Abstract
This report describes the synthesis and characterization of novel N-heterocyclic carbene (NHC) gold(I) complexes and their bioconjugation to the CCRF-CEM leukemia specific aptamer sgc8c. Confirmation of successful bioconjugation was achieved by using fluorescent tags on both the NHC-Au(I) complex and the aptamer. Cell viability assays indicate the NHC-Au(I)-aptamer conjugate is more cytotoxic than the NHC-gold complex alone. A combination of flow cytometry, confocal microscopy, and cell viability assays provide clear evidence that the NHC-Au(I)-aptamer conjugate is selective for targeted CCRF-CEM leukemia cells.
Keywords: NHC, DNA aptamer, bioconjugation, cancer cell, selectivity, carbene, cytotoxicity
Graphical Abstract
N-heterocyclic carbenes (NHCs) are an interesting class of ligands with strong σ-donating properties.[1] The resulting strong bond between transition metals and the NHCs render their metal complexes stable to air, heat, water, and acid.[2] In the context of potentially reducing the side effects caused by metallo-drug demetalation and degradation in the body, NHC-metal complexes offer a promising alternative, especially considering their ease of synthesis and vast structural diversity.[3, 4] In the last decade, NHC-Au complexes have drawn attention as a new generation of potential anticancer agents due to their impressively high cytotoxicity and stability. A series of neutral and cationic NHC-Au complexes with a wide diversity of ligand structures were synthesized and characterized in recent years.[3–5] NHC-Au(I) complexes can induce cell apoptosis by targeting mitochondrial-related cellular pathways[6, 7] that involve thioredoxin reductase (TrxR) and Estrogen Receptor (ER).[3, 7] Though NHC-Au(I) complexes inhibit the growth of tumor cells, they also interact with human normal cells, a characteristic common for metal based drugs,[8] thus limiting their potential as cancer therapeutics.[9] [10] Cell-selectivity is an obvious area in need of improvement.
This report describes a strategy to target cancer cells efficiently while potentially reducing metal-based side effects. The strategy involves covalently binding a cytotoxic NHC-Au(I) complex to a DNA aptamer. Aptamers are short single-stranded oligonucleotides which are biocompatible, stable and more importantly, they are able to specifically recognize and effectively bind to their targets—in this case—cancer cells.[11] With all these advantages, DNA-aptamers are one of the most attractive drug delivery agents.[12–14] Currently, several reports feature aptamer-drug conjugates, but most rely on noncovalent association of the drug with specific DNA sequences.[13] Non-covalent strategies have disadvantages such as complicated syntheses, uncontrollable drug loading, and uncontrollable drug release.[15] One of the reports of covalent attachment involves tethering the anti-cancer agent Doxorubicin (Dox) to an aptamer.[14] However, Dox causes side effects, such as congestive heart failure,[16] and typhlitis.[17] In addition, resistance to Dox is common, representing a major obstacle to successful treatment.[18]
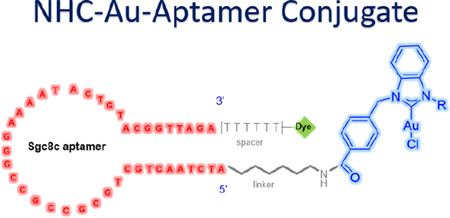
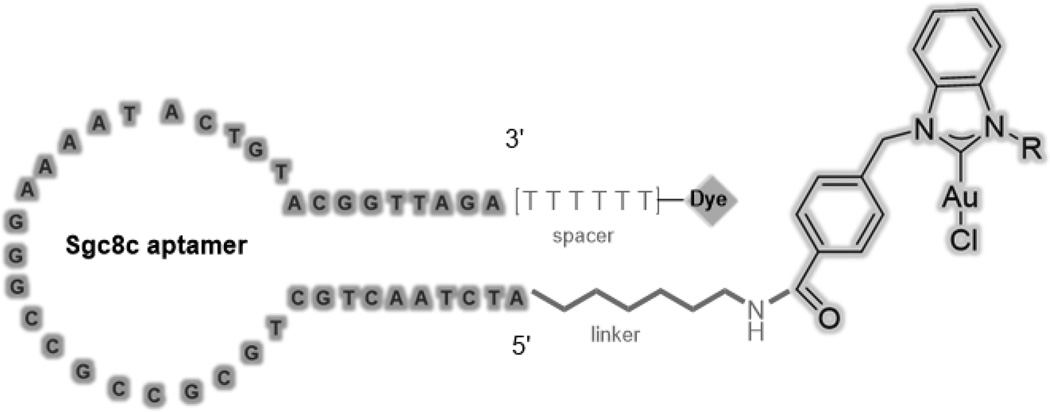
Herein, we describe the first bioconjugation of a NHC-Au(I) complex to an aptamer (Figure 1) and demonstrate its cancer-cell-specific internalization and cytotoxicity. As a model experiment, the sgc8c aptamer was chosen as the drug carrier. The sgc8c aptamer is able to recognize and target, with high binding affinity, the protein tyrosine kinase 7 (PTK-7), a transmembrane receptor protein highly expressed on CCRF-CEM leukemia cells.[19] The formation, selectivity, and cytotoxicity of the aptamer-drug conjugates were verified by fluorescence spectroscopy, high performance liquid chromatography, flow cytometric analysis, confocal fluorescence microscopy, and MTS cell proliferation assays (MTS = 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2(4-sulfophenyl)-2H-tetrazolium).
