelty paradigms (Parmentier et al., 2008) (FIGURE 2C). In such paradigms, a motor response is made to an imperative stimulus, which is preceded by a tone that signals the imperative stimulus’ impeding occurrence. If this ‘warning’ tone is replaced by an unexpected, ‘novel’ event (e.g., a tone of different frequency, Dawson et al., 1982), RT to the imperative stimulus is significantly increased. We refer to this effect as post-novelty slowing (PNS). In addition to auditory events, PNS occurs after unexpected visual (Berti and Schroger, 2004) and haptic events (Ljungberg and Parmentier, 2012; Parmentier et al., 2011). PNS occurs for manual responses (as above), and also verbal responses (Wessel and Aron, 2013). Behavioral studies aimed at identifying the nature of PNS have shown that it occurs specifically because unexpected perceptual events are surprising, and not just because they are rare (Bendixen et al., 2007; Bendixen and Schroger, 2008; Parmentier, 2014).
NEURAL CORRELATES OF UNEXPECTED EVENTS
Above, we showed that motor slowing is a ubiquitous behavioral response to unexpected events, regardless of whether they are caused by ourselves (action errors), by our interaction with the environment (unexpected action outcomes), or entirely by the environment (perceptual novelty). In the following, we review neural activity elicited by each type of unexpected event – showing that, just as for the behavioral effects, the neural substrates overlap. Crucially, as we will show, that overlap includes a well-characterized, interconnected network of brain regions that underlies a neural mechanism for action-stopping.
Event-related potentials (ERPs)
ERPs are derived from scalp EEG. They offer a temporally precise window into brain activity following specific experimental events. The ERP method flourished in the 1960s, and ERPs specific to the processing of unexpected perceptual events were among the first to be discovered. Courchesne and colleagues (1975) first showed that unexpected perceptual events elicited a complex waveform over medial prefrontal cortex, consisting of a negative deflection (the N2b) followed by a positive deflection (the P3). The N2b/P3 complex has a fronto-central radial topography on the scalp (with the N2b somewhat more anterior) and has been a valuable index of the central nervous response to perceptual novelty (reviewed by Friedman et al., 2001) (FIGURE 2F).
Interestingly, the other types of unexpected events (errors and unexpected action outcomes) have subsequently been found to evoke similar fronto-central voltage potentials, in which an initial negativity is followed by a subsequent positivity. For action errors, the early 1990s also saw the discovery of a negativity (named the ‘error-related negativity, ERN’, Falkenstein et al., 1991; Gehring et al., 1993) and a subsequent positivity (named the ‘Pe’, Falkenstein et al., 2000), which peak at the same scalp sites as the N2/P3-complex, albeit with different timing (FIGURE 2D).
Unexpected action outcomes show a similar ERP response. Our studies on unexpectedly altered action feedback (Wessel et al., 2012; Wessel et al., 2014a) showed a fronto-central N2/P3-complex after unexpected action outcomes (FIGURE 2E). These latter studies went further by directly comparing two kinds of unexpected events, and showed that the ERN/Pe-complex after action errors and the N2/P3-complex after unexpected action outcomes are non-independent brain components (i.e., they are produced by the same neural generator).
In summary, all three types of unexpected events elicit a stereotypic ERP response: a fronto-central negativity followed by a positivity. Moreover, a direct comparison of two kinds of unexpected events revealed a common EEG generator – pointing to the same underlying brain network, which is supported by the fMRI evidence presented next.
fMRI
Early fMRI studies of action errors were motivated by attempts to source-localize the abovementioned error-related negativity to a specific brain area, and pointed towards a generator in the dorso-medial prefrontal cortex, as suggested by ERP source localization (Dehaene et al., 1994). Carter and colleagues (1998) found increased BOLD activity on errors compared to correct trials in medial prefrontal cortex (BA 24/32), lateral prefrontal cortex (specifically right-lateral BA 44/45, right and left BA9, and left BA 46), and in premotor cortex (BA6). The anatomical location of the exact medial frontal region activated by errors varies across studies, which is partially a function of different naming conventions preferred by different authors. Names for what are largely the same – or overlapping – areas included (dorsal) anterior cingulate cortex, cingulate motor area, rostral cingulate zone, pre-supplementary motor area, and (anterior) midcingulate cortex (Agam et al., 2011; Hester et al., 2004; Holroyd et al., 2004; Mars et al., 2005; Picard and Strick, 1996; Ridderinkhof et al., 2004; Ullsperger and von Cramon, 2001). Terminology aside, after action errors, BOLD activity of lateral prefrontal (including right inferior frontal cortex) and posterior medial frontal regions (Ridderinkhof et al., 2004; Ullsperger et al., 2010) is clearly increased (FIGURE 2G).
Notably, a similar picture emerges for unexpected action outcomes. Unexpected action feedback engages the medial and lateral PFC (including right inferior frontal cortex), the anterior insula, and several subcortical brain regions (Wessel et al., 2012) (FIGURE 2H). This study used a conjunction analysis of fMRI data to test the hypothesis that the brain regions underlying the processing of action errors and unexpected action outcomes overlap. Significant overlap was found in the medial PFC/pre-supplementary motor area (pre-SMA), the bilateral infero-lateral PFC, the anterior insula, and several subcortical brain regions (possibly including the subthalamic nucleus, STN).
Finally, unexpected perceptual events also activate highly similar brain regions (FIGURE 2I). Across two studies, widespread prefrontal BOLD activation followed both visual and auditory perceptual unexpected events (Kiehl et al., 2001; Kiehl and Liddle, 2001). In a large dataset of N = 100, this included the bilateral inferior frontal gyri, the bilaterial anterior insula, and the dorso-medial PFC (Kiehl et al., 2005). Accordingly, another study showed that unexpected perceptual events, regardless of sensory modality (visual, auditory, haptic), engage these same brain regions, as well as the right temporo-parietal junction (Downar et al., 2002) (see for meta-analysis Levy and Wagner, 2011). This led to a popular proposal of a right-lateralized ventral frontoparietal ‘circuit breaker’ (Corbetta and Shulman, 2002), which we will revisit in later sections.
In summary, unexpected events of all three types engage overlapping brain regions. Strikingly, these are the same brain regions that have been consistently implicated by action stopping, which we cover next.
THE BRAIN’S NETWORK FOR GLOBAL MOTOR STOPPING
We now summarize a considerable literature on the neural basis of rapid action stopping. Of particular importance are observations that rapid action stopping has global motor effects. This sets the stage for our overarching theory that unexpected events affect cognition via this putative global suppressive system.
Brain signatures of stopping
In the laboratory, stopping is typically measured using the stop-signal paradigm (Logan and Cowan, 1984). On each trial, subjects initiate an action (go). On a minority of trials, they have to try to cancel that response (stop) following a stop-signal. It is possible to derive the speed of stopping, known as stop signal reaction time (SSRT). Much research across species has focused on the neural correlates of going and stopping (reviewed recently by Bari and Robbins, 2013; Jahanshahi et al., 2015; Kenemans, 2015; Schall and Godlove, 2012; Zavala et al., 2015). Stopping can be done in different ways, but when done quickly and reactively (as in the standard paradigm), the underlying brain system includes the right inferior frontal cortex (rIFC), the pre-SMA, and STN of the basal ganglia, with downstream effects on pallidum, thalamus and primary motor cortex (FIGURE 3A). As reviewed in the above papers, this is shown by fMRI, TMS, brain lesion, single unit recording, EEG, electrocorticography, and local field potential recordings from the STN in patients with Parkinson’s disease.
Figure 3.
Proposed Human Brain Circuit for Rapid Action Stopping. A) Top. Lesion, imaging and stimulation studies point to the right inferior frontal cortex (IFC) and the presupplementary motor area (preSMA) as prefrontal nodes for triggering stopping. Bottom. Right IFC (and preSMA) project via hyperdirect pathways to the subthalamic nucleus (STN). The STN is proposed to divergently excite Globus Pallidus Pars Interna (GPi), which in turn suppresses thalamocortical drive (i.e. there is reduced drive to primary motor and premotor cortex). Panels B–E represent cross-species evidence for role of STN in stopping. B) Human fMRI shows activation in vicinity of right STN for stop signal task, reproduced from Aron and Poldrack 2006, with permission from authors, permission from journal pending. White line demarcates approximate STN region based on hypointensity on structural scan. C. Single unit activity increases ~200 ms after NoGo cue in monkey prepotent Go/NoGo task, reproduced from Isoda and Hikosaka 2008, with permission from authors, permission from journal pending. D. The local field potential recorded from human STN shows a beneath-baseline reduction on Go trials (movement), and an above baseline increase on successful stop trials ~ 200 ms after the stop signal (dotted line), reproduced with permission from Ray et al. 2012. E. For rat stop signal task, STN single unit activity increases for both successful and failed stop trials, but the target (substantia nigra, akin to primate GPi) increases about 16 ms later only on successful but not failed stop trials, reproduced with permission from Schmidt et al. 2013.
The scalp EEG signatures of stopping are well-established (reviewed by Kenemans, 2015). For ERPs, successful stopping is indexed by a fronto-central P3 wave (Kok et al., 2004), whereas in time-frequency space, it corresponds to a increased power of oscillations in the delta- (2–4 Hz) and low-theta (5–8Hz) frequency bands (Huster et al., 2013). Moreover, the ERP signature is aligned in time to the supposed period when motor inhibition occurs (Wessel and Aron, 2015), and likely reflects the pre-SMA portion of the stopping network (Albert et al., 2013; Huster et al., 2011).
Rapid Action Stopping Generates Broad Skeletomotor Suppression
Two lines of evidence show that rapid action stopping has global motor effects: behavior and studies with Transcranial Magnetic Stimulation (TMS).
Behavioral Evidence
In one version of the stop-signal paradigm, participants prepare to move both their hands together. On go-trials, the two hands have to respond simultaneously, while on stop-trials, the stop-signal indicates which specific hand has to stop (while the other hand continues to respond). Several studies with this paradigm show significantly delayed RT for the continuing hand (Claffey et al., 2010; Coxon et al., 2007). This is consistent with a global stop/pause, requiring partial re-initiation of the continuing response (Aron, 2011; Bissett and Logan, 2014). Note that there are circumstances under which subjects can stop selectively rather than globally – but that is beyond the current scope (Aron, 2011).
TMS
Another way to test whether action stopping has global effects is to measure motor representations from task-irrelevant effectors. For example, the TMS coil can be placed over the motor representation of the leg, while the subject performs a task with the hand. Discharging the coil elicits the motor evoked potential (MEP) in the target muscle electromyogram, indexing corticospinal excitability (reviewed by Bestmann and Duque, 2015)) (FIGURE 4A). The first study along these lines showed that when subjects stopped their hand, there were reduced MEPs from the leg (Badry et al., 2009). This has now been replicated many times for stopping of hands, speech, and even eyes (Cai et al., 2012; Greenhouse et al., 2012; Majid et al., 2012; Wessel et al., In press; Wessel et al., 2013) (FIGURE 4B), also see (Coxon et al., 2006; MacDonald et al., 2014; Sohn et al., 2002) for similar results with related paradigms. Together, these studies clearly show that rapidly stopping one effector broadly affects the skeletomotor system. While the neural basis of this effect is still unknown, we suggest below that it relates to the impact of the STN on basal ganglia output. On this account, the stop-signal activates the STN, which broadly excites the medial global pallidus, thereby suppressing the thalamus, and temporarily interrupting thalamocortical drive – which decrements primary motor cortical representations (Stinear et al., 2009). Thus, when M1 is assayed by TMS, the reduction in drive to a particular (task irrelevant) representation is ‘read-out’ in the muscle as reduced excitability.
Figure 4.
Global motor suppression during stopping and following unexpected (perceptual) events. A) Methods of Transcranial Magnetic Stimulation (TMS). A TMS pulse over M1 induces corticospinal volleys which induce the motor evoked potential (MEP) in the hand. B) Stopping in the stop signal paradigm has global motor effects. All plots show the MEP from of a task-unrelated target muscle on the y-axis, split by trial types in the stop-signal task. Figures reproduced based on data from Wessel et al., 2013a, Majid et al., 2012, Cai et al., 2012. C) Increased global motor suppression measured with TMS relates to increased beta band power recorded from the STN. Simultaneous recordings of the MEP from the task-unrelated hand and the local-field potential (LFP) from the STN in Parkinson’s patients with a deep-brain stimulation electrode. Data reproduced from Wessel et al., in press. D) Global motor suppression from a task-unrelated effector is also evident following unexpected events. Figures reproduced based on data from Wessel & Aron, 2013.
The STN is implicated in rapid action stopping
We assume that the STN implements broad motor suppression during stopping. We now lay out the evidence for this, starting with data showing the STN is involved in motor stopping (see for larger recent reviews Jahanshahi et al., 2015; Zavala et al., 2015) (Figure 3B–E).
