Abstract
Neural cell grafting is a promising therapy for stroke, but the optimal differentiation status of the cells prior to grafting is unclear. We grafted cells at different maturity stages (days 28, 42, or 56 of in vitro neural differentiation) into the brains of eight-week-old rats one week after subcortical ischemic stroke, and assessed motor and sensory behavioral recovery over one month. We did not find a difference between the grafted or control groups on behavioral recovery, or on brain tissue outcomes including infarct size, microgliosis, or astrocytosis. Further research is needed into mechanisms of benefit of neural cell grafting for stroke.
Keywords: neural, differentiation, maturity, human, induced pluripotent stem cells, grafting, subcortical, ischemic stroke, rat, model
Introduction
Stroke is a major cause of disability, with few effective treatments available to improve recovery.[1] Cell transplantation is a promising potential treatment to improve stroke recovery.[2] Neural progenitor cells derived from human induced pluripotent stem cells are an attractive candidate cell type for further research.[3] One of the initial questions requiring clarification is how the neural differentiation maturity status of the cells prior to grafting affects behavioral and tissue responses of a stroke model. More mature neural cells may be able to more rapidly integrate into host neuronal circuitry to replace lost neurons, but may be less able to survive the grafting process, or less able to secrete beneficial factors. Less mature neural cells may take longer to replace lost neurons, but may better survive the grafting process, or may be more able to secrete beneficial factors present early in development that could aid recovery of the host tissue. We therefore sought to determine the effects of the pregraft neural differentiation maturity status of human induced pluripotent stem cells in a model of ischemic stroke.
Methods
The full methods are described in the online supplement. The induced pluripotent stem cell line iPS-DF6-9-9T was derived from postnatal human skin fibroblasts.[4] The cells were expanded as pluripotent cells and differentiated to neural lineages as previously described with modifications.[5, 6] Male 8-week-old Sprague Dawley rats, at an age widely-used for stroke research, were given small deep hemispheric infarcts similar to a human lacunar stroke by intracerebral injection of the vasoconstrictor peptide endothelin-1.
Seven days poststroke, 8 rats per group were randomly assigned to receive intracerebral grafts of approximately 250,000 cells at days 28 (named group W4), 42 (named group W6), or 56 (named group W8) of neural differentiation, or vehicle without cells for the control group (named group C). These time points represented a wide range of neural differentiation in our culture system, as previously characterized.[7] Cyclosporine was given to all the rats (cell-treated and control) starting 2 days before transplantation.
Behavioral testing occurred blind to group assignment prior to stroke and weekly thereafter. The whisker edge test assessed vibrissal sensitivity. The tape bracelet test assessed skin sensitivity. The corner test assessed gross sensorimotor symmetry. The elevated body swing test assessed gross motor symmetry. The rotarod assessed balance and walking. The staircase test assessed fine motor function.
One month after transplantation, brain sections were prepared for cresyl violet staining and immunohistochemistry with Hoechst to label nuclei. The stroke volume and thickness of the anterior commissure and the corpus callosum were measured, and cells were labeled for markers of human nuclei, human cytoplasm, human neural progenitors (nestin), neurons (MAP2, B3T), astrocytes (GFAP), dividing cells (Ki67), and microglia (CD11B). The number of labeled cells was stereologically estimated, co-labeling of cells was quantified, and optical densitometry of fluorescent marker integrated density (ID) was measured.
Data are presented as means ± standard deviation (SD). Behavioral scores were compared with one-way repeated measures analysis of variance. One-way ANOVA was used to assess for group differences in: the size of the infarct, anterior commissure, and corpus callosum; mean number of stereologically estimated labeled cells; graft cells co-labeling for secondary markers; and marker ID values.
Results
The figures are in the online supplement. There were no significant differences between groups for: any of the sensory or motor behavioral tests (figures 1 and 2); the size of the infarct, the anterior commissure, or the corpus callosum (figure 3); or tissue staining for Hoechst, GFAP, or CD11B (figures 4 and 7). Between the three grafted groups, there were no significant differences for graft cell number, or the amount of graft cells labelled for nestin, MAP2, B3T, GFAP, or Ki67 (figure 5). No seizures were seen in the rats during the month following transplantation, and we did not see graft overgrowth or tumor formation.
Figure 1.
