Abstract
Epilepsy surgery is under-utilized, but recent studies reach conflicting conclusions regarding whether epilepsy surgery rates are currently declining, increasing, or remaining steady. However, data in these prior studies are biased toward high-volume epilepsy centers, or originate from sources that do not disaggregate various procedure types.
All major epilepsy surgery procedures were extracted from the Centers for Medicare and Medicaid Services Part B National Summary Data File and the American College of Surgeons National Surgical Quality Improvement Program. Procedure rates, trends, and complications were analyzed, and patient-level predictors of postoperative adverse events were identified.
Between 2000–2013, 6200 cases of epilepsy surgery were identified. Temporal lobectomy was the most common procedure (59% of cases), and most did not utilize electrocorticography (63–64%). Neither temporal nor extratemporal lobe epilepsy surgery rates changed significantly during the study period, suggesting no change in utilization. Adverse events, including major and minor complications, occurred in 15.3% of temporal lobectomies and 55.6% of hemispherectomies.
Our findings suggest stagnant rates of both temporal and extratemporal lobe epilepsy surgery across U.S. surgical centers over the past decade. This finding contrasts with prior reports suggesting a recent dramatic decline in temporal lobectomy rates at high-volume epilepsy centers. We also observed higher rates of adverse events when both low- and high-volume centers were examined together, as compared to reports from high-volume centers alone. This is consistent with the presence of a volume-outcome relationship in epilepsy surgery.
Keywords: Epilepsy, Seizures, Epilepsy surgery, Temporal lobectomy, Complications, Demographics
1. Introduction
Recent studies suggest that epilepsy surgery is underutilized and declining in frequency, particularly anterior temporal lobectomies (ATL) (Englot et al., 2012,2013; Jehi et al, 2015; Kaiboriboon et al., 2015).This is despite randomized controlled trials documenting the superiority of ATL compared to continued medical therapy for refractory epilepsy (Engel et al., 2012; West et al, 2015; Wiebe et al, 2001). One potential confound of these studies showing declining rates of surgery is that they are drawn from study populations at historically high-volume epilepsy centers (Englot et al., 2013; Jehi et al., 2015; Kaiboriboon et al, 2015), rather than the smaller community hospitals that now also offer epilepsy surgery. This high-volume bias also holds for complication rates, which tend to be lower in high-volume academic centers (Englot et al, 2013; Rolston et al., 2015). The actual complication rates experienced by patients in the community might be significantly higher (Englot et al, 2013; Rolston et al., 2015). How these trends are manifested outside high-volume centers is unknown.
Patient registries and databases offer one method of answering these questions, but only so far as the limitations are acknowledged. For example, the Nationwide Inpatient Sample (NIS), the source of several studies on epilepsy surgery trends (Englot et al., 2013, 2012), indexes procedures based on International Classification of Diseases (ICD-9) codes. This coding obscures important differences between temporal and extratemporal surgeries, however, since it does not clearly define anatomical locations of procedures, or their indication (e.g., the ICD-9 code for selective amygdalohippocampectomy, 01.39, is the same code for evacuation of cerebral hematoma).
This ambiguity with ICD-9 procedure codes is one reason the more specific Current Procedural Terminology (CPT) codes, which are maintained by the American Medical Association (AMA), are preferred by insurers and newer databases. For instance, there are two CPT codes for ATL alone – 61537 for ATL without electrocorticography (ECoG) and 61538 for ATL with ECoG – whereas ICD-9 has no code specific to anterior temporal lobectomy, only lobectomy in general (01.53). Two databases utilizing CPT codes are of particular interest when examining epilepsy surgery.
The American College of Surgeons (ACS) started the National Surgical Quality Improvement Program (NSQIP) in 2005 as a way to reliably track adverse events in North American hospitals, including centers in Canada and Mexico. It is continually growing and currently documents nearly 3 million procedures sampled randomly from over 600 hospitals (Ingraham et al, 2010; Khuri, 2005; Rowell et al., 2007). The mix of hospitals is heterogeneous, and includes both low- and high-volume centers. Critically, it uses highly trained and frequently audited personnel to enter procedural data and follow patients for complications. Procedures and concurrent procedures are all documented with up to 21 CPT codes per case. Postoperative complications have strictly defined criteria, and are not solely based upon billing data like other nationwide databases. For example, to register in NSQIP as a deep venous thrombosis (DVT), the patient must have a confirmed diagnosis of DVT via duplex venogram, computed tomography (CT) scan, or another definitive imaging modality (including autopsy). Then the patient must be treated with anticoagulation therapy, an inferior vena cava filter, or be documented as refusing these recommended therapies. Asymptomatic events are excluded, as are events that only raise clinical suspicion but lack imaging confirmation. This makes NSQIP a remarkably reliable means of documenting complications, demographics, and procedures.
Though NSQIP contains nearly 3 million surgical procedures, the majority are not for epilepsy surgery. A useful complement, to expand the sample size, is the Centers for Medicare and Medicaid Services (CMS) Part B National Summary Data File. This dataset documents the number of allowed services for each CPT code in the United States (US) by year. These data are limited to beneficiaries of these programs (a large percentage of patients with medically intractable epilepsy do qualify and are enrolled in these programs), but they only describe the frequency of such procedures rather than patient-level characteristics. However, the size and application to such a large segment of the US population makes these data a useful means for documenting trends in surgical procedures.
Using data from multiple independent databases allows us to identify the frequency of epilepsy surgery procedures with greater confidence than one database alone, and the NSQIP dataset in particular allows us to accurately estimate the frequency of surgical complications along with their patient-level predictors. Understanding these statistics, particularly any areas of high variance, will allow us to better identify procedures and modifications requiring further study, so that better surgical guidance can be provided.
2. Materials and methods
All cases of epilepsy surgery were extracted by CPT code (Table 1) from the CMS Part B data file for the years 2000–2013 and the NSQIP database for 2005–2013. The identified CPT code could be either the primary code or any of the supplementary codes listed for the procedure (of which there are 20 in NSQIP).
Table 1.
