Abstract
Acute inflammation is a host-protective response that is mounted in response to tissue injury and infection. Initiated and perpetuated by exogenous and endogenous mediators, acute inflammation must be resolved for tissue repair to proceed and for homeostasis to be restored. Resolution of inflammation is an actively regulated process governed by an array of mediators as diverse as those that initiate inflammation. Among these, resolvins have emerged as a genus of evolutionarily conserved pro-resolving mediators that act on specific cellular receptors to regulate leukocyte trafficking and blunt production of inflammatory mediators, while promoting clearance of dead cells and tissue repair. Given that chronic unresolved inflammation is emerging as a central causative factor in the development of cardiovascular diseases, an understanding of the endogenous processes that govern normal resolution of acute inflammation is critical for determining why sterile maladaptive cardiovascular inflammation perpetuates. Here, we provide an overview of the process of resolution with a focus on the enzymatic biosynthesis and receptor-dependent actions of resolvins and related pro-resolving mediators in immunity, thrombosis and vascular biology. We discuss how nutritional and current therapeutic approaches modulate resolution and propose that harnessing resolution concepts could potentially lead to the development of new approaches for treating chronic cardiovascular inflammation in a manner that is not host-disruptive.
Keywords: Resolution of inflammation, Lipid mediators, Omega-3 fatty acids
Subject terms: Inflammation, Vascular Biology, Physiology
Inflammation and cardiovascular disease
Persistent unresolved inflammation has long been recognized to play a fundamental role in the development of chronic diseases including arthritis, autoimmune diseases, and asthma. In recent decades, it has emerged that maladaptive inflammation is causally involved in the development of cardiovascular disease (CVD) and other metabolic disorders including diabetes and obesity. Indeed, inflammation is a central feature of virtually all pathologies afflicting both the heart and the vasculature, including chronic heart failure (HF), atherosclerosis, myocardial infarction (MI), and stroke1, as discussed in greater depth in the accompanying reviews of this series. Moreover, mounting evidence indicates that the presence of other chronic inflammatory diseases (e.g., periodontitis, rheumatoid arthritis) can exacerbate CVD1. Atherosclerosis is perhaps the CVD that has been most closely linked with chronic unresolved inflammation2, 3. While the development of statins revolutionized the treatment of atherosclerosis by lowering low-density lipoprotein (LDL) cholesterol, the burden of ischemic cardiovascular conditions has continued to rise globally and CVD remains the leading cause of death among Americans4. Furthermore, intolerance and adverse effects of statins in a growing number of patients has emphasized the need for novel therapies2, 4. Many new strategies, therefore, have looked beyond lipid lowering alone and have focused instead on halting aberrant inflammatory signaling5. Several clinical trials are currently underway testing the efficacy of anti-inflammatory drugs in the context of CVD. One large trial, the Cardiovascular Inflammation Reduction Trial (CIRT), using low dose methotrexate, is currently recruiting patients and is specifically designed to assess whether inhibition of inflammation will lower vascular events rates6, 7. Another trial, the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS), has enrolled over 10,000 patients with stable coronary artery disease (CAD) and a history of MI. Preliminary results show treatment with the IL-1β receptor antagonist decreased measures of inflammation without affecting lipids4, 8. These clinical trials will provide valuable insights into the role of inflammation in CVD and may herald a new direction in treatment strategies. While invasive procedures will remain indispensable for the most severe CVD cases, innovative therapies targeting the body’s innate immune system may indeed be a promising alternative for many patients. Inherent in the discovery and development of such new, effective and less invasive therapies is a more thorough delineation of the endogenous mechanisms that govern the natural resolution of acute inflammation. A novel genus of “pro-resolving mediators” potently resolve inflammation, but also enhance certain aspects of host defense, potentially overcoming limitations to traditional therapeutic anti-inflammatory agents that can be immunosuppressive9. This review focuses on the role of these endogenous mediators in promoting the resolution of acute inflammation, highlighting their biosynthesis and cellular targets, and discusses how defective resolution may contribute to CVD.
Acute inflammation and its resolution
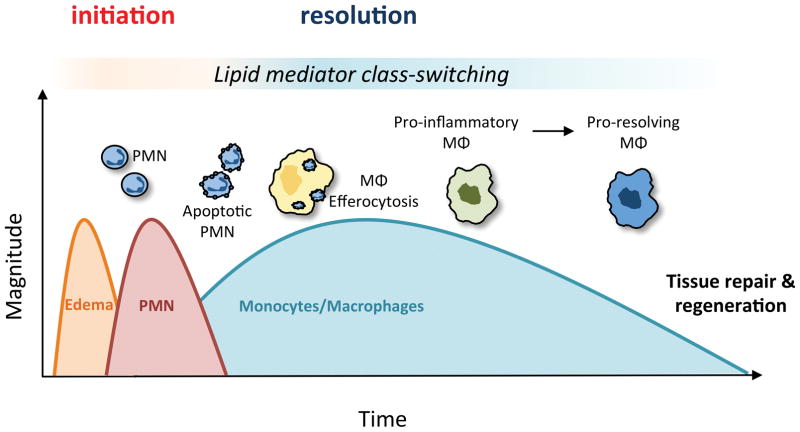
In response to injury or infection, the protective program of acute inflammation and its complete and timely resolution are critical for the restoration of tissue homeostasis10. This highly coordinated and synergistic program combines the distinct actions of multiple cell types to achieve pathogen eradication and subsequent tissue repair. The acute inflammatory response can be divided into two general phases: initiation and resolution (Fig 1). Initiation is marked by tissue edema resulting from increased blood flow and permeability of the microvasculature; processes that are mediated in part by lipid mediators (e.g., cysteinyl leukotrienes and prostaglandins) and other vasoactive products (e.g., histamine, bradykinin). Subsequently, polymorphonuclear neutrophils (PMN) migrate to the area to defend against microbial invasion. Drawn to the site of injury by exuded chemical signals including pro-inflammatory lipid mediators (e.g., leukotriene B4; LTB4) and chemokines, PMN traverse the vasculature through precise interactions with endothelial adhesion receptors and subsequently engulf and degrade pathogens within phagolysosomes11–13. The resolution phase is already being enacted at this early point as the influx of PMN is halted at a level appropriate for the insult and is accompanied by their timely apoptosis14. Monocytes subsequently infiltrate the tissue where they differentiate into macrophages that avidly respond to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) present in the injured tissue. Importantly, macrophages are highly responsive to so-called find-me and eat-me signals (e.g., nucleotides, externalized phosphatidylserine) released or presented by apoptotic cells such as PMN10, 15. Uptake of apoptotic cells by macrophages (i.e., efferocytosis) is an anti-inflammatory process associated with decreased production of inflammatory mediators, thus coupling the initiation of inflammation to its ultimate resolution (Fig. 1). The timely clearance of microbes and apoptotic cells is required to prevent bystander tissue damage and to set the stage for tissue repair and regeneration, allowing for the return to homeostasis9, 15. Indeed, active clearance of apoptotic cells is a key defining feature of resolution, as failed clearance can lead to cellular necrosis and exacerbated inflammation beyond the initial insult, impeding tissue repair. Macrophages persist in injured tissues longer than short-lived PMN, during which time they are continuously reprogrammed in response to local cues to facilitate tissue repair and orchestrate the delicate balance of fibrosis16–18. Like innate immune cells, adaptive immune cells also play critical roles in the host response to infection, resolution of inflammation and in tissue repair19, 20. Their accumulation defines the “post-resolution” phase of the inflammatory response and assures a more rapid response to subsequent exposure to the same antigens19. Interruption of this process at any point (e.g., prolonged leukocyte recruitment and survival, impairments in apoptotic cell removal, alterations in macrophage phenotype switching) could potentially lead to chronic inflammation with resultant tissue damage, excessive fibrosis and loss of function, as is seen in many CVDs like atherosclerosis and HF10, 17, 21–23.
Figure 1. The coordinated temporal events of self-limited acute inflammation.
The ideal outcome of an acute inflammatory response is complete resolution. The inflammatory response can be divided into two general phases: initiation and resolution. Critical to progressing from initiation to resolution is the temporal switch in lipid mediators that are biosynthesized by leukocytes in the tissue; a process known as lipid mediator class switching. The earliest stage of the inflammatory response is marked by tissue edema due to increased blood flow and microvascular permeability and is mediated by the release of pro-inflammatory lipid mediators including the cysteinyl leukotrienes and prostaglandins. Polymorphonuclear neutrophils (PMN) infiltrate in response to lipid mediators including leukotriene B4 and engulf and degrade pathogens. Subsequently, PMN undergo apoptosis and also switch from releasing pro-inflammatory mediators to pro-resolving mediators (e.g., resolvins) that signal the clearance of apoptotic cells by macrophages in an anti-inflammatory process termed efferocytosis. In addition to promoting efferocytosis, pro-resolving lipid mediators halt further PMN recruitment and stimulate a pro-resolving macrophage phenotype that is important for tissue repair.
By its nature, the acute inflammatory response is self-limiting in part because of inherent negative feedback regulation of inflammatory signaling pathways (e.g., transcriptional repressors, endogenous receptor antagonists) when the trigger has been eliminated. However, it has recently become evident that active resolution of inflammation involves the biosynthesis of pro-resolving mediators that, as a genus, are just as diverse as the initiators of inflammation24–33. Thus, critical to determining the fate of an inflammatory response is the balance of pro-inflammatory and pro-resolving mediators that are produced in the exudate in a temporal manner. Traditionally, it has been held that an excess production of pro-inflammatory mediators underlies chronic inflammation34, however, mounting evidence supports the view that disruptions in endogenous pro-resolving circuits may be an equally important mechanism10, 34, 35. These pro-resolving mediators actively terminate the production of pro-inflammatory mediators, but also directly stimulate macrophage phagocytosis of both apoptotic cells and bacteria, promote egress of phagocytes from sites of inflammation, regulate PMN apoptosis, promote chemokine scavenging, and stimulate tissue repair and regeneration9, 36–41. These agonist-based actions distinguish pro-resolving mediators from intrinsic negative feedback pathways and other antagonists that terminate inflammatory signaling pathways. Systems-based approaches have played a crucial role in the identification of the principal mediators of resolution9. As a result, a complex and ever-expanding network of interrelated mediators and the cellular targets and pathways that they engage has been assembled. The discovery of these novel bioactive pro-resolving mediators represents a paradigm shift in our understanding of the dynamic regulation of acute inflammation and has led to a new era of “resolution physiology”9.
Pro-resolving mediators
The discovery of bioactive mediators with potent inflammation-resolving actions in experimental models of acute inflammation was a seminal development that provided compelling evidence that resolution is an active process rather than a passive one as traditionally thought. Self-resolving inflammatory exudates were shown to contain structurally unique families of signaling molecules that are temporally produced and when added back in experimental models of acute inflammation, potently enhance the resolution phase. Like the initial phases of inflammation, enzymatically produced lipid mediators derived from polyunsaturated fatty acids (PUFA) play a central role in resolution, due in part to their rapid production by distinct immune cell subsets. During the transition from inflammation to resolution, lipid mediator class switching takes place in which initial formation of pro-inflammatory mediators induce the production of enzymes that enable the reprogramming of leukocytes to generate specialized pro-resolving lipid mediators (SPM) from the same PUFA precursors (Fig. 1)42. For instance, 5-lipoxygenase (5-LOX) is required for the generation of potent leukocyte chemoattractant, LTB4 from omega-6 PUFA arachidonic acid (AA). When 12-LOX is also expressed (e.g., during platelet:leukocyte interactions), the epoxide intermediate in LTB4 biosynthesis (i.e., LTA4) can be converted into the family of lipoxins. Lipoxins can also be produced with cooperation between 5-LOX and 15-LOX, the latter of which can be induced in PMN by prostaglandin E2 (PGE2), or in macrophages by Th2 cytokines (e.g., IL-4). Lipoxin A4 (LXA4), was the first lipid mediator shown to possess both anti-inflammatory and pro-resolving actions43 and, along with subsequently discovered members of the lipoxin family (i.e., LXB4), has been the subject of extensive and rigorous study. In addition to omega-6 PUFA serving as precursors to lipoxins, omega-3 PUFAs emerged as an additional source of novel SPM. Indeed, after the initial discovery of LXA4, the enzymatic conversion of the omega-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) to novel families of SPM – resolvins, protectins and maresins – was identified44–47. This mechanism could have evolved to ensure adequate production of SPM in organisms with different PUFA in their diets. Importantly, SPM are evolutionarily conserved from flatworms (e.g., Planaria) to humans and several studies have documented endogenous formation of these mediators in healthy individuals, with lower levels observed in patients with Alzheimer’s disease, peripheral artery disease and asthma (for review, see 25). In addition to lipid mediators of resolution, other important mediators of the resolution program have emerged and include proteins and bioactive peptides (e.g., annexin A1, galectin 1, melanocortins) and gases (nitric oxide, hydrogen sulfide, carbon monoxide). Readers are referred to recent reviews covering each of these mediators in detail24–33. Here, we focus primarily on the biosynthesis and biological actions of resolvins in the inflammation-resolution program.