Figure 1.
Design of the aptamer-NHC-Au(I) conjugate.
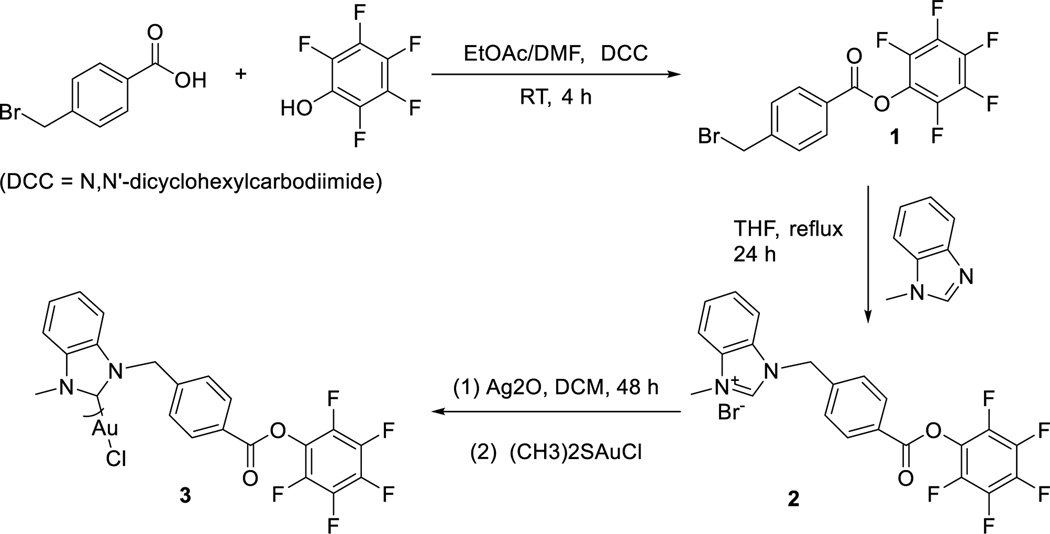
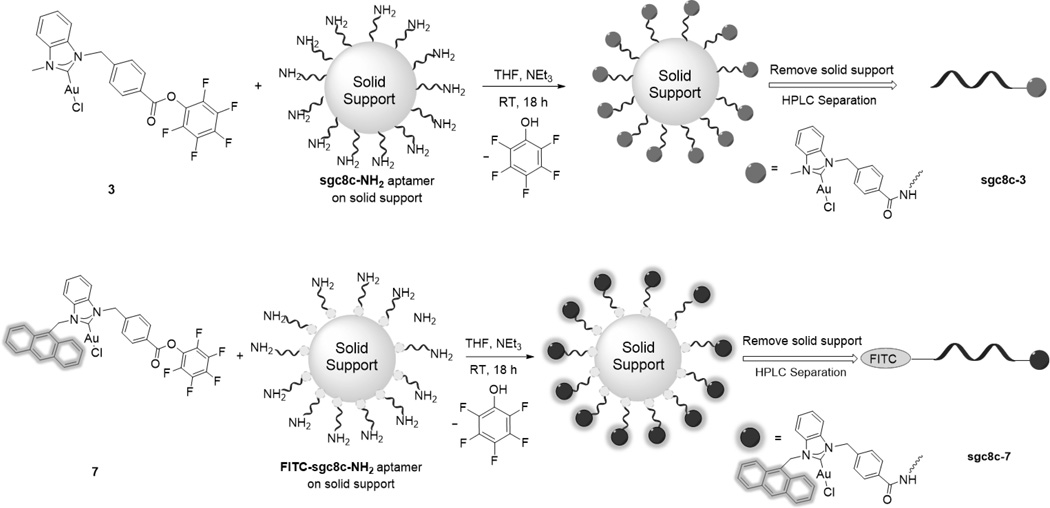
The half-life of an amide bond is approximately 600 years in pH = 7 solution and at ambient temperature.[20] The stability and biocompatibility of amide linkages make them highly attractive for site-specific bioconjugation.[21] Compared to alkyl-esters, activated esters such as pentafluorophenyl esters (Pfp-esters) react with a wider range of nucleophiles.[22] More importantly, activated esters are stable in the solid state but react rapidly with amines in solution under mild conditions with high yield and purity.[23, 24] Considering these facts, our synthetic design involves functionalizing a NHC ligand with a Pfp-ester group (Scheme 1), metalation with Au(I), and subsequent bioconjugation to an amine group functionalized on the 5’ end of the DNA aptamer sgc8c.[25]
Scheme 1.
Synthesis of Pfp-ester functionalized NHC-Au(I) complex 3.
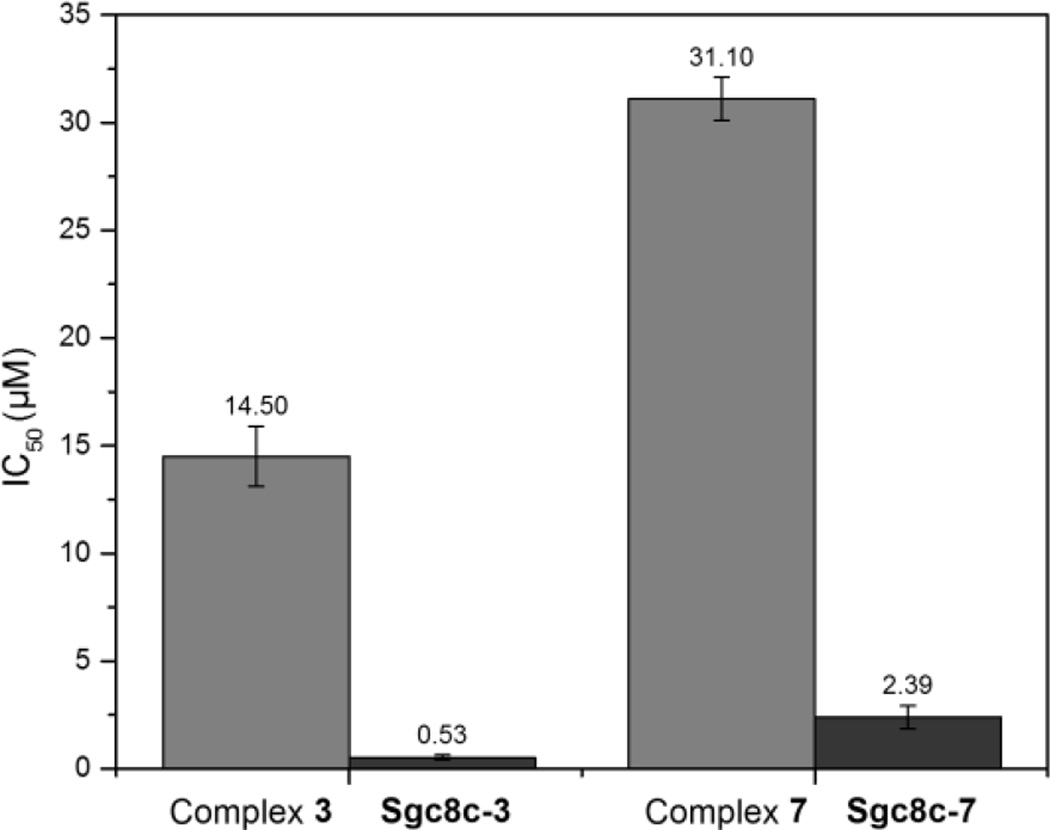
A two-step procedure yields the benzimidazolium NHC-Pfp ligand precursor 2 (Scheme 1).[23] Confirming its identity, the 1H NMR spectrum of 2 exhibits a typical downfield resonance for the imidazolium C2 proton at 11.74 ppm. A notable resonance in the 13C{1H} spectrum is the carbonyl that resonates at 166.4 