CPT codes for epilepsy surgery.
| CPT Code | Description |
|---|---|
| 61534 | Craniotomy with elevation of bone flap; for excision of epileptogenic focus without ECoG during surgery |
| 61536 | Craniotomy with elevation of bone flap; for excision of epileptic focus, with ECoG during surgery |
| 61537a | Craniotomy with elevation of bone flap; for lobectomy, temporal lobe, without ECoG during surgery |
| 61538 | Craniotomy with elevation of bone flap; for lobectomy with ECoG during surgery, temporal lobe |
| 61539 | Craniotomy with elevation of bone flap; for lobectomy other than temporal lobe, with ECoG during surgery |
| 61540a | Craniotomy with elevation of bone flap; for lobectomy other than temporal lobe, without ECoG during surgery |
| 61541 | Craniotomy with elevation of bone flap; for transection of corpus callosum |
| 61543 | Craniotomy with elevation of bone flap; for partial or subtotal hemispherectomy |
| 61566a | Craniotomy with elevation of bone flap; for selective amygdalohippocampectomy |
| 61567a | Craniotomy with elevation of bone flap; for multiple subpial transections, with electrocorticography during surgery |
Introduced in 2004.
Starting in 2009, the CMS data file censored procedures when fewer than 11 cases were documented in a single year, for privacy purposes. When relative frequencies were calculated, only the years for which all data were available were used.
Vagus nerve stimulation procedures were not indexed, since the same CPT code is used for all indications, including depression. Pulse generator placement codes also overlap with those used for deep brain stimulation, and again are not differentiated by indication (movement disorder vs. epilepsy).
Demographic data and 30-day complication data were extracted for all cases from the NSQIP database. NSQIP tracks many complications, but some might reflect preexisting conditions. For instance, one tracked complication is postoperative ventilator dependence for longer than 48 h. However, if a patient was ventilator dependent preoperatively, this postoperative event is more a reflection of a preexisting condition, rather than a new complication. We therefore excluded complications when associated preexisting conditions were present, but only when the postoperative complication was present within the first 48 h after the operation. If it took 48 h for the condition to develop, it was unlikely present at the time of surgery, and therefore included. Note that this restriction was only for possible preexisting complications—all complications without evidence of preexisting conditions were included, regardless of timing (0–30 days postoperatively). This correction for possible preexisting conditions was done for the following complications: superficial surgical site infection (SSI), deep incisional SSI, organ space SSI, pneumonia, ventilator-dependence >48h postoperatively, progressive renal insufficiency, acute renal failure, coma lasting >24h, sepsis, and septic shock.
Statistical analysis was performed with version 23 of SPSS (IBM; Armonk, NY, USA). Averages were presented with standard deviation (SD) unless otherwise specified. Multivariate regression was done using the backward Wald method, an exclusion cut-off of 0.1, and a maximum of 200 iterations.
3. Results
From 2000–2013, 6200 cases of epilepsy surgery were identified from the CMS Part B National Summary Data File (5725 cases) and the ACS NSQIP database (475 cases; Table 2). The relative frequency of each procedure was very similar across the two independent datasets, with temporal lobectomy the most frequently performed of all epilepsy surgeries at 58.6–59.2%.
Table 2.
Frequency of procedures from Medicare Part B, 2000–2013, and NSQIP, 2005–2013.
| Procedure | Medicare Part B |
NSQIP |
||
|---|---|---|---|---|
| Number of Procedures | Frequencyb | Number of Procedures | Frequency | |
| Excision of epileptic focus | 680 | 14.3 | 47 | 9.9 |
| Without ECoG | 375 | 5.6 | 11 | 2.3 |
| With ECoG | 605 | 8.7 | 36 | 7.6 |
| Temporal lobectomy | 3346 | 58.6 | 281 | 59.2 |
| Without ECoGa | 1635 | 36.3 | 177 | 37.3 |
| With ECoG | 1711 | 22.4 | 104 | 21.9 |
| Selective Amygdalohippocampectomya | 357 | 8.3 | 31 | 6.5 |
| Other lobectomy | 706 | 12.3 | 90 | 18.9 |
| Without ECoGa | 365 | 8.2 | 54 | 11.4 |
| With ECoG | 341 | 4.1 | 36 | 7.6 |
| Corpus callosotomy | 222 | 3.6 | 14 | 2.9 |
| Hemispherectomy | 72 | 1.6 | 9 | 1.9 |
| Multiple subpial transectionsa | 42 | 1.3 | 10 | 2.1 |
| Total | 5725 | 475 | ||
CPT code introduced in 2004.
Starting in 2009, data were censored if less than 11 procedures were recorded in a given year. This affected codes 61534, 61543, and 61567. Therefore, the frequency of procedures was calculated from only the five years spanning 2004–2008, when all codes were present.
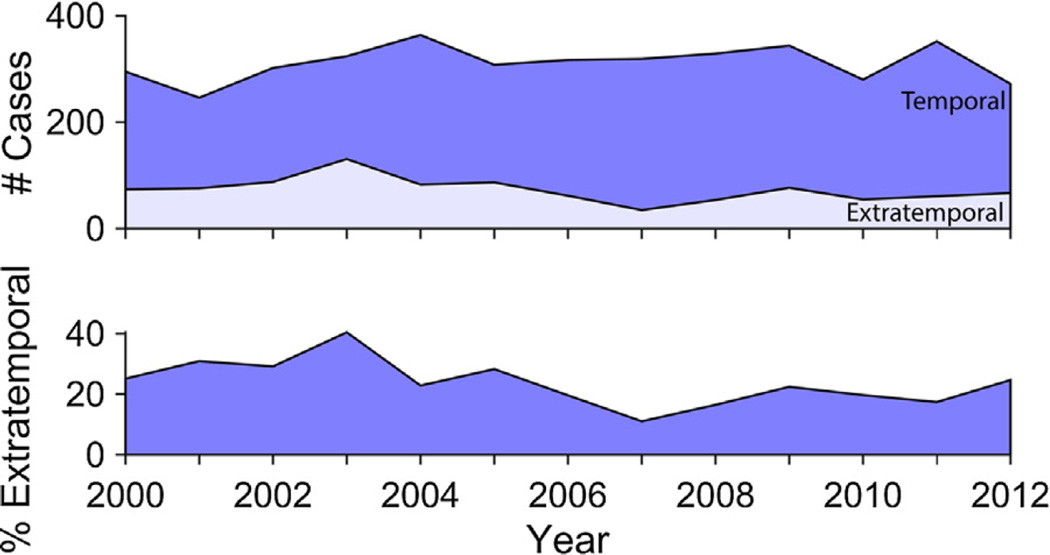
Previous reports had shown a trend for decreasing use of temporal lobectomies over the past several years (Englot et al., 2013; Jehi et al., 2015; Kaiboriboon et al., 2015), with a concomitant increase in extratemporal resections (Jehi et al., 2015; Kaiboriboon et al., 2015). We therefore examined the temporal trends of both temporal lobectomies and extratemporal resections from 2000 to 2013, using the Medicare Part B database (Fig. 1). Neither type of surgery showed a statistically significant decrease or increase over time (F = 2.59, p = 0.13 for temporal lobectomies; F = 3.38, p = 0.09 for extratemporal resections). Because the NSQIP database is not a fixed patient population, and has had a large increase in patient population over time, it cannot be used to track trends in absolute numbers of procedures. However, we compared the ratio of extratemporal resections to ATL over time, which would vary if there were relative changes of frequency between the two procedures, and found no significant change (F = 0.13, p = 0.73).