Biosynthesis of Resolvins
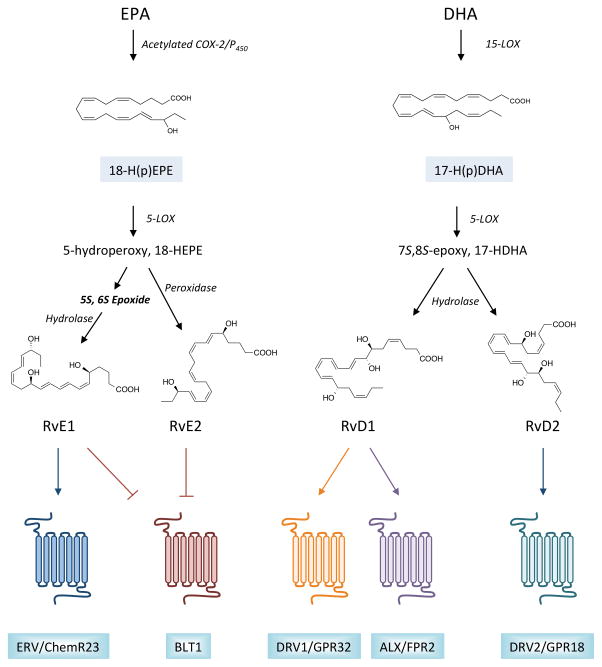
The use of liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based approaches were critical in the identification and structural elucidation of resolvins and related SPM9. Applying these methods to generate a lipid mediator profile of self-resolving inflammatory exudates in mice yielded the first evidence of a novel bioactive mediator generated from EPA during resolution44. Later named resolvin E1 (RvE1), its biosynthetic pathway proceeds via conversion of EPA to 18R-hydroperoxyeicosapentaenoic acid (18R-H(p)EPE) by acetylated cyclooxygenase-2 (COX-2) or cytochrome P450 enzymes, which is subsequently converted to RvE1 by 5-LOX (Fig. 2)44, 48. The epoxide intermediate formed during RvE1 biosynthesis is hydrolyzed by leukotriene A4 hydrolase (LTA4H), the same enzyme that produces pro-inflammatory LTB449. This mechanism provides an additional point of control in the balance between pro-inflammatory mediators and SPM. The formation of RvE1 precursor, 18-H(p)EPE, was identified in hypoxic endothelial cells and can be transformed to RvE1 through leukocyte:endothelial interactions. The complete structural elucidation of RvE1 was carried out via total organic synthesis and matching with endogenous material, which enabled assessment of the individual bioactions of RvE1. These actions include the reduction in PMN infiltration to sites of acute inflammation, blunting of inflammatory cytokine production, and the enhancement of macrophage efferocytosis44, 48. Additional members of the EPA-derived (E-series) resolvins have since been characterized. Similar to RvE1, RvE2 is generated from EPA via the intermediate 18R-HEPE and its subsequent 5-LOX-dependent conversion to a 5S-hydroperoxide50, 51. However, instead of undergoing an epoxidation step, the 5S-hydroperoxide is directly converted to RvE2 (Fig. 2). More recently, a third member, RvE3, was also identified and its structure determined52. Unlike RvE1 and RvE2, the formation of this product does not require 5-LOX.
Figure 2. Biosynthesis and receptors for resolvins.
The family of resolvins is divided primarily into two groups based on the omega-3 polyunsaturated fatty acid (PUFA) from which they are formed. For E-series resolvin biosynthesis, eicosapentaenoic acid (EPA) is utilized as a substrate for acetylated COX-2 or cytochrome P450 enzymes, giving rise to a 18-hydroperoxide intermediate that can be converted to either RvE1 or RvE2 by 5-LOX and additional enzymatic transformations, as indicated. Similarly, DHA serves as a substrate for 15-LOX, giving rise to a 17-hydroperoxide intermediate. This intermediate is subsequently converted to RvD1 or RvD2 via 5-LOX, the formation of an epoxide intermediate, and subsequent enzymatic hydrolysis. Monohydroxy fatty acids, 18-HEPE and 17-HDHA, serve as pathway markers for E-series resolvins and D-series resolvins, respectively. Once formed, resolvins elicit their potent biological actions by activating specific G-protein coupled receptors (GPCRs) in a stereoselective manner. RvE1 is an agonist for ERV/ChemR23, while both RvE1 and RvE2 block the actions of pro-inflammatory leukotriene B4 by binding to its receptor, BLT1. D-series resolvins are agonists for distinct GPCRs, with RvD1 binding to DRV1/GPR32 and ALX/FPR2, and RvD2 binding to GPR18.
In addition to EPA, the other prominent omega-3 PUFA in humans and marine oils is DHA. This fatty acid serves as the precursor to the D-series resolvins, as well as the protectins and maresins44–48. In the presence of 15-LOX, DHA is converted to a 17-hydroperoxide intermediate (17-H(p)DHA) which can subsequently be converted to D-series resolvins via 5-LOX. Specifically, the hydroperoxide formed via 5-LOX can undergo epoxidation to generate a 7S,8S epoxide that is then enzymatically hydrolyzed to produce resolvin D1 (RvD1) or resolvin D2 (RvD2; see Fig. 2)40, 53. As with the E-series resolvins, these steps can take place within a single cell (e.g., PMN or macrophages), or can occur during cell:cell interactions (e.g., leukocyte:endothelial, PMN:macrophage). Additional structurally unique D-series resolvins, including RvD3-6, have also been identified and the complete structures and stereochemical assignments of RvD3 and RvD4 have recently been reported54, 55. In murine models of acute inflammation, distinct temporal patterns of resolvin biosynthesis have been observed, highlighting additional control points in their biosynthesis (e.g., potentially novel regulation by epoxide hydrolases)54. Interestingly, within a single cell type, lipid mediator class switching can occur and pro-resolving lipid mediators can regulate each other. For instance, RvD1 reduces nuclear localization of 5-LOX in macrophages and diverts AA metabolism from pro-inflammatory lipid mediator, LTB4, to SPM, LXA4. Mechanistically, RvD1 inhibits the activation of calcium-calmodulin-dependent kinase II (CaMKII) and downstream activation of p38 and a mitogen-activated kinase (MK2), which results in reduced phosphorylation of 5-LOX56. Thus, SPM biosynthesis can be amplified by positive feed-forward mechanisms, helping to tip the balance from inflammation to resolution. Indeed, in several distinct models of acute inflammation and infection, administration of resolvins blunts production of pro-inflammatory eicosanoids and increases formation of other pro-resolving mediators9. It is important to note that, like most autacoids, resolvins are rapidly inactivated after they have elicited their biological actions in resolution. For instance, RvE1 is a substrate for 15-hydroxyprostaglandin dehydrogenase (PGDH), generating the inactive product, 18oxo-RvE1. This suggests that accelerated inactivation could potentially underlie defective resolution in some cases and as a way to circumvent metabolic inactivation from a therapeutic standpoint, a variety of stable resolvin analogs have been formulated and shown to have enhanced biological activity in vivo25.
Recently, a new family of mediators was identified wherein the epoxide intermediate in RvD1 and RvD2 biosynthesis can be enzymatically conjugated to glutathione to generate sulfido-conjugated resolvins38. They contain glutathione at the C8 position and can be subsequently converted to cysteinylglycinyl conjugates by γ-glutamyl transferase. These novel mediators were identified in infectious inflammatory exudates, human spleens and in human leukocytes (e.g., PMN and macrophages), as well as apoptotic PMN. Like other SPM, these resolvin-conjugates enhance bacterial clearance in vivo and macrophage phagocytosis. Interestingly, they have distinct roles in promoting tissue regeneration in Planaria. Given these new roles, they were named “resolvin-conjugates in tissue regeneration; RCTRs”. It is noteworthy that similar biosynthetic routes were also identified for maresin and protectin families of SPM38, 57.
Pro-resolving receptors
Like other classic lipid mediators and chemokines, SPM elicit their biological actions through activation of specific G-protein coupled receptors (GPCRs) in a stereoselective manner (Fig. 2). The pro-resolving and anti-inflammatory actions of LXA4 and RvD1 are mediated via specific signaling through ALX (also termed formyl peptide receptor 2; FPR2) and previous orphan, GPR32 (also termed D-resolvin receptor 1; DRV1)25, 58. Given that LXA4 and RvD1 are structurally similar but generated from different PUFA precursors, that they activate the same receptors and promote reciprocal biosynthesis of each other provides additional evidence that pro-resolving pathways are intimately linked. Specific binding to these receptors was unequivocally demonstrated using radiolabeled material and both homo- and heteroligand competition. Moreover, their pro-resolving actions were validated in mouse models of acute inflammation with targeted overexpression or genetic deletion of the ALX/Fpr259–61. We note that there is no murine homolog of human GPR32 identified to date. The actions of RvD1 in counter-regulating PMN recruitment during acute peritonitis are ablated in ALX/Fpr2-deficient mice59. In human macrophages, RvD1 enhances phagocytosis of both apoptotic PMN and opsonized zymosan, actions that are potentiated by overexpression of ALX/FPR2 and GPR32, and attenuated with their knockdown58. Recently, it has been shown that RvD3 and RvD5 also activate human GPR3237, 54. Given that these mediators are generated at distinct times during resolution of inflammation54, this mechanism may serve to preserve GPR32 ligands during different stages of resolution. Interestingly, ALX/FPR2 is also activated by other pro-resolving mediators, such as annexin A1, as well as pro-inflammatory mediators (e.g., serum amyloid A; SAA). Depending on the stimulus, signaling through this receptor can elicit either pro-inflammatory or pro-resolving effects. Emerging evidence indicates that dimerization underlies in part these distinct and unconventional roles62. Specifically, annexin A1 and LXA4 induce ALX homodimer formation and this leads to increased production of IL-10 in monocytes. In contrast, pro-inflammatory agonists, such as SAA, can block this homodimerization. In PMN, annexin A1, an annexin A1 peptide mimetic (Ac2-26), and LXA4 can induce formation of ALX heterodimers with FPR1. These heterodimers elicit distinct signaling pathways (JNK/caspase-3) that override pro-survival signals in PMN, such as SAA, through the same receptor62, 63. As noted, this is important because timely apoptosis and clearance of PMN is required for resolution of inflammation and prolonged PMN survival can cause excessive tissue damage.
A distinct GPCR denoted chemoattractant receptor 23 (ChemR23; also termed E-resolvin receptor, ERV), which shares 36% sequence identity with ALX/FPR2, is activated by RvE1 in a stereoselective manner48. The anti-inflammatory signaling properties of ChemR23 were also demonstrated in studies interrogating a different agonist, chemerin peptide, and in mice with genetic deletion of the receptor64. Subsequent investigations further substantiated ChemR23-mediated signaling by RvE1, including stimulation of macrophage phagocytosis via the phosphatidylinositol 3-kinase (PI3K)/Akt pathway and activation of the translational regulator, ribosomal protein S665. Interestingly, not all of RvE1’s actions can be ascribed to ChemR23. Indeed, RvE1 potently blocks PMN migration in vitro yet these cells do not express ChemR2348. Additional studies determined that RvE1 binding to human PMN is displaced by LTB4 and an antagonist to the LTB4 receptor (BLT1), but not by chemerin peptide, indicating that BLT1 is a target for RvE1 on PMN66. This study also demonstrated that the actions of RvE1 on PMN may be due, in part, to an attenuation of pro-inflammatory LTB4 signaling, which was subsequently confirmed in Blt1-deficient mice66. Like RvE1, RvE2 also binds BLT1 and blocks LTB4-stimulated actin polymerization in PMN50, 51. Importantly, although many of the downstream actions of RvD1 are similar to those of RvE1, it does not bind ChemR23 or BLT158.