Human Studies
In fMRI, stop trials activate a region in the mid-brain in the vicinity of the STN (Aron and Poldrack, 2006) (also see Jahfari et al., 2011; Li et al., 2006; Rae et al., 2015b). However, due to the small size of the STN, 3 Tesla fMRI may not convincingly demarcate it from neighboring structures (de Hollander et al., 2015). Nonetheless, there is converging evidence for the role of the STN from several other methods. Clinically-induced lesions to the STN in patients (subthalomotomies) affected stopping (Obeso et al., 2014) and several studies have shown the impact of STN deep brain stimulation (DBS) on stopping. Three of these studies found a speeding of stopping (i.e., SSRT) when stimulation was bilateral (Mirabella et al., 2011; Swann et al., 2011; van den Wildenberg et al., 2006) while one did not (Obeso et al., 2013). A caveat with these studies is that DBS and lesions are also likely to have remote effects (e.g., stimulation propagates through the wider network connected to the STN, so that such results cannot be taken to specifically implicate the STN itself). Further evidence, however, comes from STN local field potentials (LFPs) in Parkinson’s patients. Such studies generally show an increase in the power of oscillations in the beta band (13–30 Hz), before SSRT, that is greater for successful vs. failed stop trials (Bastin et al., 2014; Kuhn et al., 2004; Ray et al., 2009; Wessel et al., In press) (also see for single unit evidence Bastin et al., 2014; Benis et al., 2016)}. Note though, that while the STN beta-power increase is a well-established marker of stopping, it may not be specific to the STN itself (Leventhal et al., 2012). Overall, notwithstanding interpretative limitations, convergent evidence clearly implicates the human STN in stopping. This is further augmented by non-human studies and connectivity evidence discussed in the following.
Non-human studies
STN single-unit activity in non-human primates is increased in two types of tasks that putatively involve action-stopping, specifically, saccade countermanding and Go-NoGo (Isoda and Hikosaka, 2008). Interestingly, this STN single-unit activity was preceded by single-unit activity in the preSMA by about 10 ms, which is consistent with a possible hyperdirect cortico-STN pathway (see below). In rats, single unit recordings show that the STN is active soon after the stop signal, and, further that the firing rate of the downstream target of the STN, the substantia nigra, increased about 30 ms later (Schmidt et al., 2013). This is consistent with the idea that an STN-mediated stopping process can cancel an impending go process by reversing the neuronal firing patterns of the pallidum. Several other lesion studies of the STN have also documented impulsive responding in rodents, perhaps compatible with a stopping deficit (reviewed by Eagle et al., 2008).
The STN is connected with cortical areas known to be critical for stopping
As mentioned above, lesion, TMS, stimulation and other studies have established that two critical cortical regions for rapid action stopping are the rIFC and the preSMA (reviewed by Aron et al., 2014; Bari and Robbins, 2013). Early monkey tract tracing showed that likely homologues of these prefrontal areas connect to the STN (Inase et al., 1999) (Nambu et al., 1997) (also see Haynes and Haber, 2013). Several human studies using diffusion tensor imaging have also reported such connections, and some have shown that white matter variability in these connections relates to the speed of stopping (Aron et al., 2007; Coxon et al., 2012; Danielmeier et al., 2011; Forstmann et al., 2012; Rae et al., 2015a; Xu et al., 2016). These specific connections provide an anatomical basis for a putative ‘hyperdirect pathway’ by which the STN can be quickly activated (Nambu et al., 2002).
Taken together, these human and non-human studies provide converging evidence that rapid action stopping recruits the STN. This may be shown more definitively in future causal manipulation studies, e.g., using optogenetics.
Anatomical basis of broad STN effect on basal ganglia output
Influential models of the basal ganglia have long proposed that the STN is part of a suppression system. Mink (1996) proposed that action initiation first involves a rapid recruitment of the STN (via the hyperdirect pathway), which broadly suppresses all competing motor programs (by widely activating GPi, thereby suppressing thalamocortical drive). In this model, the recruitment of the STN is followed by direct pathway activation, which can then select the desired response by disinhibiting the relevant thalamocortical program (Nambu et al., 2002). What this and related ideas (Wiecki and Frank, 2013; Zavala et al., 2015) have in common is that the STN has a broad suppressive effect, not just on the currently engaged action, but on alternatives, competitors, and even on irrelevant response representations. For the STN to do this, it has to have a broad effect on its target structure, the GPi.
There are three kinds of evidence for this idea. First, there is the dramatic impact of lesions of the STN on the contralateral parts of the body (known as hemiballismus). In monkeys, even relatively small lesions (~20% of the STN) produce violent, ballistic, movements of contralateral limbs (Carpenter et al., 1950; e.g. Hamada and DeLong, 1992). The fact that a lesion of such a small nucleus can have such a dramatic effect points toward broad suppressive capabilities of the STN. Second, the STN-to-GPi projection appears to be relatively divergent – tracing studies from STN-GPi vs. striatum-GPi showed that STN neurons have a broader innervation (Parent and Hazrati, 1993). Third, based on the finding of widespread STN collaterals and computational modeling, it has been theorized that activation of any one part of the STN ramifies throughout the nucleus, leading to a “massive suppressive pulse” on basal ganglia output (Gillies and Willshaw, 1998).
In summary, the evidence that the STN has a broad suppressive effect on basal ganglia output is still rather preliminary. Nevertheless, this remains an influential idea, and, pending further anatomical data, remains a viable working hypothesis upon which our theory are based.
Linking STN activity to broad motor suppression
We saw that: a) rapid stopping of one effector impacts the wider skeletomotor system, b) stopping implicates the STN, and c) some anatomical data suggest the STN has a broad effect on basal ganglia output. Taken together, this suggests that the recruitment of the STN is responsible for the broad motor suppression. In a recent study, we provided correlative evidence for this framework (Wessel et al., In press) (FIGURE 4C). A group of STN-DBS patients performed a vocal stop-signal task while the MEP was measured from the task-irrelevant hand. Consistent with many studies above, the MEP from the hand was significantly reduced during successful vocal stopping (signifying global motor suppression). Furthermore, STN beta-band power was increased on successful vs. failed stop trials, also consistent with several studies reviewed above. Crucially, the global motor suppression was linked to stopping-related STN activity: On successful stop trials with greater stop-related beta-band power, there was a greater global motor suppression (the hand-MEP was reduced). This study therefore directly relates an STN signature of stopping to global motor suppression of the MEP. While this is consistent with the theory that the STN implements the broad motor suppression found during stopping, we recognize that a third process could potentially cause both of these effects. Future research with causal approaches may provide more concrete evidence.
ROLE OF GLOBAL STOPPING NETWORK IN SLOWING AFTER UNEXPECTED EVENTS
We begin by presenting evidence that a fronto-basal-ganglia system for outright stopping is recruited following both action errors and unexpected action outcomes. We then turn to unexpected perceptual events, where the theory that the stopping-network is responsible for surprise-related slowing has been tested more directly.
Post-error-slowing (PES) and slowing after unexpected action outcomes
In the section Neural Correlates of Unexpected Events, we saw that two key cortical regions of the stopping network – the preSMA and the rIFC – are active following action errors and unexpected action effects (and also unexpected perceptual events), FIGURE 2. We now review evidence that relates activity in each of these regions, and the STN, to the motor slowing that occurs following both action errors and unexpected action outcomes.
Regarding the preSMA, it is often argued that its functional role is to resolve response-conflict (Garavan et al., 2003; Nachev et al., 2007; Taylor et al., 2007; Ullsperger and von Cramon, 2001, 2003), while error-specific activity is represented more ventrally in the medial PFC, e.g., in anterior midcingulate or dorsal anterior cingulate cortex (reviewed by Shenhav et al., 2013; Ullsperger et al., 2014). However, several studies also showed error-specific activity within the preSMA itself (see meta-analyses by Hester et al., 2004; Ridderinkhof et al., 2004). More importantly, multiple fMRI studies of error-processing showed that preSMA activity covaries with PES. One such study investigated PES following erroneous eye-movements (Klein et al., 2007). Using PES as a covariate in the error vs. correct-trial BOLD contrast, the preSMA was the only brain region whose activity correlated with PES. Another study extended this using a dot-motion paradigm to investigate several post-error behaviors, amongst them PES (Danielmeier et al., 2011). Using diffusion tensor imaging, it was found that white-matter integrity, again specifically of the preSMA region, correlated with PES. Furthermore, probabilistic tractography using the preSMA as a seed region, showed that the PES-related preSMA hotspot was directly connected via fiber tracts to the STN region.
Regarding the rIFC, two fMRI studies showed right lateralized activity in error vs. correct trial contrasts (Marco-Pallares et al., 2008; Wessel et al., 2012). In a third study, rIFC activity correlated with the amount of PES across subjects (King et al., 2010). Additionally, ischemic lesions to the rIFC have been shown to significantly reduce PES in comparison to healthy controls (Molenberghs et al., 2009).
Regarding the putative target of these frontal nodes, two other studies of PES recorded the STN-LFP in DBS patients (Cavanagh et al., 2014; Siegert et al., 2014). Both studies showed that STN activity was increased following errors, and related to PES on the next trial (the studies differ slightly in the time window at which they identify STN activity that predicts PES; Siegert et al. showed that the phase-locked LFP at around 300ms following the erroneous response correlated with PES, while Cavanagh et al. showed that the event-related spectral perturbations in the theta frequency immediately before the next response related to PES). While changes in the STN-LFP do not prove that it is the STN itself that implements the function, these findings for the STN are consistent with the data from the stopping studies reviewed above.
A link between the stopping network and PES is also suggested by common signatures in the beta frequency band (13 to 30 Hz). Electrocorticography and STN LFP studies have shown such beta-band power increases in the rIFC, preSMA and the STN during stopping (Kuhn et al., 2004; Ray et al., 2009; Swann et al., 2011; Swann et al., 2009), and STN beta band increases also relate, as we saw, to the stop-induced global suppression of the skeletomotor system (Wessel et al., In press). These same beta-band signatures are also found after errors and unexpected action outcomes, moreover to an extent that is predictive of PES. In one study, increased post-error fronto-central beta-band activity on the scalp predicted PES (Marco-Pallares et al., 2008).
The evidence concerning unexpected action outcomes is much less, however, one study direcly compared these with action errors. It showed increased right-lateralized fronto-central to right-frontal beta-band coherence for both of these, and that the degree of this event-related coherence-increase directly related to PES and motor slowing after unexpected action outcomes across participants (Wessel et al., 2016). The latter study also showed that disconnections of frontal white-matter tracts in patients with small-vessel disease decreased the amount of this error/unexpectancy-related beta-band coherence, specifically to an extent that was predictive of the degree of impaired motor slowing in these participants.
Post-novel-slowing (PNS)
The above studies show that brain regions thought to be critical for outright action stopping are also activated following errors and unexpected outcomes and that their activity relates to motor slowing. While this provides converging evidence for our theory that this system underlies slowing after errors and unexpected action outcomes, this is based on reverse inference. Namely, it is incorrect to assume that a neural signature (such as preSMA, rIFC, or STN activity) of one process (e.g. stopping) in a different context (i.e., after errors and unexpected action outcomes) proves that stopping is engaged (Poldrack, 2011). By contrast, for unexpected perceptual events, several studies have directly tested whether the stopping network’s activity is related to PNS.
One study measured scalp EEG for a novelty task and a stop-signal task in the same participants (Wessel and Aron, 2013). Independent component analysis (Jutten and Herault, 1991) was used to identify components (ICs) that reflect the stopping process in the SST. This was based on a well-established EEG signature of stopping, see Brain Signatures of Stopping above. After identifying an IC in each participant that reflects this stopping-related activity, the study then tested whether the same IC was active following unexpected perceptual events (see BOX 1).
BOX 1. Independent Components Analysis.
Assume each participant in a single EEG study performs two tasks: stop signal and the cross-modal novelty task. The combined scalp EEG data are then decomposed into many Independent Components (IC) for each participant. Now the following analysis logic can be used to test if there is a process in common for the two tasks: if the stopping process (represented in the selected stopping-IC from the SST portion of the data) is not active following unexpected perceptual events, ICA will disentangle it from any other independent process that is active following unexpected perceptual events, and the stopping-IC will show no change in activity. However, if the stopping process is involved in the processing of unexpected perceptual events, the stopping-IC will show increased activity following such trials (for a theoretical review of the method, see Wessel, 2016).
The results were consistent with this: delta-, theta-, and alpha-band activity of the stopping-IC were significantly increased on unexpected vs. expected perceptual events, and the delta-band difference was significantly larger on trials with increased PNS. Hence, this study showed that activity of the stopping network relates to PNS, at least at the cortical level.