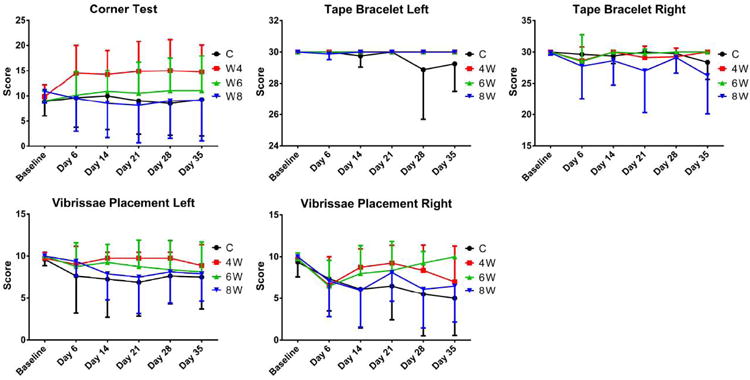
There were no significant differences between groups for the sensory behavioral tests.
Figure 2.
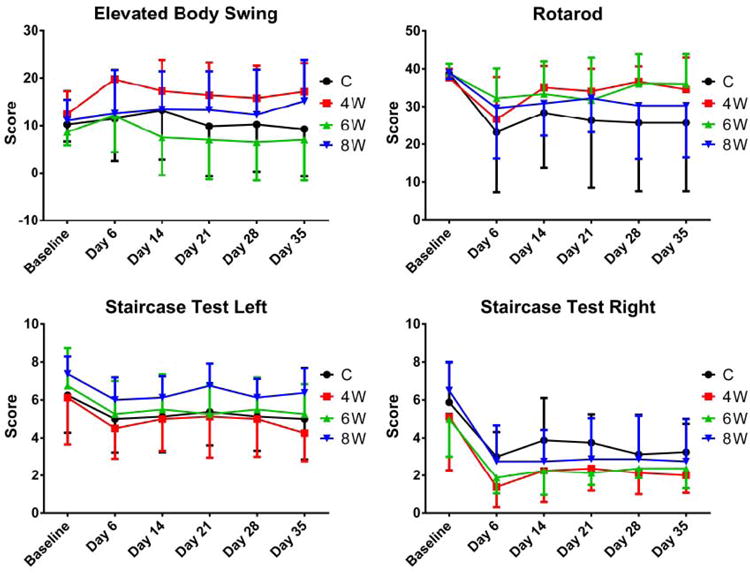
There were no significant differences between groups for the motor behavioral tests.
Figure 3.
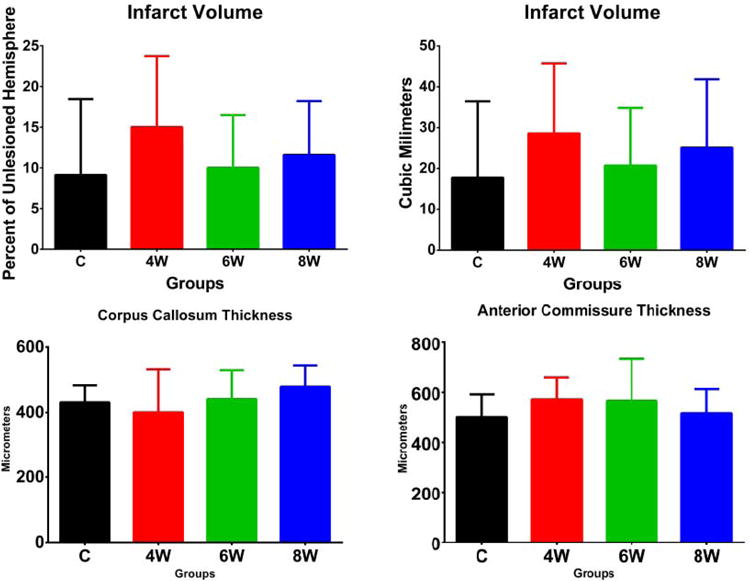
There were no significant differences between groups for the size of the infarct, the anterior commissure, or the corpus callosum.
Figure 4.
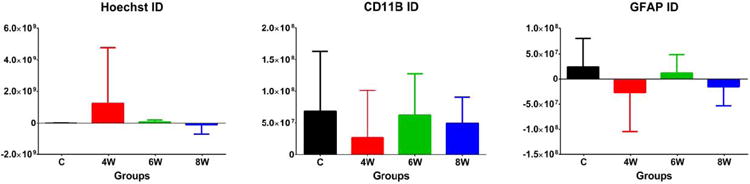
There were no significant differences between groups for tissue staining for Hoechst, GFAP, or CD11B.
Figure 7.