ppm. Silver(I) transmetalation with dimethylsulfidegold(I) chloride ((CH3)2SAuCl) provides the NHC-Au(I) complex 3. Compared to ligand 2, the downfield proton resonance at 11.74 ppm is absent in the spectrum of complex 3. In the 13C{1H} NMR spectrum of 3, the imidazole carbon resonance shifts downfield from 141.3 ppm for 2, to 174.1 ppm for 3. The carbonyl carbon maintains its position at 166.8 ppm, suggesting that the ester oxygen atoms are not interacting with the Au(I) ion. Finally, resonances at 152.41, 157.72, and 162.16 ppm in the 19F NMR spectrum confirm that complex 3 retains the Pfp-ester moiety. Employing an MTS cell proliferation assay against the human T-lymphoblastic leukemia cell line CCRF-CEM, the cytotoxicity of complex 3 was evaluated (see ESI). With an IC50 of 14.6 ± 1.40 µM, complex 3 is cytotoxicity towards the target cell.
The next step involved elucidating chemistry to conjugate complex 3 to an amine functionalized aptamer. Mimicking the amine functionalized aptamer, a test reaction with furfurylamine was examined. Treating complex 3 with furfurylamine provides the NHC-Au(I)-amide complex 4 in 91% isolated yield within 6 h (see ESI). Considering the successful test reaction, the NH2-modified sgc8c aptamer was synthesized.