This interpretation is supported by the observation that LFPs recorded from the STN and GPi, i.e., subcortical parts of the stopping network, also display increased activity following unexpected perceptual events (Bockova et al., 2011). Moreover, since we suppose that the STN is involved in generating the global effect of the stopping network’s activity (Wessel et al., In press), its putative involvement in perceptual novelty also predicts there should be global skeletomotor suppression following unexpected perceptual events. We tested this using TMS from the hand (which was task-irrelevant) during the above-mentioned cross-modal novelty task (which was done with vocal responses). Indeed, unexpected perceptual events were followed by a global suppression signature in the task-irrelevant hand muscle (Wessel and Aron, 2013), just as in outright stopping in the SST (FIGURE 4D). Furthermore, the global motor suppression signature for TMS-MEP was significantly greater on trials that showed increased PNS.
Taken together, the stopping network is engaged following unexpected perceptual events, and moreover, its activity is predictive of PNS. There is also converging evidence for the involvement of the stopping network in motor slowing following action errors and unexpected action outcomes. However, unlike the role of the stopping system in PNS, this has not been directly tested.
UNEXPECTED EVENTS MAY AFFECT COGNITION VIA THE STOPPING SYSTEM
We now turn to a different canonical effect of unexpected events – namely, that they are ‘distracting’. Everyday experience shows that unexpected events interrupt our ongoing train of thought and lead to forgetting. Accordingly, subjects also show impaired cognitive performance after unexpected events in the laboratory. For example, target detection is impaired following both unexpected perceptual events (Schroger, 1996) and errors (Houtman and Notebaert, 2013). While there are several theories about why such distraction occurs (most of which revolve around a relatively non-specific shift of attention that happens after surprise Notebaert et al., 2009) we recently tested the new idea that such distraction arises (at least partially) from the broad suppressive effect induced by unexpected events.
We tested this in following way (Wessel et al., 2016) (FIGURE 5). On each trial, subjects encoded verbal information, maintained it in working memory (WM) across a delay, and were then probed for accuracy. Crucially, the delay interval sometimes contained an unexpected perceptual (auditory) event. Behaviorally, these events led to significantly impaired WM performance, with the more surprising the event, the greater the impairment. For scalp EEG, each subject performed the above surprise-interrupts-WM task as well as a simple stop signal task. We used the ICA technique described above (see BOX 1) to identify an IC that represented activity of the stopping network. First, we showed that the more strongly the stopping-network was recruited by unexpected events, the greater was the decrement of WM. Second, the stopping-network activity following unexpected events statistically mediated the negative influence of these events on WM. Additionally, we ran the same study in DBS patients with implanted STN electrodes. As in scalp EEG, the greater the STN-LFP activity induced by the unexpected events, the greater the disruption of WM.
Figure 5.
The motor inhibition network interrupts verbal WM (adapted from Wessel et al., 2016). A) Source-level EEG. Combined EEG from the WM task and the SST were subjected to Independent Components Analysis (ICA). One IC that represented the process underlying successful stopping in the SST (the motor-suppression IC, MS-IC) was selected per subject. That component in the WM task then showed three results: a) activity was increased following surprising vs. standard tones, b) activity on surprising trials was greater the greater the decrement in WM (a single trial GLM was run in each subject, with the plot showing the group-average of the SURPRISE × WM interaction) and c) activity positively mediated the influence of the surprising tone on WM accuracy. B) STN group results. Left: increased activity in several frequency bands for surprising vs. standard tones in the WM task. Right: increased activity on surprising trials related to a greater decrement of WM (a single trial GLM was run in each subject, with the plot showing the group-average of the SURPRISE × WM interaction).
Combining these results with those from earlier sections, we propose that unexpected perceptual events recruit the fronto-basal-ganglia network (preSMA-rIFC-STN) in ‘global’ mode, i.e., the mode that is recruited to rapidly stop action and leads to the broad suppression of motor activity. We propose that this mechanism not only interrupts ongoing motor representations, but also (some kinds of) cognitive representations. We now elaborate the putative anatomical basis of this theory, consider other evidence, and other possible accounts of the data.
Anatomical basis and extension beyond verbal WM
In the section The Brain’s Network for Global Motor Stopping, we presented various results suggesting that motor suppression is implemented via a purported hyperdirect pathway from frontal areas to the STN of the basal ganglia, which then activates GPi and suppresses thalamocortical drive. We theorized that, once the STN is active, it could account for the broad skeletomotor suppression that occurs for outright stopping and unexpected perceptual events via a putatively divergent effect on the GPi. On this view, activation of the STN – whether by stop-signals or unexpected events – will suppress thalamocortical drive to the wider motor system (including many or all parts of the body). We now go further in supposing that unexpected events also interrupt cognition in the same way; at least perhaps ‘motor-based cognition’ (such as verbal WM), which might also be maintained via thalamocortical drive (FIGURE 6). This may apply to verbal WM in particular since it relies on subvocal rehearsal (Morra, 2015), and subvocal rehearsal, in turn, relies on inner speech (Pihan et al., 2000), which is probably partly motoric (furthermore, maintenance of verbal WM has been shown to activate the basal ganglia (Chang et al., 2007)).
Figure 6.
Proposed theoretical framework by which unexpected events interrupt cognition. A). Forms of working memory (cognition), is likely maintained in cortical areas via reverberating thalamocortical drive. B). An unexpected event, in this case perceptual novelty, putatively recruits the brain’s stopping system, including the STN of the basal ganglia which broadly suppresses thalamocortical drive. This erodes the cortical representation, corresponding to a concurrent loss of cognition.
Whether surprise could decrement non-motor-based WM (e.g. for a color task) via a similar putative mechanism is unknown. This would depend on a) whether such non-motor-based WM is also maintained via thalamocortical drive, or perhaps purely in cortical representations (Wolff et al., 2015), and b) whether a putative STN recruitment is broad enough to affect such non-motor basal ganglia related representations, for example, in the “associative” loop mediated by dorsolateral PFC, basal ganglia and mediodorsal thalamus (Chatham et al., 2014; Hazy et al., 2007).
There is no direct evidence to our knowledge that one part of the STN could have such a divergent projection as to affect truly different sectors of the GPi (i.e., motor and associative). Still, there are several ways this could happen. First, surprising events could recruit cortical areas other than rIFC/preSMA (such as the DLPFC, vmPFC, cingulate cortex, etc.), which then project to different sectors of the STN (motor, associative, object, limbic, etc.) as shown by monkey tract tracing (Haynes and Haber, 2013), thus resulting together in a broad suppressive effect. Second, the axonal arborization of STN neurons in the target area (GPi) could be very diffuse, so that even if a restricted part of the STN is activated by the hyperdirect pathway, the impact could still be broad. Third, the STN could have widespread dendritic arborization within itself (see for a theoretical view Gillies and Willshaw, 1998) so that again, even if a restricted part of the nucleus is activated by the hyperdirect pathway, the impact could be broad. Fourth, the impact of the STN on the GPi/SNr could be fairly focal, but the output to the thalamus could be quite widespread, thus accounting for a potential broad effect. Future tracing studies may elucidate these possibilities, analogous to recent work on the pallidal-STN connection that uses single cell labeling and stereological imaging in the target region(s) (Baufreton et al., 2009).
Other lines of evidence for broad effect of stopping system
The idea that motor stopping might also impact cognition was already foreshadowed by Logan and Cowan’s seminal paper “On the ability to inhibit thought and action: A theory of an act of control” (Logan and Cowan, 1984). However, almost all subsequent research in that line focused on the action part alone. Yet, some recent studies point to an effect of action-stopping on cognition. In one study, participants viewed pictures of faces, which were paired with either stopping or going; the faces were less likely to be remembered in a post-experimental recall phase if they had been paired with stopping (Chiu and Egner, 2015a). Using fMRI, the same authors found that faces that were not remembered in the post-experimental recall phase were associated with significantly increased rIFC activity during no-go trials (Chiu and Egner, 2015b). This result was interpreted within a resource-sharing framework, according to which a higher degree of neural activity within the stopping network (specifically, rIFC) detracts from a shared control resource that could be otherwise used to (incidentally) encode the identity of the face stimulus (which the participants were not instructed to do). However, another interpretation could be that having to stop the action interrupted the cognitive processing of the picture stimulus, thereby worsening their later recall. However, another interpretation could be that having to stop the action interrupted the cognitive processing of the picture stimulus, thereby worsening its later recall. Based on this argument, one would predict activation of the STN, which the authors did not find in their study. However, it is possible that the rapid stop-system was not activated in that study, since the experiment used equiprobable Go/Nogo trials, presented at a relatively slow pace. Hence, it is unclear whether inhibition was the relevant factor in those behavioral findings. In a different study, the same authors showed the behavioral effect for a stopsignal version of their experiment (Chiu and Egner, 2015a), and we predict that an fMRI or STN depth-electrode recording approach might elucidate the question of whether at least part of their effect related to recruitment of the stop system.
In other research, fast motor stopping has been shown to reduce the value of stimuli (Wessel et al., 2014b), see also (e.g. Houben et al., 2012; Veling et al., 2013). The mechanism underlying these devaluation effects is not yet clear. One interesting clue was that devaluation, for one behavioral paradigm, was greater in those subjects who had explicit knowledge of stimulus value (Wessel et al., 2015). This raises the possibility that stimulus value was held in a form of memory that was impacted by the stopping procedure (analogous to the impact of surprise on verbal WM). Lastly, another study demonstrated that having to stop action during a gambling task reduced the amount of risk the participants were willing to take (Stevens et al., 2015, Exp. 5). In summary, while the mechanisms underlying these behavioral effects of action-stopping on memory and value representations are still largely unknown, they provide additional behavioral evidence for a potential interaction between motor stopping and cognitive processing.
Other accounts
Our theory that unexpected events produce motor and cognitive interrupts via the putative broad impact of the STN on basal ganglia output is based on results of broad skeletomotor suppression and WM decrements related to activity in the stopping system. Yet a correlation between two variables (e.g., STN activity and global cortico-spinal suppression) does not exclude the possibility that a third process explains both of them. For example, increased arousal during successful stopping could lead to a phasic release of catecholamines such as dopamine. Notably, the dopamine system influences GABA-ergic interneurons (Gorelova et al., 2002), which could result in reduced TMS induced MEPs from M1 (i.e., what looks like global motor suppression is merely a changed cortical M1 state following neuromodulation). Such catecholamine release/arousal could also potentially lead to beta-band increases in the STN (Bockova et al., 2011). Similar arguments could apply perhaps to the locus-coeruleus/noradrenergic system (Bouret and Sara, 2005). However, we discount this view for two reasons. First, such systems are likely not fast enough to explain the effects of unexpected events on motor (or cognitive) representations that occur so shortly after event onset, i.e., in about 150 milliseconds (see Wessel and Aron, 2013). While dopamine and noradrenergic neurons may be rapidly activated by stop signals and unexpected events, and while those neurotransmitters might be rapidly released, there is a time-window of hundreds of milliseconds to seconds between release and postsynaptic changes in membrane potential and firing (Lapish et al., 2007). Second, this neuromodulatory account is inconsistent with the observation that broad skeletomotor suppression occurred more for successful than failed stop trials when the latter are likely more arousing (Cai et al., 2012; Majid et al., 2012; Wessel et al., 2013). Still, there are other ways in which the brain could produce a ‘broad suppressive effect’, for example, via brainstem nuclei (akin to the ‘motor shut down’ during REM sleep (Vetrivelan et al., 2011)). Further research is needed to test the causal role of the STN in mediating motor and cognitive interrupts.
THE RELATION BETWEEN COGNITIVE INTERRUPTIONS AND ATTENTION
The standard way of thinking about how an unexpected event interrupts cognition is that there is an attention shift – i.e., away from one’s mental contents, towards the unexpected event. This shift of attention is connoted by the phrase ‘Orienting Response,’ coined by Sokolov (1963) and also by Courchesne et al. (1975) who supposed the N2/P3 ERP potential after unexpected perceptual events indexes that orienting response. Moreover, as we saw, attention shifts indexed by the orienting response have been used to explain error-related phenomena like PES (Notebaert et al., 2009).
Our theory relates to these frameworks by positing that in order for such a shift of attention to happen, ongoing representations first need to be interrupted. As we spell out below, we suppose that the shift of attention is temporally preceded by an active disengagement from ongoing representations (the interruption), mediated by the preSMA-rIFC-STN system. This then enables attentional processing to engage the unexpected event. First, we consider how our proposed framework relates to some similar suggestions in related domains. Then, we will specifically address the interaction between our proposed interruption of cognitive processing, and the stimulus-driven shifting of attention.
Related Theories
In the field of task-switching, it has been proposed that a shift of attention towards a new task-set may involve an interruption of the previously active task-set. For example, Barcelo et al. (2006) explicitly compared neural activity for unexpected perceptual events and task-switching cues, and proposed that, unlike the interpretation put forward by pure ‘attentional-shift’ theories (see above), “novelty P3 activity should not be described as Pavlov’s “What Is It” orienting reaction (Courchesne et al., 1975) but rather as a more general “change your mind” mechanism of cognitive control”.
