Abstract
BACKGROUND
Smoking is known to vary by marital status, but little is known about its contribution to marital status differences in longevity. We examined the changing contribution of smoking to mortality differences between married and never married, divorced or widowed Finnish men and women aged 50 years and above in 1971–2010.
DATA AND METHODS
The data sets cover all persons permanently living in Finland in the census years 1970, 1975 through 2000 and 2005 with a five-year mortality follow-up. Smoking-attributable mortality was estimated using an indirect method that uses lung cancer mortality as an indicator for the impact of smoking on mortality from all other causes.
RESULTS
Life expectancy differences between the married and the other marital status groups increased rapidly over the 40-year study period because of the particularly rapid decline in mortality among married individuals. In 1971–1975 37–48% of life expectancy differences between married and divorced or widowed men were attributable to smoking, and this contribution declined to 11–18% by 2006–2010. Among women, in 1971–1975 up to 16% of life expectancy differences by marital status were due to smoking, and the contribution of smoking increased over time to 10–29% in 2006–2010.
CONCLUSIONS
In recent decades smoking has left large but decreasing imprints on marital status differences in longevity between married and previously married men, and small but increasing imprints on these differences among women. Over time the contribution of other factors, such as increasing material disadvantage or alcohol use, may have increased.
1. Introduction
Married individuals enjoy longer lives and better health than people in other marital status groups (Rendall 2011). Health behaviors, which have been shown to contribute to health disparities between married and unmarried groups, are one possible contributor to mortality differences by marital status (Dupre, Beck, and Meadows 2009; Molloy et al. 2009; Joung et al. 1995; Joung et al. 1997; Joutsenniemi et al. 2006). One of these behaviors, smoking, strongly affects mortality (Rogers et al. 2005) and it has also been shown to vary by marital status (Molloy et al. 2009; Nystedt 2006; Leinsalu, Tekkel, and Kunst 2007; Helmert and Shea 1998; Lindström 2010; Jones, Gulbis, and Baker 2010; Oh et al. 2010; Broms et al. 2004; Lee et al. 2005). The divorced are more likely to have smoked at some time than the married (Jones, Gulbis, and Baker 2010; Oh et al. 2010). Furthermore, both married men and women are least likely to be current smokers, whereas divorced men and women are most likely to be current smokers with the never married and the widowed falling in between (Molloy et al. 2009; Nystedt 2006; Helmert and Shea 1998; Lindström 2010). Differences between the married and the other marital status groups have also been found in smoking cessation among men, with married men having the highest rates of smoking cessation (Broms et al. 2004; Nystedt 2006).
However, much less is known about the contribution of smoking to health differentials between marital status groups, and the findings from these studies give partially conflicting results (Lund et al. 2002; Wyke and Ford 1992; Joutsenniemi et al. 2006), possibly resulting from differences in the choice of the health measure or differences in the stage of the smoking epidemic. In a study by Lund et al. (2002), self-reported smoking was not found to explain mortality differences by marital status in the 1990s in Denmark. However, Wyke and Ford (1992) found that about 20% of the differences in self-rated health between the married and the previously married was accounted for by smoking among men and women in the 1980s in Scotland. In Finland, Joutsenniemi et al. (2006) found that up to 33% of the differences in self-rated health was due to smoking, after controlling for education, in 1978–1980 and 2000–2001. Although previous population-based studies have made important contributions, studies based on self-reported smoking data from surveys are subject to problems related to accurately capturing lifetime smoking exposure in different marital states, due to factors such as misclassification of past and present smoking statuses, recall bias, and problems in taking into account different smoking intensities or changes in smoking status during follow-up (Ho and Elo 2013).
The mortality advantage of the married over the unmarried groups has also widened in recent decades in many high income countries, including Finland (Hu and Goldman 1990; Valkonen, Martikainen, and Blomgren 2004; Murphy, Grundy, and Kalogirou 2007; Martikainen et al. 2005; Liu 2009). Meanwhile, smoking behaviors have changed markedly since the turn of the 20th century. In Finland, smoking prevalence peaked among male cohorts born in 1911–1925; in those cohorts over 80% of men have ever smoked regularly (Martelin 1984). Among women, smoking prevalence started to increase from the cohorts born at the beginning of the 20th century and peaked at about 45% among the cohorts born in the 1950s (Helakorpi et al. 2004). Accordingly, smoking has had a large but decreasing impact on longevity among men and a smaller but increasing impact on longevity among women (Martikainen et al. 2013), with parallel findings observed in many other high income countries (Preston, Glei, and Wilmoth 2011). While smoking behaviors have changed markedly during the 20th century, no estimates exist of the changing contribution of smoking to marital status differences in mortality.
This study contributes to the current literature on the impact of smoking on mortality by examining the changing impact of smoking on life expectancy differences between married and unmarried groups in an era of increasing mortality disparities and changing patterns of marriage and divorce (Hu and Goldman 1990; Valkonen, Martikainen, and Blomgren 2004; Murphy, Grundy, and Kalogirou 2007; Martikainen et al. 2005; Liu 2009). Our data sets cover the whole population of Finland from the early 1970s to the late 2000s, and we employed an indirect estimation method based on lung cancer mortality (Preston, Glei, and Wilmoth 2010) to assess the contribution of smoking to mortality. Mortality differences between the married and the unmarried groups were measured by differences in life expectancies at age 50, and the contribution of smoking to these life expectancy differences was calculated by comparing the observed differences in life expectancies with those from which the impact of smoking was eliminated.
2. Data and methods
2.1 Data
The data sets used are based on information drawn from the Finnish population censuses and administrative registers in 1970–2010. The data sets cover all persons permanently living in Finland at the end of the census years 1970, 1975 through 2000 and 2005 and include all person-years and deaths aggregated by Statistics Finland for each five-year period following each census (1971–1975–1976–1980 through 2001–2005–2006–2010) stratified by age, gender and marital status. Individuals who emigrated during the five-year follow-up period were excluded. Marital status was measured in each census year, was fixed for the subsequent five-year follow-up period, and comprised four groups: married, never married, widowed and divorced. Person-years and deaths were allocated into age groups within each year on the basis of birthdates and further grouped into five-year categories. Analyses were restricted to people aged 50 and above since the majority of smoking-attributable deaths occur above age 50, and individuals were allowed to age into the follow-up (i.e., individuals who turn 50 in the five-year follow-up periods contribute person-years and deaths from their 50th birthday onwards).