Using an unbiased β-arrestin based screening approach, a recent study identified that RvD2 activates a distinct GPCR denoted GPR18 (also termed D-resolvin receptor 2; DRV2), which among innate immune cells, is expressed on human PMN, monocytes and macrophages67. Specific binding to GPR18 was confirmed using radiolabeled RvD2 and binding competition studies. Similar to the actions of RvD1, RvD2 increases phagocytosis of apoptotic PMN by macrophages, which is enhanced with GPR18 overexpression and decreased with GPR18 knockdown. Similarly, the potent actions of RvD2 in limiting PMN infiltration and enhancing clearance of E. coli and S. aureus in vivo are abolished in Gpr18-deficient mice67. Taken together, it is clear that the actions of resolvins have significant overlap yet remain specific and are mediated by distinct receptors. The multiplicity of cellular sites of action of resolvins reinforces the concept that these mediators are vital in orchestrating the complex, coordinated response that governs the active process of inflammation-resolution. The identification of these pro-resolving receptors has, and will continue to, illuminate the mechanisms governing resolution, including the specific signaling pathways and transcriptional programs engaged by pro-resolving mediators.
Increasing pro-resolving lipid mediators with nutritional and pharmacologic approaches
Given the potent bioactions of resolvins and related SPM during acute inflammation, there has been significant attention and effort dedicated to developing strategies for increasing their production endogenously. Perhaps the most obvious approach would be to increase the substrate. As discussed, the omega-3 PUFA EPA and DHA serve as the precursors for resolvins and other SPM. In rodent models of inflammation, administration of EPA or DHA increases resolvin production25. Mice with transgenic expression of a gene from Caenorhabditis elegans that enables endogenous generation of omega-3 PUFA from omega-6 PUFA (denoted Fat-1), are protected from inflammatory conditions such as colitis and pathological retinal angiogenesis and have elevated endogenous levels of resolvins68, 69. These protective actions were recapitulated with exogenous delivery of resolvins, establishing causality in this relationship68, 69. There have been numerous studies investigating the protective effects of increased dietary intake of omega-3s on a diverse range of inflammatory diseases in humans, including asthma and arthritis, as well as CVD, based in large part on the fact that the typical Western type diet is deficient in omega-3 PUFA70, 71. One landmark clinical trial, the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico (GISSI) Prevenzione study, enrolled more than 11,000 recent MI patients and reported that 1g of daily omega-3 supplementation for three and half years significantly reduced the risk of cardiovascular death72. More recently, GISSI also found greater survival in HF patients treated with omega-3s73. Though these trials were able to demonstrate the beneficial effects of omega-3 supplementation, the mechanism of this protection remains unclear. In humans, several studies have shown that intake of omega-3 PUFA increases production of resolvins and/or their biosynthetic pathway markers (e.g., 18-HEPE and 17-HDHA) in plasma and/or serum74–79. In a very recent study, treatment with Lovaza (omega-3 ethyl esters) in patients with stable CAD increased levels of RvD6, 17R-PD1 and RvE280. Whether production of SPM is causally related to the protective actions of omega-3s in humans requires further clinical studies and it is also important to note that some chronic inflammatory diseases are associated with defects in SPM production25, 81. Given that, unlike hormones, SPM are locally produced and have actions locally in inflammatory exudates (i.e., autacoids), the relevance of their measurement in plasma is still unclear. Given that some clinical trials have failed to show improvements in cardiovascular endpoints with omega-3 PUFA supplementation71, it is intriguing to speculate that altered downstream conversion to bioactive SPM could be related to variable results in such trials. Such variation could also arise from the diversity in dosing and preparations (e.g., ethyl esters vs. triglycerides). This is particularly relevant when one considers that the appearance of SPM is dependent upon multiple biosynthetic checkpoints and enzymatic degradation, rather than a passive linear increase with omega-3 intake. Moreover, high intake of omega-3s could also bias the utilization of EPA and DHA away from SPM and toward the formation of free radical-initiated auto-oxidation products82. While the evidence to date indicates that SPM and their intermediate precursors are indeed produced in humans in vivo and are demonstrable by LC-MS/MS74–76, 79, 83, 84, it is noteworthy that with inadequate approaches and methodology, some studies were unable to identify endogenous SPM in plasma85, which have specific stereochemistry and require the use of commercially available deuterium-labeled standards for their definitive identification in human tissues. These are important considerations for the development and implementation of new therapeutic approaches and also suggest that targeted delivery of specific SPM may be a better therapeutic approach.
One important design aspect of the GISSI trials was the instruction of enrolled patients to adhere to their recommended preventive medications in addition to the omega-3 PUFA supplement86. Included in the preventive medications for many of these patients was aspirin. Aspirin is one of the most widely used anti-inflammatory drugs, and low-dose aspirin is currently recommended by the American Heart Association (www.americanheart.org) for people at high risk of heart attack and heart attack survivors. It was traditionally held that the salubrious effects of an aspirin regimen stemmed solely from halting prostaglandin and thromboxane production87. More recently, however, additional anti-inflammatory actions of aspirin have been described. Aspirin-acetylation of COX-2 blocks the production of prostaglandin precursors, but also switches the activity of COX-2 such that it generates 15R-HETE with AA as a substrate, or 18-HEPE and 17R-HDHA with EPA and DHA as substrates, respectively. These intermediates are subsequently converted into epimeric (i.e., aspirin-triggered) lipoxins and resolvins that share the potent biological actions of the native mediators in which the chirality of the C15 or C17 hydroxyl groups in 15-HETE and 17-HDHA are predominantly in the S configuration44, 45, 49, 53, 88. Thus, aspirin could be considered to be “resolution friendly” because in addition to blocking inflammation, it jump starts resolution by shifting the lipid mediator profile from pro-inflammatory eicosanoids to SPM. Interestingly, some of these mediators (i.e., RvE1) regulate platelet activation as well (vide infra). Of interest, a recent study demonstrated that aspirin treatment enhances reverse cholesterol transport in hypercholesterolemic mice, which reduces atherosclerosis89. This was associated with increased production of lipoxins in the liver and exogenous delivery of a stable analog of LXB4 recapitulated these protective effects. These results indicate that in addition to the inflammation-resolving roles of SPM, they may have additional atheroprotective actions.
In addition to aspirin, statins are a widely prescribed class of lipid-lowering therapeutics with anti-inflammatory effects in the cardiovascular system, as discussed above4. Results of the JUPITER trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin) demonstrated that administration of Rosuvastatin decreases systemic markers of inflammation and that the observed reduction in major cardiovascular events is independent of the reduction in LDL cholesterol90. Though it is clear that statins have anti-inflammatory effects, their mechanism of action is not entirely clear. Similar to aspirin, statins have been shown to target COX-2, however rather than acetylating the enzyme, statins increase S-nitrosylation and bias the enzyme toward the formation of 15-epimeric lipoxins as well91, 92. More recently, it was demonstrated that S-nitrosylation of COX-2 via administration of Atorvastatin initiates the biosynthesis of a novel group of 13-series resolvins from omega-3 docosapentaenoic acid (DPA)93. Human and mouse leukocytes were shown to convert DPA to these novel bioactive products analogous to other SPM including the D-series resolvins93. These bioactive compounds are produced in PMN-endothelial co-cultures and are present in human and mouse tissues during sterile inflammation or infection93. The structures of four members of this family have recently been elucidated and contain conjugated triene and diene double bonds as well as an alcohol at C13 for which they have been termed 13-series resolvins (RvTs)93. Though their biosynthetic pathway remains to be fully detailed, it has been shown that endothelial COX-2 initiates their production during PMN-endothelial cell interactions and S-nitrosylation of the enzyme by Atorvastatin further increases their production. Conversely, selective COX-2 inhibitors diminish their formation93. Along these lines, with the widespread use of these and other therapeutics that target the inflammatory response, it is critical to underscore the importance of avoiding drugs that are “resolution toxic”10. Broad anti-inflammatory treatments are insufficient in that many of the signals produced during the inflammatory phase actually play key roles in initiating resolution (e.g., prostanoids-vide supra)94. Indeed, selective COX-2 inhibitors that are detrimental for CVD patients because they perturb production of protective prostanoids (i.e., prostacyclin), also impair resolution and resolvin production during acute inflammation, as do LOX inhibitors39. Ideal therapies to treat inflammatory diseases would be dual in nature and blunt the inflammatory response, while promoting the resolution program10.
Cellular actions of resolvins in the context of CVD
Neutrophils
As the first responders to sites of microbial invasion and tissue injury, the fundamental role of PMN as drivers of inflammation has been thoroughly described95. Their capacity as the initiators of resolution, however, is just beginning to be appreciated29, 96. In response to PGE2, PMN transition from releasing pro-inflammatory LTB4 to pro-resolving LXA4 in a process known as lipid mediator class switching (vide supra)42. PMN are also a prominent source of resolvins and thus, similar to macrophage subsets, PMN can be reprogrammed to promote inflammation or enact its resolution. LXA4 is critical in halting further PMN recruitment and was shown in isolated PMN to inhibit chemotaxis, as well as transendothelial and transepithelial migration (Fig. 3)97. As noted above, SPM (e.g., LXA4, RvE1) can also override survival signals in PMN, ensuring their timely apoptosis41, 63. Additionally, both lipoxins and resolvins act on macrophages to promote the efferocytosis of apoptotic PMN98, a process that reciprocally stimulates the production of both E and D-series resolvins, maresins and protectins thereby promoting the propagation of resolution rather than pro-inflammatory signaling (Fig. 3)96, 99. Resolvins also act directly on PMN to decrease chemotaxis toward chemokine gradients (e.g., IL-8) and to reduce the surface expression of adhesion receptors (e.g., CD11b/CD18) while preventing L-selectin (CD62L) shedding40, 58. These selective actions of SPM on PMN activation, rolling, and adhesion to the activated endothelium have been demonstrated both in vitro and in vivo using human endothelial cells under flow or intravital microscopy-based imaging of the inflamed microvasculature40, 59, 100. Accordingly, all SPM have been shown to blunt PMN infiltration into sites of acute inflammation, including bacterial peritonitis, dermal inflammation and lung inflammation25. In addition to blunting PMN activation and regulating apoptosis, SPM have also been shown to increase the expression of chemokine receptors (i.e., CCR5) on apoptotic PMN, thereby facilitating chemokine scavenging during resolution as these apoptotic cell/chemokine clusters are subsequently cleared by macrophages36. Interestingly, in concert with blunting PMN recruitment, SPM enhance PMN-mediated bacterial containment (Fig. 3), which translates into increased survival and reduced systemic inflammation in microbial sepsis, a condition that when uncontrolled, has detrimental cardiovascular implications37, 40.
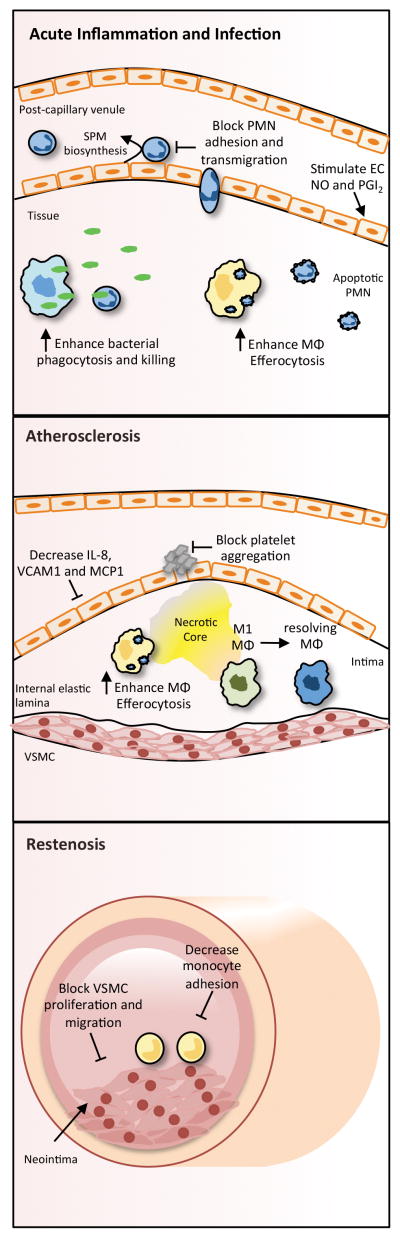
Figure 3. Biological actions of resolvins and other SPM related to cardiovascular inflammation.
