Abstract
We investigate the role of physician agency in determining health care supply and patient outcomes. We show that an increase in health care supply due to a change in private physician incentives has a theoretically ambiguous impact on patient welfare. The increase can reflect either induced demand for ineffective care or a reduction in prior rationing of effective care. Furthermore, physician market structure matters in determining the welfare effects of changes in private physician incentives. We then analyze a change to Medicare fees that caused physicians to increase their provision of chemotherapy. We find that this increase in treatment improved patient survival, extending median life expectancy for lung cancer patients by about 18%. Consistent with the model, we find that while the treatment response was larger in less concentrated markets, survival improvements were larger in more concentrated markets.
In the presence of asymmetric information, physicians may distort demand for health care in socially sub-optimal but personally beneficial ways. Although a robust literature shows that the care physicians provide responds to private financial incentives (see McGuire (2000) and Johnson (2014) for reviews), evidence on how these responses affect patient health is limited. For example, analyses of mortality, a well-defined, robust measure of health, generally find no statistically significant relationship between physician-induced changes in the supply of medical services and mortality (e.g., Heaton and Helland (2009), Clemens and Gottlieb (2014)). But given the low mortality rates in these studies, they are underpowered to assess survival and cannot rule out large effects of physician financial incentives on patient mortality. Understanding the effect of physicians’ private incentives on patient health is important for normative assessments of both past and future health policies.
While economists accept the idea that private incentives can affect behavior, physicians are generally uncomfortable with the notion that money may motivate them to behave in ways not in the best interest of their patients. More specifically, although physicians may accept the idea that clinicians sometimes offer harmless but unnecessary services for financial gain, many find it implausible that a provider might perform harmful care or withhold helpful care for financial reasons. Based on this worldview, physician agency should affect only the efficiency of health care provision and have no direct effect on patient welfare.
In this paper we provide some of the first evidence of the impact of physicians’ private financial incentives on patient welfare. We do so by first extending the theoretical literature on physician agency to make explicit the potential welfare effects of physician agency and to generate additional theoretical predictions. We extend the classic McGuire and Pauly (1991) physical agency model in two main ways. First instead of including a term in the utility function whereby “excess care” generates negative utility for the physician, we directly include patient welfare in the physician’s utility function. Such partial altruism generates a disutility for both providing unnecessary care and not providing necessary care. Further, it predicts the conditions under which health care provision is more or less elastic to physician fees.1
Second we allow market concentration to vary across markets to examine the relationship between market structure and the behavior of partially altruistic physicians. We show that market concentration affects patient outcomes through two channels: (1) the level of healthcare provided (e.g., patient-to-physician ratios), and (2) the selection of patients into treatment. While the former channel has been examined previously (Gruber and Owings (1996)), the second is novel and generates a set of testable predictions. Across markets, more concentrated markets will be 1) less elastic in the supply of care but 2) more elastic in patient welfare. Within markets, 3) a physician’s market share will be unrelated to a change in private incentives but 4) positively correlated with the magnitude of the change in patient welfare.
To test the predictions of the model, we build on Jacobson et al (2010, 2011), which study the impact of a major reform to Medicare reimbursement policy for physician-administered (Part B) drugs on chemotherapy treatment for lung cancer patients.2 This reform, which in 2005 replaced a reimbursement system based on list prices with one based on national average transaction prices, sought to remedy well known Medicare over-payments for Part B drugs. By design, it significantly reduced the profitability of chemotherapy, with the margins paid to physicians for some commonly used drugs falling by nearly a factor of five. The reform was a large negative shock to oncologist income because chemotherapy accounted for over three-quarters of oncologist practice revenues in 2005 (Akscin and Barr (2007)), the mark-ups on chemotherapy drugs accounted for more than half of oncologists’ reported net income (Butcher (2008)), and Medicare is the primary payer for many cancers.3
Leveraging our prior finding that oncologists greatly increased the supply of chemotherapy in response to the fee cuts (Jacobson et al. 2010, 2011), we study the effects of physician agency in this setting on patient outcomes. We find that the reform, which generated a 32% average decline in reimbursement rates4 and a 9% increase in the likelihood of chemotherapy treatment, reduced the likelihood of death at 3, 6, and 9 months from diagnosis by 2 to 3%. This translates into an 18% increase in median patient life expectancy.5 To our knowledge, this is among the first evidence that physician agency can affect health outcomes.6
We further consider the interaction of market concentration and partially altruistic physician preferences. Consistent with our model, we find that 1) across markets, market concentration is negatively related to the magnitude of the increase in chemotherapy but 2) positively related to changes in survival and 3) within markets physician response is unrelated to market share but 4) is strongly correlated with improvements in patient survival. These results imply that physician agency cannot be understood in isolation of market structure.
The rest of the paper is organized as follows. Section 1 presents our model of physician agency and competition and its implications for the supply of medical services in response to physician fee changes. Section 2 describes the 2005 change to Medicare fees and its impact on oncology drugs in particular. Section 3 describes our data and analytic approach to estimating the effect of the fee cuts generated by the 2005 policy. Section 4 presents our empirical results and section 5 concludes.
1 Health Care Supply
We model physician behavior in markets where physicians care about both their private utility and the welfare of their patients. We build on McGuire and Pauly (1991), who first formalized the idea that physician behavior would be modulated by physician disutility for inducing demand (i.e., over-treatment).7 We extend the model by allowing patient welfare to enter directly into the physician’s utility function. This formulation naturally generates a disutility for both inducing demand and rationing care (i.e., undertreatment). We further extend the model by examining how market concentration affects the behavior of such partially altruistic physicians. We show that while the physician-to-patient ratio is a sufficient statistic to determine market elasticity (see Gruber and Owings (1996)), concentration measures are necessary to determine welfare effects.
Let there be J physicians operating in a market k ∈ {1, K} that serves a continuum of patients of measure one. A physician j ∈ {1, J} has local monopoly power over a fraction ηj of patients in market k and can provide each patient a single unit of service q, which yields patient i a benefit b distributed over (−∞, ∞) such that b(q) is a continuous, decreasing convex function. In addition, the distribution of patients each physician sees is identical to each other and to the overall distribution of types in the market.
The physician earns a fixed price p and pays a fixed cost c for each unit of service provided. Each unit of care requires a unit of physician time such that e(q), the disutility of physician effort, is an increasing and convex function with e(0) = 0. Physician utility is assumed to be positive in net income with diminishing marginal returns. Physicians are also assumed to care directly about the welfare of their patients, so physician utility is negatively affected by any deviation from the patient’s ideal level of care (i.e., rationing and induced demand).8
Assuming additive separability, the physician utility function can be written as
| (1) |
where π = (p − c)q and α represents the relative weight the physician places on the welfare of her patients.9 Thus, a physician trades off her private utility from income with the disutility of effort and her concern for patient welfare. A physician with α = 0 is fully self interested and puts no weight on patient welfare while a physician with α→∞ is completely selfless and acts only in the interest of the patient.
1.1 Individual physician response
For notational simplicity, we define the profit margin m = (p − c). Then the first order condition for the physician is given by
| (2) |
Note that while concern for patient welfare (αb(q/η)) pushes physicians towards providing what, from the patient’s perspective, is the optimal level of care qP, in general physicians will under or over provide care relative to this optimum.10
To determine physician response to profit margin (m) changes, we take the derivative of equation 2 with respect to m. Rearranging, we get the following relationship:
| (3) |
In general, qm can be positive or negative. That is, an increase in profitability can lead to an increase or decrease in service provision. This ambiguity of changes in m on the quantity of service provided is a standard characteristic of PID (see for instance McGuire and Pauly (1991) and McGuire (2000)), and in the case of a fee cut is driven by the tradeoff between decreasing marginal returns to effort (the substitution effect) and increasing marginal returns to income (the income effect). If decreased returns to effort dominate, a cut in margins leads to a reduction in effort (i.e., services provided). When the income effect dominates, a decrease in margins generates a negatively sloped supply curve, i.e., a fee cut leads to an increase in physician effort. Thus, the change in the marginal utility of income, the relative magnitudes of Vπ and Vππ), provides some guidance as to the sign of the response. When the magnitude of the income effect Vππ is small, the substitution effect dominates and physicians display a positive supply elasticity. But when Vππ is large, it reduces the impact of the substitution effect, and if large enough, can lead to a negative medical services supply elasticity.11
As implied by the name, the literature generally frames physician agency in terms of induced demand (PID), and largely ignores the possibility that physicians might intentionally “hold down” demand or otherwise fail to provide care to those who would benefit from it. But as the FOC in equation 2 makes clear, whether the equilibrium quantity q* is above or below the patient bliss point qP is ambiguous: physician self-interest can distort the provision of services away from qP in either direction. Importantly, whether changes in care are helpful or harmful to patient welfare depends not only on the direction of the change but also the original level of service relative to qP.
Since physician response and its welfare consequences depend on initial conditions in the general case, we turn our focus to the simpler extreme cases of pure altruism and no altruism to develop some intuition about the impact of physician agency. In the case of pure altruism, physicians will supply care if and only if the patient benefits. As such neither the provision of care nor patient welfare will be affected by changes in physician profit margins. On the other extreme, if physicians care little for the welfare of their patients, then physician response is driven solely by private incentives, and physicians will be unaffected by their impact on patient welfare. Our first two empirical predictions are then:
Prediction 1) If physicians are self-interested (α ≈ 0), the magnitude of her response to changes in profit margin will be independent of either patient need or physician market share.
Prediction 2) If physicians are self-interested (α ≈ 0), the magnitude of the change in her patients’ welfare in response to a change in profit margin will be positively correlated with her market share.
The first prediction follows simply from the fact that when α = 0, both patient welfare and physician market share drop out of the physician’s utility function. As such physicians provide care until the disutility of effort equals their profit margin. The second prediction follows from the first prediction plus the assumptions that physicians 1) treat patients in order of need and 2) that within a market, physicians see identical distributions of patients. Under these assumptions, the marginal patient for physicians with larger market shares (e.g., more patients) will benefit more from treatment. Then since the magnitude of the response is the same regardless of market share, physicians with larger market share will have larger impacts on patient welfare.
1.2 Market level effect
At the market level, the interaction between physician agency and market concentration gives us the following propositions:
Proposition 1
For small α ≈ 0, if there is an internal solution to the FOC in equation 2, the magnitude of the elasticity of market response to changes in profit margin will be negatively correlated with market concentration.
Proof
See Appendix A.
Proposition 2
For small α ≈ 0, if there is an internal solution to the FOC in equation 2, the magnitude of the change in patient benefit in a market will be positively correlated with market concentration.
Proof
See Appendix A.