Fig. 1.
Trends of anterior temporal lobectomies as compared to excision of epileptic foci show no significant change overtime from 2000 to 2012. Upper panel: number of cases from the Medicare Part B database for anterior temporal lobectomies (dark blue; CPT codes 61537 and 61538) compared to the number of craniotomies for excision of epileptic foci (light blue; CPT codes 61534 and 61536). Counts include both cases with ECoG and without ECoG. The year 2013 does not include data for CPT code 61534, excision of epileptic foci without ECoG, so this time point was not included in the graph. There were no significant trends in either procedure over time (F = 2.59, p = 0.13 for temporal lobectomies; F = 3.38, p = 0.09 for extratemporal resections). Lower panel: percentage of craniotomies for epileptic foci compared to anterior temporal lobectomies. There is no significant trend over time (F = 4.751, p = 0.052).
CPT codes specify whether or not ECoG was used for excision of epileptic foci or temporal lobectomies. Analyzing these codes showed that most temporal lobectomies did not utilize ECoG (63.0–64.2%), while most craniotomies for excision of epileptic foci did (64.2–76.6%; Table 3).
Table 3.
Frequency of intraoperative ECoG usage from Medicare Part B, 2004–2013, and from NSQIP, 2005–2013.
| Procedure | Medicare Part B |
NSQIP |
||
|---|---|---|---|---|
| Number of Procedures | Frequencyb | Number of Procedures | Frequency | |
| Excision of epileptic focus | ||||
| Without ECoG | 208 | 35.8 | 11 | 23.4 |
| With ECoG | 403 | 64.2 | 36 | 76.6 |
| Temporal lobectomy | ||||
| Without ECoGa | 1635 | 64.2 | 177 | 63.0 |
| With ECoG | 913 | 35.8 | 104 | 37.0 |
| Other lobectomy | ||||
| Without ECoGa | 365 | 68.5 | 54 | 60.0 |
| With ECoG | 168 | 31.5 | 36 | 40.0 |
CPT code introduced in 2004.
Starting in 2009, data were censored if less than 11 procedures were recorded in a given year. This affected the code 61534 in 2013 only. Therefore the frequency for ECoG use for excision of epileptic foci (not for temporal lobectomy) was calculated from 2004 to 2012. Temporal lobectomy ECoG use was calculated from 2004 to 2013.
Complications in the NSQIP database are rigorously defined and tracked. In patients undergoing epilepsy surgery, complications occurred in 17.9% of cases (Table 4).The most frequent complication was return to surgery within 30 days (5.3%), followed by bleeding requiring transfusion (3.6%; Table 5). Mortality was recorded in 3.4% of cases.
Table 4.
Patient demographics from NSQIP, 2005–2013.
| Characteristic | Number (%) |
|---|---|
| Age (years ± SD) | 45.6 ± 16.8 (range 18–90) |
| Gender | |
| Male | 234 (49.3) |
| Female | 239 (50.3) |
| Unknown | 2 (0.4) |
| Race | |
| White | 385 (81.1) |
| Black or African American | 17 (3.6) |
| Asian or Pacific Islander | 16 (3.4) |
| American Indian or Alaska Native | 4 (0.8) |
| Unknown | 53 (11.2) |
| Hispanic Ethnicity | |
| No | 405 (85.3) |
| Yes | 46 (9.7) |
| Unknown | 24 (5.1) |
| ASA Classification | 2.6 ± 0.6 |
| Length of stay (days) | 6.9 ± 6.6 |
| Total operative time (min) | 224.9 ±97.4 |
| Any complication | 85 (17.9) |
| Majora | 67 (14.1) |
| Minorb | 34 (7.2) |
Major complications include death, return to OR, failure to wean from ventilator, stroke, unplanned reintubation, sepsis, DVT, organ space SSI, progressive renal insufficiency, wound dehiscence, deep SSI, septic shock, cardiac arrest requiring CPR, and myocardial infarction.
Minor complications include bleeding requiring transfusion, UTI, pneumonia, and superficial SSI.
Table 5.
Frequency of complications at 30-days postoperatively, NSQIP 2005–2013.
| Complication | Number (%) |
|---|---|
| Death | 16 (3.4) |
| Return to OR | 25 (5.3) |
| Bleeding requiring transfusion | 17 (3.6) |
| UTI | 13 (2.7) |
| Failure to wean from ventilator | 12 (2.5) |
| Stroke | 10 (2.1) |
| Unplanned reintubation | 9 (1.9) |
| Pneumonia | 7 (1.5) |
| Sepsis | 7 (1.5) |
| DVT | 5 (1.1) |
| Organ space SSI | 5 (1.1) |
| Progressive renal insufficiency | 4 (0.8) |
| Wound dehiscence | 3 (0.6) |
| Deep SSI | 2 (0.4) |
| Septic shock | 2 (0.4) |
| Cardiac arrest requiring CPR | 2 (0.4) |
| Pulmonary embolus | 2 (0.4) |
| Superficial SSI | 1 (0.2) |
| Myocardial infarction | 1 (0.2) |
Univariate analysis of patient-level predictors showed that age, ASA (American Society of Anesthesiologists) classification, male gender, preoperative ventilator dependence, hypertension requiring medication, disseminated cancer, and bleeding disorders all were associated with complications (Table 6). Multivariate logistic regression was then used to control for confounding, and four factors remained predictive: age (OR 1.04; 95% CI 1.02, 1.05), ASA class (OR 1.96; 95% CI 1.25, 3.09), male gender (OR 1.87; 95% CI 1.12, 3.14), and preexisting bleeding disorders (OR5.26; 95% CI 1.12, 24.59; Table 6).