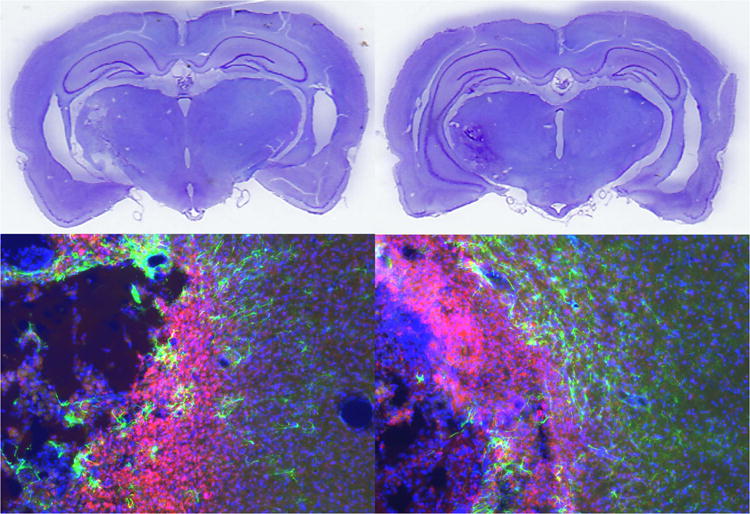
Representative histological images of a control rat (left) and grafted rat (right, group 4W). The upper images are cresyl violet stained brain sections showing subcortical infarction. The lower images are immunohistochemistry of the infarct showing astrocytosis (GFAP in green), microcytosis (CD11B in red), and overall cellularity (Hoechst in blue).
Figure 5.
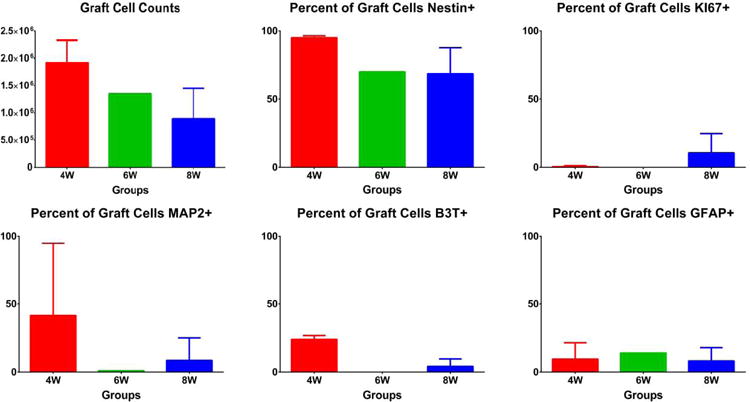
There were no significant differences between the grafted groups for graft cell number, or the amount of graft cells labelled for nestin, MAP2, B3T, GFAP, or Ki67.
Conclusions
We did not find a difference in behavioral or tissue outcomes after ischemic stroke between rats grafted with neural cells at different maturity states, or between grafted and control rats. The simplest explanation for these results is that these cells with this grafting regimen have no effect on these outcomes in this stroke model. Alternatively, a small beneficial effect may exist that would require a larger sample size to detect. It is also possible that the differentiation state of the grafted cells affects outcome, but in an unclear way that interacts with other variables of the grafting regimen, such as the dose of cells, the timing of grafting after stroke, or anatomical aspects of the intracerebral injection. Further research into the mechanisms of benefit of neural cell grafting in a wide range of stroke models may allow us optimize all variables of transplantation methods.
Supplementary Material
Figure 6.
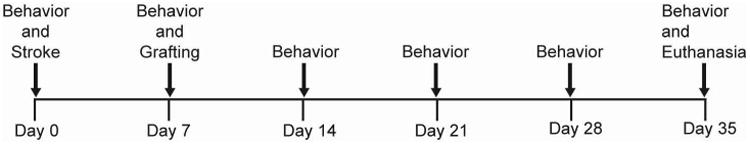
Timeline of in vivo experimental events.
Acknowledgments
Funding: Supported by NIH grants 1K08NS079622 and P30HD03352.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, et al. Circulation. 2014. Heart Disease and Stroke Statistics-2015 Update: A Report From the American Heart Association. [DOI] [PubMed] [Google Scholar]
- 2.Bliss T, et al. Cell transplantation therapy for stroke. Stroke. 2007;38(2 Suppl):817–26. doi: 10.1161/01.STR.0000247888.25985.62. [DOI] [PubMed] [Google Scholar]
- 3.Amabile G, Meissner A. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends Mol Med. 2009;15(2):59–68. doi: 10.1016/j.molmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Yu J, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen MB, et al. Survival and Differentiation of Transplanted Neural Stem Cells Derived from Human Induced Pluripotent Stem Cells in a Rat Stroke Model. J Stroke Cerebrovasc Dis. 2011 doi: 10.1016/j.jstrokecerebrovasdis.2011.09.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen MB, et al. Injected Versus Oral Cyclosporine for Human Neural Progenitor Grafting in Rats. Journal of Stem Cell Research & Therapy. 2012 doi: 10.4172/2157-7633.S10-003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu BY, et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A. 2010;107(9):4335–40. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