As before, in equilibrium, markets may under or over provide care relative to what would be demanded if all consumers were perfectly informed (qP). Additionally, the sign of any change in patient welfare cannot be determined solely by the sign of the change in the amount of care provided, but also depends on the initial level of care. Intuitively, when physicians care only about patient welfare, the level of care should be invariant to the state of the provider market - i.e., only (and all) patients who need care receive it. In contrast, when physicians care only about their own utility, the supply of health care will respond to changes in private incentives. Specifically these two propositions correspond to two predictions about the impact of a change in private physician incentives across markets. First the magnitude of the market response to a change in profit margin m (e.g., the change in the share of patients receiving care) is negatively correlated with market concentration. This is similar to a fact documented in prior studies (e.g., see Gruber and Owings (1996)), that the total amount of care increases with the physician to population ratio in a market.
Second, changes in average welfare will be positively correlated with market concentration. This result stems from the fact that the change in welfare is driven by both the mean and the distribution of physician market shares ηj while the relationship between market concentration and physician response is driven mostly by the mean physician market share (e.g., the physician to population ratio). While the mean physician market share determines how many additional patients get treated, the distribution of physician market shares determines who gets treated. Similar to the within market welfare prediction, this is a result of the fact that the benefit received by the marginal patient increases with a physician’s market share.
As a simple example, consider the case of two physicians in a market that includes 100 lung cancer patients, where each physician provides chemotherapy to 10 patients. When the two physicians have equal market shares (i.e., 50 patients each such that (η1 = η2)), the 20 most needy patients are treated. In contrast, when the market is more concentrated (e.g., physician 1 manages 75 patients and physician 2 manages 25, such that (η1 > η2)), physician 1’s marginal patient receives more benefit from chemotherapy than physician 2’s marginal patient. Thus, although the quantity of care provided in the two markets is the same, a given change in service provision has a larger impact on patient welfare in the more concentrated market. If, as in our model, patient benefit is decreasing and concave in the share of patients treated, this second effect generates an unexpected prediction: although the (relative) treatment response to a payment change will be (weakly) larger in less concentrated markets, the corresponding welfare effects will be smaller.
2 Institutional Background
Our empirical analysis focuses on the market for physician-administered chemotherapy drugs. Physicians purchase these drugs from manufacturers or wholesalers, inject or infuse them into patients in their office or clinic, and then bill Medicare and other payers for the drugs.12 Medicare Part B has covered physician-administered drugs since the program’s inception. Since at least the mid-1990s, it was well understood that Medicare overpaid physicians and outpatient hospital units for many Part B drugs (Office of Inspector General (1997a); Office of Inspector General (1997b)).
The Medicare Prescription Drug, Improvement and Modernization Act (MMA) of 2003 aimed to resolve the overpayments and reduce Medicare spending by redesigning Medicare’s reimbursements for physician-administered drugs. Prior to the reform, Medicare reimbursed physicians and outpatient hospital clinics for Part B drugs as a percentage of the average wholesale price (AWP), a list price, published in several catalogues, that reflects neither wholesale nor average prices (Berndt and Newhouse (2012)). In the trade literature, AWP is sometimes referred to as “Ain’t What’s Paid.” The MMA replaced the AWP-based system with a new average sales price (ASP) reimbursement system, whereby Part B drugs are reimbursed based on the national average of manufacturers’ sales prices, including rebates, from two quarters prior plus a 6% mark-up.13 The reform substantially reduced profit margins for many chemotherapy drugs. In 2005, the payment reform affected only physician’s offices, the primary setting for chemotherapy treatment, accounting for about 73% of episodes (defined as chemotherapy within 240 days of diagnosis) in our data. In 2006 the reform affected hospital outpatient offices, the other main setting for chemotherapy, accounting for about 19% of all chemotherapy episodes in our data.14
Our prior work, the basis for the current analysis, showed that the likelihood of chemotherapy treatment for lung cancer patients increased by about 10% in response to the reform and that the increase was specific to the physician-office setting (Jacobson et al. (2010)). Jacobson et al. (2010)) also showed that the mix of drugs used to treat patients changed in ways predicted by agency theory: drugs that lost the most margin were used less frequently among the chemotherapy-treated population and conversely, expensive drugs, which were favored by the reform’s 6% margin on all drugs, were used more often than previously.15
An obvious question is whether these changes were driven by physicians or patients; since Medicare beneficiaries face 20% coinsurance for Part B services, a decline in drug prices could have increased patient demand. In practice, however, about 90% of beneficiaries have supplemental insurance that pays this coinsurance (Kaiser Family Foundation (2010)). And anecdotal evidence suggests that oncologists were less likely to collect the coinsurance from the remaining patients when reimbursements were based on AWP, implying that the out-of-pocket costs associated with chemotherapy actually increased even as average drug costs declined for Medicare (Mullen (2007)). In addition, patient demand for life-saving or extending chemotherapy treatment is likely to be highly inelastic (e.g., see Goldman et al. (2010)). On balance, any effects from patient demand should be negligible. Consequently, this setting is well suited to examining the impact of physician agency as the reform 1) generated a large, plausibly exogenous change in the profitability of providing chemotherapy for physicians but not hospitals, 2) had little to no effect on patient costs, and 3) affected a condition (lung cancer) with significant short-run welfare variation.
3 Data and Methods
We obtained a dataset of Medicare beneficiaries with at least one claim with a lung cancer diagnosis (ICD-9 162.0–162.9) in a physician’s office or an outpatient-hospital setting between 2003 and 2005 (N=878,923) and all their claims across primary outpatient and inpatient settings from 2002 to 2006.16 These claims capture services delivered to Medicare fee-for-service patients, the services affected by the reform.17 Dates of death are from the Medicare Vital Status File through 2012.
To define a cohort of lung cancer patients, we require two or more non-institutional (i.e., physician, durable medical equipment or outpatient hospital) claims separated by at least 28 days but no more than 365 days or one institutional (short-stay or long-stay hospital, skilled nursing facility or hospice) claim with a lung cancer diagnosis. This is a common approach to defining cancer in claims data and has been shown to have high sensitivity (i.e., identify a high proportion of actual cancer cases) and very high specificity (i.e., exclude beneficiaries without cancer) in Medicare claims data (e.g., see Warren et al. (1999); Ramsey et al. (2009); Vera-Llonch et al. (2011)). We use the first claim with a lung cancer diagnosis to date the onset of disease.18
The lung cancer cohort includes 216,119 beneficiaries enrolled in Medicare Parts A and B who were diagnosed between January 2003 and November 2005. We restrict the primary analytic cohort to the 132,768 beneficiaries diagnosed between February 2004 and November 2005 to capture 11 months on either side of the payment change. We identify chemotherapy treatment using relevant diagnosis and billing codes (Warren et al. (2002)).19
3.1 Analytic Approach
To assess how the January 2005 payment change affected outcomes, we take both a graphical and regression-based approach that initially analyzes patients by month of diagnosis relative to the reform. To begin, we plot estimated month-relative-to-reform (January 2005) fixed effects along with 95 percent confidence intervals from a regression of the following basic form:
| (4) |
where Yism is the treatment or survival outcome of individual i residing in state s and diagnosed in month m, Xism are a set of patient characteristics including gender, race/ethnicity (7 categories), patient age and its square, pre-cancer comorbidities, as measured by the Deyo-Charlson score (Deyo et al. (1992); Charlson et al. (1987)), an indicator for metastatic disease at diagnosis, defined as 30 days from the date of diagnosis20; μs are state fixed effects, δm−m* are month-relative-to-reform fixed effects (e.g. mx−m* = −1 in December 2004 and +1 in February 2005) with January 2005 omitted, and εism is an error term. Plotting these month fixed effects helps verify that the time-series patterns are consistent with a causal impact of the reform. In addition, these plots guide the specification of models estimating the magnitude of the reform’s impact. We focus on a window of 11 months prior to and 11 months post reform to balance the number of pre-reform months with the limited post-reform data available to us, although using all 24 months of pre-reform data yields similar results.21 We show results for shorter windows - 9 months on either side of the reform - to hone in on the policy change.
Our measures of treatment include the likelihood that a newly diagnosed lung cancer patient received any chemotherapy, chemotherapy treatment in a specific setting (e.g., physician’s office), and treatment with specific agents conditional on receiving any chemotherapy, in each case within 30 days of diagnosis.22 Our survival analyses focus on the likelihood of death within 3, 6, 9, or 12 months of diagnosis. Because of the low rate of lung cancer survival, this follow-up period captures almost 90% of deaths in both cohorts.
We use the same basic regression framework and visual approach to analyze the characteristics of the cohorts diagnosed before and after January 2005. The goal in analyzing characteristics is to establish that individuals diagnosed just before relative to just after the payment change are similar on observable dimensions, since similarity on unobserved dimensions is a key identifying assumption for our analysis.
Based on the plots, we estimate the magnitude of the effect of the January 2005 switch to ASP-based payments as follows:
| (5) |
where Ȳt is an outcome such as the share of beneficiaries diagnosed in week t who died within 3 months, post is an indicator equal to 1 after the payment change and 0 otherwise, (t−t*) is a linear function of time relative to the reform, t*, that controls for smooth trends in outcomes around the time of the payment change and that we allow to differ on either side of the payment change. These regressions control for the share of patients by week that were male, the share by race/ethnicity, the median age and median age squared, the mean Deyo-Charlson score, and the share with metastatic disease. We compute Newey-West standard errors to allow for heteroskedasticity and autocorrelation in the error of an unknown form up to a lag of 52 weeks (Newey and West (1987)). We allow for this long a lag to account for correlation of annual events that affect treatment, such as the Christmas holidays. However, inference is not meaningfully affected by alternative lag structures.
The key analytic challenge is the pre-post design. This design assumes (1) the stability of patient characteristics across the reform and (2) that no other major changes affected treatment patterns in physician’s offices at the same time as this reform. To substantiate assumption (1), Appendix Table 1 shows pre-reform means as well as estimated changes in patient characteristics post-reform (from equation 5). The estimated changes in patient characteristics are small and generally indistinguishable from zero. Where they are statistically significant (e.g., age), these changes are not meaningful in magnitude. These results, which are discussed in more detail in Jacobson et al (2010), indicate that demographic characteristics are well balanced in the pre and post reform period. In addition, the number of newly diagnosed patients is relatively smooth across the reform, as indicated in Appendix Table 1 (last row) and Appendix Figure 1. These findings support the stability of the characteristics of newly diagnosed lung cancer patients across the reform.
To address concerns about other changes occurring at the same time as the reform, we contrast effects in physician’s offices (subject to the reform in 2006) and the outpatient hospital setting (not subject to the reform until 2006). We compare estimates from separate time-series models as well as from difference-in-differences models. Because the “control group” – hospital outpatient clinics – may be indirectly affected by practice changes in the physicians office-setting, however, the time-series model in (5) is our preferred approach. We also conduct placebo analyses of changes in outcomes for 2004, which, although subject to a small decrease in reimbursement rates (along with an increase in administration fees), did not experience a dramatic, margin-decreasing overhaul to the reimbursement system. We further take advantage of substantial geographic variation in responses to the payment change to assess whether the survival response was larger in states with a larger treatment response. Finally, we assess differences in survival by age, since older patients are more likely to be under-treated (Davidoff et al. (2010); Booth C. M. et al. (2010)).23 Together, the results indicate that reform led physicians to increase chemotherapy treatment and that this change lead to a meaningful increase in patient survival.