Table 6.
Predictors of complications, NSQIP 2005–2013. Characteristics significant in univariate analysis are shown in bold. For the multivariate model, all characteristics with significant univariate associations were included, except ventilator dependence, since there were not enough data to include it for multivariate regression. n/s = not significant; n/a= not applicable.
| Characteristic | No complication (%) | Complication (%) | Univariate Odds Ratio (95% CI) or t-test p-value | Multivariate Odds Ratio (95% CI) |
|---|---|---|---|---|
| Age (years) | 43.4 ±16.1 | 56.0 ±16.3 | p < 0.0001 | 1.04 (1.02, 1.05) |
| Height (in.) | 66.7 ±4.1 | 67.1 ±4.0 | p = 0.382 | |
| Weight (lbs.) | 177.9 ±45.6 | 181.4±51.9 | p = 0.528 | |
| ASA | 2.6 ±0.6 | 3.0 ±0.6 | p< 0.0001 | 1.96 (1.25, 3.09) |
| Gender | ||||
| Female | 206 (52.8) | 33 (38.8) | 1 [reference] | |
| Male | 182 (46.7) | 52 (61.2) | 1.78 (1.10, 2.88) | 1.87 (1.12, 3.14) |
| Hispanic Ethnicity | ||||
| No | 329 (84.4) | 76 (89.4) | 1 [reference] | |
| Yes | 39 (10.0) | 7 (8.2) | 0.78 (0.33, 1.80) | |
| Race | ||||
| White | 313 (80.3) | 72 (84.7) | 1 [reference] | |
| Black or African American | 14 (3.6) | 3 (3.5) | 0.93 (0.26, 3.33) | |
| American Indian or Alaska Native | 2 (0.5) | 2 (2.4) | 4.35 (0.60, 31.38) | |
| Asian or Pacific Islander | 12 (3.1) | 4 (4.7) | 1.45 (0.45, 4.62) | |
| Diabetes | ||||
| No | 375 (96.2) | 78 (91.8) | 1 [reference] | |
| Yes | 14 (3.6) | 6 (7.1) | 2.06 (0.77, 5.53) | |
| Dyspnea | ||||
| No | 381 (97.7) | 81 (95.3) | 1 [reference] | |
| Yes | 8 (2.1) | 4 (4.7) | 2.35 (0.69, 8.00) | |
| Ventilator dependent | n/a | |||
| No | 389 (99.7) | 77 (90.6) | 1 [reference] | |
| Yes | 1 (0.3) | 8 (9.4) | 40.42 (4.98, 327.81) | |
| Hypertension | n/s | |||
| No | 313 (80.3) | 49 (57.6) | 1 [reference] | |
| Yes | 77 (19.7) | 36 (42.4) | 2.99 (1.82, 4.91) | |
| Chronic steroid use | ||||
| No | 365 (93.6) | 75 (88.2) | 1 [reference] | |
| Yes | 25 (6.4) | 10 (11.8) | 1.95 (0.90, 4.22) | |
| Disseminated cancer | n/s | |||
| No | 375 (96.2) | 75 (88.2) | 1 [reference] | |
| Yes | 15 (3.8) | 10 (11.8) | 3.33 (1.44, 7.70) | |
| Bleeding disorder | ||||
| No | 385 (98.7) | 78 (91.8) | 1 [reference] | 1 [reference] |
| Yes | 5 (1.3) | 7 (8.2) | 6.91 (2.14, 22.34) | 5.26 (1.12, 24.59) |
We also evaluated the frequency of complications within each surgical type (Table 7). Hemispherectomies had the highest frequency of complications (5 of 9 cases; 55.6%), followed by craniotomies for excision of epileptic foci without ECoG (4 of 7 cases; 36.4%). The lowest rate was for craniotomies for excision of epileptic foci with ECoG (1 of 36 cases; 2.8%).
Table 7.
Predictors of complications, NSQIP 2005–2013. Complications were split into major and minor categories. Risk ratios were computed for any complication.
| Procedure | Age (mean±SD) |
No Complication (%) |
Minor Complication (%)a |
Major Complication (%)b |
Any Complication (%) |
Risk Ratio (95% CI) |
Mortality Rate (%) |
|---|---|---|---|---|---|---|---|
| Temporal lobectomy | 45.6 ± 16.5 | 238 (84.7) | 15 (5.3) | 34 (12.1) | 43 (15.3) | 1 [reference] | 6 (2.1) |
| Selective amygdalohippocampectomy | 40.4 ± 12.4 | 29 (93.5) | 1 (3.2) | 1 (3.2) | 2 (6.5) | 0.42 (0.11, 1.66) | 0 |
| Excision of focus | 39.5 ± 15.4 | 42 (89.4) | 3 (6.4) | 4 (8.5) | 5 (10.6) | 0.70 (0.29, 1.66) | 0 |
| Other lobectomy | 50.1 ±17.8 | 62 (68.9) | 13 (14.4) | 20 (22.2) | 28 (31.1) | 2.03 (1.35, 3.07) | 8 (8.9) |
| Corpus callosotomy | 36.3 ± 14.8 | 12 (85.7) | 1 (7.1) | 2 (14.3) | 2 (14.3) | 0.93 (0.25, 3.47) | 0 |
| Hemispherectomy | 56.1 ±20.4 | 4 (44.4) | 3 (33.3) | 4 (44.4) | 5 (55.6) | 3.63 (1.90, 6.92) | 1 (11.1) |
| Multiple subpial transections | 44.3 ± 20.9 | 8 (80.0) | 0 | 2 (20.0) | 2 (20.0) | 1.31 (0.37, 4.65) | 1 (10.0) |
Major complications include death, return to OR, failure to wean from ventilator, stroke, unplanned reintubation, sepsis, DVT, organ space SSI, progressive renal insufficiency, wound dehiscence, deep SSI, septic shock, cardiac arrest requiring CPR, and myocardial infarction.