[26, 27] To accomplish the bioconjugation reaction, 0.1 µmol (1 equiv) of solid supported aptamer suspended in 500 µL of THF was treated with complex 3 (10 µmol, 100 equiv) followed by slow addition of 1.4 µL of Et3N (Scheme 3). The mixture was shaken at ambient temperature for 18 h, then the solid support was removed and the NHC-Au(I)-aptamer conjugate sgc8c-3 was purified via HPLC separation (Scheme 2). The MTS assay of the aptamer-drug conjugate reveals an impressive increase in cytotoxicity compared to complex 3 alone. The IC50 value decreased from 14.6 ± 1.40 for 3 to 0.54 ± 0.85 µM for sgc8c-3. Possible explanations for the increased cytotoxicity include high affinity and efficient internalization of the aptamer conjugate to the target cells, and the enhanced hydrophilic nature of the drug after conjugation to the aptamer.[14, 28]
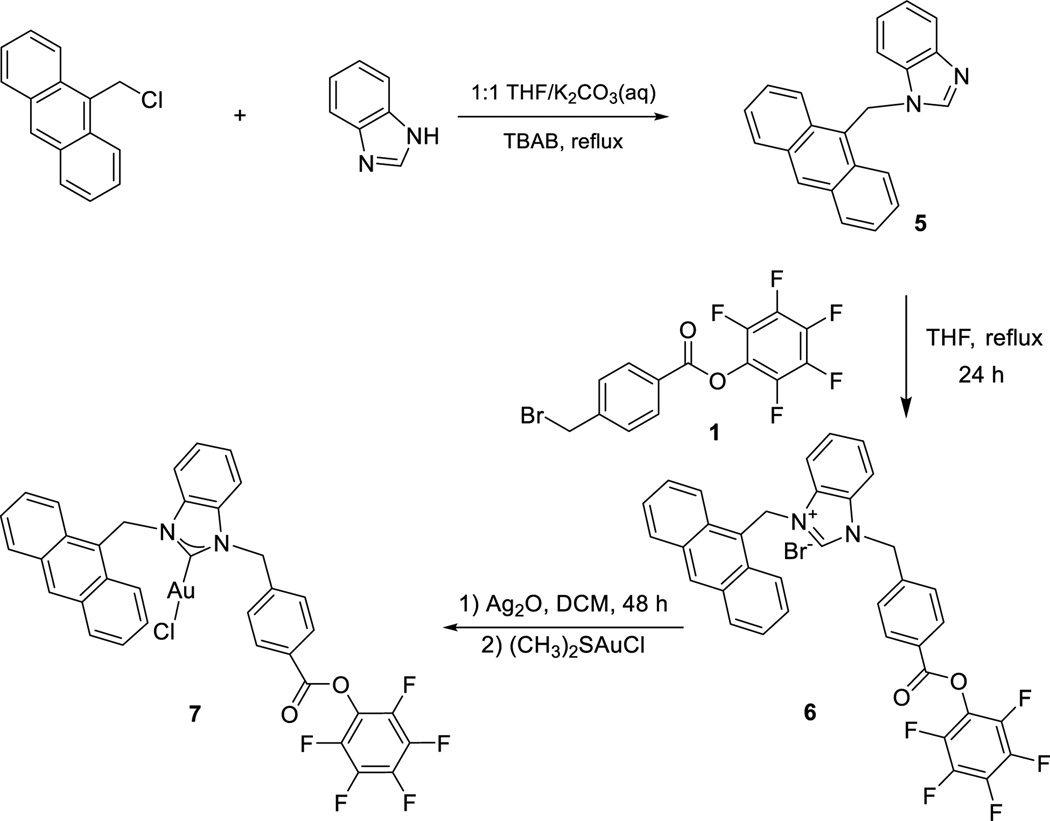
Scheme 3.
Synthesis of fluorescent Pfp-ester functionalized complex 7.
Scheme 2.
Synthesis strategy of aptamer-NHC-Au conjugates sgc8c-3 and sgc8c-7
As a tool for monitoring the fate of the aptamer and the NHC-Au conjugate independently, we constructed a dual-tagged aptamer-NHC-Au conjugate. The anthracenyl tag was chosen as a fluorescent signalling agent for the NHC-Au(I) fragment.