4 Results
4.1 Bite of the Reform
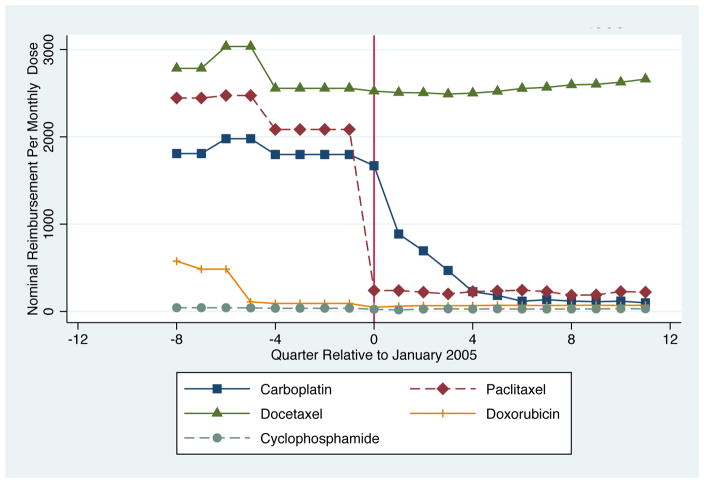
Table 1 and Figure 1, which show payment rates for several drugs commonly used to treat lung cancer, demonstrate that the reform had bite. Payment rates declined after the reform for carboplatin, paclitaxel, and etoposide. The changes were most striking for carboplatin and paclitaxel; reimbursement rates for a standard monthly dose declined from over $2,270 to $225 for paclitaxel and from $1,845 to $930 for carboplatin. As shown in Figure 1, the changes in carboplatin and paclitaxel reimbursements occurred around the time the new payment system took effect (time 0)). A complication for carboplatin is that the drug went off patent in October 2004, the quarter before ASP implementation. To ensure that our treatment results are not driven entirely by carboplatin, which would combine the effect of a patent expiration and the payment reform, we examine chemotherapy use of regimens with and without carboplatin.24 For the other agents shown, payments were relatively flat, reflecting the differential effect of the reform on payment rates, depending on pre-reform mark-ups.
Table 1.
Quarterly Reimbursement Rates Pre versus Post Jan 2005 Reform
| Full Period | 2004 | 2005 | |
|---|---|---|---|
| Quarterly Reimbursement Rates Per Standard Monthly Dose ($) | |||
| carboplatin | 1540 (153) | 1845 (29) | 930 (261) |
| paclitaxel | 1590 (294) | 2272 (70.9) | 225 (9.28) |
| docetaxel | 2657 (58.4) | 2732 (74.8) | 2506 (6.87) |
| etoposide | 77.6 (27.8) | 111(36.8) | 11.4(0.369) |
| gemcitabine | 1311 (13.5) | 1313 (20.6) | 1305 (1.71) |
Notes: Standard deviations are in parentheses. The pre-period is 2004 and the post-period is 2005; the full period captures the average across quarters in both years. Reimbursement rates are from the CMS website. In the post-reform period, payment rates are updated quarterly. Pre-reform, the rates are reported by fiscal year; variation within a calendar year is generated by the difference between the calendar and the fiscal year and, in some cases, because the same drug corresponds to several dosage-specific reimbursement (or HCPC) codes.
Figure 1.
Change in Nominal Quarterly Chemotherapy Payments Rates for Select Drugs Relative to January 2005
Both Table and Figure 1 show that the reform impacted reimbursement rates for chemotherapy in a very discrete way. On average the decline in reimbursement was about 32%. Given how much of oncologist income is driven by the mark-up on physician-administered drugs – more than 50% based on some assessments – it is plausible that the reform affected physician behavior (Butcher (2008)).
4.2 Changes in Treatment
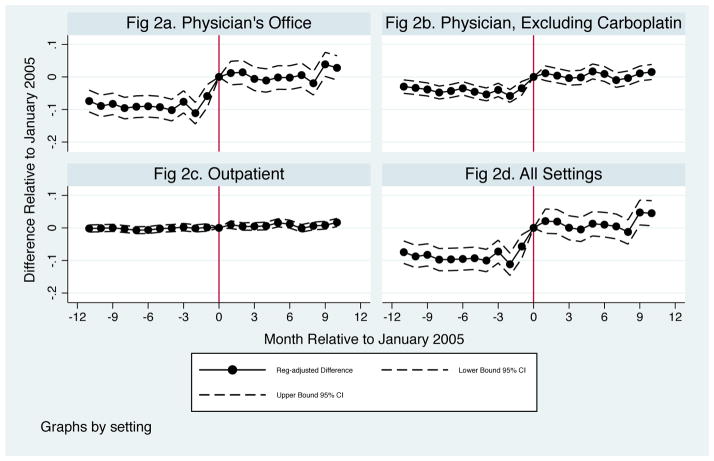
Jacobson et al. (2010) analyzed the reform’s effect on treatment. Appendix Figs 2a – 2d summarize the main findings from that work. For those diagnosed just after relative to just before the reform, the likelihood of chemotherapy treatment within 1 month of diagnosis: (1) increased in physician offices, (2) increased when excluding regimens containing carboplatin, (3) was unchanged in the outpatient hospital setting, where payment rates did not change until the following year, and (4) increased overall, reflecting the concentration of treatment in physician’s offices.
The estimated increase in chemotherapy, based on equation (5) and using 11 (col 2) or 9 months (col 3) on either side of the payment change, is shown in Table 2, Panel A. The change in the likelihood of chemotherapy treatment in the physician office is about 1.6 percentage points, representing a 12.3% increase in the likelihood of treatment in response to the reform. This finding is largely insensitive to including relative month trends or calendar month fixed effects instead of relative-week trends or using calendar month fixed effects and controlling for the share of patients by year of age instead of using an age quadratic (see Appendix Table 2).25 The relative increase in chemotherapy treatment within 3-months is quite similar – about 14%–when accounting for the partial overlap in the treatment window and the reform for those diagnosed in the quarter before the reform took effect (see Appendix Table 3).
Table 2.
Changes in Chemotherapy Treatment after the Jan 2005 Payment Reform
| Pre-Jan 2005 mean | Sample Window (Months) | ||
|---|---|---|---|
| +/−11 | +/−9 | ||
| Panel A: Share of Patients Receiving Chemotherapy Treatment… | |||
| within 1 month of diagnosis, in a Physician’s Office | 0.130 | 0.016 (0.003) | 0.016 (0.003) |
| within 1 month, Physician excluding carboplatin | 0.052 | 0.015 (0.002) | 0.014 (0.001) |
| within 1 month, in an outpatient hospital clinic | 0.028 | −0.001 (0.001) | −0.001 (0.001) |
| within 1 month, all settings | 0.166 | 0.015 (0.003) | 0.014 (0.002) |
|
| |||
| Panel B: Percent Change in Chemotherapy Service Counts… | |||
| within 1 month of diagnosis, in a Physician’s Office | 0.916 | 0.347 (0.047) | 0.340 (0.046) |
| within 1 month, Physician excluding carboplatin | 0.274 | 0.203 (0.024) | 0.209 (0.023) |
| within 1 month, in an outpatient hospital clinic | 0.087 | 0.013 (0.003) | 0.013 (0.004) |
| within 1 month, all settings | 1.00 | 0.339 (0.046) | 0331 (0.044) |
|
| |||
| Panel C: Share of Chemotherapy-treated patients receiving within 1 month… | |||
| carboplatin | 0.557 | −0.039 (0.008) | −0.026 (0.008) |
| paclitaxel | 0.300 | −0.074 (0.007) | −0.061 (0.006) |
| docetaxel | 0.083 | 0.026 (0.004) | 0.026 (0.006) |
| etoposide | 0.210 | −0.005 (0.010) | 0.004 (0.007) |
| gemcitabine | 0.100 | 0.007 (0.003) | 0.008 (0.004) |
|
| |||
| Number of Observations (weeks) | 48 | 96 | 72 |
Notes: Means in column 1 are for the dependent variable prior to ASP implementation, Feb–Dec 2004. Columns (2) and (3) contain coefficients from separate time-series regressions using data from Feb 2004–Nov 2005 and April 2004–Sept 2005, respectively. Estimates are the coefficients on an indicator for the pot-reform/ASP payment period. Regressions control for mean patient characteristics (see Table 1) and relative-week trends that are allowed to differ on either side of the payment change. Excluding carboplatin” means the share of patients treated with chemotherapy and without carboplatin. Newey-West standard errors allowing for autocorrelation up to 52-week lags are given in parentheses.
Appendix B discusses several new checks of our past findings, including estimates from a difference-in-differences specification (in Appendix Table 4), a structural break test (in Appendix Figure 3), and a placebo analysis (in Appendix Figures 4a–4d and Appendix Table 5). All provide strong evidence that the reform generated a sharp change in chemotherapy treatment.
We further consider changes in chemotherapy service counts, which were not analyzed in prior work. Service counts, meaning the number of units of a chemotherapy drug administered, measure both extensive and intensive margin changes. As shown in Figure 2, we find a relatively large increase in chemotherapy service counts outside of the outpatient clinic setting, where the change is again essentially zero. As shown in Table 2, Panel B, the mean number of chemotherapy services within 30 days was 0.916 prior to the reform; among those diagnosed after the reform, services in physician offices increased almost 35%. Given the 12.3% increase in treatment on the extensive margin, these results suggest that chemotherapy services increased even among those who would have received treatment absent the reform. This increase is robust to excluding treatments that include carboplatin. Models with relative-month trends or calendar month fixed effects yield similar results (see Appendix Table 6). The mix of drugs administered also changed (see Panel C of Table 2 and Jacobson et al (2010) for details), with drugs that lost the most margin used less and expensive agents favored by the 6% margin used more. These intensive margin changes are relevant as they imply that any changes in patient survival will combine the effects of extensive and intensive margin changes in treatment.
Figure 2.
Percent Change in Chemotherapy Service Count by Month of Diagnosis Relative to the January 2005 ASP Implementation Date
Although cancer treatment is constantly evolving, no other change in management was so suddenly implemented that could account for either the discrete nature of the treatment changes we observe or the full pattern of results, i.e., the change by setting, the change in drug mix, and so on.26 Rather, physicians changed their practice in response to a change in the private profitability of treatment. These findings are consistent with a sizable body of evidence on the role of private incentives in determining the provision of healthcare. But, as such behavior can be consistent with physicians as perfect altruists or as perfectly selfish, changes in services alone are informative only about the role of physician agency in the efficiency of health care and do not measure the impact of physician agency on patient welfare. To address this issue, in the next section we analyze the impact of the increase in chemotherapy treatment on patient outcomes.