Minor complications include bleeding requiring transfusion, UTI, pneumonia, and superficial SSI.
Thirty-day mortality rate was also attributed to each procedure type (Table 7). The highest rate was for hemispherectomies (11.1%), while temporal lobectomies had a rate of 2.1%.
Univariate analysis was used to detect a relationship between ECoG usage and complications (Table 8). While there was a statistically significant protective effect of ECoG during excision of epileptic foci (RR 0.8; 95% CI 0.01, 0.61), there was no statistically significant difference for ECoG use in temporal lobectomies (RR 0.74; 95% CI 0.4, 1.35) or other lobectomies (RR 1.13; 95% CI 0.61, 2.09). Additionally, if ECoG usage was added to the multivariate model in Table 6, it failed to show a significant association with complications.
Table 8.
Effect of intraoperative ECoG on complications.
| Procedure | No complication (%) | Complication (%) | Risk Ratio (95% CI) |
|---|---|---|---|
| Excision of focus | |||
| Without ECoG | 7 (63.6) | 4 (36.4) | 1 [reference] |
| With ECoG | 35 (97.2) | 1 (2.8) | 0.08 (0.01, 0.61) |
| Temporal lobectomy | |||
| Without ECoG | 147 (83.1) | 30 (16.9) | 1 [reference] |
| With ECoG | 91 (87.5) | 13 (12.5) | 0.74 (0.4, 1.35) |
| Other lobectomy | |||
| Without ECoG | 38 (70.4) | 16 (29.6) | 1 [reference] |
| With ECoG | 24 (66.7) | 12 (33.3) | 1.13 (0.61, 2.09) |
4. Discussion
Here, we used multiple independent databases – Medicare Part B and ACS NSQIP – to describe epilepsy surgery as practiced by the North American community of neurosurgeons, in both academic and private centers, and both high- and low-volume centers.
4.1. Rates of epilepsy surgery
The overall rates and ratios of the different surgeries we described did not significantly change over time (Fig. 1). This is in comparison to recent data from the National Association of Epilepsy Centers (NAEC), where temporal lobectomies decreased by >65% between 2006 and 2010, and extratemporal surgeries more than doubled (Kaiboriboon et al., 2015). It also differs from a recent major study across nine high-volume centers in North America, Germany, and Australia, which showed a 48% drop in temporal lobectomies as of 2011, from a baseline in either 1991 or 2001 (the baseline was chosen to maximize the difference for each center), along with a parallel increase in extratemporal resections (Jehi et al, 2015).
As in our data, the large changes in procedural frequency seen in the NAEC dataset (Kaiboriboon et al, 2015) and the high-volume centers in the Jehi et al. study (Jehi et al., 2015) were not identified in the Nationwide Inpatient Sample (Englot et al, 2013, 2012). Though, when procedures were selected based on hospital volume, there was a trend for higher-volume centers to begin performing fewer procedures than lower-volume community hospitals over time (Englot et al., 2013).
As noted, the two databases we examined showed a stable rate of epilepsy surgery since 2000. Since our databases include a heterogeneous mix of hospital types, and are not limited to large academic or high-volume centers, one potential explanation is that low-volume centers are performing more surgeries. The sources of such a shift to low-volume centers are unclear.
However, it should also be noted that our data includes years before and after the 2001 randomized trial by Wiebe et al. (2001) showing the superiority of ATL over continued medical management for refractory epilepsy, and also before and after the 2003 American Academy of Neurology (AAN) practice guidelines recommending referral to epilepsy surgery centers for refractory patients (Engel et al., 2003). The fact that rates have remained stagnant again supports the finding that epilepsy surgery is not increasing in frequency despite the publication of these trials and guidelines (Englot et al, 2012; Rolston et al., 2015).
Despite being independently constructed, both databases showed very similar results for the type and frequency of epilepsy surgeries. As anticipated, temporal lobectomies were the most commonly performed procedure, accounting for 59% of all procedures. The second most common surgery in our study was for extratemporal lobectomy (12.3–18.9%), followed by craniotomy for excision of epileptic focus 9.9–14.3%, selective amygdalohippocampectomy (6.5–8.3%), corpus callosotomy (2.9–3.6%), hemispherectomy (1.6–1.9%), and MST (1.3–2.1%). The rate of temporal lobectomies we observed is actually lower than 2010–2011 data from the United Kingdom, where the rate was 75.2% (Neligan et al, 2013). Also of note, extratemporal resections were more common in the UK (21.9%) than in our datasets, and the other procedures much less common: hemispherectomies at 1.7%, corpus callosotomies at 0.8%, and MST at 0.4%, which accounted for just one of their 242 procedures (Neligan et al, 2013).
An interesting question is whether or not these rates of different surgery types correspond to the prevalence of different types of epilepsy. For example, is the rate of extratemporal resections proportional to the number of patients with refractory extratemporal epilepsy? If mismatches were present, it would identify areas of distinct underutilization. Recent epidemiological data is hard to find in this regard, especially when looking for refractory cases that would ultimately be surgical candidates, but would be a fruitful area of research.
4.2. Usage of intraoperative ECoG
Intraoperative ECoG is used to identify interictal epileptiform activity during surgery in order to identify putatively epileptogenic areas for resection. As described in the introduction, the literature is mixed on the utility of intraoperative ECoG. For MRI-identified mesial temporal sclerosis, several studies suggest that it is non-informative and should not be routinely used (Schwartz et al., 1997; Yang et al., 2014), although others maintain that it is useful for tailoring the extent of temporal lobe resection (McKhann et al., 2000). For nonlesional epilepsy, the findings are also mixed, with studies showing both improvement when ECoG is used (Berger et al, 1993), and a detriment when it was used (Tripathi et al., 2010). The rate of ECoG usage we observed, 35.8–37.0%, shows that over one-third of surgeons are using ECoG for temporal lobectomies (Table 3). These practices reinforce the need for better data to guide surgeons on the utility of this technique.