[29] Ligand 6 and NHC-Au complex 7 were synthesized according to Scheme 3. In the 1H NMR spectrum of 6, a resonance downfield at 12.03 ppm is attributable to the C2 proton on the imidazole. Indicative of successful metalation, the C2 proton resonance is absent in the spectrum of complex 7. Also, in the 13C{1H} NMR spectrum of complex 7, the carbene carbon resonance appears at 180.5 ppm, indicative of a NHC-Au(I) bond.
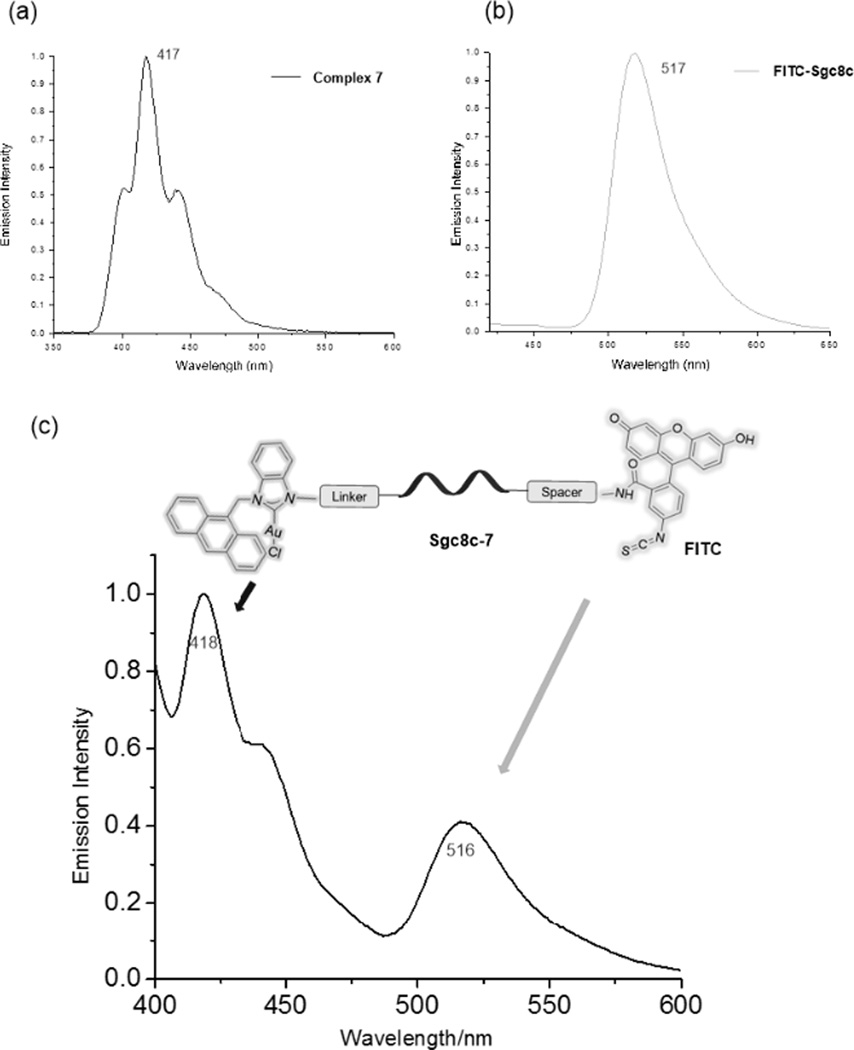
Similar to the previous method described for the synthesis of sgc8c-3, Scheme 2 (bottom) depicts the conjugation between complex 7 and FITC-modified sgc8c (FITC = fluorescein isothiocyanate). Several key data confirm the successful bioconjugation. The FITC-sgc8c aptamer alone has a HPLC retention time of 16 min while the retention time for complex 7 is 35 min. After the conjugation reaction, a significant peak appears with a retention time of 24 min. The material exiting the column at 24 min was collected, dried and analysed. Figure 2 depicts the emission spectrum of the NHC-Au(I)-FITC-sgc8c conjugate sgc8c-7. Clearly, emission maxima for the NHC fragment at 418 nm and the FITC dye at 516 nm are strong evidence for the successful conjugation reaction and formation of sgc8c-7. As depicted in Figure 3, sgc8c-7 also shows an enhanced cytotoxicity (IC50 = 2.39 ± 1.50 µM) compared to complex 7 alone (IC50 =31.1 ± 2.11 µM) against the targeted CCRF-CEM cell line.
Figure 2.