4.3 Changes in Survival
We analyze changes in survival to address the impact of physician agency on patient welfare in this setting. A benefit to using survival as our measure of welfare in this context is that lung cancer survival is (unfortunately) quite low, which provides considerable statistical power. A limitation is that it does not capture changes in the quality of life, which could deteriorate in response to harsh chemotherapy treatment (e.g., see Ballatori et al. (2007)). Despite the common view that chemotherapy generally decreases quality of life, for advanced lung cancer (the illness we examine here) the evidence suggest that this is not the case. Specifically a recent randomized trial in patients with advanced lung cancer found that, compared to placebo, chemotherapy not only increased survival but also improved some measures of quality of life (e.g., the worsening of pain and coughing up of blood) while leaving others unchanged (Belani C. P. et al. (2012)).
Appendix Figure 5 plots the unadjusted Kaplan-Meier survival curves for patients diagnosed in the 11 months before and after the reform. Median survival based on the Kaplan-Meier estimators increased by about 3 weeks from 261 to 284 days for those diagnosed in the pre and post-reform periods, respectively. To assess whether this difference is related to the reform, Figures 3a–3d plot regression-adjusted mean changes in the proportion of patients dying within 3, 6, 9 and 12 months of diagnosis by month of diagnosis relative to the January 2005 reform. These figures show a discrete decline in the likelihood of dying within each interval for the post-reform cohorts. This decline corresponds closely to the timing of ASP implementation and the previously demonstrated increase in chemotherapy utilization.
Figure 3.
Change in the Proportion Dying by Month of Diagnosis Relative to the January 2005 ASP Implementation Date
Table 3 quantifies the mortality changes in Figure 3. Among patients diagnosed just after relative to just before the reform, the likelihood of death within 3 months of diagnosis decreased about 1 percentage point or about 4% relative to the base of 33.9% dying in this interval. The results are virtually identical using patients diagnosed 9 months before to 9 months after the payment change (col 3). At 6 months from diagnosis, the reduction in the likelihood of death is about 1.6 percentage points or 3.4% relative to the base rate of 46.9%. At 9 months the effect is 1.1 percentage points, a relative decrease in mortality of about 2.1%. At 1 year, the survival effects disappear. Thus, while the increase in chemotherapy induced by the change in physician fees increased survival, it did not increase the rate of full remission.
Table 3.
Change in the Share of Patients Dying within 3, 6, 9 or 12 Months of Diagnosis
| Pre-Jan 2005 mean | Sample Window (Months) | ||
|---|---|---|---|
| +/−11 | +/−9 | ||
| Share of Patients Dying within… | |||
| 3 months of diagnosis | 0.339 | −0.009 (0.004) | −0.010 (0.005) |
| 6 months of diagnosis | 0.469 | −0.016 (0.002) | −0.016 (0.004) |
| 9 months of diagnosis | 0.527 | −0.011 (0.002) | −0.011 (0.004) |
| 1 year of diagnosis | 0. 619 | −0.001 (0.003) | −0.001 (0.003) |
| Number of Observations (weeks) | 48 | 96 | 72 |
Notes: Means in column 1 are for the dependent variable prior to ASP implementation, Feb–Dec 2004. Columns (2) and (3) contain coefficients from separate time-series regressions using data from Feb 2004–Nov 2005 and April 2004–Sept 2005, respectively. Estimates are the coefficients on an indicator for the pot-reform/ASP payment period. Regressions control for mean patient characteristics (see Table 1) and include relative_week trends. Newey-West standard errors allowing for autocorrelation up to 52-week lags are given in parentheses.
Appendix Table 7 shows sensitivity checks that use relative-month instead of relative-week trends (cols (1) and (2)) or calendar-month of diagnosis fixed effects (cols. (3) and (4)) or calendar month fixed effects and the share of patients by year of age (cols (5) and (6)). The specifications using calendar-month fixed effects are especially relevant here given seasonality in mortality. These specifications yield results that are quite similar to those in Table 3, with larger point estimates when calendar-month fixed effects are included.
While the reductions in the likelihood of death at 3 to 9 months post-diagnosis are small, these estimates average survival across all patients, whereas the reform increased chemotherapy in about 2 out of every 100 patients. The reform also changed the intensity of treatment and the drugs used, making it difficult to isolate the specific source of the survival change. As an alternative approach, Appendix Table 8 reports hazard ratios from Cox and Weibull Proportional Hazard Models of the impact of the reform on mortality rates. Both show that median survival is about 6% longer for those diagnosed after the reform, irrespective of the value of other covariates.27 Assuming the improvement is specific to only the 1/3 of patients receiving chemotherapy in a physician’s office, this implies an 18% improvement in median life expectancy.
4.4 Further Linking Survival Changes to the Reform
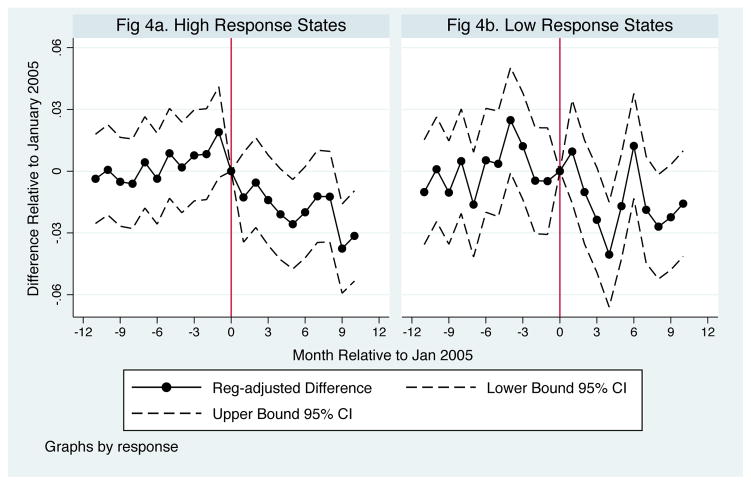
To subject the pre-post analysis to greater scrutiny, we next leverage the fact that after the reform the likelihood of chemotherapy treatment was virtually unchanged in some states (e.g., CA and MO), increased more than twice the national average in others (e.g., MN, CT) and even declined in a few states (e.g., OK, ID) (Jacobson et al. (2011)). If reform-related changes in chemotherapy improved survival, these effects should be concentrated in the most responsive states.
Table 4 reports estimates of changes in the the likelihood of death within 3–12 months of diagnosis for patients in states with above versus below median 30-day chemotherapy treatment changes. The likelihood of death declined by 3%–6% within 3–6 months of diagnosis for patients in the most responsive states but, if anything, increased for those in the least responsive states. At 9 months from diagnosis, the estimates imply a 1.5%–3% decline in the likelihood of death in the most responsive states and essentially no change in the likelihood of death in the least responsive states. These changes, which are shown at 9-months in Figures 4a and 4b, support the view that the increase in chemotherapy generated by the reform improved survival.
Table 4.
Change in the Share of Patients Dying: Above vs. Below Median Responsive States
| Above Median Response | Below Median Response | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean Prior to Jan 2005 | +/− 11 Months | +/− 9 Months | Mean Prior to Jan 2005 | +/− 11 Months | +/− 9 Months | |
| Change in Share Dying within… | ||||||
| 3 months | 0.338 | −0.011 (0.006) | −0.023 (0.003) | 0.340 | 0.014 (0.002) | 0.014 (0.003) |
| 6 months | 0.466 | −0.015 (0.004) | −0.023 (0.004) | 0.473 | 0.003 (0.005) | 0.006 (0.007) |
| 9 months | 0.524 | −0.008 (0.004) | −0.017 (0.004) | 0.531 | −0.0001 (0.006) | 0.003 (0.007) |
| 1 year | 0. 615 | −0.0001 (0.003) | −0.007 (0.004) | 0.623 | 0.009 (0.007) | 0.007 (0.008) |
| Obs (weeks) | 48 | 96 | 72 | 48 | 96 | 72 |
Notes: Means in column 1 and 4 are for the dependent variable for states with above or below median changes in chemotherapy treatment in the period prior to the reform. See notes to Tables 3 for other details.
Figure 4.
Change in Share Dying within 9 Months by Month of Diagnosis Relative to the January 2005 ASP Implementation Date
As a final robustness check, we split the sample by above and below median age patients. Since older patients are more likely to be undertreated (Davidoff et al. (2010); Booth C. M. et al. (2010)), they should benefit most from increased treatment. Although treatment increases sharply for both groups (in all but the outpatient setting), a sharp reform-related decline in survival is clearest for the above-median age patients. Table 5 shows the estimated changes in treatment and survival. Above-median age patients, who are almost half as likely to receive chemotherapy within 30 days, experience about an 8% increase in the likelihood of treatment; the increase is about 11% for the younger group. Service counts also increase sharply for both groups (not shown).
Table 5.
Change in Treatment and Survival: Above vs. Below Median Age Patients
| Above Median Age Patients | Below Median Age Patients | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean Prior to Jan 2005 | +/− 11 Months | +/− 9 Months | Mean Prior to Jan 2005 | +/− 11 Months | +/− 9 Months | |
| Share of Patients Receiving Chemotherapy Treatment within 1 month… | ||||||
| In Physician’s office | 0.094 | 0.014 (0.002) | 0.016 (0.002) | 0.164 | 0.024 (0.004) | 0.023 (0.004) |
| In Physician’s Office excl. Carboplatin | 0.039 | 0.013 (0.002) | 0.014 (0.002) | 0.065 | 0.019 (0.002) | 0.017 (0.002) |
| In outpatient hospital | 0.018 | −0.001 (0.001) | −0.001 (0.001) | 0.036 | 0.003 (0.001) | 0.004 (0.002) |
| All settings | 0.119 | 0.014 (0.002) | 0.014 (0.003) | 0.210 | 0.024 (0.003) | 0.020 (.003) |
| Share of Patients Dying Within… | ||||||
| 3 months of diagnosis | 0.397 | −0.023 (0.006) | −0.024 (0.005) | 0.285 | −0.0059 (0.0035) | −0.0063 (0.0036) |
| 6 months of diagnosis | 0.530 | −0.029 (0.004) | −0.032 (0.005) | 0.411 | −0.015 (0.002) | −0.020 (0.0023) |
| 9 months of diagnosis | 0.588 | −0.026 (0.003) | −0.031 (0.004) | 0.470 | −0.012 (0.003) | −0.017 (0.003) |
| 1 year of diagnosis | 0. 675 | −0.015 (0.002) | −0.018 (0.003) | 0.567 | 0.002 (0.003) | −0.006 (0.0038) |
| Observations (weeks) | 48 | 96 | 72 | 48 | 96 | 72 |
Notes: Means in column 1 and 4 are for the dependent variable for patients above versus below the median age patient at diagnosis in the period prior to the reform, Feb–Nov 2004. See notes to Table 3 for other details.