Intraoperative ECoG preferences are reversed for extra-temporal resections, where 64.2–76.6% of cases used this technique. This shows that at least two-thirds of surgeons use ECoG for extra-temporal cases, as compared to one-third for temporal lobectomies (Table 3). The evidence for ECoG in extra-temporal epilepsy is, on balance, stronger than that for temporal lobe epilepsy, whether lesional (Berger et al., 1993; Palmini et al., 1995; Salanova et al., 1992; Tripathi et al., 2010) or not (Jayakar et al., 2008). This likely explains the greater usage of this technique in extratemporal cases. But again, usage is not universal, with one-quarter to one-third of surgeons operating without ECoG. As with temporal lobectomies, these practices show that more studies are needed to provide clear guidance on the use of ECoG.
4.3. Complications
Across all surgery types, complications were present in 17.9% of cases in the NSQIP database (Table 7), with 15.3% in temporal lobectomies. This rate is higher than previous estimates from high-volume centers. For example, Wiebe et al. (2001) report a 10% rate of complications from their randomized clinical trial of temporal lobectomies. Behrens et al. (1997) report a surgical complication rate of 8.4% (though they also separate this from their 5.4% rate of “neurological” complications). Using billing data in the Nationwide Inpatient Sample, Englot et al. (2013) found a rate of 6.1% for adverse events at high-volume epilepsy centers, and 12.9% at low-volume centers for temporal lobectomies specifically.
Mortality was observed in 16 patients (3.4%). Half of these were from extratemporal lobectomies (8 of 90 cases, 8.9% rate). The highest rate among the different surgeries was for hemispherectomies (1 of 9 cases; 11.1%), and deaths occurred in 6 cases of temporal lobectomy (2.1%). As with other adverse events, these rates are higher than those reported in academic series from high-volume centers. For example, (Behrens et al., 1997), (Wiebe et al., 2001), and (Engel et al., 2012) report 0% mortality in their respective series of temporal lobectomies. Englot et al. found a 0.3% rate of mortality for temporal lobectomies at medium-volume centers in the Nationwide Inpatient Sample, but none at low- or high-volume centers (Englot et al., 2013). Other studies concur with this rate less then or around 1%: Hader et al. report a rate of 0.4% for temporal lobectomies amongst a systematic review of academic center reports, and a rate of 1.2% for extratemporal resections (Hader et al., 2013), and a large systematic review from Ontario found a rate of 0.1–0.5% (Health Quality Ontario, 2012) for temporal lobectomies. While the cause of death is not recorded for these 16 patients, 2 had documented cardiac arrest requiring CPR, 3 had prolonged ventilator dependence postoperatively, 1 had sepsis, 1 had septic shock, and 1 had pneumonia requiring reintubation postoperatively. The other 8 had no additional recorded complications.
What explains the rate of complications we observed, which was higher than prior studies? One reason is likely the inclusion of low-volume and non-academic centers. Volume-outcome relationships are commonplace throughout neurosurgery (Davies et al., 2015), and specifically in epilepsy surgery (Englot et al., 2013; Rolston et al., 2015). For example, the rate of adverse events for temporal lobectomies at low-volume centers was more than twice that of high-volume centers (12.9 vs. 6.1%) amongst patients in the Nationwide Inpatient Sample (Englot et al., 2013), and mortality was significantly lower for hemispherectomies in higher-volume hospitals than low-volume hospitals (Lin et al, 2014). While we cannot specifically disaggregate our data by hospital size or volume, it is likely that the inclusion of many low-volume centers might partially account for our observed rate of complications.
Secondly, the NSQIP database has stringent criteria for defining each complication, and uses a well-trained and frequently audited staff to identify and enter these complications. It also includes many medical complications, such as pneumonias and urinary tract infections, that surgeons might inadvertently neglect in lieu of more “surgical” complications, like strokes, hematomas, and infections. As such, NSQIP tends to capture more complications than retrospective reviews and even many clinical trials. This could be one reason that our rate is greater than prior reports.
The most frequent complication we observed was an unplanned return to surgery within 30 days, occurring in 5.3% of cases (Table 5). Causes for returns to surgery were only documented beginning in 2012, providing us with postoperative diagnoses for 11 of these 25 patients (Table 9). The most common cause for return to OR was hemorrhage, but also included spinal fluid leaks and 2 respiratory issues (one of which required a tracheostomy and the other a gastrostomy).
Table 9.
Causes of return to OR, 2012–2013. NSQIP began tracking ICD-9 codes for returns to the operating room starting in 2012. 11 of the 25 cases with returns to the OR were in this time period, and their postoperative diagnoses (for the reoperation) are included below.
| Complication | Number |
|---|---|
| Cerebral or cerebellar hemorrhage | 2 |
| Subdural hemorrhage | 1 |
| Subarachnoid hemorrhage | 1 |
| Hematoma, unspecified | 1 |
| Stroke | 1 |
| Hydrocephalus | 1 |
| Respiratory failurea | 1 |
| Aspiration pneumoniab | 1 |
| Spinal fluid leak | 1 |
| Pseudomeningocele | 1 |
Return to OR for tracheostomy.
Return to OR for gastrostomy tube placement.
Significant predictors of complications were age, gender, ASA classification, and the presence of a preexisting bleeding disorder (Table 6). There were too few cases of each complication to describe a reliable breakdown of complications for each surgery type (e.g., hemispherectomy vs. temporal lobectomy), and there did not appear to be any obvious trends. For example, the complications for hemispherectomy were one each of a superficial SSI, pneumonia, urinary tract infection, stroke, bleeding requiring transfusion, deep venous thrombosis, return to OR in 30 days, and postoperative death.
As expected by the physical size of the operation, hemispherectomies had the highest rate of complications (55.6%), and a significantly elevated relative risk of 3.63 (95% CI 1.90, 6.92) as compared to temporal lobectomies (Table 7). This rate is remarkably similar to the 56% rate of complications for hemispherectomies observed in the Nationwide Inpatient Sample (Vadera et al., 2015).
Overall, while complications appear more frequently in the above data, it is likely a reflection of more stringent surveillance by NSQIP and the inclusion of low-volume centers and consequent volume-outcome effects (Englot et al, 2013; Lin et al., 2014). Randomized-clinical trials and academic case series continue to show that epilepsy surgery is safe and effective at those centers (Engel et al., 2012; Hader et al, 2013; Health Quality Ontario, 2012; West et al., 2015; Wiebe et al, 2001).