Another related theory by Corbetta and Shulman (2002) proposed that a right-lateralized ‘circuit breaker’, prominently featuring the rIFC, interrupts ongoing cognition after unexpected events. However, they did not distinguish between this putative interruptive function and the subsequent re-orienting of attention, likely because much of their theory was motivated by fMRI data – yet the time-resolution of fMRI cannot tease apart these two closely-related stages.
Our theory builds upon these ideas (FIGURE 7). Specifically, we propose that: 1) in the surprise -> interruption -> reorienting sequence, it is the preSMA-rIFC-STN system (part of the wider network of anatomical regions underlying Corbetta and Shulman’s ‘circuit breaker’), that performs the ‘interruption’ part [footnote1], 2) this interrupt system is independent from attentional re-orienting, and 3) a global interrupt by the stopping network temporally precedes the attentional reorienting after unexpected events. As we have already discussed above, unexpected events recruit the stopping network. We now elaborate.
Figure 7.
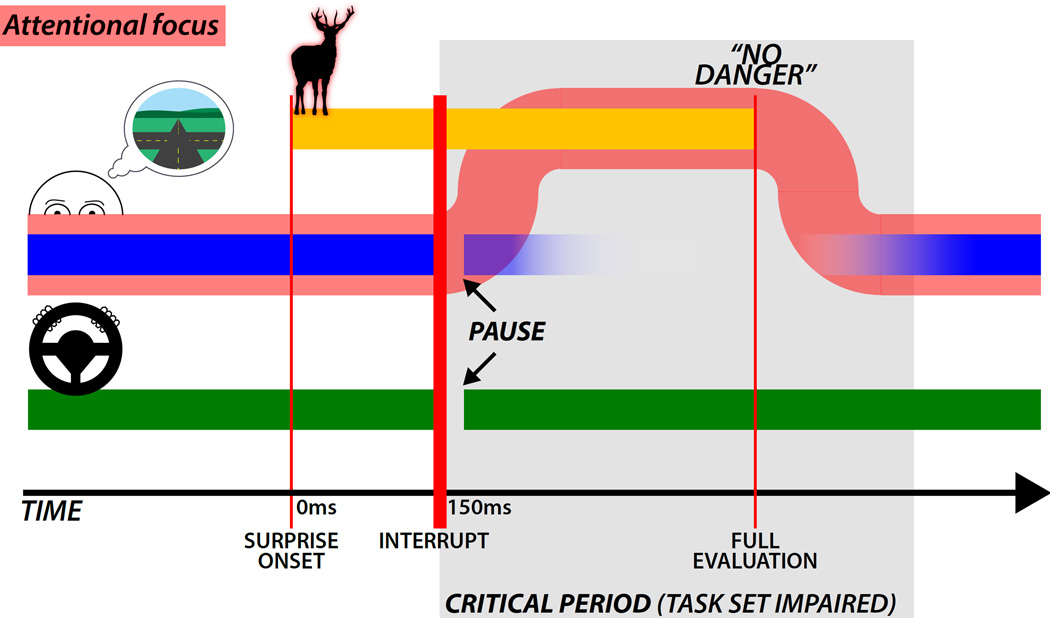
Proposed time-course of the surprise -> interruption -> attentional re-orienting sequence. Before the unexpected event (red line denoted “surprise onset”), the task set (broad blue line) is maintained in working memory - in this case the driver is representing the route ahead, and the skeletomotor system (green line) is active for postural, task and task-irrelevant muscles. Immediately following the unexpected event, both the working memory and motor systems are interrupted. The motor interruption is evident at 150 ms but is short-lived (Wessel and Aron, 2013). The interrupt allows the attention focus (broad, red line) to shift away from the current task set, and towards the unexpected event. The task-set representation decays (fading blue line) – due both to the global suppression induced by the interruption, which erodes, for example subvocally maintained if-then rules, and also the attention shift. Once the unexpected event is processed (“No Danger”), attentional focus can shift back onto the previous task set, and the task-set can be re-established. The time period between the initial interrupt, and the re-establishment of the task-set is a critical period (gray highlighting). If, for example in an experimental setting in the laboratory, the next relevant stimulus falls into this critical interval, we hypothesize that this would lead to a delay of RT on the next trial, as well as a reduction of accuracy. However, if the next stimulus appears after the task-set is reestablished, performance should be unimpaired (both RT and accuracy).
Separating motor suppression from attention
One key proposition of our theory is that motor suppression and attentional orienting are separate (although likely tightly connected) processes.
Motor suppression and attention are independent processes
In a scalp EEG study, we saw evidence for dissociable putative signatures of motor suppression and attentional detection of relevant signals (Wessel and Aron, 2014). We used two different tasks in the same group of participants: a simple stop-signal task (which required the detection of a simple visual stop-signal and the subsequent outright cancellation of an action), and a complex stop-signal task, in which go-stimuli resembled the stop-signal to different degrees, leading to different degrees of motor slowing. Two independent neural components occurred after stop-signals in the simple task: a fronto-central low-frequency component that indexes motor suppression (see above), and a posterior-occipital alpha-band component that has been identified elsewhere for stimulus-driven shifts of visual attention (Sauseng et al., 2005; Yamagishi et al., 2003). Crucially, in the complex task, the level of activity of the fronto-central (response suppression) component related to the amount of response slowing (putatively the application of a ‘brake’), while the posterior-occipital alpha (attention) component did not change in relation to motor slowing (Wessel and Aron, 2014). This suggests dissociable brain components for inhibitory control and attentional processing.
High temporal resolution methods
The above-mentioned studies of the relation between unexpected events, motor suppression and WM disruption used the high-resolution methods of scalp EEG, intracranial EEG, and TMS-probed MEPs. These provide a temporally precise window into the chronological order of putative psychological processes, arguing for the following sequence: surprise -> interruption -> reorienting.
Once the unexpected event occurs, the event must be perceived as surprising (i.e., discriminated from an expected event). Studies of early sensory perception have shown that such differential perception of expected vs. unexpected stimuli happens as early as 50ms in the auditory domain (Gamble and Woldorff, 2015) and as early as ~100ms in the visual domain (Pazo-Alvarez et al., 2003). Notably, these neural responses occur in (or near) primary sensory areas, and are largely independent of attention (Czigler, 2007).
Next, there is interruption of ongoing processing, putatively implemented by the preSMA-rIFC-STN network. We propose this occurs before orienting. This is supported by two main lines of evidence. First, STN beta-band activity starts to significantly increase following unexpected auditory stimuli (compared to expected stimuli) around 60–70ms following the onset of the tone (Wessel et al., 2016) – i.e., immediately after the early auditory potentials that signify stimulus detection (Gamble and Woldorff, 2015). In contrast to that, potentials such as the scalp N2/P3 complex, which have been proposed to reflect a shift of attention / attentional re-orienting (Barcelo et al., 2002; Courchesne et al., 1975; Donchin, 1981) begin later – i.e., around 250 – 300ms following stimulus onset. Hence, interruption is situated chronologically after stimulus detection, but before attentional re-orienting. Second, a TMS study that probed motor excitability following unexpected events provided a temporal indicator of the global interruption, at least on the motor level (Wessel and Aron, 2013). After unexpected tones, there was global motor suppression at 150ms, but not at later time points (175 and 200ms). Since motor suppression was not measured before 150ms, it is unclear how early this global suppression begins. However, an above-mentioned study (FIGURE 4C) also showed that during successful stopping of speech there is increased STN beta band power before the global motor suppression measured with TMS-MEP (Wessel et al., in press). Thus, the suppressive influence may be present as early as the significant increase of STN beta-band activity at 60–70ms following stimulus onset. Taken together, these studies show that motor suppression signatures are present within 150 ms (and perhaps earlier) following the onset of an unexpected event, again arguing that the putative motor suppression mediated interrupt occurs before attentional re-orienting.
Surprise interrupts tapping
A related study examined the impact of a single unexpected visual event on continuous finger tapping (Horstmann, 2006). In 78% of participants, tapping was interrupted with a latency of 214 ms after the unexpected event. This is roughly comparable to the timing (150 ms) of our above-mentioned effect of surprise on task irelevant effectors (Wessel and Aron, 2013) – the slightly longer latency may reflect a visual vs. auditory signal. Because tapping is a classic example of a simple action that is barely demanding (Pashler, 1994) that result speaks against the possibility that the action interruption was a mere consequence of attending elsewhere; instead it is compatible with our theory that surprise interrupts the skeletomotor and cognitive systems, footnote2.
GLOBAL INTERRUPTION: ADAPTIVE OR MALADAPTIVE?
Our theory explains the two typical consequences of unexpected events: the ubiquitous slowing of RTs following any type of unexpected event and the ‘distraction’ associated with them. We propose that both effects have a common underlying mechanism – unexpected events recruit a fronto-basal-ganglia network that broadly disrupts ongoing representations maintained in thalamocortical loops. As we will argue, this mechanism serves an adaptive purpose: it interrupts ongoing cognitive and motor processes to allow the re-orienting of attention towards the unexpected event, effectively freeing up resources to properly adapt if necessary.
A unifying theory
Our framework provides a unified explanation for seemingly incompatible phenomena, and helps inculcate a re-appreciation of classic theories of control. We take the example of error processing research. As we saw in the section Motor Slowing After Action Errors the classic view is that PES is an adaptive process aimed at avoiding further errors (Botvinick et al., 2001; Danielmeier and Ullsperger, 2011; Miller and Cohen, 2001; Ridderinkhof et al., 2004; Ullsperger et al., 2014). However, as discussed in that section, the pattern of changes in response accuracy after errors challenges this standard model. If errors lead to adaptive processes (like PES) that aim to prevent future errors, error rates should be decreased on trials immediately following errors. While some studies indeed find such a reduction (Danielmeier et al., 2011; Laming, 1968; Marco-Pallares et al., 2008), others find an increase in error rate after errors (Fiehler et al., 2005; Houtman and Notebaert, 2013; Rabbitt and Rodgers, 1977). The standard model of error-related cognitive control has been unable to account for these findings. As we saw, one study found that under conditions in which PES (an ostensibly adaptive process) is greatest, post-error accuracy is actually worst (Jentzsch and Dudschig, 2009). The authors explained this using the ‘attentional bottleneck’ account, according to which error monitoring draws upon a limited resource for information processing, which negatively affects performance on the next trial when time is short. However, this bottleneck account cannot explain why post-trial slowing occurs after correct trials when errors are the expected outcome (Notebaert et al., 2009).
Crucially, these paradoxical findings can be reconciled in our theory. We propose that errors – because they are unexpected events – recruit the fronto-basal-ganglia network to interrupt ongoing motor representations (thereby producing PES). Further, the fronto-basal-ganglia network interrupts ongoing cognitive representations (at least some kinds of cognition such as verbal WM). In the case of an RT task, the ongoing cognitive representation is the task set (i.e., a set of stimulus-response mappings stored in WM). If this is declarative WM (i.e., explicit conditional rules) then the task-set could be maintained via subvocal rehearsal, which is likely motor based; and if it is procedural WM, this could also be motor-based, e.g. via fronto-striatal action plans (Hazy et al., 2007; Oberauer, 2009; Wiecki and Frank, 2013). To the extent that task-set is maintained via reverberating thalamocortical drive, it will also be susceptible to momentary interruptions by the stopping network. This could explain why conditions that produce greater PES sometimes lead to more errors. On our theory, the momentary interruption of motor and cognitive representations is part of a combined effort to free up resources to deal with the unexpected event.
Thus, we propose that the unexpected event recruits the suppression process within 150 ms (maybe earlier) and this is short lived – because, in some circumstances it would be adaptive, after the stop, to quickly (within a few hundred ms) make a new action, such as moving away from danger. Even though the suppressive process is brief, its impact can last because it erodes the task set (which could be implemented in cognitive alone or cognitive/motor systems). In the laboratory, this could affect accuracy on the next trial. However, if the ITI is long enough, this interruption could benefit processing: if the source and nature of the unexpectedness (error) can be fully resolved, the task-set could be re-instantiated and the next trial could be performed more accurately and with a reduced RT delay. Note that our theory of a short-lived motor suppression also explains why inserting a NoGo (stopping) condition after an unexpected event does not necessarily improve stopping (Leiva et al., 2015). Note also that our theory predicts that an unexpected event induces a two-stage ‘hit’ on task-set (working memory) (Figure 7). The first ‘hit’ is from the global suppression process, which we suppose impacts the task-set itself, and the second ‘hit’ is from attention being ‘moved’ to the unexpected event (also see Chiu and Egner, 2015a). We suppose both of these would decrement the task-set. Note that while, according to our theory, the unexpected event will induce a brief period of motor inhibition, delayed reaction times after unexpected events (such as PES) do not necessarily stem from motor inhibition per se, but from an interruption of all ongoing processing after unexpected events (see also Figure 7). Importantly, we do not mean to imply that any and all interruptions of task sets (or, more generally, attentional/cognitive processing) necessarily recruit the STN-mediated mechanism. When adaptations are more strategic or deliberate (such as in explicit task-switching paradigms), or when unexpected events do immediately necessitate the adaptation of ongoing thoughts and actions, the inhibitory mechanism may not be involved.