In contrast, the survival effects are substantially larger for the older group. The likelihood of death within 3 months declines by about 0.6 percentage points for the younger group but 2.3 percentage points for the older group. At 6 and 9 months from diagnosis, the declines are 2.6–3.2 percentage points in the older group and 1.2–2 percentage points in the younger group. The differences in treatment and survival at 3–9 months across above and below median age patients are statistically distinguishable. 28 Relative to baseline survival, these changes represent a decrease in the likelihood of death within 3–9 months of 5–6% for the older group compared to 2–4% for the younger group. In other words, consistent with the idea that the older patients are more likely to be under-treated, we find that they experience a disproportionately large increase the survival despite a slightly smaller increase in chemotherapy overall. And, as can be seen in Appendix Figures 6a–6d, the timing of the changes in both treatment and survival line up directly with the reform.
Taken together these tests should alleviate the concern that our survival results are picking up unobserved, contemporaneous changes in other factors, such as the efficacy of cancer treatment. In particular, for an unobserved factor to explain these results would require that 1) the timing of the change corresponds closely with the date of the policy change, 2) the impact of the change is correlated with the responsiveness of physicians in a state to the change in reimbursement, and 3) the change caused by this unobserved factor disproportionately affects older individuals.
4.5 Market Concentration Interactions
We next examine the relationship between market structure (concentration) and outcomes. First, we examine whether within markets, physician market share impacts the supply and effectiveness of chemotherapy. Specifically, within each market (defined as county) we compare the differential change in outcomes of patients treated by physicians with above versus below median market share in the county. As shown in Table 6, the average patient resides in a county where the median provider treats about a third of the patients in the county. The distribution of this variable is highly skewed, with the median patient residing in a county where the median share is just 0.016 and ranges between nearly 0 to well over 1, given that physicians treat patients across counties. In addition, about 45% of patients are treated by physicians that treat more than the median share of patients in their county. In other words, the median patient is treated by a small provider.
Table 6.
Summary Statistics for Provider Size and Concentration Measures by Patient
| Mean | Median | |
|---|---|---|
| Median Share of County Patients Treated by a Provider | 0.342 | 0.016 |
| Share of Patients treated by “Large” Providers | 0.453 | -- |
| Herfindahl-Hirschman Index (HHI) | 0.140 | 0.085 |
| Highly Concentrated (HHI>0.25) | 0.156 | -- |
Notes: Data are at the patient level and include the 122,597 patients analyzed in Tables 7 and 8. Large is an indicator set equal to 1 if the patient is seen most frequently by a physician who has more than the median share of patients within a county. The HHI is defined as the sum of each provider’s share of a county’s chemotherapy administrations pre-reform.
The results of the market share analysis, shown in Table 7, are consistent with our theoretical model. While the chemotherapy increase is identical across the two groups, (i.e., the interaction term is zero, implying a 1.7 percentage point increase in treatment using the 9-month window for both groups), the welfare benefit is larger for the patients of physicians with above median market share. Based on a 9 month window of diagnoses pre and post reform, the estimates indicate a 2.8 vs. 1.4 percentage point reduction in the likelihood of death within 9-months for patients treated by physicians with above versus below median market share. As a sensitivity check, we consider physicians with above versus below mean instead of median market share. The results, which are presented in Appendix Table 9, show no differential treatment for patients with providers who have above mean market share of patients in the county. The survival benefit for patients is considerably larger among those treated by physicians with above mean market share, although the precision of the estimates falls when we limit to the 9-month window. Together these results indicate that, although treatment does not increase differentially for patients of high concentration providers, their survival increase is about twice as large.29
Table 7.
Differential Change in Chemotherapy Treatment and Survival for Patients Treated by Providers with more than Median Market-Share of Patients in a County
| Sample Window (Months) | ||
|---|---|---|
| +/−11 | +/−9 | |
| Panel A: Chemotherapy Treatment within 1 month of diagnosis in a Physician’s Office… | ||
| Post-reform | 0.020 (0.003) | 0.017 (0.003) |
| Post-reform × Large Practice | −0.0005 (0.004) | 0.001 (0.005) |
| Large Practice | 0.107 (0.004) | 0.107 (0.004) |
|
| ||
| Panel B: Death within 6-months of Diagnosis | ||
| Post reform | −0.011 (0.004) | −0.012 (0.004) |
| Post-reform × Large Practice | −0.016 (0.005) | −0.014 (0.006) |
| Large Practice | −0.035 (0.004) | −0.034 (0.004) |
|
| ||
| Panel C: Death within 9-months of Diagnosis | ||
| Post reform | −0.012 (0.004) | −0.014 (0.005) |
| Post-reform × Large Practice | −0.014 (0.004) | −0.014 (0.004) |
| Large Practice | −0.017 (0.005) | −0.013 (0.006) |
| Observations | 122597 | 99980 |
All regression are estimated at the individual level. Estimates for post-reform are the coefficients on an indicator for the post-reform/ASP payment period. Large is an indicator set equal to 1 if the patient’s is seen most frequently by a physician who has more than the median share of patients within a county. Columns (2) and (3) contain use data from Feb 2004–Nov 2005 and April 2004–Sept 2005, respectively. Regressions control for patient characteristics (see Table 1), calendar month fixed effects (i.e., Jan, Feb, …) and county fixed effects. Standard errors are clustered at the county level.
Second, we test whether across markets the changes in treatment and survival are related to market concentration. We compare the response of low concentration (i.e., more competitive) and high concentration (i.e., less competitive) markets in response to the policy change. Our primary measure of market concentration is a county level Herfindahl-Hirshman Index (HHI) for chemotherapy providers. Specifically we calculate the HHI as the sum of each provider’s share of a county’s chemotherapy administrations pre-reform.30 Following the US DOJ Horizontal Merger Guidelines (2010), we define a county as high concentration if the HHI is greater than 0.25. The median pre-reform HHI across counties in the dataset is 0.22, with a mean of 0.3 (not shown). In other words, the median county is not highly concentrated in terms of chemotherapy provision, although there is a thick tail of highly concentrated counties.
If we look at the distribution at the patient, and not county level, the fact that more populous counties tend to have a more competitive market environment means that the average market concentration is lower. Specifically, as shown in the last two rows of Table 6, the average patient resides in a market with a pre-reform chemotherapy HHI of 0.14 and the median patient is in an even more competitive market (HHI= 0.085). By this measure, roughly 16% of patients are treated by providers that, according to DOJ guidelines, operate in very concentrated markets.
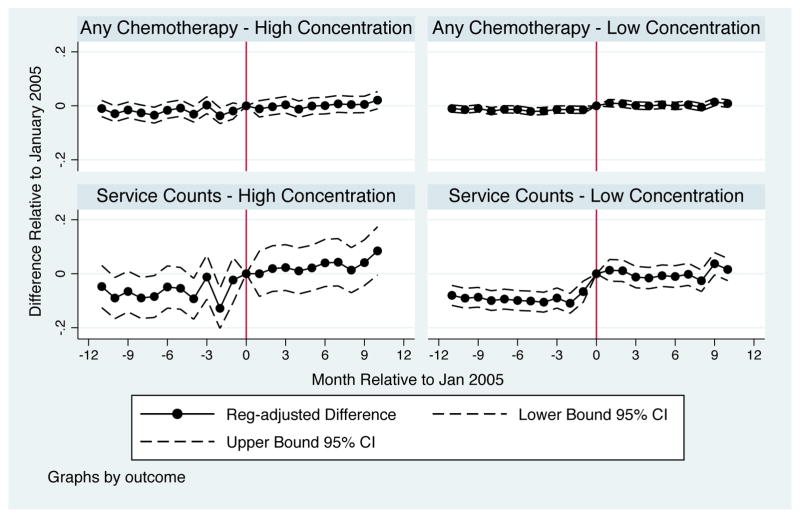
We contrast 30-day chemotherapy treatment changes for patients in high concentration counties versus medium/low concentration counties. On the extensive margin (any use), we see no compelling evidence of a change in treatment rates in high concentration markets but an increase right after the reform in less concentrated counties. The estimates shown in Table 8 and pictured in Figure 5 indicate that the changes in chemotherapy treatment on the extensive margin are small and generally insignificant in high concentration counties but imply about a 12% increase in 30-day treatment probabilities in less concentrated counties. For service counts, a mix of intensive and extensive margin changes, we see a small increase in treatment in more concentrated markets but a sharper and larger increase in less concentrated markets (see the bottom panels of Figure 5). Specifically, chemotherapy service counts increase by about 20% in more concentrated markets but by almost 40% in less concentrated markets. The differences in treatment rates and service counts are in all cases statistically different across market types.
Table 8.
Change in Treatment and Survival: Counties with a High vs. Low Concentration of Providers Administering Chemotherapy Treatment
| High Concentration Counties | Low Concentration Counties | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean Prior to Jan 2005 | +/− 11 Months | +/− 9 Months | Mean Prior to Jan 2005 | +/− 11 Months | +/− 9 Months | |
| Chemotherapy Treatment within 1 month… | ||||||
| Share Treated in Physician’s Office | 0.121 | 0.008 (0.005) | 0.010 (0.006) | 0.132 | 0.019 (0.003) | 0.019 (0.003) |
| Percent Change in Services in Physician’s Office | 0.329 | 0.242 (0.046) | 0.231 (0.044) | 0.298 | 0.389 (0.034) | 0.391 (0.033) |
| Share Receiving Any | 0.169 | −0.0004 (0.005) | 0.001 (0.007) | 0.165 | 0.020 (0.002) | 0.019 (0.003) |
| Percent Change in Services | 0.984 | 0.203 (0.045) | 0.185 (0.045) | 1.01 | 0.389 (0.031) | 0390 (0.030) |
| Share Dying Within… | ||||||
| 3 months of diagnosis | 0.351 | −0.017 (0.006) | −0.010 (0.004) | 0.337 | −0.006 (0.0036) | −0.008 (0.004) |
| 6 months of diagnosis | 0.482 | −0.028 (0.008) | −0.022 (0.006) | 0.466 | −0.012 (0.002) | −0.014 (0.002) |
| 9 months of diagnosis | 0.541 | −0.027 (0.008) | −0.019 (0.005) | 0.524 | −0.004 (0.003) | −0.007 (0.002) |
| 1 year of diagnosis | 0. 635 | −0.008 (0.008) | 0.0003 (0.006) | 0.616 | 0.006 (0.005) | 0.0001 (0.002) |
| Observations (weeks) | 48 | 96 | 72 | 48 | 96 | 72 |
Notes: Means in column 1 and 3 are for the dependent variable for patients in counties with low vs. high concentration in the period prior to the reform, Feb–Nov 2004. The mean for service counts is without logs. High concentration is defined as an HHI greater than 0.25. See notes to Table 3 for other details.
Figure 5.