2.2 Methods
To assess whether and how much smoking has contributed to life expectancy differences by marital status, we used the method developed by Preston, Glei, and Wilmoth (2010; 2011; ‘PGW method’ hereafter) to estimate smoking-attributable mortality. The method uses lung cancer mortality as an indicator of the population-level damage from smoking and thus does not rely on self-reported information on smoking histories in the study population. Instead, the method uses the association between lung cancer mortality and mortality from all other causes besides lung cancer at the cross-country level and the expected lung cancer death rates among non-smokers to estimate the overall fraction of deaths attributable to smoking in a particular age and sex group. The association between lung cancer mortality and mortality from all other causes was determined by Preston, Glei, and Wilmoth (2010) using a negative binomial regression model which was fitted to a data set containing information from 20 high-income countries, including Finland, 1950–2006, for the population aged 50 years and older. We used the age- and sex-specific coefficients characterizing the association between lung cancer mortality and mortality from all other causes published in Preston, Glei, and Wilmoth (2011) and the age- and sex-specific lung cancer mortality rates among non-smokers taken from the American Cancer Society’s Cancer Prevention Study II (CPS-II) (Thun et al. 1997). The model coefficients apply to the year 2003, and for the analyses in this study, they were modified to apply to each period using a time trend based on interactions between lung cancer mortality and year from the regression model described above (Preston, Glei, and Wilmoth 2011).
We used period life table methods (Preston, Heuveline, and Guillot 2001) and age-specific mortality rates in five-year age intervals to calculate life expectancies with and without smoking at age 50 by marital status, period, and gender. The contribution of smoking to the life expectancy differences was calculated by comparing the observed differences in life expectancies with those constructed assuming no exposure to smoking at all in the populations under study (i.e., in a scenario that would prevail if smoking were eliminated entirely). Because gender differences in the levels of and trends in smoking-attributable mortality have been significant over the study period (Martikainen et al. 2013), the analyses were conducted separately for men and women.
3. Results
3.1 Differences in life expectancy by marital status in 1971–2010
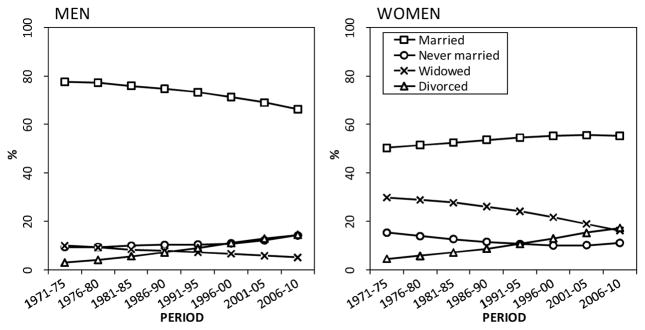
Table 1 shows the distribution of person-years by marital status and gender in 1971–1975 and 2006–2010. Among men in the earliest period, the married accounted for almost 80% of all person-years, but the proportion declined during the study period to 66% in 2006–2010. Among women, the married accounted for about half of the person-years in the first period, and during the study period this proportion remained quite stable. The proportion of person-years lived by the divorced substantially increased among both genders, and the proportion lived by the widowed was halved among men and women, but from clearly higher baseline levels among women. The proportion of person-years lived by those never married increased among men and decreased among women.
Table 1.
Distribution of person-years by marital status (%, age-adjusted1) among Finnish men and women aged 50 and above in 1971–1975 and 2006–2010
| Men
|
Women
|
|||
|---|---|---|---|---|
| 1971–1975 | 2006–2010 | 1971–1975 | 2006–2010 | |
| Married | 78 | 66 | 50 | 55 |
| Never married | 9 | 14 | 15 | 11 |
| Widowed | 10 | 5 | 30 | 16 |
| Divorced | 3 | 14 | 5 | 17 |
| All | 100 | 100 | 100 | 100 |
| Person-years (in 1,000s) | 2,432 | 4,627 | 3,463 | 5,486 |
The age standard was the combined population of men and women during the whole study period.
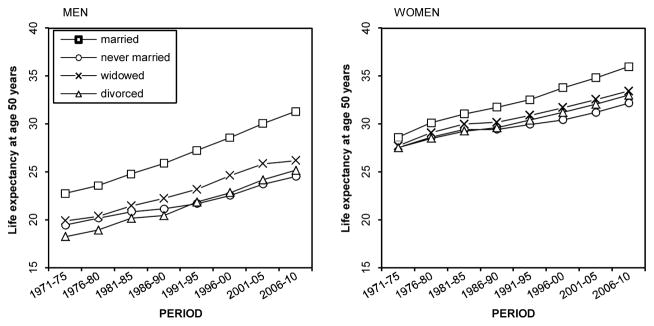
Life expectancy at age 50 increased in every marital status group between 1971–1975 and 2006–2010 among both men and women (Figure 1, Tables 2 and 3). In 1971–1975 the married had the highest life expectancy at age 50 (22.8 and 28.6 years among men and women respectively), and married men and women also experienced the most rapid increases in life expectancy (8.6 and 7.4 years, respectively). These changes led to the widening of the life expectancy differences between the married and unmarried groups (increases of 1.7–3.5 years among men and 1.7–2.7 years among women). Among the unmarried men, the divorced had the lowest life expectancy in the first period (18.3 years) but experienced the largest increase (6.9 years), whereas those never married had the smallest increase (5.1 years) in life expectancy. In the 1970s women in the three unmarried groups had similar life expectancies, and the difference in life expectancy in comparison with the married was about one year. Life expectancy increased among all unmarried women, although the increase was somewhat unequal. It was most rapid among the widowed women (5.7 years) and slowest among the never married women (4.6 years).
Figure 1.
Life expectancy at age 50 and above by period and marital status, Finnish men and women in 1971–2010
Table 2.