Crucially, in addition to explaining why short ITI conditions produce decreased accuracy despite greater PES, our theory can also explain why post-trial slowing occurs when errors are the expected outcome (Notebaert et al., 2009). In this scenario, the fronto-basal-ganglia network should become recruited after correct trials, as those become surprising within the expectation space established by the task parameters. Hence, in such scenarios, PES and impaired cognitive processing on short ITI trials, should occur after correct trials too.
While this example deals with error processing (the most comprehensive area of the control literature, at least regarding motor effects), our theory similarly applies to other types of unexpected events, and unites those literatures under a common framework. This motivates many concrete hypotheses for future study, at the center of which is the proposition that error commission, unexpected action outcomes, unexpected perceptual events, and motor stopping all have comparable effects on motor and cognitive processing.
Relation to theories of conflict: uncertainty as an overarching concept?
According to an influential theory by Frank and colleagues, response or decision-conflict recruit the STN to ‘hold back’ responding in order to buy more time to accumulate evidence about which response to make (Frank, 2006; Wiecki and Frank, 2013). It was proposed that this is effected by a “global NoGo” signal, which was, however, conceived as a suppression of all possible responses for the current task, rather than the wider motor system. While that theory places more of an emphasis on uncertainty about what to do (resulting from the incompatibility of two motor responses), it is possible that “unexpectedness”, the core concept of our theory, could also be viewed as resulting in uncertainty. Thus, while a conflict signal causes uncertainty about which response to make (e.g., to withhold the action for vanilla ice cream and instead choose chocolate), an unexpected event could cause uncertainty about whether to change entire behaviors/cognitive states (e.g., keep walking on the current route). In both cases, motor suppression could serve the function of buying time to make that decision. In this scheme, uncertainty could be the overarching rubric, with conflict and unexpectedness being particular instantiations3. While this might imply that conflict and unexpectedness operate by similar principles, and might involve overlapping circuitry, they are not the same thing. For example, conflict may recruit the STN to pause and suppress motor activity (Brittain et al., 2012), but should ideally not result in a temporary cognitive impairment. By contrast, we are proposing that unexpected events can indeed lead such cognitive effects. Future studies could examine whether this relates to a difference in intensity of recruitment, a breadth of recruitment (e.g., conflict might be directed more narrowly at STN-related motor circuitry, while unexpected events might simultaneously recruit many STN-related circuits with impacts on the cognitive and limbic), or the involvement of differential circuitry for conflict and/or unexpectedness.
Evolutionarily old?
The putative fronto-basal-ganglia network that is triggered by unexpected events may have arisen from an evolutionary benefit. In a scenario in which a prehistoric human is returning from the hunt with his/her mind set on preparing the meal ahead, it would be beneficial if the first thing that happens following an unexpected event (e.g., a rustling noise that could reflect danger) is to quickly stop motor and cognitive processing. In such a scenario, it is highly adaptive to free up both cognitive and motor resources prior to the full evaluation of the stimulus (i.e., the noise). The prefrontal cortex, specifically the rIFC, which is part of Corbetta and Shulman’s proposed ‘circuit breaker’, might be particularly well situated for this function – receiving inputs from the ventral visual stream and auditory system (Romanski and Goldman-Rakic, 2002; Sakagami et al., 2001), and even more quickly via thalamus; connected to the preSMA via the ‘aslant tract’ (Catani et al., 2012); being adjacent to lateral premotor cortex; and with a likely hyperdirect input to the STN (Aron et al., 2014). While this putatively ancient brain system may have evolved for rapid detection of surprise/deviance/exception and prediction errors (to quickly put the brake on motor output) (see discussion in Horstmann, 2006; Wiecki and Frank, 2013), modern requirements such as stopping to an explicit signal may leverage it. This speaks to a ‘hard wiring’ for a system that turns on for exceptions, and has the function of pausing, stopping and clearing. This may occur before any attention shift, and indeed perhaps be a requisite for it. It may facilitate a kind of ‘reset’ that overcomes the ‘stickiness’ of the prior task set. If this is so, variations in how well this system works could relate to motor and cognitive disorders.
CLINICAL IMPLICATIONS
Our over-arching theory is that some forms of cognition, such as verbal WM, are maintained via reverberant thalamocortical drive, and this can be interrupted via an STN-mediated system which also induces a broad skeletomotor pause. This broad interruption could have the function of ‘clearing cognition’. If an STN-related stopping system is key for such clearing then deficiencies in that system could lead to mental inflexibility. This makes a direct connection to patients with Parkinson’s Disease (PD) who are typically ‘stuck in set’, especially with regard to switching behavior (in addition to their classic motor symptoms) (Bowen et al., 1975; Lees and Smith, 1983; Taylor et al., 1986). PD is characterized by over-synchrony between basal ganglia and cortex (Brown, 2003; George et al., 2013; Swann et al., 2015); and it is thought that this ‘locking’ prevents cortico-basal-ganglia circuits from operating properly. Indeed, multiple studies show PD patients off treatment are impaired at stopping (George et al., 2013; Obeso et al., 2014; Obeso et al., 2011; Swann et al., 2011). We hypothesize that the mental inflexibility in PD is at least partly explained by a system that is not properly interruptible by new information (because the STN-mediated interrupt is ineffective due to oversynchrony). This leads to the prediction that PD patients Off their medication will be less interrupted by surprising events than patients On their medication, c.f. (Cools et al., 2010), and for the reason that the STN-mediated system cannot be readily triggered.
The flip side of mental inflexibility is mental-overflexibility – i.e. switching set and attention too readily. This is epitomized by Attention Deficit Hyperactivity Disorder (ADHD). While there are different forms of the disorder, and it is likely that no simple systems-level disruption will explain all aspects (Castellanos and Tannock, 2002), we conjecture that part of the distractibility could relate to an over-interruptible system at the level of the fronto-STN mediated stop. This leads to the prediction that ADHD patients Off their medication will be more interrupted by surprising events than patients On their medication, and that this is because the STN-mediated system is triggered too readily. While several studies of ADHD have documented alterations in novelty processing, including hyperactivation of ventral prefrontal and other brain regions (Cortese et al., 2012; Konrad et al., 2006; Tegelbeckers et al., 2015), we are not yet aware of any research specifically speaking to this issue.
CONCLUSIONS, CAVEATS, AND FUTURE DIRECTIONS
Unexpected events are everyday occurrences. Variations in how well we deal with them could explain much about individual differences as well as disorders characterized by over- and under-distractibility. Here we considered three kinds: action errors, unexpected action outcomes and unexpected perceptual events. All produce post-event motor slowing and can also produce cognitive effects such as post-event accuracy reductions and working memory loss. There is a rich literature on these effects and several theories abound. We proposed a unifying, anatomically grounded, theory. We argued that the same (or a closely analogous) brain system that is recruited by outright action stopping is also recruited by the three types of unexpected events. This brain system includes the rIFC, preSMA and STN of the basal ganglia as affirmed by studies with many methods – all showing commonality of signatures.
Whether stopping and unexpected events recruit literally the same circuit, or perhaps a closely analogous circuit in slightly different ways remains to be established. For example, there are hyperdirect pathways to different parts of the STN from different prefrontal sectors, and these could mediate a similar pause, but in slightly different ways (Zavala et al., 2015). Moreover, while evidence from fMRI, EEG and STN LFP points to recruitment of the STN and preSMA for unexpected events as well as outright stopping, and while fMRI points to rIFC for both, it is unclear if the same sector of rIFC is recruited for unexpected events as stopping. For example, a meta-analysis showed that oddball events activated a more dorsal part of rIFC compared to stopping (which was more ventral and caudal/opercular) (Levy and Wagner, 2011). Although oddball events are infrequent as opposed to unexpected, this raises the prospect that different sectors of rIFC implement different aspects of processing (Aron et al., 2015; Hampshire and Sharp, 2015). Future research is required to examine these questions more closely; for example, whether a putative rIFC-mediated brake is recruited in the same way by external (stop signals, perceptual events) vs. internal (action errors) triggers.
The STN of the basal ganglia is in a position to suppress thalamocortical drive – perhaps broadly, although the anatomical evidence is so far preliminary. Recruitment of the STN by all these scenarios could thus explain the observation of broad motor effects, including on task irrelevant effectors – this is because, we suppose, all parts of the skeletomotor system receive thalamocortical drive, even if they are not currently task relevant. This induces a pause in the skeletomotor system that could help outright stopping (if it is needed), and unexpected events (and in a different literature conflict processing Wiecki and Frank, 2013; Zavala et al., 2015). Unexpected events can also decrement cognition and perception – most obviously in the case of unexpected perceptual events (e.g. cell phone chirping), when we forget our train of thought; but also following action errors and unexpected action outcomes (Purcell and Kiani, 2016; Ullsperger and Danielmeier, 2016). We propose that such distraction relates to the same STN-mediated pause that produces the broad motor suppression. This is because cognition may also depend on thalamocortical drive during maintenance. This may only apply to motor based working memory, such as verbal and visuomotor; or it may apply more broadly to perhaps visual WM – an interesting topic for future research. This raises the question of how exactly a putative STN-mediated pause could ‘straddle’ both motor and non-motor domains – something that is still unknown. We also note that our theory that unexpected events impact cognition via a fronto-basal-ganglia network certainly does not explain everything about the effects of unexpected events – there are surely many other factors including arousal changes, the attention shift itself, and subsequent effector-specific changes (reviewed by Ullsperger et al., 2014). Note also, that while we suppose that global suppression is an obligatory consequence of unexpected events, there are situations where global suppression can be induced without unexpected events. This is the case in the standard stop signal paradigm, where, as we have seen, stop signals (which are expected, albeit infrequent), induce global motor suppression. Further, there are scenarios in which stop signals do not induce global motor suppression – for example when the subject is incentivized to prepare to stop a particular effector in advance of the stop signal (Cai et al., 2011). However, doing so is effortful and slow, and we expect participants typically default to the fast, putative hyperdirect, system that comes along with a brief and global suppression. Various lines of research suggest that mechanistically selective (i.e., non-global) stopping involves a fronto-striatal pathway, rather than a fronto-STN pathway (Majid et al., 2013). Mechanistically, selective stopping is possibly implemented via the so-called indirect pathway, which would also include the STN. It is an enduring mystery how the STN could be putatively recruited broadly by the hyperdirect pathway and more selectively via the indirect pathway – something that could probably only be elucidated by non-human animal research. Yet, regarding unexpected events, we suppose that they always recruit the stopping system in global mode, because, as we outlined above, surprise may tap into an evolutionary ancient trigger.
The timing of the processes proposed in this theory is key. For example, post-error slowing is highly dependent on the timing of the next stimulus after an action error (Jentzsch and Dudschig, 2009), and the effect of unexpected events on subsequent inhibitory control of motor activity highly depends on the relative timing of the processes (Leiva et al., 2015). We propose that signal detection, inhibition, attentional orienting, and the potential suppression of ongoing cognitive representations (such as task sets) are all part of a tightly linked cascade of interacting neural processes. Future studies may use methods with high temporal resolution to disentangle the neural mechanisms underlying these processes, and to characterize their interaction following unexpected events. It will be important to understand the effects of the stopping-network on different types of representations and events, specifically with respect to the exact time the stopping-network is active in relation to ongoing cognitive or motor activity.
An important implication of our theory is that it puts research that was formerly in different domains into the same framework. Whereas cognitive neuroscience has tended to proceed with different camps studying different tasks, or different camps studying different putative functions such as response inhibition, working memory, and attention, our theory provides fruitful links between these, and also links to the non-human animal research on fronto-basal-ganglia circuits. A striking development in the wider field was the discovery that both conflict and stopping implicate a similar brain system (Frank, 2006; Wiecki and Frank, 2013; Zavala et al., 2015); now we extend that to unexpected events (and speculate about how both conflict and unexpectedness may fit under an overall rubric of ‘uncertainty’). This may help refocus the wider field on core cognitive ‘primitives’ that are grounded in neural circuitry, even as they have many different signatures in terms of ERPs, oscillatory band, and patterns of TMS-induced MEPs. Such cognitive primitives, such as the putative function of the fronto-basal-ganglia system that is triggered in the way we describe, may be building blocks for a richer understanding of cognition that is somewhat task-independent. Similarly, a focus on the circuit starts to cut across artificial boundaries between working memory and attention – here, for example, one implication of our theory is that attention reorienting may be preceded by a fronto-basal-ganglia broad motor interrupt (c.f. Krauzlis et al., 2014); and another implication is that working memory can be ‘reset’ by this same mechanism. We look forward to future studies aimed at testing these ideas (Box 2), as well as related theories that pertain to the basic neural building blocks of cognitive control.