Change in Treatment by Provider Concentration Relative to the January 2005 ASP Implementation Date
In contrast to the change in treatment, survival improved more in both absolute and relative terms in more concentrated markets (see the last panel of Table 8). The likelihood of death within 6 months declines by 2.2 percentage points or 4.6% in highly concentrated counties but 1.4 percentage points or 3% in less concentrated counties. The survival difference across market types is even starker for the likelihood of death within 9 months, with a 1.9 percentage point or 3.5% decline in highly concentrated markets and a 0.007 percentage point or 1.3% decline in competitive markets. This difference in 9-month survival is statistically distinguishable across market types. As a sensitivity check, Appendix Table 10 shows results that break out counties by whether they are above or below the mean HHI across counties. The results are quite similar, with larger treatment responses in counties that have below mean HHI but clearer improvements in survival in counties with below mean HHI.
In summary, while the reform increased chemotherapy (both intensive and extensive) more in less concentrated markets, patients in more concentrated markets received larger average survival benefits. While this may seems counterintuitive (i.e., a smaller response had a bigger effect), it is consistent with the simple model in section 1 and suggests that the matching of physicians to patients is both important and systematically related to market structure. In other words, physician treatment response to the payment change is not enough to infer welfare effects. Rather, these treatment responses must be understood in the context of local market conditions.
5 Conclusion
Prior work has demonstrated time and again that private physician incentives affect the quantity and type of care provided to patients. Less well understood (and generally more controversial) is whether these incentives affect patient health. We study this question among U.S. oncologists in the context of a Medicare reform that decreased physician profit margins for many chemotherapy drugs. We find that, at least in this setting, physician agency is not only an important determinant of the supply of heath care but also of survival. In response to the reform, oncologists increased treatment, as measured by chemotherapy use on both the intensive and extensive margin. This increase, which was driven by changes in private physician incentives, had an economically and statistically significant effect on patient survival. In dollar terms, using the U.S. EPA value for a statistical life (VSL) of $4.8 million, the 23 day increase in median survival is worth just over $300,000 per patient.31 While the total cost of care measured over a 3-month period increases by about $650 per patient, due to increased inpatient care, this cost is swamped by the survival improvement. In other words, the increase in survival dominates any change in the cost of care.
We also investigate the interaction between physician agency and market structure. We find empirical support for using standard agency models to understand physician behavior. Consistent with this model, we find that while increases in care are independent of physician market share, increases in patient survival are concentrated in patients of physicians with larger than median market share.
While our results provide strong evidence for physicians as imperfectly altruistic agents, we caution against overly simplistic translation of these results to policy. For example cross-sectionally, the treatment and survival increases are larger in areas where the pre-reform mix of chemotherapy drugs meant a larger average decrease in margins from the reform. These results indicate that, in this instance, an overly generous payment scheme led to physician rationing - i.e., the underprovision of care - and that better aligning payments to costs led to better outcomes at lower total cost.32 But, without knowing both the sign of the elasticity of care, and whether a given condition is over or under treated, one cannot predict whether a similar policy would have a beneficial or harmful effect. Payment changes are generally blunt instruments with ambiguous effects on care. Both theoretically and empirically, payment cuts can increase or decrease care and improve or harm health. The results presented here serve mainly to highlight that the health effects of physician agency can be significant. Finally, an important issue for policymakers not considered here should be the long-run supply effects that changes in payment policy may engender. In the current setting, for example, lower margins may affect the long-run supply of oncologists, which could reduce access to care and treatment.
Supplementary Material
Figure 6.
Proportion Dying within 6 or 9 Months by Provider Concentration Relative to the January 2005 ASP Implementation Date
Highlights.
We study the effect of physician agency on health care supply and patient outcomes.
An increase in supply due to a change in private physician incentives need not capture induced demand for ineffective care but rather could reflect a reduction in prior rationing of effective care.
Market structure matters in determining the welfare effects of changes in private physician incentives.
An increase in chemotherapy treatment due to a change in regulated Medicare payment rates is shown to increase survival for lung cancer patients by about 18%.
The survival improvements are larger in more concentrated markets.
Appendix A: Proofs
Proof of Proposition 1
Proof
Let the change in the share of patients treated by physician j in response to a change in m. Then for α ≈ 0, equation 3 can be rewritten as
| (6) |
Denote the inverse of the change in the share of patients treated as where . The market level measure of h is then given by the weighed averages of the individual h(ηj):
| (7) |
An internal solution for equation 2 implies that κ is invariant with respect to η, so Hm can be rewritten as
| (8) |
So the change in the share of patients treated in a market m is equivalent to a scaled version of the Herfindahl-Hirschman Index (HHI). And since HHI belongs to the family of “allowable” concentration indices as described in Encaoua and Jacquemin (1980), Hm will be positively correlated to any other such allowable concentration index (e.g. the entropy index).
Then since Hm is positively correlated to market concentration, its inverse (i.e. the magnitude of the change in the share of patients treated in a market) will be negatively correlated with measures of market concentration.
Proof of Proposition 2
Proof
The change in patient welfare generated by a physician j’s response to a change in profit margin m is given by . The magnitude of the market level change in patient welfare can thus be written as , which for small α ≈ 0 can be rewritten as
| (9) |
Encaoua and Jacquemin (1980) show that for a concentration index defined as
| (10) |
where the αis represents the market share of firm i, if g(α) is any arbitrary function that is weakly increasing in α, if αg(α) is convex in α, it belongs to a family of “allowable” concentration indices. Such allowable indices (which include HHI and the entropy index) satisfies the following properties: it is invariant to permutations of market shares between firms i, it satisfies the Lorentz condition that a mean pressuring spread increases ε, and the concentration of symmetric firms decreases in the number of firms. Thus it is sufficient to show that h(η) is increasing in η and ηh(η) is convex in η to prove the proposition.
To show h(η) is weakly increasing in η, we take the derivative of h with respect to η and get
| (11) |
Then since b′≤ 0, equation 11 is positive and h(η) is weakly increasing in η.
To show convexity of ηh(η) we take the second derivative and get (after some simplification) the expression
| (12) |
Since by assumption b″ > 0, .
Two-Payer Model
In this section, we analyze the model presented in the paper, with the addition of a second, private payer. Here we show that the two propositions in the paper hold in a two payer model, and generate two addition predictions regarding how the share for public vs. private patients affects physician response to a fee cut.
In addition the assumptions of the main model, we incorporate a second payer for medical care. In each market k, for patients of type b, a fraction γ are public (i.e. Medicare) patients while the remaining (1 − γ) are private payers. Notationally, we denote the number of public patients treated by q and the number of private payer patients treated by q̃. Similarly the profit margin for treating each type of patient is denoted by m and m̃ respectively.
Physician utility function can then be written as
| (13) |
where π = mq+ m̃q̃ and α represents the weight physicians place on patient benefit. The FOCs for utility maximization are then given by
| (14) |
| (15) |
As before concern for patient welfare (αb) pushes physicians towards providing what, from the patient’s perspective, is the optimal level of care qP, but in general physicians may either under or over provide care relative to this optimum: in the limit α→∞, q* → qP.
To determine physician response in q to a change in profit margin m, we take the derivative of equation 14 with respect to m. Rearranging, we get the following relationship:
| (16) |
As before qm can be positive or negative. That is, it cannot be determined ex-ante whether an increase in profitability leads to an increase or decrease in provision of the service experiencing a price cut. But now qm is driven by the tradeoff between decreasing marginal returns (the substitution effect) and the increasing marginal returns to income (the income effect), as well as “cross-service effects” (i.e. changes in the marginal cost of effort of q due to changes in q̃).
Note that in the case where q̃m ≈ 0, equation 16 is identical to equation 3, with ηγ, the Medicare market share, taking taking the place of overall market share η. This is the case described in McGuire and Pauly (1991): “When income effects are absent and either the marginal utility of leisure is unchanged... we get a surprising conclusing: there will be no effects” on the quantity of service provided to the private payer in response to a change in public payer margins. In such a case the ”bite of a price cut will decline with market share”. That is doctors in markets with higher share of Medicare patients will be more affected by the price cut than those in low Medicare patient share markets.
In this case, in addition to proposition 1 and 2, and we get the following corollaries:
Corollary 3
If Proposition 3 holds, is increasing in γ.
Proof
Since
Corollary 4
If Proposition 4 holds, is increasing in γ.
Proof
. Since b′ < 0,
These corollaries simply state that when the the share of public patients is larger, a cut in public patient margins will have a larger impact on physician behavior.
In the case where there are significant “cross service effects” (i.e. ||q̃m|| ≫ 0), the expressions that describe physician response are quite complex, and will depend on the specific values of the various parameters. In such a case, while proposition 1 and 2 hold, the two corollaries need not, and will depend on the specific form of the utility function, as well as the parameter values.
While the predictions regarding share Medicare patient share and physician response are straightforward, data on the share of Medicare patients vs. private payer patients is largely unavailable, and so we were unable to empirically test these additional predictions in this paper.
Appendix B: Checks of Treatment Results
In this section we present the results of several robustness and sensitivity checks. First, we conduct a difference-in-differences model, exploiting the fact that the payment change took effect for care in the physician office setting (the treatment group) a year prior to the hospital-outpatient setting (the control group). Estimates from a difference-in-differences specification in Appendix Table 4, which uses treatment in the outpatient setting as a control, are slightly larger. These estimates imply a 17% and 19% increase in the probability of treatment in a physician’s office within one and three months of diagnosis, respectively. Given that the treatment in the outpatient setting may respond to changes in in the physicians office-setting, however, the time-series approach is retained as our preferred specification.
Second, as a formal test of a structural break in treatment, we employ a Quandt likelihood ratio (QLR) test (Quandt 1960). The QLR can be used to test for structural breaks at all possible dates between two time periods, τ0 to τ1. Operationally, the test is a modified set of sequential Chow tests based on equation (2), where a post-period is defined at each cutoff date τ between the two time periods, i.e. post = 1(t ≥ τ*) is an indicator equal to zero for all weeks before τ* and one for all subsequent weeks; this indicator is also interacted with the linear trend. Under the null hypothesis of no break, the coefficients on postτ and postτ * (t−t*) are jointly equal to zero. The QLR statistic, also known as the sub-Wald statistic, is just the maximal of the F-statistics testing the null of no break at all dates between τ0 to τ1; it provides an estimate of the structural break date. To implement this test, we adopt the conventional choice for τ0 to τ1 of 0.15T and 0.85T or −34 weeks and + 33 weeks relative to the first week, i.e. week 0, in January 2005. In this way, the post period cutoff or break date, τ*, is sequentially defined over the inner 70% of the 96 week sample. We take the critical values of the QLR statistic for the endpoints 0.15T and 0.85T and two restrictions tested from Stock and Watson (2003), which are adapted from Andrews (2003). The QLR critical values are larger than the critical value for a single F-test; they are effectively corrected for multiple-hypothesis testing induced by searching over many F-tests.