During acute inflammation or infection, SPM are generated during leukocyte:endothelial interactions and directly block PMN chemotaxis. By directly targeting the endothelial cells of the vasculature (e.g., post-capillary venules), SPM also prevent the capture, rolling and adhesion of PMN to the endothelial monolayer and block their extravasation across the endothelium. Additionally, they stimulate production of nitric oxide (NO) and prostacyclin (PGI2) from endothelial cells. In the tissue, SPM promote the protective actions of leukocytes by enhancing the phagocytosis of bacteria and cellular debris and macrophage efferocytosis of apoptotic PMN. In the context of atherosclerosis, SPM decrease endothelial cell production of leukocyte chemoattractants and adhesion molecules, potentially halting further recruitment of leukocytes to the vessel wall. They also potently prevent platelet aggregation and thrombosis. In the subendothelium, enhanced macrophage efferocytosis and promotion of a resolving macrophage phenotype contributes to a more stable plaque with decreased necrosis. During pathologic inward remodeling (i.e. restenosis), SPM combat neointimal hyperplasia by inhibiting vascular smooth muscle cell (VSMC) proliferation and migration while simultaneously preventing monocyte adhesion and inflammatory signaling.
In response to a sterile ischemic injury in tissues such as the heart, PMN rapidly infiltrate as they would during an infection. During this process, however, excessive generation of pro-inflammatory mediators, as well as reactive oxygen species (ROS), are released and cause further injury to the surviving myocytes101. Therefore, promoting a timely removal of PMN is paramount in protecting the healthy myocardium during ischemia. Recently, it was demonstrated that treatment with RvD1 after MI in mice reduced accumulation of PMN in the ischemic myocardium, reduced fibrosis and improved fractional shortening, compared with untreated mice102. These actions were associated with increased SPM biosynthesis in the spleen and reduced splenic PMN numbers at day 5 post-MI. Similarly, it was previously shown that RvE1 decreases infarct size in a rat model of MI/reperfusion injury103. Pro-resolving mediators have also been described to be protective in ischemia/reperfusion injury outside the heart. In the kidney, in response to reperfusion after an ischemic insult, there is increased production of D-series resolvins and protectins. Treatment with resolvins prior to ischemia decreases PMN infiltration, tissue levels of myeloperoxidase (MPO), and tissue fibrosis, while administration shortly after reperfusion was also organ protective104. In a model of hind limb ischemia/reperfusion, resolvins protect against second-organ reperfusion injury by blocking PMN recruitment in the lung67, 105. The endogenous protective role of SPM was demonstrated in mice lacking the ALX/Fpr2 receptor, in which ischemia/reperfusion resulted in excessive leukocyte adhesion and emigration in the mesenteric microcirculation106. As noted above, there is also emerging evidence that PMN participate in distinct phases of tissue repair as well29. For instance, a very recent study demonstrated that PMN depletion impairs the development of a tissue reparative macrophage phenotype, resulting in increased apoptotic cell accumulation in the heart after MI107. Along these lines, recent studies by Gronert et al. identified a distinct tissue resident and lymphoid homing PMN subset that produces SPM to blunt activation of Th1 and Th17 T cells in a murine model of immune-driven dry eye disease108. Clearly, the multifaceted roles of PMN in inflammation, resolution and tissue repair deserve further study.
While mature human atherosclerotic plaques largely contain macrophages and T cells, recent studies have begun to elucidate the roles of PMN in the initiation and progression of atherosclerotic plaques, as well as in plaque rupture109. In humans, peripheral blood PMN counts correlate with coronary syndromes110. Initiation of atherosclerosis is marked by sub-endothelial retention of LDL and endothelial activation at sites of disturbed flow11. This dysfunction is accompanied by the upregulation of adhesion molecules and production of chemokines by endothelial cells (EC) and leukocytes (e.g., interleukin 8, C-C chemokine ligand 3), which subsequently recruit PMN (in addition to other leukocytes; vide infra) to the vessel wall. In mice, atherosclerotic lesion size is positively correlated with circulating PMN levels and conversely smaller lesions with PMN depletion111. Importantly, recruitment of monocytes is diminished in the absence of PMN, which is due in part to PMN-derived granule protein, cathelicidin112. Diabetes exacerbates atherosclerosis in part by increasing PMN production of S100A8/S100A9, which leads to enhanced myelopoiesis and monocyte accumulation in lesions113. Moreover, a recent study found that cholesterol crystals active PMN to release neutrophil extracellular traps (NETs), which amplify immune cell activation in atherosclerosis109, 114. As noted above, PMN are a rich source 5-LOX-derived LTB4, which promotes further leukocyte recruitment. Mice lacking the LTB4 receptor, BLT1 have a significant reduction in plaque formation and macrophage recruitment115 as did mice that received bone marrow transplants with genetically reduced levels of 5-LOX116. Importantly, SPM including RvD1 block LTB4-stimulated expression of adhesion molecules and actin polymerization in PMN, and the E-series resolvins block activation of BLT1 by LTB458, 66. PMN have also been linked with latter stages of atherosclerosis, including plaque destabilization. Plaques that are highly inflamed will continue to attract PMN to infiltrate, and the pro-inflammatory mediators and oxidants they produce may contribute to endothelial erosion and rupture of the fibrous cap. While most studies have primarily focused on other cell types (i.e., macrophages, vascular smooth muscle cells) to combat atherosclerosis, targeting PMN may offer valuable insights into halting the initiation or the eventual rupture of a plaque. All of these aforementioned features are indicative of defective resolution. In this context, it has previously been shown that RvE1 reduces PMN activation and tissue destruction in periodontitis, which is a risk factor for atherosclerosis117. In rabbits, periodontitis increases atherosclerosis and administration of RvE1 simultaneously decreases both periodonitits and atherosclerosis118. Further studies revealed that RvE1 reduces atherosclerosis even in absence of periodontitis, which was associated with a reduction in systemic levels of C-reactive protein (CRP). Interestingly, platelet:PMN aggregates formed during inflammation lead to SPM biosynthesis, which have been shown to reduce human platelet:PMN aggregates119, 120. Given that platelet:PMN aggregates contribute to plaque inflammation121, these results highlight that SPM may have additional protective roles in regulating PMN activation during inflammation associated with atherosclerosis.
Macrophages
Both tissue macrophages and monocytes that differentiate into macrophages after being recruited to the site of injury biosynthesize and respond to SPM during resolution. Several SPM, including E-series and D-series resolvins potently enhance macrophage phagocytosis of bacteria and efferocytosis, essential functions fundamental to host-defense and resolution (Fig. 3)25. Stimulation of phagocytosis by SPM is at least partially dependent upon downstream activation of Akt, PI3K and ERK, and involves cytoskeletal remodeling associated with activation of Rho-GTPases (i.e., Cdc42, RhoA, Rac) in a receptor-dependent manner 65, 122–125. Interestingly, during efferocytosis, SPM (e.g., RvD1, RvD2, RvE2) production increases, which potentiates further clearance of debris and apoptotic cells in an autocrine and paracrine manner39, 99. However, in several chronic inflammatory diseases (e.g., asthma, obesity/diabetes), SPM production is disrupted and these conditions are also associated with defective resolution and efferocytosis81. Indeed, in diabetic wounds, apoptotic cells accumulate and are associated with defective wound healing126. RvD1 enhances wound healing in diabetic mice in part by enhancing efferocytosis125. The delayed clearance of apoptotic cells in the thymus was also rescued by RvD1 and in isolated diabetic macrophages, RvD1 rescues defective phagocytosis in a receptor (ALX/Fpr2) and PI3K-dependent manner125. The specific mechanisms underlying impaired SPM production and efferocytosis in these diseases are incompletely understood. It is known however that the cellular location of the key 5-LOX enzyme plays an important role in dictating the predominant lipid mediators that macrophages produce. As noted above, RvD1 promotes cytoplasmic localization of 5-LOX (rather than nuclear) and shifts the production in favor of pro-resolving LXA4 rather than the pro-inflammatory LTB456. More recently, the mechanism of this nuclear localization was found to be governed by signaling through the myeloid-epithelial-reproductive tyrosine kinase (MerTK) receptor, mechanistically coupling efferocytosis to SPM biosynthesis127. In mice genetically engineered to resist cleavage (and therefore inactivation) of this receptor, SPM biosynthesis is increased and resolution of acute inflammation and efferocytosis are improved127. In acute MI, mice deficient in efferocytosis receptors, Mertk and/or Mfge8, have decreased cardiac function and remodeling that was specifically due to abrogation of efferocytosis and not to an alteration in the circulating or tissue resident macrophages, nor the ability of monocytes to infiltrate the tissue128, 129. It is, therefore, reasonable to consider that defective efferocytosis in CVD could be related to defective production of SPM, but that these alterations could be potentially rescued by exogenous administration of SPM. Whether there is a causal relationship between SPM production and efferocytosis in the heart during MI requires further study.
Defective efferocytosis has also been implicated as a crucial underlying contributor to the pathophysiology of atherosclerosis22. Macrophages play essential roles in all aspects of the life of an atherosclerotic plaque (Fig. 3). They are important both in the pathology of the lesion from initiation of formation to expansion, necrosis and eventual rupture, as well as in the resolution and regression of lesions3, 130. Monocytes infiltrate the subendothelium of the arterial wall in response to inflammatory cytokines. Once in the plaque environment, monocytes differentiate into macrophages and attempt to clear excess oxidized lipoproteins and cholesterol from the tissue22. At this early stage of lesion development efferocytosis seems to function normally and the majority of plaques do not progress to a vulnerable state. In the plaques that do progress, however, both key events of resolution (cessation of additional immune cell entry and efferocytosis) are impaired22. This precipitates the generation of lipid-laden foam cells that fail to clear from the plaque, continue to secrete pro-inflammatory mediators and eventually undergo post-apoptotic secondary necrosis22. Atherosclerosis could, therefore, be viewed as a state of failed resolution of inflammation and defective clearance of plaque macrophages may underlie the progression of advanced atherosclerotic lesions, characterized by macrophage necrosis (Fig. 3)22. In a mouse model of diabetic atherosclerosis, isolated peritoneal macrophages were shown to have impaired phagocytosis and in atherosclerotic lesions, there was defective macrophage efferocytosis that was reversed by a fish-oil diet rich in SPM precursors, EPA and DHA21. Interestingly, it was also recently shown that RvD1, acting through ALX/FPR2, protects macrophages from oxidative stress-induced apoptosis during efferocytosis, in part by regulating NADPH oxidase activation and expression of apoptotic proteins, Bcl-xL and Bcl-2131. In the context of atherosclerotic lesions, the ability of macrophages to survive and continue to phagocytose debris despite the highly oxidative environment is critically important. Furthermore, atherosclerotic apoE-null mice lacking SPM biosynthetic enzyme 12/15-LOX display exacerbated lesion formation compared with apoE-null mice and macrophage-specific overexpression of 12/15-LOX protects against lesion development132. Macrophages isolated from these mice produce higher amounts of SPM132. Similarly, in rabbit models of atherosclerosis, it was shown that macrophage-specific overexpression of 15-LOX is atheroprotective, resulting in decreased lesion area133. It should be noted that these enzymes produce both pro-inflammatory and pro-resolving mediators in a temporal manner and both atheroprotective and atherogenic roles of this enzyme have been reported134. However, as noted above, RvE1 used as an oral/topical agent decreases aortic atherogenesis, the intima/media ratio, and inflammatory cell infiltration into plaques118. Additionally, recent studies documented that the ALX/FPR2 receptor has an endogenous anti-atherosclerotic role and that selective targeting of this receptor improves lesion stability and reduces lesion necrosis in mice with pre-existing atherosclerosis135, 136. These results collectively demonstrate that chronic macrophage-mediated inflammation in atherosclerosis may be due in part to a failure of endogenous resolution programs.