(a) The emission spectrum of complex 7 (acetonitrile/H2O=1:1); (b) The emission spectrum of complex FITC-sgc8c (acetonitrile/H2O=1:1); (c) The emission spectrum of HPLC-separated aptamer-drug conjugate sgc8c-7 exhibiting both the emission of the NHC-Au(I) and FITC-aptamer fragments (acetonitrile:H2O=1:1).
Figure 3.
The comparison of the cytotoxicity between NHC-Au complexes 3 and 7 and their corresponding conjugates sgc8c-3 and sgc8c-7.
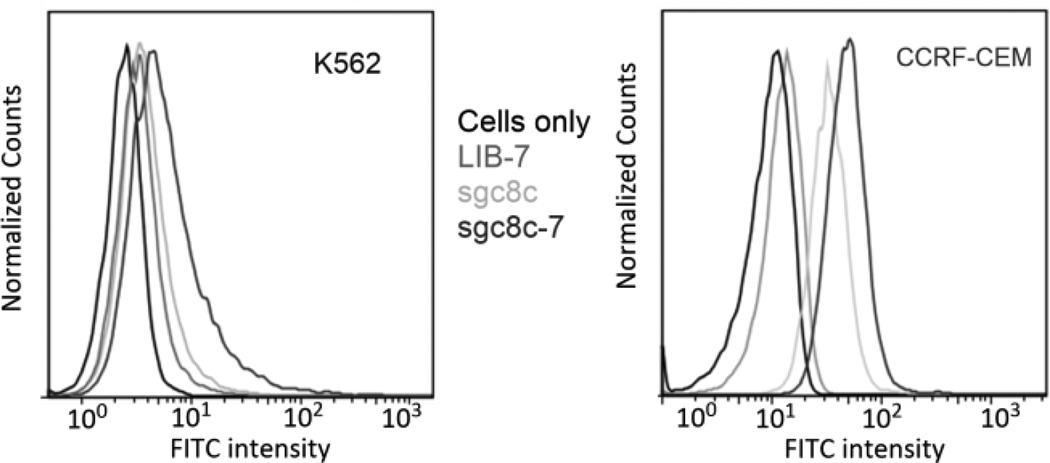
CCRF-CEM leukemia cells express the PTK-7 protein, which is recognized by the sgc8c-7 aptamer conjugate. In contrast, K562 leukemia cells do not over-express PTK-7, and therefore should not have any appreciable binding affinity for sgc8c-7. To demonstrate the selectivity of sgc8c-7 between these cell lines, a flow cytometry experiment was performed. Figure 4 depicts the fluorescence intensity results of K562 cells (left) and CCRF-CEM cells (right). Sgc8c aptamer alone was used as a positive control and complex 7 conjugated to a library of random sequences tagged with FITC (LIB-7) was used as negative control. As shown in Figure 4_right, the enhancement of the fluorescence intensity indicates sgc8c-7 has a specific binding affinity to the CCRF-CEM target cells, whereas in the case of K562 cells (left), neither LIB-7 nor sgc8c-7 caused any obvious fluorescence intensity change.