Contribution of smoking-attributable mortality to life expectancy differences between the married and the unmarried groups, Finnish men aged 50 and above in 1971–1975 and 2006–2010
| Life expectancy at age 50 (e50)
|
Difference in e50
|
Contribution of smoking to the difference
|
||||
|---|---|---|---|---|---|---|
| Observed | Without smoking | Observed | Without smoking | Years1 | % | |
| Period 1971–1975 | ||||||
| Married | 22.8 | 25.6 | ref. | ref. | ||
| Never married | 19.5 | 22.8 | 3.3 | 2.9 | 0.4 | 12 |
| Widowed | 19.9 | 23.9 | 2.8 | 1.8 | 1.1 | 37 |
| Divorced | 18.3 | 23.3 | 4.5 | 2.3 | 2.2 | 48 |
| Period 2006–2010 | ||||||
| Married | 31.3 | 32.7 | ref. | ref. | ||
| Never married | 24.5 | 26.7 | 6.8 | 6.0 | 0.7 | 11 |
| Widowed | 26.2 | 28.1 | 5.1 | 4.6 | 0.6 | 11 |
| Divorced | 25.2 | 27.7 | 6.1 | 5.0 | 1.1 | 18 |
| Change from 1971–1975 to 2006–2010 | ||||||
| Married | 8.6 | 7.0 | ||||
| Never married | 5.1 | 3.9 | 3.5 | 3.2 | 0.3a | 10 |
| Widowed | 6.3 | 4.3 | 2.3 | 2.8 | −0.5a | −22 |
| Divorced | 6.9 | 4.3 | 1.7 | 2.7 | −1.1a | −64 |
“Observed difference in life expectancy” minus “difference in life expectancy without smoking”
“Observed change in life expectancy difference” minus “change in life expectancy difference without smoking”
Table 3.
Contribution of smoking-attributable mortality to life expectancy differences between the married and the unmarried groups, Finnish women aged 50 and above in 1971–1975 and 2006–2010
| Life expectancy at age 50 (e50)
|
Difference in e50
|
Contribution of smoking to the difference
|
||||
|---|---|---|---|---|---|---|
| Observed | Without smoking | Observed | Without smoking | Years1 | % | |
| Period 1971–1975 | ||||||
| Married | 28.6 | 28.7 | ref. | |||
| Never married | 27.6 | 27.6 | 1.1 | 1.1 | 0.0 | 0 |
| Widowed | 27.7 | 27.8 | 0.9 | 0.9 | 0.0 | 5 |
| Divorced | 27.5 | 27.8 | 1.1 | 0.9 | 0.2 | 16 |
| Period 2006–2010 | ||||||
| Married | 36.0 | 36.3 | ref. | |||
| Never married | 32.2 | 32.9 | 3.8 | 3.4 | 0.4 | 10 |
| Widowed | 33.4 | 34.3 | 2.6 | 2.0 | 0.5 | 21 |
| Divorced | 33.0 | 34.2 | 3.0 | 2.1 | 0.9 | 29 |
| Change from 1971–1975 to 2006–2010 | ||||||
| Married | 7.4 | 7.6 | ||||
| Never married | 4.6 | 5.3 | 2.7 | 2.3 | 0.4a | 14 |
| Widowed | 5.7 | 6.4 | 1.7 | 1.2 | 0.5a | 30 |
| Divorced | 5.5 | 6.4 | 1.9 | 1.2 | 0.7a | 37 |
”Observed difference in life expectancy” minus “difference in life expectancy without smoking”
“Observed change in life expectancy difference” minus “change in life expectancy difference without smoking”
3.2 The contribution of smoking among men
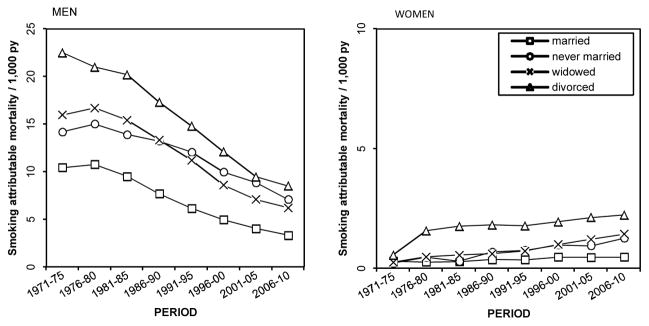
Throughout the study period among men, age-adjusted smoking-attributable mortality clearly differed between the married and the unmarried groups; the married had the lowest rates and the divorced the highest (Figure 2). These patterns were reflected in the contribution of smoking-attributable mortality to marital-status differences in life expectancy at age 50 throughout the study period 1971–2010 (Table 2). The largest life expectancy difference in 1971–1975 was between the divorced and the married (4.5 years), but it would have been 2.2 years smaller without smoking. Thus, smoking accounted for 48% of the observed life expectancy difference between divorced and married men. Smoking also contributed substantially to the life expectancy difference between the widowed and the married men (1.1 years or 37%), but it made a smaller contribution to the difference between the never married and married men (0.4 years or 12%).
Figure 2.
Age-adjusted smoking-attributable mortality (/1,000 person-years) by period and marital status, Finnish men and women aged 50 and above in 1971–2010
Over the study period, smoking-attributable mortality at least halved in each marital status group among men (Figure 2). The relative decline was fastest among the married (68% decline), slowest among the never married (50% decline) and similar among the divorced and widowed men (about 61% decline). However, in absolute terms, the decline was most rapid among the divorced due to their very high level of smoking-attributable mortality in the beginning of the study period; over time their rate converged with that of the other unmarried groups. The absolute decline was also faster among widowers than among the married, whereas the pace of the decline did not differ between those never married and the married. Smoking-attributable mortality as a share of all-cause mortality declined in each marital status group, as the proportional decline in age-adjusted smoking-attributable mortality was more rapid than the proportional decline in other-cause mortality (results not shown here).
Due to the rapid decrease in smoking-attributable mortality across marital status groups, the contribution of smoking to the life expectancy differences among men diminished to less than a fifth by 2006–2010 (Table 2). By 2006–2010 the contribution of smoking-attributable mortality to the life expectancy difference between the divorced and the married had diminished to 18% (1.1 years) and between the widowed and the married to 11% (0.6 years). In absolute terms, the contribution of smoking to life-expectancy differences between never married and married men increased slightly, but as a percentage it remained stable and was 11% (0.7 years) in 2006–2010.
The rapid decrease in smoking-attributable mortality among the divorced and widowed men slowed down the increase of the life expectancy differentials. In the absence of smoking, the increase in the life expectancy difference between the divorced and married men would have been 64% larger (1.1 years) than the observed increase (Table 2). Similarly, the increase between widowed and married men would have been 22% larger (0.5 years) without smoking. In contrast, smoking accounted for 10% of the observed increase in the life expectancy difference between never married and married men.