Box 2. Predictions and Open Questions.
Predictions
-
-
Attentional shifts after unexpected events are preceded by (and may even require) global (putative STN-mediated) motor inhibition.
-
-
Clinical disorders in which global motor inhibition is impaired will show a relative ‘overpreservation’ of ongoing cognition in the face of unexpected events (i.e., malfunctioning neural circuitry underlying motor inhibition may impair the suppression of ongoing cognitive processes).
-
-
Motor slowing following unexpected events is explained by a temporary disruption of the task-set. This will be more dramatic for task-sets that are more abstract and less practiced.
-
-
Unexpected action outcomes and action errors will lead to global motor suppression, and this will be evident in the motor evoked potential.
Open questions
-
-
Do unexpected events in different sensory modalities (auditory, visual, haptic) affect cognitive and motor representations by recruiting the same neural circuitry?
-
-
Are working memory contents that are not maintained in motor-based, phonological-loop-type systems susceptible to interruption via the same neural system that leads to a suppression of verbal WM after unexpected events?
-
-
If stopping-system interruptions of cognitive processes (e.g., working memory) reflect a ‘refreshing’ of the cognitive state, does this lead to improved ability to take in new information?
-
-
Can both conflict and unexpectedness be thought of as instantiations of uncertainty?
-
-
Can the stopping system be ‘voluntarily’ recruited to suppress cognitive representations, e.g., in the Think/NoThink paradigm?
In brief.
Wessel and Aron provide a new perspective on unexpected events. They argue that unexpected events interrupt action and cognition, partly by recruiting a specific fronto-basal-ganglia mechanism heretofore related to action stopping. Their theory links diverse literatures and has clinical implications.
Acknowledgments
Thanks to Tom Hnasko for discussion, to Eleonora Bartoli, Claudia Danielmeier, Dawn Finzi, Joy Geng, and Andrew Hollingworth for comments on the manuscript, and to Milena Gavala and Navarre Guitterez-Reed for artwork. JRW is supported by the Aging Mind and Brain Initiative at the University of Iowa. ARA is supported by NIH DA026452 and James S McDonnell grant #220020375.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Corbetta and Shulman’s analysis does not include the preSMA and STN. While some of the studies underlying the meta-analysis in Corbetta and Shulman do in fact show increased preSMA activity following surprising events, the absence of reported activation in the vicinity of the STN could be explained by generally lower field strength of 1.5 Tesla fMRI. Even 3T is probably inadequate to confidently localize the STN (de Hollander et al. 2015).
[Note that the timing and motor impact of unexpected events is quite distinct from the startle reflex, and very probably describes a distinct phenomenon, Horstmann, 2006].
We thank a reviewer for making this point.
REFERENCES
- Agam Y, Hamalainen MS, Lee AK, Dyckman KA, Friedman JS, Isom M, Makris N, Manoach DS. Multimodal neuroimaging dissociates hemodynamic and electrophysiological correlates of error processing. Proc Natl Acad Sci U S A. 2011;108:17556–17561. doi: 10.1073/pnas.1103475108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert J, Lopez-Martin S, Hinojosa JA, Carretie L. Spatiotemporal characterization of response inhibition. NeuroImage. 2013;76:272–281. doi: 10.1016/j.neuroimage.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69:e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Cai W, Badre D, Robbins TW. Evidence Supports Specific Braking Function for Inferior PFC. Trends Cogn Sci. 2015;19:711–712. doi: 10.1016/j.tics.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 2014;18:177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Badry R, Mima T, Aso T, Nakatsuka M, Abe M, Fathi D, Foly N, Nagiub H, Nagamine T, Fukuyama H. Suppression of human cortico-motoneuronal excitability during the Stop-signal task. Clin Neurophysiol. 2009;120:1717–1723. doi: 10.1016/j.clinph.2009.06.027. [DOI] [PubMed] [Google Scholar]
- Band GP, Kok A. Age effects on response monitoring in a mental-rotation task. Biol Psychol. 2000;51:201–221. doi: 10.1016/s0301-0511(99)00038-1. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Perianez JA, Knight RT. Think differently: a brain orienting response to task novelty. Neuroreport. 2002;13:1887–1892. doi: 10.1097/00001756-200210280-00011. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Bastin J, Polosan M, Benis D, Goetz L, Bhattacharjee M, Piallat B, Krainik A, Bougerol T, Chabardes S, David O. Inhibitory control and error monitoring by human subthalamic neurons. Translational psychiatry. 2014;4:e439. doi: 10.1038/tp.2014.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baufreton J, Kirkham E, Atherton JF, Menard A, Magill PJ, Bolam JP, Bevan MD. Sparse but selective and potent synaptic transmission from the globus pallidus to the subthalamic nucleus. Journal of neurophysiology. 2009;102:532–545. doi: 10.1152/jn.00305.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendixen A, Roeber U, Schroger E. Regularity extraction and application in dynamic auditory stimulus sequences. J Cogn Neurosci. 2007;19:1664–1677. doi: 10.1162/jocn.2007.19.10.1664. [DOI] [PubMed] [Google Scholar]
- Bendixen A, Schroger E. Memory trace formation for abstract auditory features and its consequences in different attentional contexts. Biol Psychol. 2008;78:231–241. doi: 10.1016/j.biopsycho.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Benis D, David O, Piallat B, Kibleur A, Goetz L, Bhattacharjee M, Fraix V, Seigneuret E, Krack P, Chabardes S, Bastin J. Response inhibition rapidly increases single-neuron responses in the subthalamic nucleus of patients with Parkinson's disease. Cortex. 2016;84:111–123. doi: 10.1016/j.cortex.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Berti S, Schroger E. Distraction effects in vision: behavioral and eventrelated potential indices. Neuroreport. 2004;15:665–669. doi: 10.1097/00001756-200403220-00018. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Duque J. Transcranial Magnetic Stimulation: Decomposing the Processes Underlying Action Preparation. Neuroscientist. 2015 doi: 10.1177/1073858415592594. [DOI] [PubMed] [Google Scholar]
- Bissett PG, Logan GD. Selective stopping? Maybe not. J Exp Psychol Gen. 2014;143:455–472. doi: 10.1037/a0032122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockova M, Chladek J, Jurak P, Halamek J, Balaz M, Rektor I. Involvement of the subthalamic nucleus and globus pallidus internus in attention. J Neural Transm (Vienna) 2011;118:1235–1245. doi: 10.1007/s00702-010-0575-4. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28:574–582. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Bowen FP, Kamienny RS, Burns MM, Yahr M. Parkinsonism: effects of levodopa treatment on concept formation. Neurology. 1975;25:701–704. doi: 10.1212/wnl.25.8.701. [DOI] [PubMed] [Google Scholar]
- Brittain JS, Watkins KE, Joundi RA, Ray NJ, Holland P, Green AL, Aziz TZ, Jenkinson N. A role for the subthalamic nucleus in response inhibition during conflict. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:13396–13401. doi: 10.1523/JNEUROSCI.2259-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA. Toddler fearfulness is linked to individual differences in error-related negativity during preschool. Dev Neuropsychol. 2014;39:1–8. doi: 10.1080/87565641.2013.826661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2003;18:357–363. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- Cai W, Oldenkamp CL, Aron AR. A proactive mechanism for selective suppression of response tendencies. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:5965–5969. doi: 10.1523/JNEUROSCI.6292-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Oldenkamp CL, Aron AR. Stopping speech suppresses the taskirrelevant hand. Brain and language. 2012;120:412–415. doi: 10.1016/j.bandl.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MB, Whittier JR, Mettler FA. Analysis of choreoid hyperkinesia in the Rhesus monkey; surgical and pharmacological analysis of hyperkinesia resulting from lesions in the subthalamic nucleus of Luys. J Comp Neurol. 1950;92:293–331. doi: 10.1002/cne.900920303. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attentiondeficit/ hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Catani M, Dell'acqua F, Vergani F, Malik F, Hodge H, Roy P, Valabregue R, Thiebaut de Schotten M. Short frontal lobe connections of the human brain. Cortex. 2012;48:273–291. doi: 10.1016/j.cortex.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Sanguinetti JL, Allen JJ, Sherman SJ, Frank MJ. The Subthalamic Nucleus Contributes to Post-error Slowing. J Cogn Neurosci. 2014 doi: 10.1162/jocn_a_00659. [DOI] [PubMed] [Google Scholar]
- Chang C, Crottaz-Herbette S, Menon V. Temporal dynamics of basal ganglia response and connectivity during verbal working memory. NeuroImage. 2007;34:1253–1269. doi: 10.1016/j.neuroimage.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Chatham CH, Frank MJ, Badre D. Corticostriatal output gating during selection from working memory. Neuron. 2014;81:930–942. doi: 10.1016/j.neuron.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YC, Egner T. Inhibition-Induced Forgetting Results from Resource Competition between Response Inhibition and Memory Encoding Processes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015a;35:11936–11945. doi: 10.1523/JNEUROSCI.0519-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YC, Egner T. Inhibition-induced forgetting: when more control leads to less memory. Psychol Sci. 2015b;26:27–38. doi: 10.1177/0956797614553945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claffey MP, Sheldon S, Stinear CM, Verbruggen F, Aron AR. Having a goal to stop action is associated with advance control of specific motor representations. Neuropsychologia. 2010;48:541–548. doi: 10.1016/j.neuropsychologia.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Miyakawa A, Sheridan M, D'Esposito M. Enhanced frontal function in Parkinson's disease. Brain : a journal of neurology. 2010;133:225–233. doi: 10.1093/brain/awp301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalography and clinical neurophysiology. 1975;39:131–143. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow W. Selective inhibition of movement. Journal of neurophysiology. 2007 doi: 10.1152/jn.01284.2006. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. Intracortical inhibition during volitional inhibition of prepared action. Journal of neurophysiology. 2006 doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Van Impe A, Wenderoth N, Swinnen SP. Aging and inhibitory control of action: cortico-subthalamic connection strength predicts stopping performance. The Journal of neuroscience. 2012;32:8401–8412. doi: 10.1523/JNEUROSCI.6360-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czigler I. Visual mismatch negativity: violation of nonattended environmental regularities. Journal of Psychophysiology. 2007;21:224–230. [Google Scholar]
- Danielmeier C, Eichele T, Forstmann BU, Tittgemeyer M, Ullsperger M. Posterior medial frontal cortex activity predicts post-error adaptations in task-related visual and motor areas. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:1780–1789. doi: 10.1523/JNEUROSCI.4299-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielmeier C, Ullsperger M. Post-error adjustments. Front Psychol. 2011;2:233. doi: 10.3389/fpsyg.2011.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Beers JR, Kelly A. Allocation of cognitive processing capacity during human autonomic classical conditioning. J Exp Psychol Gen. 1982;111:273–295. doi: 10.1037//0096-3445.111.3.273. [DOI] [PubMed] [Google Scholar]
- de Hollander G, Keuken MC, Forstmann BU. The subcortical cocktail problem; mixed signals from the subthalamic nucleus and substantia nigra. PLoS One. 2015;10:e0120572. doi: 10.1371/journal.pone.0120572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a Neural System for Error-Detection and Compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Donchin E. Surprise… surprise? Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. Journal of neurophysiology. 2002;87:615–620. doi: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- Dutilh G, Vandekerckhove J, Forstmann BU, Keuleers E, Brysbaert M, Wagenmakers EJ. Testing theories of post-error slowing. Atten Percept Psychophys. 2012;74:454–465. doi: 10.3758/s13414-011-0243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology. 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- Endrass T, Reuter B, Kathmann N. ERP correlates of conscious error recognition: aware and unaware errors in an antisaccade task. Eur J Neurosci. 2007;26:1714–1720. doi: 10.1111/j.1460-9568.2007.05785.x. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and clinical neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Fiehler K, Ullsperger M, von Cramon DY. Electrophysiological correlates of error correction. Psychophysiology. 2005;42:72–82. doi: 10.1111/j.1469-8986.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Keuken MC, Jahfari S, Bazin PL, Neumann J, Schafer A, Anwander A, Turner R. Cortico-subthalamic white matter tract strength predicts interindividual efficacy in stopping a motor response. NeuroImage. 2012;60:370–375. doi: 10.1016/j.neuroimage.2011.12.044. [DOI] [PubMed] [Google Scholar]