Based on the QLR statistic, a structural break in treatment occurs two weeks prior to January 2005 for (1) treatment in a physician’s office (see Appendix Figure 3) and (2) any chemotherapy treatment, irrespective of setting. Excluding carboplatin, the break occurs one week prior to ASP implementation. The pre-implementation break is not worrisome given that we are analyzing 30-day treatment and patients diagnosed at the end of 2004 likely began treatment after the new payment system took effect on January 1 rather than during the Christmas holidays. In all three cases, the maximal F-statistic exceeds the 1% critical value of 7.78, implying we can reject the null of no structural break in treatment. In contrast, we cannot reject the null at any point for chemotherapy treatment in the outpatient setting.
Third, we conduct a placebo analysis that uses January 2004 as the reform date. In actuality, there was a small change in payment rates, from 95 to 85% of AWP in 2004. However, this change was much less dramatic and was easy to predict, conditional on firms not changing AWP. As can be seen in Appendix Figures 4a – 4d, treatment was relatively smooth across January 2004, although there may have been a slight retiming of physician effort relative to the January 2004 AWP change, with patients treated 4-months before the reform having a higher likelihood of treatment and 1 month before a lower likelihood of treatment. As shown in Appendix Table 5, however, the net change in treatment for those diagnosed in the 12 months before versus after January 2004 is essentially zero.33
Footnotes
Mary Price provided excellent programming to create the original cohort used in this work. We thank the JEBO editor, William Neilson, and two anonymous referees for very helpful comments and insights. We also thank Ernie Berndt, Srikanth Kadiyala, Emmett Keeler, David I. Levine, Tom McGuire, Nolan Miller, Giuseppe Ragusa, Heather Royer, James P. Smith, Tim Vogelsang and seminar participants at USC’s Sol Price School of Public Policy, the NBER Spring Health Care Program meetings, the Quintiles seminar at the Schaeffer Center at USC, RAND, and ASHEcon 2014 for many helpful comments. Support for this analysis was made possible by grants from the Robert Wood Johnson Foundation’s Changes in Health Care Financing and Organization (HCFO) program and the National Cancer Institute, 1R01CA152043-01. All remaining errors are our own.
The generally negative relationship between the elasticity of care and patient welfare may be part of the reason why positive evidence for private physician incentives affecting patient health has been so elusive.
This is a particularly good setting to study how physician agency affects health because the impact of this policy on physician incentives is large and lung cancer survival is low, both of which increase statistical power. In addition, since some evidence suggests that chemotherapy does not negatively affect quality of life in lung cancer patients (Ballatori et al. (2007)), lifespan may be a good measure of utility.
Oncologists maintained that Medicare fees for other services, e.g., evaluation and management and drug administration, did not cover expenses. However, the Government Accountability Office found to the contrary, that these payments exceed expenses by 4% on average, which is similar to the mark-up received by other specialists on these services (Government Accountability Office (2001)).
The change in reimbursement is the weighted average change in reimbursements for a standardized monthly dose of drugs, where the weights are based on the distribution of drugs used in the 11 months prior to the reform. Payment rates are from the Center for Medicare and Medicaid Services (CMS) for the last quarter of 2004 and the first quarter of 2005. We assumed each drug was given according to the most common dose and schedule recommended in the Dana-Farber Cancer Institute’s Oncology Protocol System and that each patient had a body surface area of 1.7m2 (except for carboplatin which is dosed according to the area under the curve (AUC) of a patient’s kidney function. See the appendix to Jacobson et al. (2010) for more details on dosing.
We calculate the increase in life expectancy for the 1/3 of all patients (irrespective of treatment) who received chemotherapy in a physician’s office because that is the site affected by the reform. Unconditionally, the increase in median life expectancy is about 6%.
The only other paper we are aware of is Johnson and Rehavi (2016), which finds that physician-mothers, who should face relatively little asymmetric information, are less likely to have cesarean sections than non-physician mothers and have fewer birth complications.
In related work, Miller et al. (2006) focus on provider’s private incentives to stint on quality instead of over-providing quantity.
While the idea of physician induced demand (PID) is complex (see McGuire (2000)), we define it here as distortions away from the care that would be demanded by the mythical, fully informed patient. To follow the terminology currently employed in the literature, we refer to the provision of services to a patient who receives a non-positive benefit, net of any out-of-pocket patient cost, as PID. If, on the other hand, the patient would have received a net positive benefit from a service, we call such a distortion physician rationing (PR).
We omit the j subscript for physicians for notational simplicity.
In the limit α→∞, q* → qP, where q* is the physician’s private optimum.
The empirical literature has confirmed the ambiguity of the sign of physician response to financial incentives, with some papers showing a positive relationship between fee changes and health care supply (e.g., see Clemens and Gottlieb (2014)) and many others showing backward bending supply curves, i.e. volume offsets (see the reviews in McGuire (2000) and Johnson (2014)).
Some private insurers have begun to purchase the drugs themselves and deliver them to the physician’s office or clinic, reimbursing simply for administration. This practice is still quite rare
The ASP calculation does not take into account 340(B) discounts for Medicaid patients. Since about 2009 it is believed that hospitals began leveraging these discounts to purchase oncology drugs for all their patients and thereby increase the mark-ups they receive on oncology drugs administered to Medicare and commercially insured patients. This is a relatively recent practice that is unlikely to have occurred during our study period.
Perhaps surprisingly, the setting of chemotherapy administration (physician office or outpatient hospital) was largely unchanged (see Friedman JY (2007) and Jacobson et al. (2010).)
Similarly, androgen-deprivation therapy (ADT) or medical castration, which is reimbursed as a Part-B drug, declined for prostate cancer treatment while surgical castration, which did not face a payment change, increased (Weight et al. (2007)). Although the ADT decline was largest among those for whom the benefits were unclear (Shainian et al. (2010)), these welfare implications may not generalize to other cancers. Specific to chemotherapy, Colla et al. (2012) finds that in response to the reform treatment declined in the last 14 days of life.
The data come from the Medicare Carrier, Outpatient, Durable Medical Equipment (DME), Medicare Provider Analysis and Review (MedPAR) and Hospice files.
Patients in Medicare Advantage (MA) plans, Medicare-approved private health plans that are paid a lump-sum on a risk-adjusted per-enrollee per month basis, are not in our data. Although in principle such patients could serve as controls, there is no comparable source of national data for them. We did gain access to Kaiser Northern California’s MA data. However, the FFS treatment response to the MMA in Northern California was small, making this comparison less relevant. The cause of this geographic variation is unclear, although it is widespread in Medicare (Skinner (2012)).
To ensure diagnosis and treatments that were not influenced by prior clinical decisions, we exclude patients for whom we could not verify a 12-month cancer free period. We exclude beneficiaries with more than one confirmed primary cancer as well as those who were enrolled in a Medicare Advantage plan at any point during the study period since they lack complete billing data. See Jacobson et al. (2010) for further details on the construction of the analytic dataset
We use ICD-9-CM procedure code 99.25 or diagnosis codes V58.1, V66.2 or V67.2, diagnosis-related group code 410, or HCPC codes 96400–96549, J9000–J9999 or Q0083 through Q0085, C8953–C8955 (2006 only), G0345–G0362 and revenue center codes 0331, 0332 and 0335.
To confirm metastasis we require one institutional claim or two or more non-institutional claims separated by at least 28 and no more than 365 days with a secondary cancer diagnosis [ICD-9 codes: 197–199]. Although this algorithm is likely to miss many beneficiaries with secondary cancer, it does have reasonable specificity (Earle et al. (2002)). Results are not sensitive to excluding this measure.
In principle we have 12 months of post-reform data but the cohort counts show a sharp drop off in December, suggesting the data were incomplete.
We choose a 30-day treatment period because it enables us to relatively cleanly define treatment (diagnosed post-reform) and control (diagnosed pre-reform) cohorts. Results using a more commonly defined treatment window of 3-months are quite similar (see Appendix Table 3).
We also consulted several academic oncologists to understand whether any clinical changes may have been coincident with the reform. We could identify none that would be consistent with the sharp changes in treatment we show below and in our prior work.
Upon its patent expiration in October 2004, at least 5 generic manufacturers had secured FDA approval to enter the carboplatin market. The gradual decline in carboplatin’s payment rates in Figure 1, which may capture switching to generics along with the lag in ASP determination – quarterly reimbursements are based on wholesale prices two quarters prior – suggests carboplatin maintained a margin above the legislated 6% for longer than the first quarter of 2005.
The effects are slightly larger when using calendar month fixed effects, which is the specification used in Jacobson et al. (2010), implying a 14% increase in treatment in physician offices. However, the patterns in Appendix Figure 2. suggest relative trends that vary on either side of January 1, 2005 may better capture treatment trends before and after the reform.
As one example, evidence for adjuvant chemotherapy was emerging in the mid-2000s but the timing of major presentations and publications on this subject are unlikely to have caused a sudden change in practice in January 2005 (Le Chevalier (2003); Arriagada et al. (2004); Winton et al. (2004); Winton et al. (2005); Strauss et al. (2004); Strauss et al. (2006); Strauss et al. (2008); Douillard et al. (2005); Douillard et al. (2006)). Furthermore, evidence on uptake of adjuvant chemotherapy suggests utilization was stable across the policy change (Booth C. M. et al. (2010)), and our own data (not shown) show no increase in the use of cisplatin-based regimens, as would be expected if the diffusion of adjuvant chemotherapy were to explain our findings. In addition, such evidence-based changes in treatment would not explain why such changes correlate to the policy driven change in profitability of the agents.
In other words, considering those of median age, conditioning on metastatic/non-metastatic, white/non-white, male/female, etc., yields relative survival improvements that are similar even though the levels (median survival length) are quite different.
Using the 9-month window, the chemotherapy difference is only distinguishable at the 10% level.
While these results are consistent with our model, they are also consistent with patient sorting to higher market share physicians based on need. We have no a priori reason to believe this is what happens. However, if it does, such sorting could contribute to the finding that increased treatment has greater welfare effects among physicians with higher market share and thus in higher concentration markets.
Since our data capture all billing-providers, irrespective of whether she is an oncologist, we restrict to those who billed for chemotherapy for any patient in the unrestricted sample of over 800,000 beneficiaries. Because we have only a patient’s county, providers can contribute to the HHI of multiple counties. Further measurement error arises because we only have data for Medicare fee-for-service patients and because we are relying on a sample of lung cancer patients whereas many oncologists treat different types of cancer patients.
Note that this calculation does not include any quality of life adjustments.
While we discuss under-provision in terms of rationing, the results are also consistent with doctors mistakenly believing they were providing the optimal amount of care prior to the reform and thus intentionally providing excess care post-reform. This alternative scenario is consistent with our model, but, if true, suggests an additional policy lever. Specifically efforts to promote evidence-based medicine might improve outcomes even in the absence of payment reform, although without the cost-savings generated by the reform.