Figure 4.
Flow cytometry assays of conjugate sgc8c-7 with the K562 cell line (left) and the CCRF-CEM cell line (right). Random aptamer sequences conjugated to 7 to create LIB-7 were used as negative control and do not exhibit any fluorescence intensity change. The aptamer sgc8c and the conjugate sgc8c-7, which can target CCRF-CEM cells exhibit fluorescence intensity increases (See ESI for more information).
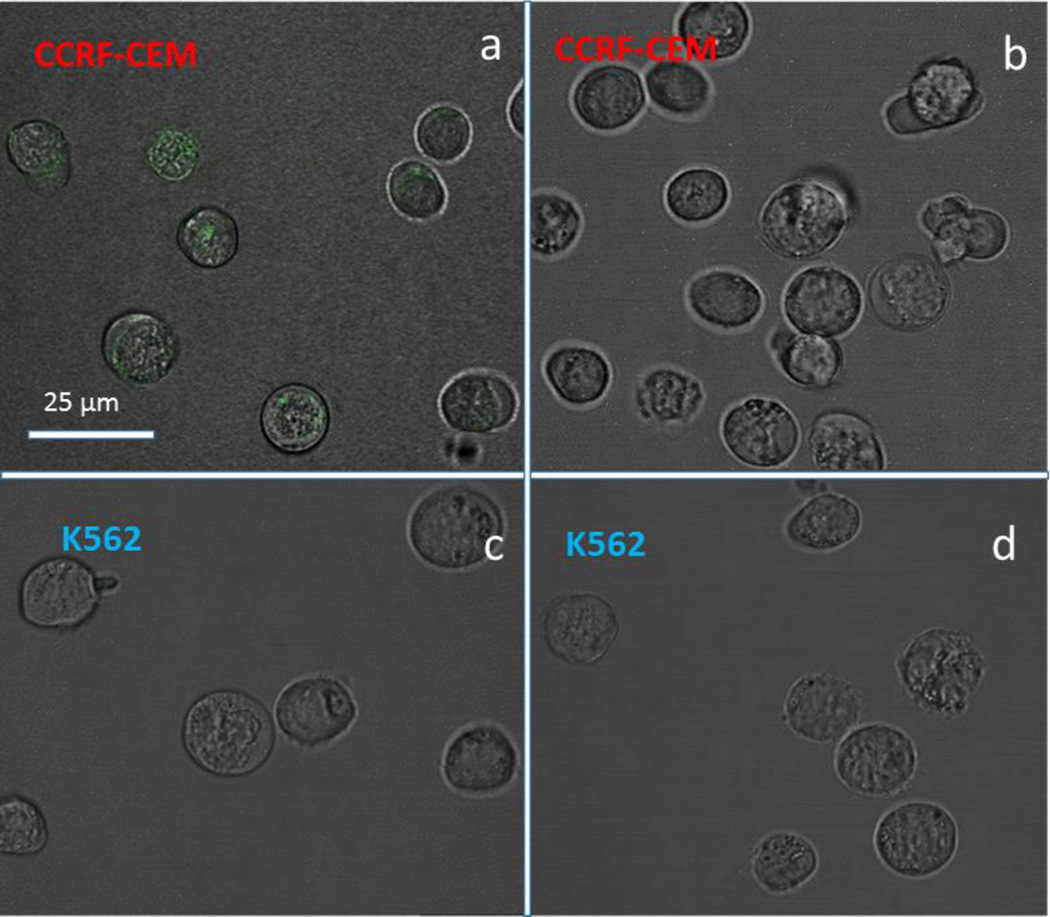
Besides specific recognition, a drug delivery agent should be able to internalize into cancer cells. In earlier work, aptamer sgc8c was reported to be internalized into target CCRF-CEM leukemia cells selectively.[27] In our current study, sgc8c-7 and LIB-7 were incubated with CCRF-CEM cells and K562 cells for 4 h. Figure 5a exhibits strong green fluorescence in the cytoplasm of CEM cells treated with sgc8c-7. In contrast, CEM cells treated with LIB-7, K562 cells treated with sgc8c-7 and LIB-7 displayed negligible fluorescence, thus demonstrating the specificity of sgc8c-7 recognition and internalization into targeted CCRF-CEM cancer cells.