- Frank M. Hold you horses: A dynamic computational role for the subthalamic nucleus in decision making. Neural Netw. 2006 doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain's evaluation of novelty. Neuroscience and biobehavioral reviews. 2001;25:355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Friedman D, Nessler D, Cycowicz YM, Horton C. Development of and change in cognitive control: a comparison of children, young adults, and older adults. Cogn Affect Behav Neurosci. 2009;9:91–102. doi: 10.3758/CABN.9.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble ML, Woldorff MG. The Temporal Cascade of Neural Processes Underlying Target Detection and Attentional Processing During Auditory Search. Cereb Cortex. 2015;25:2456–2465. doi: 10.1093/cercor/bhu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. NeuroImage. 2003;20:1132–1139. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological science. 1993;4:385–390. [Google Scholar]
- Gentsch A, Ullsperger P, Ullsperger M. Dissociable medial frontal negativities from a common monitoring system for self- and externally caused failure of goal achievement. NeuroImage. 2009;47:2023–2030. doi: 10.1016/j.neuroimage.2009.05.064. [DOI] [PubMed] [Google Scholar]
- George JS, Strunk J, Mak-McCully R, Houser M, Poizner H, Aron AR. Dopaminergic therapy in Parkinson's disease decreases cortical beta band coherence in the resting state and increases cortical beta band power during executive control. Neuroimage Clin. 2013;3:261–270. doi: 10.1016/j.nicl.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies AJ, Willshaw DJ. A massively connected subthalamic nucleus leads to the generation of widespread pulses. Proc Biol Sci. 1998;265:2101–2109. doi: 10.1098/rspb.1998.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. Journal of neurophysiology. 2002;88:3150–3166. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- Greenhouse I, Oldenkamp CL, Aron AR. Stopping a response has global or nonglobal effects on the motor system depending on preparation. Journal of neurophysiology. 2012;107:384–392. doi: 10.1152/jn.00704.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada I, DeLong MR. Excitotoxic acid lesions of the primate subthalamic nucleus result in transient dyskinesias of the contralateral limbs. Journal of neurophysiology. 1992;68:1850–1858. doi: 10.1152/jn.1992.68.5.1850. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Sharp D. Inferior PFC Subregions Have Broad Cognitive Roles. Trends Cogn Sci. 2015;19:712–713. doi: 10.1016/j.tics.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Haynes WI, Haber SN. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:4804–4814. doi: 10.1523/JNEUROSCI.4674-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O'Reilly RC. Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philos Trans R Soc Lond B Biol Sci. 2007;362:1601–1613. doi: 10.1098/rstb.2007.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Fassbender C, Garavan H. Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cereb Cortex. 2004;14:986–994. doi: 10.1093/cercor/bhh059. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Nystrom L, Mars RB, Coles MG, Cohen JD. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nat Neurosci. 2004;7:497–498. doi: 10.1038/nn1238. [DOI] [PubMed] [Google Scholar]
- Horstmann G. Latency and duraction of the action interruption in surprise. Cognition and Emotion. 2006;20:242–273. [Google Scholar]
- Houben K, Havermans RC, Nederkoorn C, Jansen A. Beer a no-go: learning to stop responding to alcohol cues reduces alcohol intake via reduced affective associations rather than increased response inhibition. Addiction. 2012;107:1280–1287. doi: 10.1111/j.1360-0443.2012.03827.x. [DOI] [PubMed] [Google Scholar]
- Houtman F, Notebaert W. Blinded by an error. Cognition. 2013;128:228–236. doi: 10.1016/j.cognition.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Eichele T, Enriquez-Geppert S, Wollbrink A, Kugel H, Konrad C, Pantev C. Multimodal imaging of functional networks and event-related potentials in performance monitoring. NeuroImage. 2011;56:1588–1597. doi: 10.1016/j.neuroimage.2011.03.039. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Enriquez-Geppert S, Lavallee CF, Falkenstein M, Herrmann CS. Electroencephalography of response inhibition tasks: functional networks and cognitive contributions. Int J Psychophysiol. 2013;87:217–233. doi: 10.1016/j.ijpsycho.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Inase M, Tokuno H, Nambu A, Akazawa T, Takada M. Corticostriatal and corticosubthalamic input zones from the presupplementary motor area in the macaque monkey: comparison with the input zones from the supplementary motor area. Brain Res. 1999;833:191–201. doi: 10.1016/s0006-8993(99)01531-0. [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. In The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008:7209–7218. doi: 10.1523/JNEUROSCI.0487-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Obeso I, Rothwell JC, Obeso JA. A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nat Rev Neurosci. 2015;16:719–732. doi: 10.1038/nrn4038. [DOI] [PubMed] [Google Scholar]
- Jahfari S, Waldorp L, van den Wildenberg WP, Scholte HS, Ridderinkhof KR, Forstmann BU. Effective connectivity reveals important roles for both the hyperdirect (fronto-subthalamic) and the indirect (fronto-striatal-pallidal) fronto-basal ganglia pathways during response inhibition. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:6891–6899. doi: 10.1523/JNEUROSCI.5253-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedema HP, Carter MD, Dugan BP, Gurnsey K, Olsen AS, Bradberry CW. The acute impact of ethanol on cognitive performance in rhesus macaques. Cereb Cortex. 2011;21:1783–1791. doi: 10.1093/cercor/bhq244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentzsch I, Dudschig C. Why do we slow down after an error? Mechanisms underlying the effects of posterror slowing. Q J Exp Psychol (Hove) 2009;62:209–218. doi: 10.1080/17470210802240655. [DOI] [PubMed] [Google Scholar]
- Jutten C, Herault J. Blind separation of sources, part I: An adaptive algorithm based on neuromimetic architecture. Signal processing. 1991;24:1–10. [Google Scholar]
- Kenemans JL. Specific proactive and generic reactive inhibition. Neuroscience and biobehavioral reviews. 2015;56:115–126. doi: 10.1016/j.neubiorev.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Duty TL, Forster BB, Liddle PF. Neural sources involved in auditory target detection and novelty processing: an event-related fMRI study. Psychophysiology. 2001;38:133–142. [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF. An event-related functional magnetic resonance imaging study of an auditory oddball task in schizophrenia. Schizophr Res. 2001;48:159–171. doi: 10.1016/s0920-9964(00)00117-1. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Stevens MC, Laurens KR, Pearlson G, Calhoun VD, Liddle PF. An adaptive reflexive processing model of neurocognitive function: supporting evidence from a large scale (n = 100) fMRI study of an auditory oddball task. NeuroImage. 2005;25:899–915. doi: 10.1016/j.neuroimage.2004.12.035. [DOI] [PubMed] [Google Scholar]
- King JA, Korb FM, von Cramon DY, Ullsperger M. Post-error behavioral adjustments are facilitated by activation and suppression of task-relevant and task-irrelevant information processing. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:12759–12769. doi: 10.1523/JNEUROSCI.3274-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M. Neural correlates of error awareness. NeuroImage. 2007;34:1774–1781. doi: 10.1016/j.neuroimage.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Kok A, Ramautar JR, De Ruiter MB, Band GP, Ridderinkhof KR. ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology. 2004;41:9–20. doi: 10.1046/j.1469-8986.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Hanisch C, Fink GR, Herpertz-Dahlmann B. Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: evidence from an event-related functional magnetic resonance imaging study. Biol Psychiatry. 2006;59:643–651. doi: 10.1016/j.biopsych.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Bollimunta A, Arcizet F, Wang L. Attention as an effect not a cause. Trends Cogn Sci. 2014;18:457–464. doi: 10.1016/j.tics.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, Yarrow K, Brown P. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain : a journal of neurology. 2004;127:735–746. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- Laming DRJ. Information theory of choice-reaction times. London: Academic P.; 1968. [Google Scholar]
- Lapish CC, Kroener S, Durstewitz D, Lavin A, Seamans JK. The ability of the mesocortical dopamine system to operate in distinct temporal modes. Psychopharmacology. 2007;191:609–625. doi: 10.1007/s00213-006-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees AJ, Smith E. Cognitive deficits in the early stages of Parkinson's disease. Brain : a journal of neurology. 1983;106(Pt 2):257–270. doi: 10.1093/brain/106.2.257. [DOI] [PubMed] [Google Scholar]
- Leiva A, Parmentier FB, Elchlepp H, Verbruggen F. Reorienting the mind: The impact of novel sounds on go/no-go performance. J Exp Psychol Hum Percept Perform. 2015;41:1197–1202. doi: 10.1037/xhp0000111. [DOI] [PubMed] [Google Scholar]
- Leventhal DK, Gage GJ, Schmidt R, Pettibone JR, Case AC, Berke JD. Basal ganglia beta oscillations accompany cue utilization. Neuron. 2012;73:523–536. doi: 10.1016/j.neuron.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci. 2011;1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and postresponse processing. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg JK, Parmentier FB. Cross-modal distraction by deviance: functional similarities between the auditory and tactile modalities. Exp Psychol. 2012;59:355–363. doi: 10.1027/1618-3169/a000164. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the Ability to Inhibit Thought and Action: A Theory of an Act of Control. Psych Rev. 1984;91:295–327. [Google Scholar]
- Logan GD, Crump MJ. Cognitive illusions of authorship reveal hierarchical error detection in skilled typists. Science. 2010;330:683–686. doi: 10.1126/science.1190483. [DOI] [PubMed] [Google Scholar]
- MacDonald HJ, Coxon JP, Stinear CM, Byblow WD. The fall and rise of corticomotor excitability with cancellation and reinitiation of prepared action. Journal of neurophysiology. 2014;112:2707–2717. doi: 10.1152/jn.00366.2014. [DOI] [PubMed] [Google Scholar]
- Majid A, Cai W, Corey-Bloom J, Aron AR. Proactive selective response suppression is implemented via the basal ganglia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013 doi: 10.1523/JNEUROSCI.5651-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid DS, Cai W, George JS, Verbruggen F, Aron AR. Transcranial magnetic stimulation reveals dissociable mechanisms for global versus selective corticomotor suppression underlying the stopping of action. Cereb Cortex. 2012;22:363–371. doi: 10.1093/cercor/bhr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco-Pallares J, Camara E, Munte TF, Rodriguez-Fornells A. Neural mechanisms underlying adaptive actions after slips. J Cogn Neurosci. 2008;20:1595–1610. doi: 10.1162/jocn.2008.20117. [DOI] [PubMed] [Google Scholar]
- Mars RB, Coles MG, Grol MJ, Holroyd CB, Nieuwenhuis S, Hulstijn W, Toni I. Neural dynamics of error processing in medial frontal cortex. NeuroImage. 2005;28:1007–1013. doi: 10.1016/j.neuroimage.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mink JW. The Basal Ganglia: Focused Selection and Inhibition of Competing Motor Programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mirabella G, Iaconelli S, Romanelli P, Modugno N, Lena F, Manfredi M, Cantore G. Deep Brain Stimulation of Subthalamic Nuclei Affects Arm Response Inhibition In Parkinson's Patients. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr187. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Gillebert CR, Schoofs H, Dupont P, Peeters R, Vandenberghe R. Lesion neuroanatomy of the Sustained Attention to Response task. Neuropsychologia. 2009;47:2866–2875. doi: 10.1016/j.neuropsychologia.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Morra S. How do subvocal rehearsal and general attentional resources contribute to verbal short-term memory span? Front Psychol. 2015;6:145. doi: 10.3389/fpsyg.2015.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Wydell H, O'Neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. NeuroImage. 2007;36(Suppl 2):T155–T163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Inase M, Takada M. Corticosubthalamic input zones from forelimb representations of the dorsal and ventral divisions of the premotor cortex in the macaque monkey: comparison with the input zones from the primary motor cortex and the supplementary motor area. Neurosci Lett. 1997;239:13–16. doi: 10.1016/s0304-3940(97)00877-x. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the corticosubthalamo- pallidal 'hyperdirect' pathway. Neurosci Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Neuronal correlates of post-error slowing in the rat dorsomedial prefrontal cortex. Journal of neurophysiology. 2008;100:520–525. doi: 10.1152/jn.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notebaert W, Houtman F, Opstal FV, Gevers W, Fias W, Verguts T. Post-error slowing: an orienting account. Cognition. 2009;111:275–279. doi: 10.1016/j.cognition.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Oberauer K. Design for a Working Memory. In: Ross BH, editor. The Psychology of Learning and Motivation. 2009. pp. 45–100. [Google Scholar]
- Obeso I, Wilkinson L, Casabona E, Speekenbrink M, Luisa Bringas M, Alvarez M, Alvarez L, Pavon N, Rodriguez-Oroz MC, Macias R, et al. The subthalamic nucleus and inhibitory control: impact of subthalamotomy in Parkinson's disease. Brain : a journal of neurology. 2014;137:1470–1480. doi: 10.1093/brain/awu058. [DOI] [PubMed] [Google Scholar]