3.3 The contribution of smoking among women
Among women in the 1970s, the level of smoking-attributable mortality was very low in all marital status groups (Figure 2). In 1971–1975 the contribution of smoking to life expectancy differences at age 50 by marital status was negligible, except for the difference between the divorced and the married. Smoking accounted for 16% of this life expectancy difference in this period (Table 3). After the early 1970s, age-adjusted smoking-attributable mortality began to increase in every marital status group, and the levels between groups diverged (Figure 2). Smoking-attributable mortality increased least among married women in both absolute and relative terms. Accordingly, by 2006–2010 the contribution of smoking to the increased life expectancy differences had also grown, and it ranged from 10% (0.4 years) between the never married and the married to 29% (0.9 years) between the divorced and the married in 2006–2010 (Table 3).
Among women (Table 3), diverging and increasing rates of smoking-attributable mortality contributed to the increase in all-cause mortality differences between the married and each of the unmarried groups. In the absence of smoking-attributable deaths, the increase in life expectancy differences would have been 14%, 30% and 37% smaller than the observed increases between the married and the never married, widowed and divorced, respectively.
4. Discussion
The aim of this study is to quantify the contribution of smoking to marital status differences in mortality in Finland from 1971 to 2010 and to assess how smoking has contributed to changes in these differences. Life expectancies were used to measure mortality differences, and the indirect PGW method was used to estimate the impact of smoking on mortality. Previous literature quantifying the contribution of smoking to marital status differences in mortality is scarce, and to the best of our knowledge, this study is the first to apply the PGW method to study the contribution of smoking to marital status differences in life expectancy and, in particular, is the first study to assess changes in the contribution of smoking to marital status differences over a 40-year period.
4.1 Summary of the main findings
We found that in the past 40 years marital status differences in life expectancy among those aged 50 years and above rapidly increased due to more rapid increases in the life expectancy of the married than the unmarried population. During this period, smoking-attributable mortality declined among men in all marital status groups, but particularly strongly among the divorced and widowed. These changes were significant drivers of trends in life expectancy differentials by marital status. In particular, in the absence of smoking, life expectancy differences between the married and divorced men would have increased by 64% more than they did (2.7 years as opposed to the observed increase of 1.7 years). However, smoking made a minor contribution to the increasing life expectancy differential between married and never married men, mainly because of relatively similar absolute trends in smoking-attributable mortality. At the end of the study period in 2006–2010, smoking-attributable mortality accounted for 11–18% of the life expectancy differences between married and unmarried men.
Among women, smoking-attributable mortality was negligible at the beginning of the 1970s. Over the study period, it increased in all groups, but least among the married. As a consequence, the contribution of smoking to life-expectancy differences increased over time, and life expectancy differences between married women and each of the other marital status groups would have increased by up to 37% less in the absence of smoking. At the end of the study period in 2006–2010, smoking-attributable mortality accounted for up to 29% of marital status differences in life expectancy at age 50 among women. As to gender differences, the relative (%) contribution of smoking to marital status differences in life expectancy was similar or larger among women than among men in the most recent period. However, as marital status differences have been larger among men, in absolute terms, the contribution remains larger among men.
4.2 Comparison to previous studies
In this study we focus on life expectancy and document an increase in the absolute differences in life expectancy between the married and each of the unmarried groups resulting from more rapid life expectancy increases in the married population among both men and women. These results are in line with a previous study by Martikainen et al. (2005) showing widening differences in relative mortality by marital status in the working aged and elderly Finnish population in recent decades, and with the observed widening of absolute differences in life expectancy between the married and the unmarried groups during the 1970s and 1980s among the Finnish elderly (Martelin 1994).
The finding that the divorced had the highest rates of smoking-attributable mortality is in line with previous literature showing that divorced men and women consistently have the highest smoking prevalence and lung cancer mortality rates in Finland (Martikainen et al. 2005; Martelin 1984) and in other high income countries (Molloy et al. 2009; Nystedt 2006; Helmert and Shea 1998; Lindström 2010; Berntsen 2011; Joung et al. 1996; Kallan 1997; Ben-Shlomo et al. 1993). The lowest rates of lung cancer mortality and smoking prevalence are observed among the married in Finland (Martikainen et al. 2005; Martelin 1984). Similarly in our study population, smoking-attributable mortality was lowest among the married, followed by either the never married or the widowed (depending on the time period).
In the previous literature, insights into causes behind marital status differences in mortality and changes in these differences have been drawn from cause-specific mortality analyses and survey-based cohort studies. The findings from previous studies showing that the divorced have the highest lung cancer mortality rates among both men and women (Martikainen et al. 2005; Berntsen 2011; Joung et al. 1996; Kallan 1997; Ben-Shlomo et al. 1993) and that lung cancer mortality has decreased among men and increased among women in Finland (Martikainen et al. 2005), and our similar findings on smoking-attributable mortality reflect the same differences and trends in exposure to smoking in different population subgroups. However, although studies focusing on lung cancer mortality provide important insight into the contribution of smoking, such analyses will significantly underestimate the total impact of smoking. In our data, 32% of all smoking-attributable deaths among men and 27% among women were estimated to be due to lung cancer in 2006–2010. As lung cancer mortality makes up a relatively small (less than a third) fraction of all smoking-attributable mortality, we were able to provide a fuller and more accurate estimate of the overall contribution of smoking to mortality differentials by marital status by using the PGW method, which captures all smoking-attributable mortality.
Few studies provide direct estimates of the contribution of smoking to mortality differences by marital status. We are aware of only one such cohort study based on a survey with mortality follow-up that shows no effect of smoking on marital status differences in mortality after controlling for self-rated health and functional ability (Lund et al. 2002). However, in the study by Lund et al. (2002), the measure for smoking included only two classes, differentiating those who have never smoked from those who have ever smoked, thus inaccurately estimating lifetime smoking exposure. Moreover, adjustment for self-rated health and functional ability prior to smoking adjustment may partially mask the true effects of smoking on mortality differentials, since smoking-related illness may lead to lower self-rated health and impaired functional ability. Overall, by using the PGW method, we avoided the problems related to accurately capturing lifetime smoking exposure that survey-based methods may suffer from, including recall bias, misclassification of past and present smoking statuses, and difficulties in taking into account different smoking intensities or changes in smoking status during follow-up (Ho and Elo 2013).