In addition to macrophage efferocytosis, the phenotype of both monocytes and macrophages is an important determinant of resolution (Fig. 3). Recruited by specific chemokines (e.g., C-C chemokine ligand 2; CCL2) in the injured myocardium or atherosclerotic lesions, inflammatory Ly6Chigh monocytes infiltrate the tissue shortly after the initial PMN wave, as occurs during any normal acute inflammatory response101. After this first phase of recruitment, monocytes can differentiate into Ly6Clow monocytes/macrophages to facilitate tissue repair in a process dependent in part upon the transcription factor, Nr4a1137. These monocytes/macrophages facilitate repair because they are a rich source of growth factors and cytokines that promote tissue vascularization and matrix remodeling, while inflammatory monocytes/macrophages secrete inflammatory cytokines (e.g., IL-1β, IL-6, TNF-α) and are highly microbicidal138. Too many Ly6Chigh cells or a delayed switch into reparative macrophages can lead to excessive proteolysis of the healthy myocardium surrounding the infarct, while increased production of IL-1β enhances proliferation of hematopoietic stem and progenitor cells to promote leukocytosis139, 140. In addition, a population of embryonic-derived resident cardiac macrophages may contribute to homeostasis, as well as tissue repair in the heart during injury, and can be replaced by circulating monocytes and through local proliferation17, 141. Similar to the heart, the phenotype of lesional macrophages in atherosclerosis has become an important indicator of the overall plaque environment. Macrophages have traditionally been lumped into general classically-activated (M1) or alternatively-activated (M2) designations based largely on in vitro model systems in which macrophages are polarized with LPS/IFNγ or IL-4, respectively, although this nomenclature has been revised because macrophages largely exist in a phenotype spectrum dependent upon the local milieu16. This is true for disease states like atherosclerosis, where distinct M1 vs. M2 populations are difficult to discern130. With this inherent plasticity, agents that induce pro-resolving characteristics in macrophages may be beneficial in breaking the feed forward pro-inflammatory cycle occurring in the lesion. Notably, SPM biosynthetic enzyme, ALOX15, is highly induced in macrophages stimulated with IL-4 and these IL-4 stimulated macrophages make more SPM overall than LPS/IFNγ-polarized macrophages99. SPMs have also been implicated in promoting a switch from an inflammatory macrophage phenotype to a tissue reparative phenotype. For instance, it was recently shown that RvD1 blunts macrophage chemotaxis toward CCL2, while enhancing phagocytic function. In human macrophages, RvD1 potently blocks production of pro-inflammatory cytokines (e.g., IL-1β, IL-8 and CCL2) in a GPR32-dependent manner, which could help to prevent further recruitment of inflammatory monocytes142. In inflamed obese adipose tissue, RvD1 decreases the proportion of inflammatory macrophages and enhances macrophage phagocytosis and expression of arginase 1143, 144. In isolated macrophages, RvD1 increases expression of Arg1, Ym1, IL-10 and CD206, typical of an anti-inflammatory macrophage phenotype143. RvD2 similarly increases surface expression of CD206, as well as CD163, in human macrophages in a GPR18-dependent manner67. In murine peritonitis, both RvE1 and RvD1 have been shown to decrease CD11b expression on macrophages. CD11blow macrophages have been reported to express lower levels of iNOS, COX-2 and MMP-9 and engulf more apoptotic cells compared with CD11bhigh macrophages145. There have also been reports of a previously undescribed resolution-phase macrophage (rM) subset with similar features of the CD11blow population and possessing a hybrid of the M1/M2 phenotypes (M2: mannose receptor expression, IL-10, TGF-β and arginase 1; M1: high iNOS and COX-2 expression)146. These rM also express Alox15, indicating that they may contribute to SPM production during resolution. As critical regulators of the resolution program, targeting the actions of macrophages may be an effective strategy to address chronic maladaptive inflammation in CVD.
Endothelial cells
As the interface between the blood and surrounding tissues, the vascular endothelium plays an important early role in the inflammatory response by regulating the passage of macromolecules and immune cells across the vessel wall. As such, the temporal regulation of endothelial permeability and activation is a critical determinant of resolution of the inflammatory response. Indeed, CVDs such as atherosclerosis are associated with EC dysfunction and chronic vascular inflammation11. Analogous to the dynamic regulation of leukocytes during resolution, EC activation is a self-limited process that is uniquely positioned to control the magnitude and duration of the acute inflammatory response. Indeed, continued EC activation, characterized by expression of specific leukocyte adhesion receptors such as vascular cell adhesion molecule 1 (VCAM-1), is associated with chronic inflammatory diseases such as atherosclerosis and diabetes11. Accordingly, EC participate in the local biosynthesis of SPM during acute inflammation and are also direct cellular targets of SPM (Fig. 3). As noted above, the biosynthesis of most SPM occurs during cell:cell interactions in which the biosynthetic intermediates, such as 18-HEPE, can be generated in EC and transformed by adhering leukocytes expressing 5-LOX to generate SPM such as RvE144, 45. Furthermore, it was in the co-culture of human PMN and EC that the novel 13-series resolvins (RvTs) were recently identified93. A recent study documented that RvD1 and RvE1 are also biosynthesized during co-culture of choroid-retinal (CR) EC with leukocytes (both PMN and monocytes), but not by CREC alone147. This mechanism likely serves as a local circuit to ensure timely termination of leukocyte adhesion to EC. Indeed, EC express the known receptors for SPM, including ALX/FPR2, GPR32 and GPR1858, 148, 149. Acting through these receptors, SPM regulate both recruitment and adhesion of leukocytes. AA-derived LXA4 downregulates VCAM-1 and additional adhesion receptors, E-selectin150 and ICAM-1151, 152, but also increases nitric oxide (NO) and prostacyclin (PGI2) that potently counter-regulate leukocyte:EC interactions and platelet activation153, 154. Lipoxins have further EC-specific effects including the downregulation of NADPH oxidase, which results in the decreased generation of ROS155. Similarly, resolvins have also been shown to regulate leukocyte:EC interactions. For instance, RvD2 limits leukocyte:EC adhesion in a NO-dependent manner and the regulation of PMN recruitment during microbial peritonitis by RvD2 is attenuated in mice deficient in endothelial nitric oxide synthase (eNOS)40. Both RvD1 and RvD2 limit PMN interaction with human EC pre-stimulated with inflammatory cytokines (e.g., TNFα, IL-1β) under shear conditions at a physiological flow rate in a receptor-specific manner40, 59. In this context, resolvins block PMN capture, rolling and firm adhesion to EC and they attenuate EC production of chemokines (e.g., IL-8 and CCL2) that recruit additional leukocytes132, 147. A recent study of the mechanisms whereby resolvins regulate inflammatory signaling in human EC uncovered that D-series resolvins stimulate the activation of PI3K and Akt in a receptor-dependent manner and thereby regulate the production of developmental endothelial locus 1 (Del-1), an anti-inflammatory protein secreted by EC that prevents PMN transmigration156.
In addition to regulating EC-dependent adhesion of leukocytes during inflammation, resolvins also regulate permeability of the microvasculature. In vitro, RvD1 protects against endotoxin-induced impairment of barrier function in human EC157 and aspirin-triggered-RvD1 enhances the restitution of endothelial barrier function following acute lung injury in vivo158. These results build on prior studies showing that lipoxins counter-regulate leukocyte-mediated microvascular permeability in vivo during acute inflammation stimulated by pro-inflammatory lipid mediators159. Permeability of the microvasculature is increased by several inflammatory mediators that also regulate angiogenesis. Along these lines, LXA4 decreases vascular endothelial growth factor receptor 2 (VEGFR-2) phosphorylation and its downstream signaling in human EC, thereby blocking VEGF-induced angiogenesis152. Similarly, in a model of murine retinal neovascularization, administration of exogenous SPM (LXA4, RvD1, RvE1 and PD1) protects against pathologic angiogenesis69, 160, 161. This is in contrast to some pro-inflammatory lipid mediators (i.e., 12-HETE and PGE2) that promote angiogenesis162, 163. Collectively, EC have emerged as important cellular targets of SPM during resolution, suggesting that engagement of SPM receptors might offer a new approach to reversing EC dysfunction that is associated with CVD. Future studies will be required to understand fully the role of SPM in EC biology and whether dysfunctional EC in chronic diseases is associated with deficits in resolution circuits at the level of EC:leukocyte interactions.
Vascular smooth muscle cells
In addition to their physiological role in regulating blood vessel tone, vascular smooth muscle cells (VSMC) also contribute to the pathophysiology of atherosclerosis and restenosis. In response to vascular injury, VSMC undergo a phenotype switch from a contractile to a synthetic phenotype; synthetic VSMC are more proliferative and chemotactic and are characterized by a decreased expression of contractile proteins and increased production of pro-inflammatory mediators164. During chronic vascular inflammation (e.g., atherosclerosis) this pro-inflammatory environment can lead to successive contractile to synthetic phenotype switching thereby propagating the pro-inflammatory environment. Moreover, VSMC can assume a phenotype similar to macrophages and express macrophage markers, such as CD68 and F4/80 in lesions164. Interruption of this pro-inflammatory signaling cycle may, therefore, be a useful strategy to combat inflammatory diseases of the vessel wall. Receptors for SPM, including ALX/FPR2 and ChemR23, are expressed in human saphenous vein SMC and administration of RvE1 and 15-epi-LXA4 counter-regulate platelet-derived growth factor (PDGF)-stimulated VSMC migration in a dose-dependent manner (Fig. 3)165. These actions are due in part to direct regulation of PDGF receptor phosphorylation. Building on these in vitro studies, it was recently found that 15-epi-LXA4 inhibits VSMC migration and intimal hyperplasia following murine carotid artery ligation and this protection afforded by 15-epi-LXA4 is abolished in mice lacking the ALX/Fpr2 receptor166. Interestingly, genetic deficiency of ALX/Fpr2 decreases stability of atherosclerotic lesions, effects that were attributed to decreased collagen production by VSMC167. Given that VSMC can also contribute to stability of atherosclerotic lesions164 and that it is their phenotype that dictates their contribution to atherosclerosis, these results suggest that SPM may regulate the balance of VSMC phenotypes important for regulating lesion stability. More recently, it was demonstrated that RvD1 and RvD2 inhibit proliferation, migration, monocyte adhesion, superoxide production and pro-inflammatory gene expression in human168, rat169 and mouse170 VSMC. Similar results were observed with another SPM, maresin 1 (Mar1)171. In a rabbit model of balloon angioplasty, local delivery of RvD2 reduces cell proliferation, leukocyte recruitment and neointimal hyperplasia168. Interestingly, RvD2 and Mar1 increase M2 macrophage polarization during vascular injury, which may help to promote vessel remodeling and repair170. Additionally, RvD1, delivered perivascularly via a novel biodegradable wrap reduces carotid artery neointimal formation without promoting thrombosis after carotid angioplasty in rats169. In a model of human pulmonary artery hypertension, RvD1 and RvE1 are able to normalize arterial hyper-reactivity induced by pro-inflammatory mediators172, 173. These results add further support to the concept that most CVDs are driven by abhorrent inflammation and that stimulating resolution of inflammation could potentially restore physiological function of the vasculature.
Platelets
In response to blood vessel damage, platelets are rapidly activated and are a critical component of the coagulation process that serves to re-establish a physical barrier and prevent blood loss. This process is tightly coupled with the inflammatory program and like the regulation of leukocyte trafficking, platelet activation must be tightly controlled to prevent further damage to the host. Platelet aggregation and ensuing thrombosis are significant vascular events that are linked to inflammation and atherogenesis and causative to the clinical manifestations of atherosclerosis, namely stroke and MI174. In a randomized clinical trial involving healthy subjects receiving aspirin, pro-thrombotic thromboxane was reduced and there was a concomitant increase in 15-epi LXA4175, 176. Plasma levels of 15-epi-LXA4 were increased most significantly with low dose aspirin (i.e., 81mg), the currently recommended dose for patients with CVD. Interestingly, human platelets express ChemR23 and RvE1 blocks ADP and thromboxane-stimulated platelet aggregation (Fig. 3)100, 177. Further mechanistic studies revealed that RvE1 blocks ADP-mediated signals downstream of its receptor, P2Y12, and that the effects of RvE1 are ChemR23-dependent177. In a mouse burn wound model, exogenous systemic administration of RvD2 prevents thrombosis of the deep dermal vascular network and subsequent dermal necrosis. Treatment with RvD2 allowed enhanced neutrophil access to the dermis while preventing neutrophil-mediated damage due to an abrogation of pro-inflammatory mediator secretion178. Lastly, it was very recently shown that SPM increase macrophage uptake of blood clots in vitro, indicating that SPM may play a role in clot remodeling during resolution80. As noted above, SPM are biosynthesized during interactions of platelets and leukocytes during inflammation. Thus, these results collectively demonstrate that SPM may play key roles in regulating the resolution of thrombosis during inflammation, which could have important therapeutic implications for CVD.