- Obeso I, Wilkinson L, Jahanshahi M. Levodopa medication does not influence motor inhibition or conflict resolution in a conditional stop-signal task in Parkinson's disease. Exp Brain Res. 2011;213:435–445. doi: 10.1007/s00221-011-2793-x. [DOI] [PubMed] [Google Scholar]
- Obeso I, Wilkinson L, Rodriguez-Oroz MC, Obeso JA, Jahanshahi M. Bilateral stimulation of the subthalamic nucleus has differential effects on reactive and proactive inhibition and conflict-induced slowing in Parkinson's disease. Exp Brain Res. 2013;226:451–462. doi: 10.1007/s00221-013-3457-9. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Anatomical aspects of information processing in primate basal ganglia. Trends Neurosci. 1993;16:111–116. doi: 10.1016/0166-2236(93)90135-9. [DOI] [PubMed] [Google Scholar]
- Parmentier FB. The cognitive determinants of behavioral distraction by deviant auditory stimuli: a review. Psychol Res. 2014;78:321–338. doi: 10.1007/s00426-013-0534-4. [DOI] [PubMed] [Google Scholar]
- Parmentier FB, Elford G, Escera C, Andres P, San Miguel I. The cognitive locus of distraction by acoustic novelty in the cross-modal oddball task. Cognition. 2008;106:408–432. doi: 10.1016/j.cognition.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Parmentier FB, Ljungberg JK, Elsley JV, Lindkvist M. A behavioral study of distraction by vibrotactile novelty. J Exp Psychol Hum Percept Perform. 2011;37:1134–1139. doi: 10.1037/a0021931. [DOI] [PubMed] [Google Scholar]
- Pashler H. Dual-task interference in simple tasks: data and theory. Psychol Bull. 1994;116:220–244. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- Pazo-Alvarez P, Cadaveira F, Amenedo E. MMN in the visual modality: a review. Biol Psychol. 2003;63:199–236. doi: 10.1016/s0301-0511(03)00049-8. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Pihan H, Altenmuller E, Hertrich I, Ackermann H. Cortical activation patterns of affective speech processing depend on concurrent demands on the subvocal rehearsal system. A DC-potential study. Brain : a journal of neurology. 2000;123(Pt 11):2338–2349. doi: 10.1093/brain/123.11.2338. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron. 2011;72:692–697. doi: 10.1016/j.neuron.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell BA, Kiani R. Neural Mechanisms of Post-error Adjustments of Decision Policy in Parietal Cortex. Neuron. 2016;89:658–671. doi: 10.1016/j.neuron.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt P, Rodgers B. What Does a Man Do after He Makes an Error - Analysis of Response Programming. Q J Exp Psychol. 1977;29:727–743. [Google Scholar]
- Rabbitt PMA. Error Correction Time without External Error Signals. Nature. 1966;212:438-&. doi: 10.1038/212438a0. [DOI] [PubMed] [Google Scholar]
- Rae CL, Hughes LE, Anderson MC, Rowe JB. The Prefrontal Cortex Achieves Inhibitory Control by Facilitating Subcortical Motor Pathway Connectivity. Journal of Neuroscience. 2015a;35:786–794. doi: 10.1523/JNEUROSCI.3093-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CL, Hughes LE, Anderson MC, Rowe JB. The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015b;35:786–794. doi: 10.1523/JNEUROSCI.3093-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray NJ, Jenkinson N, Brittain J, Holland P, Joint C, Nandi D, Bain GP, Yousif N, Green A, Stein JS, Aziz TZ. The role of the subthalamic nucleus in response inhibition: Evidence from deep brain stimulation for Parkinson's disease. Neuropsychologia. 2009 doi: 10.1016/j.neuropsychologia.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR. Micro- and macro-adjustments of task set: activation and suppression in conflict tasks. Psychol Res. 2002;66:312–323. doi: 10.1007/s00426-002-0104-7. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Span MM, van der Molen MW. Perseverative behavior and adaptive control in older adults: performance monitoring, rule induction, and set shifting. Brain Cogn. 2002;49:382–401. doi: 10.1006/brcg.2001.1506. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Goldman-Rakic PS. An auditory domain in primate prefrontal cortex. Nat Neurosci. 2002;5:15–16. doi: 10.1038/nn781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami M, Tsutsui K, Lauwereyns J, Koizumi M, Kobayashi S, Hikosaka O. A code for behavioral inhibition on the basis of color, but not motion, in ventrolateral prefrontal cortex of macaque monkey. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:4801–4808. doi: 10.1523/JNEUROSCI.21-13-04801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Stadler W, Schabus M, Doppelmayr M, Hanslmayr S, Gruber WR, Birbaumer N. A shift of visual spatial attention is selectively associated with human EEG alpha activity. Eur J Neurosci. 2005;22:2917–2926. doi: 10.1111/j.1460-9568.2005.04482.x. [DOI] [PubMed] [Google Scholar]
- Schall JD, Godlove DC. Current advances and pressing problems in studies of stopping. Curr Opin Neurobiol. 2012;22:1012–1021. doi: 10.1016/j.conb.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Leventhal DK, Mallet N, Chen F, Berke JD. Canceling actions involves a race between basal ganglia pathways. Nature Neuroscience. 2013;16:1118–1124. doi: 10.1038/nn.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroger E. A neural mechanism for involuntary attention shifts to changes in auditory stimulation. J Cogn Neurosci. 1996;8:527–539. doi: 10.1162/jocn.1996.8.6.527. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert S, Herrojo Ruiz M, Brucke C, Huebl J, Schneider GH, Ullsperger M, Kuhn AA. Error signals in the subthalamic nucleus are related to post-error slowing in patients with Parkinson's disease. Cortex. 2014 doi: 10.1016/j.cortex.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Wiltz K, Hallett M. Effect of volitional inhibition on cortical inhibitory mechanisms. Journal of neurophysiology. 2002;88:333–338. doi: 10.1152/jn.2002.88.1.333. [DOI] [PubMed] [Google Scholar]
- Sokolov EN. Higher nervous functions; the orienting reflex. Annu Rev Physiol. 1963;25:545–580. doi: 10.1146/annurev.ph.25.030163.002553. [DOI] [PubMed] [Google Scholar]
- Stevens T, Brevers D, Chambers CD, Lavric A, McLaren IP, Mertens M, Noel X, Verbruggen F. How does response inhibition influence decision making when gambling? J Exp Psychol Appl. 2015;21:15–36. doi: 10.1037/xap0000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear CM, Coxon JP, Byblow WD. Primary motor cortex and movement prevention: where Stop meets Go. Neuroscience and biobehavioral reviews. 2009;33:662–673. doi: 10.1016/j.neubiorev.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Summerfield C, de Lange FP. Expectation in perceptual decision making: neural and computational mechanisms. Nat Rev Neurosci. 2014;15:745–756. doi: 10.1038/nrn3838. [DOI] [PubMed] [Google Scholar]
- Swann N, Poizner H, Houser M, Gould S, Greenhouse I, Cai W, Strunk J, George J, Aron AR. Deep brain stimulation of the subthalamic nucleus alters the cortical profile of response inhibition in the beta frequency band: a scalp EEG study in Parkinson's disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:5721–5729. doi: 10.1523/JNEUROSCI.6135-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann N, Tandon N, Canolty R, Ellmore TM, McEvoy LK, Dreyer S, DiSano M, Aron AR. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:12675–12685. doi: 10.1523/JNEUROSCI.3359-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, de Hemptinne C, Aron AR, Ostrem JL, Knight RT, Starr PA. Elevated synchrony in Parkinson disease detected with electroencephalography. Ann Neurol. 2015;78:742–750. doi: 10.1002/ana.24507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AE, Saint-Cyr JA, Lang AE. Frontal lobe dysfunction in Parkinson's disease. The cortical focus of neostriatal outflow. Brain : a journal of neurology. 1986;109(Pt 5):845–883. doi: 10.1093/brain/109.5.845. [DOI] [PubMed] [Google Scholar]
- Taylor PC, Nobre AC, Rushworth MF. Subsecond changes in top down control exerted by human medial frontal cortex during conflict and action selection: a combined transcranial magnetic stimulation electroencephalography study. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:11343–11353. doi: 10.1523/JNEUROSCI.2877-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegelbeckers J, Bunzeck N, Duzel E, Bonath B, Flechtner HH, Krauel K. Altered salience processing in attention deficit hyperactivity disorder. Hum Brain Mapp. 2015;36:2049–2060. doi: 10.1002/hbm.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, Danielmeier C. Reducing Speed and Sight: How Adaptive Is Post-Error Slowing? Neuron. 2016;89:430–432. doi: 10.1016/j.neuron.2016.01.035. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Danielmeier C, Jocham G. Neurophysiology of performance monitoring and adaptive behavior. Physiol Rev. 2014;94:35–79. doi: 10.1152/physrev.00041.2012. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Harsay HA, Wessel JR, Ridderinkhof KR. Conscious perception of errors and its relation to the anterior insula. Brain Struct Funct. 2010;214:629–643. doi: 10.1007/s00429-010-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. NeuroImage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:4308–4314. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wildenberg WPM, van Boxtel GJ, van der Molen MIW, Bosch DA, Speelman JD, Brunia CHM. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson’s disease. Journal Cognitive Neuroscience. 2006 doi: 10.1162/jocn.2006.18.4.626. [DOI] [PubMed] [Google Scholar]
- Veling H, Aarts H, Stroebe W. Stop signals decrease choices for palatable foods through decreased food evaluation. Front Psychol. 2013;4:875. doi: 10.3389/fpsyg.2013.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrivelan R, Chang C, Lu J. Muscle tone regulation during REM sleep: neural circuitry and clinical significance. Arch Ital Biol. 2011;149:348–366. doi: 10.4449/aib.v149i4.1272. [DOI] [PubMed] [Google Scholar]
- Wessel J, Ghahremani A, Udupa K, Saha U, Kalia SK, Hodaie M, Lozano AM, Aron A, Chen R. Stop-related subthalamic beta activity indexes global motor suppression in Parkinson’s Disease. Movement Disorders. doi: 10.1002/mds.26732. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR. Testing Multiple Psychological Processes for Common Neural Mechanisms Using EEG and Independent Component Analysis. Brain Topogr. 2016 doi: 10.1007/s10548-016-0483-5. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Aron AR. Unexpected events induce motor slowing via a brain mechanism for action-stopping with global suppressive effects. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:18481–18491. doi: 10.1523/JNEUROSCI.3456-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Aron AR. Inhibitory motor control based on complex stopping goals relies on the same brain network as simple stopping. NeuroImage. 2014;103:225–234. doi: 10.1016/j.neuroimage.2014.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Aron AR. It's not too late: the onset of the frontocentral P3 indexes successful response inhibition in the stop-signal paradigm. Psychophysiology. 2015;52:472–480. doi: 10.1111/psyp.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Danielmeier C, Morton JB, Ullsperger M. Surprise and error: common neuronal architecture for the processing of errors and novelty. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:7528–7537. doi: 10.1523/JNEUROSCI.6352-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Jenkinson N, Brittain JS, Voets SH, Aziz TZ, Aron AR. Surprise disrupts cognition via a fronto-basal ganglia suppressive mechanism. Nature communications. 2016;7:11195. doi: 10.1038/ncomms11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Klein TA, Ott DV, Ullsperger M. Lesions to the prefrontal performance-monitoring network disrupt neural processing and adaptive behaviors after both errors and novelty. Cortex. 2014a;50:45–54. doi: 10.1016/j.cortex.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Wessel JR, O'Doherty JP, Berkebile MM, Linderman D, Aron AR. Stimulus devaluation induced by stopping action. J Exp Psychol Gen. 2014b;143:2316–2329. doi: 10.1037/xge0000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Reynoso HS, Aron AR. Saccade suppression exerts global effects on the motor system. Journal of neurophysiology. 2013 doi: 10.1152/jn.00229.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Tonnesen AL, Aron AR. Stimulus devaluation induced by action stopping is greater for explicit value representations. Front Psychol. 2015;6(1640) doi: 10.3389/fpsyg.2015.01640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiecki TV, Frank MJ. A computational model of inhibitory control in frontal cortex and basal ganglia. Psychol Rev. 2013;120:329–355. doi: 10.1037/a0031542. [DOI] [PubMed] [Google Scholar]
- Wolff MJ, Ding J, Myers NE, Stokes MG. Revealing hidden states in visual working memory using electroencephalography. Front Syst Neurosci. 2015;9:123. doi: 10.3389/fnsys.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Sandrini M, Wang WT, Smith JF, Sarlls JE, Awosika O, Butman JA, Horwitz B, Cohen LG. PreSMA stimulation changes task-free functional connectivity in the fronto-basal-ganglia that correlates with response inhibition efficiency. Hum Brain Mapp. 2016 doi: 10.1002/hbm.23236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi N, Callan DE, Goda N, Anderson SJ, Yoshida Y, Kawato M. Attentional modulation of oscillatory activity in human visual cortex. NeuroImage. 2003;20:98–113. doi: 10.1016/s1053-8119(03)00341-0. [DOI] [PubMed] [Google Scholar]
- Zavala B, Zaghloul K, Brown P. The subthalamic nucleus, oscillations, and conflict. Movement disorders : official journal of the Movement Disorder Society. 2015;30:328–338. doi: 10.1002/mds.26072. [DOI] [PMC free article] [PubMed] [Google Scholar]