4.3 Interpretation of the findings
4.3.1 All-cause mortality
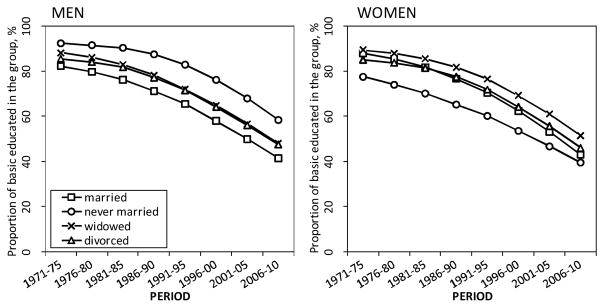
Marital status differences in mortality and changes in these differences may partly be due to differences and changes in the socioeconomic or demographic characteristics of the groups, for instance, education. Because the unmarried groups in our study were systematically less educated than the married among men, but not among women (see Appendix, Figure A-1), and because education is related to all-cause and smoking-attributable mortality (Martikainen et al. 2013), some of the mortality differences between the married and the unmarried groups found in our study may have arisen from differences in the socioeconomic structure between the marital status groups, especially among men (see Appendix, Figure A-1). In a study by Martikainen et al. (2005) between 12% and 26% of the relative all-cause mortality differences between the married and the unmarried groups were explained by social class and education in the 1970s and the 1990s among working-aged men, but they explained less among elderly men and women of all ages.
The decline in the proportion of those with basic education only (up to 9 years of schooling) seems to have been fastest among the married among both genders in our study population, and slowest among the never married men (Appendix, Figure A-1). If any strengthening of the contribution of education to life expectancy differences occurred over time, this has been most likely to have happened between never married and married men. In the study by Martikainen et al. (2005), increases in the relative differences could not be explained by changes in the distribution of socioeconomic position, nor household size, economic activity or number of children. However, because these characteristics were not taken into account in this study, the contribution of these factors to the results remains unclear.
First marriage rates have decreased for cohorts born since the late 1940s and divorce rates have increased in Finland (Pitkänen and Jalovaara 2007). These trends are presumably reflected in the increase in divorced men and women and never married men seen in this study (see Appendix, Figure A-2) and may indicate changes in the factors that contribute to marital status differences in mortality. For example, the decreasing trend of the first marriage rates may suggest strengthened positive selection into marriage, which may have improved the average health of the married. Meanwhile, the health of the divorced may have improved due to lessened negative selection as their relative size strongly increased during the study period from very small proportions. However, more detailed data would be needed to assess the possible changes in the causation-selection related mechanisms.
4.3.2 The contribution of smoking
Among men, the contribution of smoking to life expectancy differences by marital status has diminished over time, and increases in life expectancy differentials would have been even larger without smoking. Between the never married and married men, the contribution of smoking remained most persistent. The declining rates of smoking-attributable mortality in our study reflect the aging of the cohorts of men among whom smoking peaked, that is, male cohorts born around 1911–1925, of which about 80% ever smoked regularly (Martelin 1984), the replacement of these cohorts with successive younger cohorts among whom smoking initiation started to decline, and an overall increase in cessation over time (Laaksonen et al. 1999). Overall, increases in cessation and deterring initiation have been highly successful among Finnish men and have helped dampen increasing inequality in life expectancy. The success of addressing smoking is reflected in its disappearing contribution among men, which also appears to have opened the door for the contribution of other factors.
Marrying and living in a stable marriage have been found to be associated with increased cessation among Finnish men in the 1980s (Broms et al. 2004). In this study, the contribution of smoking to life expectancy differences in the most recent period, 2006–2010, was of similar magnitude in all the unmarried groups (11% to 18%), a finding consistent with Nystedt’s (2006) hypothesis about the importance of the presence of a spouse for men in success in quitting smoking. It may be more difficult for an unmarried person to quit because of the lack of, for instance, spousal support or control available for smoking reduction and cessation (Westmaas, Wild, and Ferrence 2002; Umberson 1992). Married individuals may also be in a better position to bear the stresses associated with quitting smoking, such as the withdrawal symptoms, due to the overall stress buffering effects that a stable marriage may bring, or they may have stronger incentives (Osler and Prescott 1998) to quit because of, for instance, parenting responsibilities. In addition, difficulties in smoking abstinence during the processes of losing a spouse may contribute to higher overall lifetime smoking exposure among divorced or widowed men (Nystedt 2006).
The contribution of smoking to the life expectancy difference between married and divorced men was especially pronounced in the beginning of the study period, and this contribution also showed the largest decrease over time. Besides cessation differences, differences in initiation may also partly account for these findings; the proportion of those who have ever smoked has been found to be consistently higher only among divorced men when compared with the married in the Finnish population (see Appendix, Figure A-3; Martelin 1984). Part of these differences may be related to marital conflict, i.e., marital frictions induce smoking initiation, but as smoking usually starts during adolescence or early adulthood (Khuder, Dayal, and Mutgi 1999; Chen and Millar 1998) and marriage usually occurs later (Pitkänen and Jalovaara 2007), it is also possible that processes related to marital selection (Fu and Goldman 2000) contribute to these initiation differences. The low proportion of the divorced and their high smoking-attributable mortality in the first study periods may also imply selection (Hu and Goldman 1990) based on characteristics related to both smoking and higher risk of divorce, for instance, heavy alcohol use (Laaksonen et al. 2002; Leonard, Smith, and Homish 2014; Torvik et al. 2013). As divorce became more common during the study period, it is possible that the divorced became more like the average in these respects, and selection on these factors became less relevant.
Among men, those never married experienced the slowest relative decline in smoking-attributable mortality, and in contrast to the other unmarried groups, smoking also contributed to the increase in life expectancy differences between the men who never married and the married. It is unclear whether changes in smoking initiation between those never married and married contributed to this trend, but as previous studies do not suggest higher initiation among men who have never married when compared with married men in Finland (Martelin 1984) or elsewhere (Leinsalu, Tekkel, and Kunst 2007; Ben-Shlomo et al. 1993), differences in success in smoking cessation (Martelin 1984; Nystedt 2006; Tillgren et al. 1996; Leinsalu, Tekkel, and Kunst 2007) may have been the stronger driver here. Men who never married may have been the slowest to terminate the habit of smoking, and this may be related to their lifetime exclusion from relationships that may be, on average, important sources of support, control and enhanced incentive to preserve health.