We use 12 months because of the availability of data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mireille G. Jacobson, UCI, The Paul Merage School of Business and NBER
Tom Y. Chang, USC Marshall School of Business, Department of Finance and Business Economics
Craig C. Earle, The Ontario Institute for Cancer Research, Toronto, Canada
Joseph P. Newhouse, Department of Health Care Policy, Harvard Medical School, Department of Health Policy and Management, Harvard School of Public Health, Harvard Kennedy School and NBER
References
- Akscin J, Barr TR. Key practice indicators in office-based oncology practices: 2007 report on 2006 data. Journal of Oncology Practice. 2007;3(4):200–203. doi: 10.1200/JOP.0743001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews DW. Tests for parameter instability and structural change with unknown change point: A corrigendum. Econometrica. 2003;71(1):395–397. [Google Scholar]
- Arriagada R, Bergman B, Dunant A, Le C, Pignon J, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. New England Journal of Medicine. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- Ballatori E, Roila F, Ruggeri B, Betti M, sarti S, Soru G, Cruciani G, Maio MD, Andrea B, Deuson R. The impact of chemotherapy-induced nausea and vomiting on health-related quality of life. Supportive Care in Cancer. 2007;15(2):179–185. doi: 10.1007/s00520-006-0109-7. [DOI] [PubMed] [Google Scholar]
- Belani CP, et al. Quality of life in patients with advanced non-small-cell lung cancer given maintenance treatment with pemetrexed versus placebo (h3e-mc-jmen): Results from a randomised, double-blind, phase 3 study. The Lancet Oncology. 2012;13(3):292–299. doi: 10.1016/S1470-2045(11)70339-4. [DOI] [PubMed] [Google Scholar]
- Berndt E, Newhouse JP. Pricing and reimbursement in u.s. pharmaceutical markets. In: Danzon PM, Nicholson S, editors. The Oxford Handbook of the Economics of the Biopharmaceutical Industry. Oxford UP; 2012. pp. 201–265. [Google Scholar]
- Booth CM, et al. Adoption of adjuvant chemotherapy for non-small-cell lung cancer: a population-based outcomes study. Journal of Clinical Oncology. 2010;28(21):3472–3478. doi: 10.1200/JCO.2010.28.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher L. Insurers and oncologists forge a better cancer drug policy. Managed Care Magazine. 2008 Apr; [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Disease. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Clemens J, Gottlieb J. Do physicians’ financial incentives affect medical treatment and patient health? American Economic Review. 2014;104(4):1320–49. doi: 10.1257/aer.104.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colla CH, Morden NE, Skinner JS, Hoverman JR, Meara E. Impact of payment reform on chemotherapy at the end of life. Journal of Oncology Practice. 2012;8(3s):e6s–e13s. doi: 10.1200/JOP.2012.000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff AJ, Tang M, Seal B, Edelman MJ. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. Journal of Clinical Oncology. 2010;28:2191–2197. doi: 10.1200/JCO.2009.25.4052. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with icd-9 cm administrative databases. Journal of Clinical Epidemiology. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- Douillard J, Rosell R, De L. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage ib-iiia non-small-cell lung cancer (adjuvant navelbine international trialist association [anita]): a randomised controlled trial. Lancet Oncology. 2006;7(9):719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- Douillard J, Rosell R, Delena M. Phase iii adjuvant vinorelbine (n) and cisplatin (p) versus observation (obs) in completely resected (stage i–iii) non-small-lung cancer (nsclc) patients (pts): Final results after 70-month follow-up. on behalf of the adjuvant navelbine international trialist association. Journal of Clinical Oncology. 2005;23(16S) [Google Scholar]
- Earle CC, Nattinger AB, Potosky AL. Identifying cancer relapse using seer-medicare data. Medical Care. 2002;40(8 Supplemental):IV, 75–81. doi: 10.1097/00005650-200208001-00011. [DOI] [PubMed] [Google Scholar]
- Encaoua D, Jacquemin A. Degree of monopoly, indices of concentration and threat of entry. Internation Economic Review. 1980;21(1):87–105. [Google Scholar]
- Friedman JY, Curtis LH, HB, et al. The medicare modernization act and reimbursement for outpatient chemotherapy: do patients perceive changes in access to care? Cancer. 2007;110(10):2304–12. doi: 10.1002/cncr.23042. [DOI] [PubMed] [Google Scholar]
- Goldman DP, Jena AB, Lakdawalla DN, Malkin JL, Sun E. The value of specialty oncology drugs. Health Services Research. 2010;45(1):115–132. doi: 10.1111/j.1475-6773.2009.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government Accountability Office. Medicare physician fee schedule: Practice payments to oncologists indicate need for overall refinements. GAO-02-53 2001 [Google Scholar]
- Gruber J, Owings M. Physician financial incentives and cesarean delivery. The RAND Journal of Economics. 1996;27(1):99–123. [PubMed] [Google Scholar]
- Heaton P, Helland E. Does treatment respond to reimbursement rates? evidence from trauma care. RAND Working Paper WR-648-ICJ 2009 [Google Scholar]
- Jacobson M, Earle CC, Newhouse JP. How medicare’s payment cuts for cancer chemotherapy drugs changed patterns of treatment. Health Affairs. 2010;29:1391–1399. doi: 10.1377/hlthaff.2009.0563. [DOI] [PubMed] [Google Scholar]
- Jacobson M, Earle CC, Newhouse JP. Geographic variation in physicians’ response to a reimbursement change. New England Journal of Medicine. 2011;365:2049–2052. doi: 10.1056/NEJMp1110117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. Physician-Induced Demand, Volume 3 of Encyclopedia of Health Economics. Elsevier; 2014. pp. 77–82. [Google Scholar]
- Johnson E, Rehavi M. Physicians treating physicians: Information and incentives in childbirth. American Economic Journal: Economic Policy. 2016;8(1):115–41. [Google Scholar]
- Kaiser Family Foundation. Kaiser family foundation medicare chartbook. 2010. [Google Scholar]
- Le Chevalier T. Results of the randomized international adjuvant lung cancer trial (ialt): cisplatin-based chemotherapy (ct) vs no ct in 1867 patients (pts) with resected non-small cell lung cancer (nsclc) Proceedings of the American Society of Clinical Oncology. 2003;22 (abstr 5) [Google Scholar]
- McGuire TG. Physician agency. In: Culyer AJ, Newhouse JP, editors. Handbook of Health Economics. North-Holland; 2000. pp. 462–217. [Google Scholar]
- McGuire TG, Pauly MV. Physician response to fee changes with multiple payers. Journal of Health Economics. 1991;10(4):385–410. doi: 10.1016/0167-6296(91)90022-f. [DOI] [PubMed] [Google Scholar]
- Miller N, Eggleston K, Zeckhauser R. Provider choice of quality and surplus. Int J Health Care Finance Econ. 2006;6:103–117. doi: 10.1007/s10754-006-7107-7. [DOI] [PubMed] [Google Scholar]
- Mullen P. The arrival of average sales price. Biotechnology Healthcare. 2007 Jun;:48–53. [PMC free article] [PubMed] [Google Scholar]
- Newey W, West K. A simple, positive semi-definite, heteroscedastic and autocorrelation consistent covariance matrix. Econometrica. 1987;55:703–708. [Google Scholar]
- Office of Inspector General. Department of health and human services. excessive medicare payment for prescription drugs, no. oei-03-97-00290 1997a Dec; [Google Scholar]
- Office of Inspector General. Department of health and human services. excessive medicare payment for prescription drugs, no. oei-03-97-00290 1997b Dec; [Google Scholar]
- Ramsey SD, Scoggins JF, Blough DK, McDermott CL, Reyes CM. Sensitivity of administrative claims to identify incident cases of lung cancer: a comparison of 3 health plans. Journal of Managed Care Pharmacy. 2009;15(8):659–668. doi: 10.18553/jmcp.2009.15.8.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shainian VB, Kuo Y, Gilbert S. Reimbursement policy and androgen-deprivation therapy for prostate cancer. New England Journal of Medicine. 2010;363:1822–1832. doi: 10.1056/NEJMsa0910784. [DOI] [PubMed] [Google Scholar]
- Skinner J. Causes and consequences of regional variations in health care. In: Barros PP, McGuire TG, Pauly MV, editors. Handbook of Health Economics. Vol. 2. Amsterdam: North-Holland; 2012. [Google Scholar]
- Stock JH, Watson MW. Introduction to Econometrics. Pearson Education, Inc; 2003. [Google Scholar]
- Strauss G, Herndon J, Maddaus M. Randomized clinical trial of adjuvant chemotherapy with paclitaxel and carboplatin following resection in stage ib non-small cell lung cancer (nsclc): Report of cancer and leukemia group b (calgb) protocol 963. Journal of Clinical Oncology. 2004;22(14S) (7019) [Google Scholar]
- Strauss G, Herndon J, Maddaus M. Adjuvant chemotherapy in stage ib non-small cell lung cancer (nsclc): Update of cancer and leukemia group b (calgb) protocol 9633. Journal of Clinical Oncology. 2006;24(18S) [Google Scholar]
- Strauss G, Herndon J, Maddaus M. Adjuvant paclitaxel plus carboplatin compared with observation in stage ib non-small-cell lung cancer: Calgb 9633 with the cancer and leukemia group b, radiation therapy oncology group, and north central cancer treatment group study groups. Journal of Clinical Oncology. 2008;26(31):5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Llonch M, Weycker D, Glass A. Healthcare cost in patients with metastatic lung cancer receiving chemotherapy. BMC Health Services Research. 2011;11:305. doi: 10.1186/1472-6963-11-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JL, Feuer E, Potosky AL, Riley GF, Lynch CF. Use of medicare hospital and physician data to assess breast cancer incidence. Medical Care. 1999;37(5):445–456. doi: 10.1097/00005650-199905000-00004. [DOI] [PubMed] [Google Scholar]
- Warren JL, Harlan LC, Fahey A, Virnig BA, Freeman JL, Klabunde CN, Cooper GS, Knoft KB. Utility of the seer-medicare data to identify chemotherapy use. Medical Care. 2002;40(Supplemental):IV, 55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- Weight CJ, Klein EJ, Jones JS. Androgen deprivation falls as orchiectomy rates rise after changes in reimbursement in the u.s. medicare population. Cancer. 2007;110:2304–2312. doi: 10.1002/cncr.23421. [DOI] [PubMed] [Google Scholar]
- Winton T, Livingston R, Johnson D. A prospective randomised trial of adjuvant vinorelbine (vin) and cisplatin (cis) in completely resected stage 1b and ii non small cell lung cancer (nsclc) intergroup jbr.10. Journal of Clinical Oncology. 2004;22(14S) (7018) [Google Scholar]
- Winton T, Livingston R, Johnson D. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. New England Journal of Medicine. 2005;352(25):2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.