4.4. Limitations
Individual databases will always be limited in their applicability to broader populations, since there is inevitably selection bias in their construction. This is true with the Medicare database, which includes only Medicare patients (and is therefore a limited subsampling of all epilepsy patients), and the NSQIP database, which is a limited subset of all North American hospitals. These limitations also apply to other studies of restricted populations, like those limited to NAEC hospitals (Kaiboriboon et al, 2015) or high-volume epilepsy centers (Jehi et al., 2015). In terms of sample size, the Medicare database is far larger than that analyzed in the study of high-volume centers (5725 patients in Medicare vs. 1349 patients) (Jehi et al, 2015), but smaller than the NAEC dataset (though this also includes pediatric epilepsy surgery, which accounted for 41.5% of the surgical volume in 2012) (Kaiboriboon et al., 2015). Selection bias is one reason we included data from multiple databases in our analyses, so they might complement one another and highlight generalities across datasets. When comparing our databases, individual metrics like the rate of various types of surgery (e.g., temporal lobectomy vs. hemispherectomy) were remarkably consistent—and this was true even when compared to datasets from other countries (Neligan et al., 2013). These similarities in independently constructed databases give us more confidence in the generalizability of their findings. Nevertheless, the results of the present paper should be viewed alongside analyses drawn from different datasets, like the NIS, NAEC, or high-volume centers.
Another limitation is the available adverse event data present in the NSQIP database. Because NSQIP is a general surgical database, many of the specific complications of neurosurgical procedures (e.g., CSF leaks, visual field deficits) are not tracked. Additionally, the database only tracks complications for 30 days. Databases and registries specific to neurosurgery and epilepsy surgery would obviously address these issues, but unfortunately do not yet exist. On the other hand, there are some advantages of tracking general complications, like UTIs and cardiac events, in neurosurgical procedures, since these oftentimes are overlooked by neurosurgeons who tend to focus solely on neurological complications at the expense of other organs. For example, when looking at extra-operative ECoG case series, no article had ever reported UTI as a complication, though it appears to happen in roughly 1 in every 50 cases (Rolston et al, 2016).
5. Conclusions
Using two independent North American databases, which draw from both low- and high-volume hospitals, we found that the rate of epilepsy surgery is overall stable. This contrasts with recent reports from high-volume centers showing a decline in temporal lobectomies and a possible increase in extratemporal surgeries. It also highlights the stagnation of epilepsy surgery rates despite multiple randomized clinical trials and practice guidelines advocating its use. We also found a higher rate of adverse events than previously reported by high-volume centers, suggesting the presence of a volume-outcome relationship for epilepsy surgery. Further research should attempt to understand the sources of these trends in surgical volume, and also the best way to reduce surgical complications across all practice settings.
Acknowledgments
The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors. JDR was supported by a fellowship (F32DC013953-01) from the National Institute on Deafness and Other Communication Disorders.
References
- Behrens E, Schramm J, Zentner J, König R. Surgical and neurological complications in a series of 708 epilepsy surgery procedures. Neurosurgery. 1997;41:1–9. doi: 10.1097/00006123-199707000-00004. discussion 9–10. [DOI] [PubMed] [Google Scholar]
- Berger MS, Ghatan S, Haglund MM, Dobbins J, Ojemann GA. Low-grade gliomas associated with intractable epilepsy: seizure outcome utilizing electrocorticography during tumor resection. J. Neurosurg. 1993;79:62–69. doi: 10.3171/jns.1993.79.1.0062. [DOI] [PubMed] [Google Scholar]
- Davies JM, Ozpinar A, Lawton MT. Volume-outcome relationships in neurosurgery. Neurosurg. Clin. N. Am. 2015;26 doi: 10.1016/j.nec.2014.11.015. http://dx.doi.org/10.1016/j.nec.2014.11.015,207-18-viii. [DOI] [PubMed] [Google Scholar]
- Engel J, Wiebe S, French J, Sperling M, Williamson P, Spencer D, Gumnit R, Zahn C, Westbrook E, Enos B. Quality Standards Subcommittee of the American Academy of Neurology, American Epilepsy Society, American Association of Neurological Surgeons, Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003 doi: 10.1212/01.wnl.0000055086.35806.2d. http://dx.doi.org/10.1212/01,WNL.0000055086.35806.2D. [DOI] [PubMed]
- Engel J, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, Sperling MR, Gardiner I, Erba G, Fried I, Jacobs M, Vinters HV, Mintzer S, Kieburtz K Early Randomized Surgical Epilepsy Trial (ERSET) Study Group. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012;307:922–930. doi: 10.1001/jama.2012.220. http://dx.doi.org/10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Ouyang D, Garcia PA, Barbaro NM, Chang EF. Epilepsy surgery trends inthe United States, 1990–2008. Neurology. 2012;78:1200–1206. doi: 10.1212/WNL.0b013e318250d7ea. http://dx.doi.org/10.1212/WNL.0b013e318250d7ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Ouyang D, Wang DD, Rolston JD, Garcia PA, Chang EF. Relationship between hospital surgical volume, lobectomy rates, and adverse perioperative events at US epilepsy centers. J. Neurosurg. 2013;118:169–174. doi: 10.3171/2012.9.JNS12776. http://dx.doi.org/10.3171/2012.9.JNS12776. [DOI] [PubMed] [Google Scholar]
- Hader WJ, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L, Wiebe S, Kwon C-S, Jette N. Complications of epilepsy surgery: a systematic review of focal surgical resections and invasive EEG monitoring. Epilepsia. 2013;54:840–847. doi: 10.1111/epi.12161. http://dx.doi.org/10.1111/epi.12161. [DOI] [PubMed] [Google Scholar]