In contrast to men, the contribution of smoking to life expectancy differences by marital status among women has increased over time, and smoking has contributed to increases in these differences. Rates of smoking-attributable mortality were lower than those among men and increasing because of the time lag between the genders at the start of the smoking epidemic (Lopez, Collishaw, and Piha 1994). These trends among women reflect the aging of the cohorts of women among whom the prevalence of smoking started to increase, that is, the cohorts born in the beginning of the 20th century (Martelin 1984). Besides increased smoking initiation in successive birth cohorts, cessation has also increased among women across cohorts over time (Laaksonen et al. 1999). The slower increase in smoking-attributable mortality among the married seen in our study could then be due to slower increases in initiation in the successive birth cohorts of married women and higher levels of smoking cessation and possibly larger increases in cessation over time among the married. Here women are experiencing the typical story of an adverse risk factor being distributed in a way that favors the theoretically most advantaged group, married women.
In the literature on smoking behavior, systematically lower rates of cessation in comparison with married women have been found only among divorced women in Finland (Martelin 1984). Evidence on cessation differences from other countries also show that marital breakdown, especially that leading to divorce, is related to sustained smoking among women (Nystedt 2006). Smoking-attributable mortality was increased about twofold among divorced women in the 2000s when compared with the other unmarried groups in our study, which may partly reflect stresses related to marital conflict and dissolution hindering smoking cessation and abstinence. Comparable to our findings for men, initiation differences may also have contributed to the higher rates of smoking-attributable mortality among divorced women (see Appendix, Figure A-3; Martelin 1984; Oh et al. 2010).
4.4 Methodological considerations
The data used in this study is of high quality in terms of its representativeness of the population and completeness of the follow-up; the data includes the entire population of Finland and has no loss of follow-up information. In addition, the quality of cause of death information has been assessed to be high in Finland (Mathers et al. 2005). The study also benefitted from using the PGW method when compared with studies based solely on lung cancer mortality and survey-based cohort studies, as discussed in the previous sections.
However, this study also has its limitations. First, the indirect method assumes that the expected lung cancer death rates among non-smokers reported in the U.S. CPS-II study do not differ by marital status. This assumption would not hold if marital status was associated with factors other than smoking that increase lung cancer risk, such as exposure to asbestos or other carcinogens in occupational settings or in living environments. However, there is no strong reason to believe that differences in these exposures by marital status are large. It was also assumed that the association between lung cancer mortality and mortality from all other causes does not differ by marital status. This assumption would not hold if, for instance, similar levels of lung cancer mortality across marital status groups were associated with lower levels of mortality from all other causes among the married than among the unmarried groups. The unmarried may have higher mortality due to smoking-related causes other than lung cancer because of, for instance, higher co-morbidity and higher levels of other risk factors, such as heavy alcohol use.
Marital status was measured in each census year, and was not updated during the subsequent five-year follow-up period. At ages above 50, (re)marriage rates have been low in past decades but are increasing among the divorced and never married; 5% of the divorced and 1% of the never married in 1990 (re)married during 1991–1995 among men, while during 2006–2010 these figures were 8% for divorced and 4% for men who never married in 2005 (source: individual level data from Statistics Finland, TK-53-339-13). Among women, (re)marriage rates have been lower than among men but are also increasing. The proportion of married individuals at ages above 50 who recently lost their spouse through bereavement or divorce has been stable at about 6% from 1991–1995 to 2006–2010 among men and has decreased among women from about 13% to 11%. Taking these changes into account would probably lead to wider life expectancy differences than observed here, especially towards the end of the study period – and possibly larger estimates of the contribution of smoking to life expectancy differences. In this respect, the results presented in this study are likely to be conservative.
5. Conclusions
In Finland, marital status differences in life expectancy have increased in recent decades. Our study demonstrates the importance of smoking in shaping these differences. Due to differences in the timing of the smoking epidemic between men and women, smoking has left large but decreasing imprints on marital status differences in longevity between married and previously married men and small but increasing imprints on these differences among women. Smoking now accounts for a relatively larger proportion of life expectancy differences by marital status among women than among men, but its contribution in absolute terms still remains larger for men. Because changes in smoking-attributable mortality did not explain or explained only a part of the increases in life expectancy differences between the married and the three unmarried groups, over time the contribution of other factors, such as increasing material disadvantage or alcohol use, may have increased.
Acknowledgments
Riina Peltonen and Pekka Martikainen received support from the Academy of Finland and the Signe and Ane Gyllenberg Foundation. Jessica Ho received support from the National Institute on Aging (NIA) Grant T32 AG000139 to the Population Research Institute at Duke University and from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) of the National Institutes of Health under Award Number K99 HD083519. Irma Elo received support from the National Institute of Aging Centers on Demography and Economics P30 program, AG-AG-012836 at the University of Pennsylvania.
Appendix
Figure A-1.
The proportion (%) of the population with only basic education (up to nine years of schooling) by marital status, Finnish men and women aged 50 and above in 1971–2010
Figure A-2.
The distribution of person-years by marital status (%, age-adjusted) among Finnish men and women aged 50 and above in 1971–2010
Figure A-3.
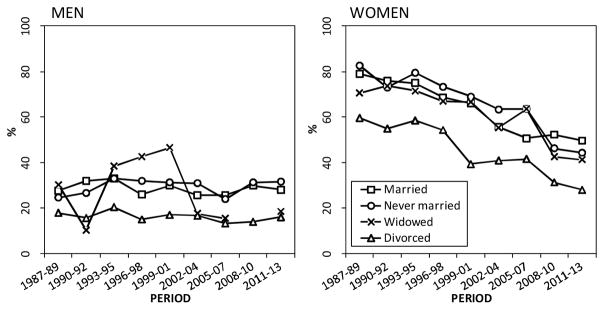
The proportion of never smokers (three-year averages, %) in marital status groups, Finnish men and women aged 55–64 in 1987–2013
Calculated on the basis of: Helldán et al. (2013).