Summary and future directions
A significant effort has been dedicated to the structural elucidation of pro-resolving mediators and the identification of their receptors9. It is becoming apparent that alterations in specific resolution pathways are associated with a wide range of chronic diseases. Chronic unresolved inflammation is now widely documented to play a causal role in the development of CVD. As such, a large effort is underway to determine whether targeting specific pro-inflammatory pathways could be beneficial for clinical management of CVD beyond the current standard of care (e.g., aspirin, statins, anti-platelet therapies)4. In contrast to traditional anti-inflammatory strategies that blunt the production of inflammation “initiators” and could potentially lead to immunosuppression, pro-resolving mediators described in this review activate distinct cellular programs to resolve inflammation without compromising host-defense9. In fact, pro-resolving mediators enhance host defense to both viral and bacterial infections and lower the threshold for antibiotic therapy37, 179. Thus, new strategies to promote resolution of inflammation may offer unique opportunities to combat chronic inflammation associated with CVD. Pro-resolving mediators blunt excessive inflammation that can be detrimental to healthy tissues, particularly in sterile inflammation, and they also stimulate distinct processes necessary for tissue repair and regeneration. They have multiple cellular targets in the inflammatory response, including immune cells, platelets and vascular cells. Collectively, the structural elucidation of these mediators and identification of the signaling pathways that they engage could inform the development of therapeutic strategies encompassing a novel “resolution pharmacology” approach. Indeed, clinical trials with pro-resolving mediators in humans with other inflammatory pathologies are currently underway180.
In regards to therapeutically stimulating resolution of inflammation in CVD, there are several things to consider. First, can SPM be effectively targeted to sites of local inflammation? In a rabbit model of atherosclerosis, delivery of RvE1 to the oral cavity decreased lesion formation in the aorta, suggesting that resolvins can get absorbed and have systemic effects118. Our group has also shown that microparticles released from activated immune cells carry SPM and as a form of biomimicry, we formulated humanized nanoparticles that showed enhanced bioactivity; these could potentially be vehicles for targeted delivery181. Moreover, recent studies have begun to highlight other methodologies for local sustained SPM delivery (e.g., vascular wraps) that could be used in vascular surgery for example169. Second, what are the mechanisms underlying failed resolution of inflammation? As noted in this review, several chronic diseases are associated with lower levels of SPM. Does this occur because of altered biosynthesis or enhanced metabolic inactivation? How are SPM receptors modulated in chronic diseases like CVD? Lastly, are nutritional based approaches (e.g., omega-3 PUFA) sufficient to promote resolution, or are there contexts in which resolution is perturbed regardless of substrate availability? These are all important questions for future work in the area and given that the field of “resolution physiology” is relatively new, an understanding of the mechanisms underlying failed resolution of inflammation in CVD is likely to be a fruitful area of future investigation.
Acknowledgments
Sources of Funding
The authors acknowledge the support of NIH grants HL106173 and GM095467.
Non-standard Abbreviations and Acronyms
- LXA4
lipoxin A4, 5S,6R,15S-trihydroxy-7,9,13-trans-11-cis-eicosatetraenoic acid
- LXB4
lipoxin B4, 5S,14R,15S-trihydroxy-6,10,12-trans-8-cis-eicosatetraenoic acid
- RvD1
resolvin D1, 7S,8R,17S-trihydroxy-docosa-4Z,9E,11E,13Z,15E,19Z-hexaenoic acid
- RvD2
resolvin D2, 7S,16R,17S-trihydroxy-docosa-4Z,8E,10Z,12E,14E,19Z-hexaenoic acid
- RvE1
resolvin E1, 5S,12R,18R-trihydroxy-eicosa-6Z,8E,10E,14Z,16E-pentaenoic acid
- RvE2
resolvin E2, 5S,18R-dihydroxy-eicosa-6E,8Z,11Z,14Z,16E-pentaenoic acid
- AA
arachidonic acid
- EPA
eicosapentaenoic acid
- DHA
docosahexaenoic acid
- SPM
specialized pro-resolving lipid mediators
- LOX
lipoxygenase
- COX
cyclooxygenase
- PMN
polymorphonuclear neutrophils
- PUFA
polyunsaturated fatty acids
- CVD
cardiovascular disease
- EC
endothelial cells
- VSMC
vascular smooth muscle cells
Footnotes
Disclosures
None
References
- 1.Libby P, Nahrendorf M, Swirski FK. Leukocytes Link Local and Systemic Inflammation in Ischemic Cardiovascular Disease: An Expanded “Cardiovascular Continuum”. J Am Coll Cardiol. 2016;67:1091–103. doi: 10.1016/j.jacc.2015.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P, Bornfeldt KE, Tall AR. Atherosclerosis: Successes, Surprises, and Future Challenges. Circ Res. 2016;118:531–4. doi: 10.1161/CIRCRESAHA.116.308334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P, Tabas I, Fredman G, Fisher EA. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res. 2014;114:1867–79. doi: 10.1161/CIRCRESAHA.114.302699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro MD, Fazio S. From Lipids to Inflammation: New Approaches to Reducing Atherosclerotic Risk. Circ Res. 2016;118:732–49. doi: 10.1161/CIRCRESAHA.115.306471. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ Res. 2016;118:145–56. doi: 10.1161/CIRCRESAHA.115.306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT) J Thromb Haemost. 2009;7(Suppl 1):332–9. doi: 10.1111/j.1538-7836.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 7.Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA, Glynn RJ, Ridker PM. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199–207. e15. doi: 10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, Thuren T, Group CPI. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–48. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 9.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–32. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gimbrone MA, Jr, Garcia-Cardena G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res. 2016;118:620–36. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–75. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 13.Granger DN, Rodrigues SF, Yildirim A, Senchenkova EY. Microvascular responses to cardiovascular risk factors. Microcirculation. 2010;17:192–205. doi: 10.1111/j.1549-8719.2009.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 2014;40:315–27. doi: 10.1016/j.immuni.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–80. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frangogiannis NG. Inflammation in cardiac injury, repair and regeneration. Curr Opin Cardiol. 2015;30:240–5. doi: 10.1097/HCO.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen HB, Mosser DM. Extrinsic and intrinsic control of macrophage inflammatory responses. J Leukoc Biol. 2013;94:913–9. doi: 10.1189/jlb.0413236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016 doi: 10.1038/nrd.2016.39. [DOI] [PubMed] [Google Scholar]
- 20.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4:211–22. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Sun Y, Liang CP, Thorp EB, Han S, Jehle AW, Saraswathi V, Pridgen B, Kanter JE, Li R, Welch CL, Hasty AH, Bornfeldt KE, Breslow JL, Tabas I, Tall AR. Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of ob/ob mice and reversal by a fish oil diet. Circ Res. 2009;105:1072–82. doi: 10.1161/CIRCRESAHA.109.199570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–19. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 24.Perretti M. The resolution of inflammation: New mechanisms in patho-physiology open opportunities for pharmacology. Semin Immunol. 2015;27:145–8. doi: 10.1016/j.smim.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin Immunol. 2015;27:200–15. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Headland SE, Norling LV. The resolution of inflammation: Principles and challenges. Semin Immunol. 2015;27:149–60. doi: 10.1016/j.smim.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Gilroy D, De Maeyer R. New insights into the resolution of inflammation. Semin Immunol. 2015;27:161–8. doi: 10.1016/j.smim.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Crean D, Godson C. Specialised lipid mediators and their targets. Semin Immunol. 2015;27:169–76. doi: 10.1016/j.smim.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Colgan SP. Neutrophils and inflammatory resolution in the mucosa. Semin Immunol. 2015;27:177–83. doi: 10.1016/j.smim.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viola J, Soehnlein O. Atherosclerosis - A matter of unresolved inflammation. Semin Immunol. 2015;27:184–93. doi: 10.1016/j.smim.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Haworth O, Buckley CD. Pathways involved in the resolution of inflammatory joint disease. Semin Immunol. 2015;27:194–9. doi: 10.1016/j.smim.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Montero-Melendez T. ACTH: The forgotten therapy. Semin Immunol. 2015;27:216–26. doi: 10.1016/j.smim.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Wallace JL, Ianaro A, Flannigan KL, Cirino G. Gaseous mediators in resolution of inflammation. Semin Immunol. 2015;27:227–33. doi: 10.1016/j.smim.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–82. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 35.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–7. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 36.Ariel A, Fredman G, Sun YP, Kantarci A, Van Dyke TE, Luster AD, Serhan CN. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol. 2006;7:1209–16. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–8. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalli J, Ramon S, Norris PC, Colas RA, Serhan CN. Novel proresolving and tissue-regenerative resolvin and protectin sulfido-conjugated pathways. FASEB J. 2015;29:2120–36. doi: 10.1096/fj.14-268441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–74. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–91. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Kebir D, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc Natl Acad Sci U S A. 2012;109:14983–8. doi: 10.1073/pnas.1206641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–9. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 43.Serhan CN, Hamberg M, Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci U S A. 1984;81:5335–9. doi: 10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–87. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 48.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–22. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest. 2011;121:569–81. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, Arita M, Serhan CN. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, Serhan CN. Resolvin E2 formation and impact in inflammation resolution. J Immunol. 2012;188:4527–34. doi: 10.4049/jimmunol.1103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, Taguchi R, Masuda K, Sasaki K, Urabe D, Inoue M, Arai H. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J Biol Chem. 2012;287:10525–34. doi: 10.1074/jbc.M112.340612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282:9323–34. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 54.Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, Chiang N, Petasis NA, Serhan CN. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol. 2013;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winkler JW, Orr SK, Dalli J, Cheng CY, Sanger JM, Chiang N, Petasis NA, Serhan CN. Resolvin D4 stereoassignment and its novel actions in host protection and bacterial clearance. Sci Rep. 2016;6:18972. doi: 10.1038/srep18972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fredman G, Ozcan L, Spolitu S, Hellmann J, Spite M, Backs J, Tabas I. Resolvin D1 limits 5-lipoxygenase nuclear localization and leukotriene B4 synthesis by inhibiting a calcium-activated kinase pathway. Proc Natl Acad Sci U S A. 2014;111:14530–5. doi: 10.1073/pnas.1410851111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dalli J, Chiang N, Serhan CN. Identification of 14-series sulfido-conjugated mediators that promote resolution of infection and organ protection. Proc Natl Acad Sci U S A. 2014;111:E4753–61. doi: 10.1073/pnas.1415006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–5. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arterioscler Thromb Vasc Biol. 2012;32:1970–8. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Devchand PR, Arita M, Hong S, Bannenberg G, Moussignac RL, Gronert K, Serhan CN. Human ALX receptor regulates neutrophil recruitment in transgenic mice: roles in inflammation and host defense. FASEB J. 2003;17:652–9. doi: 10.1096/fj.02-0770com. [DOI] [PubMed] [Google Scholar]