- Health Quality Ontario. Epilepsy surgery: an evidence summary. Ont. Health Technol. Assess. Ser. 2012;12:1–28. [PMC free article] [PubMed] [Google Scholar]
- Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv. Surg. 2010;44:251–267. doi: 10.1016/j.yasu.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Jayakar P, Dunoyer C, Dean P, Ragheb J, Resnick T, Morrison G, Bhatia S, Duchowny M. Epilepsy surgery in patients with normal or nonfocal MRI scans: integrative strategies offer long-term seizure relief. Epilepsia. 2008;49:758–764. doi: 10.1111/j.1528-1167.2007.01428.x. http://dx.doi.org/10.1111/j.1528-1167.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- Jehi L, Friedman D, Carlson C, Cascino G, Dewar S, Elger C, Engel J, Knowlton R, Kuzniecky R, McIntosh A, O’Brien TJ, Spencer D, Sperling MR, Worrell G, Bingaman B, Gonzalez Martinez J, Doyle W, French J. The evolution of epilepsy surgery between 1991 and 2011 in nine major epilepsy centers across the United States, Germany, and Australia. Epilepsia. 2015;56 doi: 10.1111/epi.13116. http://dx.doi.org/10.1111/epi.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiboriboon K, Malkhachroum AM, Zrik A, Daif A, Schiltz NM, Labiner DM, Lhatoo SD. Epilepsy surgery in the United States: analysis of data from the National Association of Epilepsy Centers. Epilepsy Res. 2015:105–109. doi: 10.1016/j.eplepsyres.2015.07.007. http://dx.doi.org/10.1016/j.eplepsyres.2015.07.007. [DOI] [PubMed]
- Khuri SF. The NSQIP: a new frontier in surgery. Surgery. 2005;138:837–843. doi: 10.1016/j.surg.2005.08.016. http://dx.doi.org/10.1016/j.surg.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Lin Y, Harris DA, Curry DJ, Lam S. Trends in outcomes, complications, and hospitalization costs for hemispherectomy in the United States for the years 2000–2009. Epilepsia. 2014;56:139–146. doi: 10.1111/epi.12869. http://dx.doi.org/10.1111/epi.12869. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Schoenfeld-McNeill J, Born DE, Haglund MM, Ojemann Ga. Intraoperative hippocampal electrocorticography to predict the extent of hippocampal resection in temporal lobe epilepsy surgery. J. Neurosurg. 2000;93:44–52. doi: 10.3171/jns.2000.93.1.0044. http://dx.doi.org/10.3171/jns.2000.93.1.0044. [DOI] [PubMed] [Google Scholar]
- Neligan A, Haliasos N, Pettorini B, Harkness WFJ, Solomon JK. A survey of adult and pediatric epilepsy surgery in the United Kingdom. Epilepsia. 2013;54:e62–e65. doi: 10.1111/epi.12148. http://dx.doi.org/10.1111/epi.12148. [DOI] [PubMed] [Google Scholar]
- Palmini A, Gambardella A, Andermann F, Dubeau F, da Costa JC, Olivier A, Tampieri D, Gloor P, Quesney F, Andermann E. Intrinsic epileptogenicity of human dysplastic cortex as suggested by corticography and surgical results. Ann. Neurol. 1995;37:476–487. doi: 10.1002/ana.410370410. http://dx.doi.org/10.1002/ana.410370410. [DOI] [PubMed] [Google Scholar]
- Rolston JD, Ouyang D, Englot DJ, Wang DD, Chang EF. National trends and complication rates for invasive extraoperative electrocorticography in the USA. J. Clin. Neurosci. 2015;22:823–827. doi: 10.1016/j.jocn.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolston JD, Englot DJ, Cornes S, Chang EF. Major and minor complications in extraoperative electrocorticography: a review of a national database. Epilepsy Res. 2016;122:26–29. doi: 10.1016/j.eplepsyres.2016.02.004. http://dx.doi.org/10.1016/j.eplepsyres.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell KS, Turrentine FE, Hutter MM, Khuri SF, Henderson WG. Use of national surgical quality improvement program data as a catalyst for quality improvement. J. Am. Coll. Surg. 2007;204:1293–1300. doi: 10.1016/j.jamcollsurg.2007.03.024. http://dx.doi.org/10.1016/j.jamcollsurg.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Salanova V, Andermann F, Olivier A, Rasmussen T. Occipital lobe epilepsy: electroclinical manifestations, electrocorticography, cortical stimulation and outcome in 42 patients treated between 1930 and 1991. Brain. 1992;115:1655–1680. doi: 10.1093/brain/115.6.1655. http://dx.doi.org/10.1093/brain/115.6.1655. [DOI] [PubMed] [Google Scholar]
- Schwartz TH, Bazil CW, Walczak TS, Chan S, Pedley TA, Goodman RR. The predictive value of intraoperative electrocorticography in resections for limbic epilepsy associated with mesial temporal sclerosis. Neurosurgery. 1997;40:302–309. doi: 10.1097/00006123-199702000-00014. [DOI] [PubMed] [Google Scholar]
- Tripathi M, Garg A, Gaikwad S, Bal CS, Chitra S, Prasad K, Dash HH, Sharma BS, Chandra PS. Intraoperative electrocorticography in lesional epilepsy. Epilepsy Res. 2010;89:133–141. doi: 10.1016/j.eplepsyres.2009.12.007. http://dx.doi.org/10.1016/j.eplepsyres.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Vadera S, Griffith SD, Rosenbaum BP, Seicean A, Kshettry VR, Kelly ML, Weil RJ, Bingaman W, Jehi L. National Trends and in-hospital complication rates in more than 1600 hemispherectomies from 1988–2010. Neurosurgery. 2015;77:185–191. doi: 10.1227/NEU.0000000000000815. http://dx.doi.org/10.1227/NEU.0000000000000815. [DOI] [PubMed] [Google Scholar]
- West S, Nolan SJ, Cotton J, Gandhi S, Weston J, Sudan A, Ramirez R, Newton R. Surgery for epilepsy. Cochrane Database Syst. Rev. 2015;7 doi: 10.1002/14651858.CD010541.pub2. http://dx.doi.org/10.1002/14651858,CD010541.pub2://dx.doi.org/10.1002/14651858,CD010541.pub2. [DOI] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N. Engl. J. Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. http://dx.doi.org/10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Yang T, Hakimian S, Schwartz TH. Intraoperative ElectroCorticoGraphy (ECog): indications, techniques, and utility in epilepsy surgery. Epileptic. Disord. 2014;16:271–279. doi: 10.1684/epd.2014.0675. http://dx.doi.org/10.1684/epd.2014.0675. [DOI] [PubMed] [Google Scholar]