References
- Ben-Shlomo Y, Smith GD, Shipley M, Marmot MG. Magnitude and causes of mortality differences between married and unmarried men. Journal of Epidemiology and Community Health. 1993;47(3):200–205. doi: 10.1136/jech.47.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsen KN. Trends in total and cause-specific mortality by marital status among elderly Norwegian men and women. BMC Public Health. 2011;11:537. doi: 10.1186/1471-2458-11-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broms U, Silventoinen K, Lahelma E, Koskenvuo M, Kaprio J. Smoking cessation by socioeconomic status and marital status: The contribution of smoking behavior and family background. Nicotine & Tobacco Research. 2004;6(3):447–455. doi: 10.1080/14622200410001696637. [DOI] [PubMed] [Google Scholar]
- Chen J, Millar WJ. Age of smoking initiation: Implications for quitting. Health Reports. Statistics Canada. 1998;9(4):39–46. [PubMed] [Google Scholar]
- Dupre ME, Beck AN, Meadows SO. Marital trajectories and mortality among US adults. American Journal of Epidemiology. 2009;170(5):546–555. doi: 10.1093/aje/kwp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Goldman N. The association between health-related behaviors and the risk of divorce in the USA. Journal of Biosocial Science. 2000;32(1):63–88. doi: 10.1017/S0021932000000638. [DOI] [PubMed] [Google Scholar]
- Helakorpi S, Martelin T, Torppa J, Patja K, Vartiainen E, Uutela A. Did Finland’s Tobacco Control Act of 1976 have an impact on ever smoking? An examination based on male and female cohort trends. Journal of Epidemiology and Community Health. 2004;58(8):649–654. doi: 10.1136/jech.2003.015925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helldán A, Helakorpi S, Virtanen S, Uutela A. Health behavior and health among the Finnish adult population. Helsinki: National Institute for Health and Welfare; 2013. [and earlier publications during 1987–2013] https://www.thl.fi/fi/web/thlfi-en/research-and-expertwork/population-studies/health-behaviour-and-health-among-the-finnish-adult-population-avtk. [Google Scholar]
- Helmert U, Shea S. Family status and self-reported health in West Germany. Sozial- und Präventivmedizin. 1998;43(3):124–132. doi: 10.1007/BF01359720. [DOI] [PubMed] [Google Scholar]
- Ho JY, Elo IT. The contribution of smoking to black-white differences in U.S. mortality. Demography. 2013;50(2):545–568. doi: 10.1007/s13524-012-0159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YR, Goldman N. Mortality differentials by marital status: An international comparison. Demography. 1990;27(2):233–250. doi: 10.2307/2061451. [DOI] [PubMed] [Google Scholar]
- Jones A, Gulbis A, Baker EH. Differences in tobacco use between Canada and the United States. International Journal of Public Health. 2010;55(3):167–175. doi: 10.1007/s00038-009-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joung IM, Stronks K, van de Mheen H, Mackenbach JP. Health behaviors explain part of the differences in self reported health associated with partner/marital status in the Netherlands. Journal of Epidemiology and Community Health. 1995;49(5):482–488. doi: 10.1136/jech.49.5.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joung IM, Glerum JJ, van Poppel FW, Kardaun JW, Mackenbach JP. The contribution of specific causes of death to mortality differences by marital status in the Netherlands. European Journal of Public Health. 1996;6(2):142–149. doi: 10.1093/eurpub/6.2.142. [DOI] [Google Scholar]
- Joung IM, Stronks K, van de Mheen H, van Poppel FW, van der Meer JB, Mackenbach JP. The contribution of intermediary factors to marital status differences in self-reported health. Journal of Marriage and Family. 1997;59(2):476–490. doi: 10.2307/353484. [DOI] [Google Scholar]
- Joutsenniemi KE, Martelin TP, Koskinen SV, Martikainen PT, Härkänen TT, Luoto RM, Aromaa AJ. Official marital status, cohabiting, and self-rated health – time trends in Finland, 1978–2001. European Journal of Public Health. 2006;16(5):476–483. doi: 10.1093/eurpub/cki221. [DOI] [PubMed] [Google Scholar]
- Kallan J. Effects of sociodemographic variables on adult mortality in the United States: Comparisons by sex, age, and cause of death. Social Biology. 1997;44(1–2):136–147. doi: 10.1080/19485565.1997.9988940. [DOI] [PubMed] [Google Scholar]
- Khuder SA, Dayal HH, Mutgi AB. Age at smoking onset and its effect on smoking cessation. Addictive Behaviors. 1999;24(5):673–677. doi: 10.1016/S0306-4603(98)00113-0. [DOI] [PubMed] [Google Scholar]
- Laaksonen M, Uutela A, Vartiainen E, Jousilahti P, Helakorpi S, Puska P. Development of smoking by birth cohort in the adult population in eastern Finland 1972–97. Tobacco Control. 1999;8(2):161–168. doi: 10.1136/tc.8.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaksonen M, Luoto R, Helakorpi S, Uutela A. Associations between health-related behaviors: A 7-year follow-up of adults. Preventive Medicine. 2002;34(2):162–170. doi: 10.1006/pmed.2001.0965. [DOI] [PubMed] [Google Scholar]
- Lee S, Cho E, Grodstein F, Kawachi I, Hu FB, Colditz GA. Effects of marital transitions on changes in dietary and other health behaviors in US women. International Journal of Epidemiology. 2005;34(1):69–78. doi: 10.1093/ije/dyh258. [DOI] [PubMed] [Google Scholar]
- Leinsalu M, Tekkel M, Kunst AE. Social determinants of ever initiating smoking differ from those of quitting: a cross-sectional study in Estonia. European Journal of Public Health. 2007;17(6):572–578. doi: 10.1093/eurpub/ckm030. [DOI] [PubMed] [Google Scholar]
- Leonard KE, Smith PH, Homish GG. Concordant and discordant alcohol, tobacco, and marijuana use as predictors of marital dissolution. Psychology of Addictive Behaviors. 2014;28(3):780–789. doi: 10.1037/a0034053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström M. Social capital, economic conditions, marital status and daily smoking: A population-based study. Public Health. 2010;124(2):71–77. doi: 10.1016/j.puhe.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Liu H. Till death do us part: Marital status and U.S. mortality trends, 1986–2000. Journal of Marriage and Family. 2009;71(5):1158–1173. doi: 10.1111/j.1741-3737.2009.00661.x. [DOI] [Google Scholar]
- Lopez AD, Collishaw NE, Piha T. A descriptive model of the cigarette epidemic in developed countries. Tobacco Control. 1994;3:242–247. doi: 10.1136/tc.3.3.242. [DOI] [Google Scholar]