- 61.Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, Murphy PM International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61:119–61. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJ, Flower RJ, Perretti M. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci U S A. 2013;110:18232–7. doi: 10.1073/pnas.1308253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El Kebir D, Jozsef L, Khreiss T, Pan W, Petasis NA, Serhan CN, Filep JG. Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: a novel mechanism for resolution of inflammation. J Immunol. 2007;179:616–22. doi: 10.4049/jimmunol.179.1.616. [DOI] [PubMed] [Google Scholar]
- 64.Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, Hendrick AG, Carlton MB, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med. 2008;205:767–75. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem. 2010;285:3451–61. doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–7. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 67.Chiang N, Dalli J, Colas RA, Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med. 2015;212:1203–17. doi: 10.1084/jem.20150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, Serhan CN, Kang JX. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci U S A. 2006;103:11276–81. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N, Jr, Serhan CN, Smith LE. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–73. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851:469–84. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 71.De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–50. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 72.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 73.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G, Gissi HFI. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–30. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 74.Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol. 2014;307:C39–54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barden A, Mas E, Croft KD, Phillips M, Mori TA. Short-term n-3 fatty acid supplementation but not aspirin increases plasma proresolving mediators of inflammation. J Lipid Res. 2014;55:2401–7. doi: 10.1194/jlr.M045583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin Chem. 2012;58:1476–84. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- 77.Barden AE, Mas E, Mori TA. n-3 Fatty acid supplementation and proresolving mediators of inflammation. Curr Opin Lipidol. 2016;27:26–32. doi: 10.1097/MOL.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 78.Grenon SM, Owens CD, Nosova EV, Hughes-Fulford M, Alley HF, Chong K, Perez S, Yen PK, Boscardin J, Hellmann J, Spite M, Conte MS. Short-Term, High-Dose Fish Oil Supplementation Increases the Production of Omega-3 Fatty Acid-Derived Mediators in Patients With Peripheral Artery Disease (the OMEGA-PAD I Trial) J Am Heart Assoc. 2015;4:e002034. doi: 10.1161/JAHA.115.002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barden AE, Moghaddami M, Mas E, Phillips M, Cleland LG, Mori TA. Specialised pro-resolving mediators of inflammation in inflammatory arthritis. Prostaglandins Leukot Essent Fatty Acids. 2016;107:24–9. doi: 10.1016/j.plefa.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 80.Elajami TK, Colas RA, Dalli J, Chiang N, Serhan CN, Welty FK. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J. 2016 doi: 10.1096/fj.201500155R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spite M, Claria J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014;19:21–36. doi: 10.1016/j.cmet.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roberts LJ, 2nd, Montine TJ, Markesbery WR, Tapper AR, Hardy P, Chemtob S, Dettbarn WD, Morrow JD. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 1998;273:13605–12. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- 83.Arnardottir H, Orr SK, Dalli J, Serhan CN. Human milk proresolving mediators stimulate resolution of acute inflammation. Mucosal Immunol. 2016;9:757–66. doi: 10.1038/mi.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sasaki A, Fukuda H, Shiida N, Tanaka N, Furugen A, Ogura J, Shuto S, Mano N, Yamaguchi H. Determination of omega-6 and omega-3 PUFA metabolites in human urine samples using UPLC/MS/MS. Anal Bioanal Chem. 2015;407:1625–39. doi: 10.1007/s00216-014-8412-5. [DOI] [PubMed] [Google Scholar]
- 85.Murphy RC. Specialized pro-resolving mediators: do they circulate in plasma? J Lipid Res. 2015;56:1641–2. doi: 10.1194/jlr.C062356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marchioli R, Schweiger C, Tavazzi L, Valagussa F. Efficacy of n-3 polyunsaturated fatty acids after myocardial infarction: results of GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico. Lipids. 2001;36(Suppl):S119–26. doi: 10.1007/s11745-001-0694-8. [DOI] [PubMed] [Google Scholar]
- 87.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003;110:255–8. doi: 10.1016/s0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 88.Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92:9475–9. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Demetz E, Schroll A, Auer K, Heim C, Patsch JR, Eller P, Theurl M, Theurl I, Theurl M, Seifert M, Lener D, Stanzl U, Haschka D, Asshoff M, Dichtl S, Nairz M, Huber E, Stadlinger M, Moschen AR, Li X, Pallweber P, Scharnagl H, Stojakovic T, Marz W, Kleber ME, Garlaschelli K, Uboldi P, Catapano AL, Stellaard F, Rudling M, Kuba K, Imai Y, Arita M, Schuetz JD, Pramstaller PP, Tietge UJ, Trauner M, Norata GD, Claudel T, Hicks AA, Weiss G, Tancevski I. The arachidonic acid metabolome serves as a conserved regulator of cholesterol metabolism. Cell Metab. 2014;20:787–98. doi: 10.1016/j.cmet.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 91.Birnbaum Y, Ye Y, Lin Y, Freeberg SY, Nishi SP, Martinez JD, Huang MH, Uretsky BF, Perez-Polo JR. Augmentation of myocardial production of 15-epi-lipoxin-a4 by pioglitazone and atorvastatin in the rat. Circulation. 2006;114:929–35. doi: 10.1161/CIRCULATIONAHA.106.629907. [DOI] [PubMed] [Google Scholar]
- 92.Planaguma A, Pfeffer MA, Rubin G, Croze R, Uddin M, Serhan CN, Levy BD. Lovastatin decreases acute mucosal inflammation via 15-epi-lipoxin A4. Mucosal Immunol. 2010;3:270–9. doi: 10.1038/mi.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dalli J, Chiang N, Serhan CN. Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat Med. 2015;21:1071–5. doi: 10.1038/nm.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 95.Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood. 2014;124:710–9. doi: 10.1182/blood-2014-03-453217. [DOI] [PubMed] [Google Scholar]
- 96.Jones HR, Robb CT, Perretti M, Rossi AG. The role of neutrophils in inflammation resolution. Semin Immunol. 2016 doi: 10.1016/j.smim.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 97.Fierro IM, Colgan SP, Bernasconi G, Petasis NA, Clish CB, Arita M, Serhan CN. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit human neutrophil migration: comparisons between synthetic 15 epimers in chemotaxis and transmigration with microvessel endothelial cells and epithelial cells. J Immunol. 2003;170:2688–94. doi: 10.4049/jimmunol.170.5.2688. [DOI] [PubMed] [Google Scholar]
- 98.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–7. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 99.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, Cheng G, von Andrian UH, Serhan CN. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–55. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–6. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J Mol Cell Cardiol. 2015;84:24–35. doi: 10.1016/j.yjmcc.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keyes KT, Ye Y, Lin Y, Zhang C, Perez-Polo JR, Gjorstrup P, Birnbaum Y. Resolvin E1 protects the rat heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;299:H153–64. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- 104.Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–11. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 105.Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol. 2008;181:8677–87. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dufton N, Hannon R, Brancaleone V, Dalli J, Patel HB, Gray M, D’Acquisto F, Buckingham JC, Perretti M, Flower RJ. Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J Immunol. 2010;184:2611–9. doi: 10.4049/jimmunol.0903526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Weber C, Soehnlein O, Steffens S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw002. [DOI] [PubMed] [Google Scholar]
- 108.Gao Y, Min K, Zhang Y, Su J, Greenwood M, Gronert K. Female-Specific Downregulation of Tissue Polymorphonuclear Neutrophils Drives Impaired Regulatory T Cell and Amplified Effector T Cell Responses in Autoimmune Dry Eye Disease. J Immunol. 2015;195:3086–99. doi: 10.4049/jimmunol.1500610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Doring Y, Drechsler M, Soehnlein O, Weber C. Neutrophils in atherosclerosis: from mice to man. Arterioscler Thromb Vasc Biol. 2015;35:288–95. doi: 10.1161/ATVBAHA.114.303564. [DOI] [PubMed] [Google Scholar]
- 110.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–15. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 111.Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–45. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 112.Doring Y, Drechsler M, Wantha S, Kemmerich K, Lievens D, Vijayan S, Gallo RL, Weber C, Soehnlein O. Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ Res. 2012;110:1052–6. doi: 10.1161/CIRCRESAHA.112.265868. [DOI] [PubMed] [Google Scholar]
- 113.Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, Schmidt AM, Orchard TJ, Fisher EA, Tall AR, Goldberg IJ. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17:695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316–20. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Subbarao K, Jala VR, Mathis S, Suttles J, Zacharias W, Ahamed J, Ali H, Tseng MT, Haribabu B. Role of leukotriene B4 receptors in the development of atherosclerosis: potential mechanisms. Arterioscler Thromb Vasc Biol. 2004;24:369–75. doi: 10.1161/01.ATV.0000110503.16605.15. [DOI] [PubMed] [Google Scholar]
- 116.Mehrabian M, Allayee H, Wong J, Shi W, Wang XP, Shaposhnik Z, Funk CD, Lusis AJ. Identification of 5-lipoxygenase as a major gene contributing to atherosclerosis susceptibility in mice. Circ Res. 2002;91:120–6. doi: 10.1161/01.res.0000028008.99774.7f. [DOI] [PubMed] [Google Scholar]
- 117.Dave S, Van Dyke T. The link between periodontal disease and cardiovascular disease is probably inflammation. Oral Dis. 2008;14:95–101. doi: 10.1111/j.1601-0825.2007.01438.x. [DOI] [PubMed] [Google Scholar]
- 118.Hasturk H, Abdallah R, Kantarci A, Nguyen D, Giordano N, Hamilton J, Van Dyke TE. Resolvin E1 (RvE1) Attenuates Atherosclerotic Plaque Formation in Diet and Inflammation-Induced Atherogenesis. Arterioscler Thromb Vasc Biol. 2015;35:1123–33. doi: 10.1161/ATVBAHA.115.305324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abdulnour RE, Dalli J, Colby JK, Krishnamoorthy N, Timmons JY, Tan SH, Colas RA, Petasis NA, Serhan CN, Levy BD. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc Natl Acad Sci U S A. 2014;111:16526–31. doi: 10.1073/pnas.1407123111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Norris PC, Arnardottir H, Sanger JM, Fichtner D, Keyes GS, Serhan CN. Resolvin D3 multi-level proresolving actions are host protective during infection. Prostaglandins Leukot Essent Fatty Acids. 2016 doi: 10.1016/j.plefa.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gerdes N, Seijkens T, Lievens D, Kuijpers MJ, Winkels H, Projahn D, Hartwig H, Beckers L, Megens RT, Boon L, Noelle RJ, Soehnlein O, Heemskerk JW, Weber C, Lutgens E. Platelet CD40 Exacerbates Atherosclerosis by Transcellular Activation of Endothelial Cells and Leukocytes. Arterioscler Thromb Vasc Biol. 2016;36:482–90. doi: 10.1161/ATVBAHA.115.307074. [DOI] [PubMed] [Google Scholar]
- 122.Maderna P, Cottell DC, Toivonen T, Dufton N, Dalli J, Perretti M, Godson C. FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis. FASEB J. 2010;24:4240–9. doi: 10.1096/fj.10-159913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maderna P, Cottell DC, Berlasconi G, Petasis NA, Brady HR, Godson C. Lipoxins induce actin reorganization in monocytes and macrophages but not in neutrophils: differential involvement of rho GTPases. Am J Pathol. 2002;160:2275–83. doi: 10.1016/S0002-9440(10)61175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reville K, Crean JK, Vivers S, Dransfield I, Godson C. Lipoxin A4 redistributes myosin IIA and Cdc42 in macrophages: implications for phagocytosis of apoptotic leukocytes. J Immunol. 2006;176:1878–88. doi: 10.4049/jimmunol.176.3.1878. [DOI] [PubMed] [Google Scholar]