- Lund R, Due P, Modvig J, Holstein BE, Damsgaard MT, Andersen PK. Cohabitation and marital status as predictors of mortality – an eight year follow-up study. Social Science & Medicine. 2002;55(4):673–679. doi: 10.1016/S0277-9536(01)00219-2. [DOI] [PubMed] [Google Scholar]
- Martelin T. Tupakointitapojen kehitys Suomessa haastattelu ja kyselytutkimusten valossa. Helsinki: Lääkintöhallitus; 1984. [In Finnish only] [Google Scholar]
- Martelin T. Sociodemographic mortality differences among the Finnish elderly. Helsinki: Finnish Demographic Society; 1994. Differential mortality at older ages. [Google Scholar]
- Martikainen P, Martelin T, Nihtilä E, Majamaa K, Koskinen S. Differences in mortality by marital status in Finland from 1976 to 2000: Analyses of changes in marital-status distributions, socio-demographic and household composition, and cause of death. Population Studies. 2005;59(1):99–115. doi: 10.1080/0032472052000332737. [DOI] [PubMed] [Google Scholar]
- Martikainen P, Ho JY, Preston S, Elo IT. The changing contribution of smoking to educational differences in life expectancy: Indirect estimates for Finnish men and women from 1971 to 2010. Journal of Epidemiology and Community Health. 2013;67(3):219–224. doi: 10.1136/jech-2012-201266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: An assessment of the global status of cause of death data. Bulletin of the World Health Organization. 2005;83(3):171–177. [PMC free article] [PubMed] [Google Scholar]
- Molloy GJ, Stamatakis E, Randall G, Hamer M. Marital status, gender and cardiovascular mortality: Behavioural, psychological distress and metabolic explanations. Social Science & Medicine. 2009;69(2):223–228. doi: 10.1016/j.socscimed.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Grundy E, Kalogirou S. The increase in marital status differences in mortality up to the oldest age in seven European countries, 1990–99. Population Studies. 2007;61(3):287–298. doi: 10.1080/00324720701524466. [DOI] [PubMed] [Google Scholar]
- Nystedt P. Marital life course events and smoking behaviour in Sweden 1980–2000. Social Science & Medicine. 2006;62(6):1427–1442. doi: 10.1016/j.socscimed.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Oh DL, Heck JE, Dresler C, Allwright S, Haglund M, Del Mazo SS, Kralikova E, Stucker I, Tamang E, Gritz ER, Hashibe M. Determinants of smoking initiation among women in five European countries: A cross-sectional survey. BMC Public Health. 2010;10:74. doi: 10.1186/1471-2458-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osler M, Prescott E. Psychosocial, behavioural, and health determinants of successful smoking cessation: A longitudinal study of Danish adults. Tobacco Control. 1998;7(3):262–267. doi: 10.1136/tc.7.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen K, Jalovaara M. Perheet ja perheenmuodostus. In: Koskinen S, Martelin T, Notkola I, Notkola V, Pitkänen K, Jalovaara M, Mäenpää E, Ruokolainen A, Ryynänen M, Söderling I, editors. Suomen väestö. Helsinki: Gaudeamus Helsinki University Press; 2007. pp. 115–167. [In Finnish only] [Google Scholar]
- Preston S, Heuveline P, Guillot M. Demography: Measuring and modeling population processes. Oxford: Wiley-Blackwell; 2001. [Google Scholar]
- Preston SH, Glei DA, Wilmoth JR. A new method for estimating smoking-attributable mortality in high-income countries. International Journal of Epidemiology. 2010;39(2):430–438. doi: 10.1093/ije/dyp360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SH, Glei DA, Wilmoth JR. Contribution of smoking to international differences in life expectancy. In: Crimmins EM, Preston SH, Cohen B, editors. International differences in mortality at older ages: Dimensions and sources. Washington, D.C: The National Academies Press; 2011. pp. 105–131. [PubMed] [Google Scholar]
- Rendall MS, Weden MM, Favreault MM, Waldron H. The protective effect of marriage for survival: a review and update. Demography. 2011;48(2):481–506. doi: 10.1007/s13524-011-0032-5. [DOI] [PubMed] [Google Scholar]
- Rogers RG, Hummer RA, Krueger PM, Pampel FC. Mortality attributable to cigarette smoking in the United States. Population and Development Review. 2005;31(2):259–292. doi: 10.1111/j.1728-4457.2005.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thun MJ, Day-Lally C, Myers DG, Calle EE, Flanders WD, Zhu BP, Namboodiri MM, Heath CW. Trends in tobacco smoking and mortality from cigarette use in cancer prevention studies I (1959 through 1965) and II (1982 through 1988) In: Burns DM, Garfinkel L, Samet JM, editors. Changes in cigarette-related disease risks and their implications for prevention and control. Bethesda, MD: Cancer Control and Population Sciences, National Cancer Institute, U.S. National Institutes of Health; 1997. pp. 305–382. NIH publication no. 97–4213. [Google Scholar]
- Tillgren P, Haglund BJ, Lundberg M, Romelsjö A. The sociodemographic pattern of tobacco cessation in the 1980s: Results from a panel study of living condition surveys in Sweden. Journal of Epidemiology and Community Health. 1996;50(6):625–630. doi: 10.1136/jech.50.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torvik FA, Røysamb E, Gustavson K, Idstad M, Tambs K. Discordant and concordant alcohol use in spouses as predictors of marital dissolution in the general population: Results from the Hunt study. Alcoholism, Clinical and Experimental Research. 2013;37(5):877–884. doi: 10.1111/acer.12029. [DOI] [PubMed] [Google Scholar]
- Umberson D. Gender, marital status and the social control of health behavior. Social Science & Medicine. 1992;34(8):907–917. doi: 10.1016/0277-9536(92)90259-S. [DOI] [PubMed] [Google Scholar]
- Valkonen T, Martikainen P, Blomgren J. Increasing excess mortality among non-married elderly people in developed countries. Demographic Research Special Collection. 2004;S2(12):305–330. doi: 10.4054/DemRes.2004.S2.12. [DOI] [Google Scholar]
- Westmaas JL, Wild TC, Ferrence R. Effects of gender in social control of smoking cessation. Health Psychology. 2002;21(4):368–376. doi: 10.1037/0278-6133.21.4.368. [DOI] [PubMed] [Google Scholar]
- Wyke S, Ford G. Competing explanations for associations between marital status and health. Social Science & Medicine. 1992;34(5):523–532. doi: 10.1016/0277-9536(92)90208-8. [DOI] [PubMed] [Google Scholar]