- 125.Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes. 2013;62:618–27. doi: 10.2337/db12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, Bhasker V, Gordillo GM, Sen CK, Roy S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5:e9539. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cai B, Thorp EB, Doran AC, Subramanian M, Sansbury BE, Lin C, Spite M, Fredman G, Tabas I. MerTK cleavage limits pro-resolving mediator biosynthesis and exacerbates tissue inflammation. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1524292113. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S, Iwata S, Han X, Homma S, Drosatos K, Lomasney J, Engman DM, Miller SD, Vaughan DE, Morrow JP, Kishore R, Thorp EB. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res. 2013;113:1004–12. doi: 10.1161/CIRCRESAHA.113.301198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Howangyin KY, Zlatanova I, Pinto C, Ngkelo A, Cochain C, Rouanet M, Vilar J, Lemitre M, Stockmann C, Fleischmann BK, Mallat Z, Silvestre JS. Myeloid-Epithelial-Reproductive Receptor Tyrosine Kinase and Milk Fat Globule Epidermal Growth Factor 8 Coordinately Improve Remodeling After Myocardial Infarction via Local Delivery of Vascular Endothelial Growth Factor. Circulation. 2016;133:826–39. doi: 10.1161/CIRCULATIONAHA.115.020857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tabas I, Bornfeldt KE. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ Res. 2016;118:653–67. doi: 10.1161/CIRCRESAHA.115.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee HN, Surh YJ. Resolvin D1-mediated NOX2 inactivation rescues macrophages undertaking efferocytosis from oxidative stress-induced apoptosis. Biochem Pharmacol. 2013;86:759–69. doi: 10.1016/j.bcp.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 132.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shen J, Herderick E, Cornhill JF, Zsigmond E, Kim HS, Kuhn H, Guevara NV, Chan L. Macrophage-mediated 15-lipoxygenase expression protects against atherosclerosis development. J Clin Invest. 1996;98:2201–8. doi: 10.1172/JCI119029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wittwer J, Hersberger M. The two faces of the 15-lipoxygenase in atherosclerosis. Prostaglandins Leukot Essent Fatty Acids. 2007;77:67–77. doi: 10.1016/j.plefa.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 135.Drechsler M, de Jong R, Rossaint J, Viola JR, Leoni G, Wang JM, Grommes J, Hinkel R, Kupatt C, Weber C, Doring Y, Zarbock A, Soehnlein O. Annexin A1 counteracts chemokine-induced arterial myeloid cell recruitment. Circ Res. 2015;116:827–35. doi: 10.1161/CIRCRESAHA.116.305825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fredman G, Kamaly N, Spolitu S, Milton J, Ghorpade D, Chiasson R, Kuriakose G, Perretti M, Farokhzad O, Tabas I. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci Transl Med. 2015;7:275ra20. doi: 10.1126/scitranslmed.aaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherer-Crosbie M, Hedrick CC, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114:1611–22. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Varga T, Mounier R, Horvath A, Cuvellier S, Dumont F, Poliska S, Ardjoune H, Juban G, Nagy L, Chazaud B. Highly Dynamic Transcriptional Signature of Distinct Macrophage Subsets during Sterile Inflammation, Resolution, and Tissue Repair. J Immunol. 2016;196:4771–82. doi: 10.4049/jimmunol.1502490. [DOI] [PubMed] [Google Scholar]
- 139.Sager HB, Heidt T, Hulsmans M, Dutta P, Courties G, Sebas M, Wojtkiewicz GR, Tricot B, Iwamoto Y, Sun Y, Weissleder R, Libby P, Swirski FK, Nahrendorf M. Targeting Interleukin-1beta Reduces Leukocyte Production After Acute Myocardial Infarction. Circulation. 2015;132:1880–90. doi: 10.1161/CIRCULATIONAHA.115.016160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629–38. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schmid M, Gemperle C, Rimann N, Hersberger M. Resolvin D1 Polarizes Primary Human Macrophages toward a Proresolution Phenotype through GPR32. J Immunol. 2016;196:3429–37. doi: 10.4049/jimmunol.1501701. [DOI] [PubMed] [Google Scholar]
- 143.Titos E, Rius B, Gonzalez-Periz A, Lopez-Vicario C, Moran-Salvador E, Martinez-Clemente M, Arroyo V, Claria J. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J Immunol. 2011;187:5408–18. doi: 10.4049/jimmunol.1100225. [DOI] [PubMed] [Google Scholar]
- 144.Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J. 2011;25:2399–407. doi: 10.1096/fj.10-178657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schif-Zuck S, Gross N, Assi S, Rostoker R, Serhan CN, Ariel A. Saturated-efferocytosis generates pro-resolving CD11b low macrophages: modulation by resolvins and glucocorticoids. Eur J Immunol. 2011;41:366–79. doi: 10.1002/eji.201040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bystrom J, Evans I, Newson J, Stables M, Toor I, van Rooijen N, Crawford M, Colville-Nash P, Farrow S, Gilroy DW. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood. 2008;112:4117–27. doi: 10.1182/blood-2007-12-129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tian H, Lu Y, Sherwood AM, Hongqian D, Hong S. Resolvins E1 and D1 in choroid-retinal endothelial cells and leukocytes: biosynthesis and mechanisms of anti-inflammatory actions. Invest Ophthalmol Vis Sci. 2009;50:3613–20. doi: 10.1167/iovs.08-3146. [DOI] [PubMed] [Google Scholar]
- 148.Chiang N, Arita M, Serhan CN. Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot Essent Fatty Acids. 2005;73:163–77. doi: 10.1016/j.plefa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 149.Offertaler L, Mo FM, Batkai S, Liu J, Begg M, Razdan RK, Martin BR, Bukoski RD, Kunos G. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- 150.Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Villela CG, Fierro IM. Novel lipid mediator aspirin-triggered lipoxin A4 induces heme oxygenase-1 in endothelial cells. Am J Physiol Cell Physiol. 2005;289:C557–63. doi: 10.1152/ajpcell.00045.2005. [DOI] [PubMed] [Google Scholar]
- 151.Souza MC, Padua TA, Torres ND, Souza Costa MF, Candea AP, Maramaldo T, Seito LN, Penido C, Estato V, Antunes B, Silva L, Pinheiro AA, Caruso-Neves C, Tibirica E, Carvalho L, Henriques MG. Lipoxin A4 attenuates endothelial dysfunction during experimental cerebral malaria. Int Immunopharmacol. 2015;24:400–7. doi: 10.1016/j.intimp.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 152.Baker N, O’Meara SJ, Scannell M, Maderna P, Godson C. Lipoxin A4: anti-inflammatory and anti-angiogenic impact on endothelial cells. J Immunol. 2009;182:3819–26. doi: 10.4049/jimmunol.0803175. [DOI] [PubMed] [Google Scholar]
- 153.Brezinski ME, Gimbrone MA, Jr, Nicolaou KC, Serhan CN. Lipoxins stimulate prostacyclin generation by human endothelial cells. FEBS Lett. 1989;245:167–72. doi: 10.1016/0014-5793(89)80214-5. [DOI] [PubMed] [Google Scholar]
- 154.Paul-Clark MJ, Van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med. 2004;200:69–78. doi: 10.1084/jem.20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Fierro IM. Aspirin-triggered lipoxin A4 blocks reactive oxygen species generation in endothelial cells: a novel antioxidative mechanism. Thromb Haemost. 2007;97:88–98. [PubMed] [Google Scholar]
- 156.Maekawa T, Hosur K, Abe T, Kantarci A, Ziogas A, Wang B, Van Dyke TE, Chavakis T, Hajishengallis G. Antagonistic effects of IL-17 and D-resolvins on endothelial Del-1 expression through a GSK-3beta-C/EBPbeta pathway. Nat Commun. 2015;6:8272. doi: 10.1038/ncomms9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang X, Wang T, Gui P, Yao C, Sun W, Wang L, Wang H, Xie W, Yao S, Lin Y, Wu Q. Resolvin D1 reverts lipopolysaccharide-induced TJ proteins disruption and the increase of cellular permeability by regulating IkappaBalpha signaling in human vascular endothelial cells. Oxid Med Cell Longev. 2013;2013:185715. doi: 10.1155/2013/185715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Eickmeier O, Seki H, Haworth O, Hilberath JN, Gao F, Uddin M, Croze RH, Carlo T, Pfeffer MA, Levy BD. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol. 2013;6:256–66. doi: 10.1038/mi.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1998;101:819–26. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jin Y, Arita M, Zhang Q, Saban DR, Chauhan SK, Chiang N, Serhan CN, Dana R. Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators. Invest Ophthalmol Vis Sci. 2009;50:4743–52. doi: 10.1167/iovs.08-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Leedom AJ, Sullivan AB, Dong B, Lau D, Gronert K. Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am J Pathol. 2010;176:74–84. doi: 10.2353/ajpath.2010.090678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nie D, Tang K, Diglio C, Honn KV. Eicosanoid regulation of angiogenesis: role of endothelial arachidonate 12-lipoxygenase. Blood. 2000;95:2304–11. [PubMed] [Google Scholar]
- 163.Finetti F, Donnini S, Giachetti A, Morbidelli L, Ziche M. Prostaglandin E(2) primes the angiogenic switch via a synergic interaction with the fibroblast growth factor-2 pathway. Circ Res. 2009;105:657–66. doi: 10.1161/CIRCRESAHA.109.203760. [DOI] [PubMed] [Google Scholar]
- 164.Bennett MR, Sinha S, Owens GK. Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ho KJ, Spite M, Owens CD, Lancero H, Kroemer AH, Pande R, Creager MA, Serhan CN, Conte MS. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am J Pathol. 2010;177:2116–23. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Petri MH, Laguna-Fernandez A, Tseng CN, Hedin U, Perretti M, Back M. Aspirin-triggered 15-epi-lipoxin A(4) signals through FPR2/ALX in vascular smooth muscle cells and protects against intimal hyperplasia after carotid ligation. Int J Cardiol. 2015;179:370–2. doi: 10.1016/j.ijcard.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Petri MH, Laguna-Fernandez A, Gonzalez-Diez M, Paulsson-Berne G, Hansson GK, Back M. The role of the FPR2/ALX receptor in atherosclerosis development and plaque stability. Cardiovasc Res. 2015;105:65–74. doi: 10.1093/cvr/cvu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Miyahara T, Runge S, Chatterjee A, Chen M, Mottola G, Fitzgerald JM, Serhan CN, Conte MS. D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J. 2013;27:2220–32. doi: 10.1096/fj.12-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wu B, Mottola G, Chatterjee A, Lance KD, Chen M, Siguenza IO, Desai TA, Conte MS. Perivascular delivery of resolvin D1 inhibits neointimal hyperplasia in a rat model of arterial injury. J Vasc Surg. 2016 doi: 10.1016/j.jvs.2016.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Akagi D, Chen M, Toy R, Chatterjee A, Conte MS. Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. FASEB J. 2015;29:2504–13. doi: 10.1096/fj.14-265363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chatterjee A, Sharma A, Chen M, Toy R, Mottola G, Conte MS. The pro-resolving lipid mediator maresin 1 (MaR1) attenuates inflammatory signaling pathways in vascular smooth muscle and endothelial cells. PLoS One. 2014;9:e113480. doi: 10.1371/journal.pone.0113480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hiram R, Rizcallah E, Sirois C, Sirois M, Morin C, Fortin S, Rousseau E. Resolvin D1 reverses reactivity and Ca2+ sensitivity induced by ET-1, TNF-alpha, and IL-6 in the human pulmonary artery. Am J Physiol Heart Circ Physiol. 2014;307:H1547–58. doi: 10.1152/ajpheart.00452.2014. [DOI] [PubMed] [Google Scholar]
- 173.Hiram R, Rizcallah E, Marouan S, Sirois C, Sirois M, Morin C, Fortin S, Rousseau E. Resolvin E1 normalizes contractility, Ca2+ sensitivity and smooth muscle cell migration rate in TNF-alpha- and IL-6-pretreated human pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2015;309:L776–88. doi: 10.1152/ajplung.00177.2015. [DOI] [PubMed] [Google Scholar]
- 174.Foley JH, Conway ME. Cross Talk Pathways Between Coagulation and Inflammation. Circ Res. 2016 doi: 10.1161/CIRCRESAHA.116.306853. In Press. [DOI] [PubMed] [Google Scholar]
- 175.Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci U S A. 2004;101:15178–83. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chiang N, Hurwitz S, Ridker PM, Serhan CN. Aspirin has a gender-dependent impact on antiinflammatory 15-epi-lipoxin A4 formation: a randomized human trial. Arterioscler Thromb Vasc Biol. 2006;26:e14–7. doi: 10.1161/01.ATV.0000196729.98651.bf. [DOI] [PubMed] [Google Scholar]
- 177.Fredman G, Van Dyke TE, Serhan CN. Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler Thromb Vasc Biol. 2010;30:2005–13. doi: 10.1161/ATVBAHA.110.209908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bohr S, Patel SJ, Sarin D, Irimia D, Yarmush ML, Berthiaume F. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound Repair Regen. 2013;21:35–43. doi: 10.1111/j.1524-475X.2012.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, Watanebe T, Sakabe S, Daidoji T, Nakamura S, Kadowaki A, Ohto T, Nakanishi H, Taguchi R, Nakaya T, Murakami M, Yoneda Y, Arai H, Kawaoka Y, Penninger JM, Arita M, Imai Y. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–25. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 180.Auven Therapeutics. http://www.auventherapeutics.com/auven/products/RX10045.php.
- 181.Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN. Cutting edge: Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. 2011;186:5543–7. doi: 10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]