Figure 5.
Confocal microscopy images of a): CEM cells incubated with Sgc8c-7; b): CEM cells incubated with LIB-7; c): K562 cells incubated with Sgc8c-7; d): K562 cells incubated with LIB-7 for 4 h at 37 °C. Sgc8c aptamer and LIB sequences were labelled with fluorescein. The scale bars correspond to 25 µm. Only the cells treated with Sgc8c-7 exhibit appreciable internalization (green color in panel a).
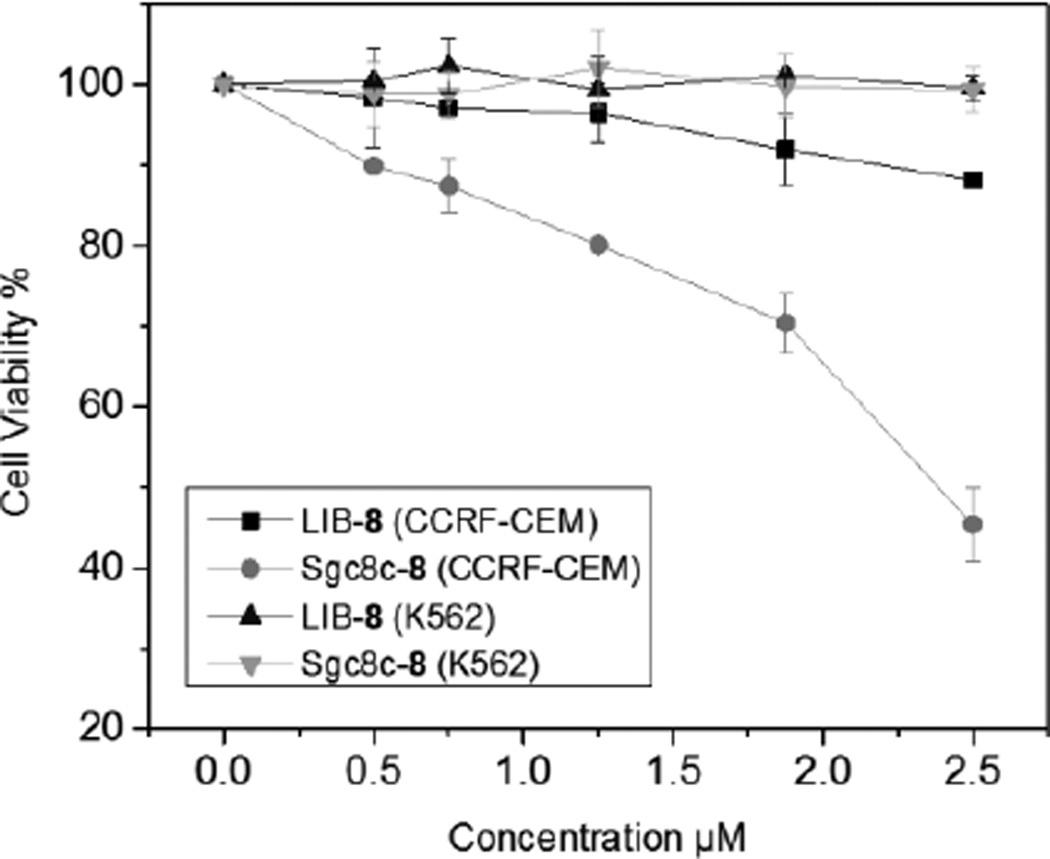
As sgc8c-7 was validated for selective cancer cell recognition and internalization, we subsequently studied its selective cytotoxicity. The target CCRF-CEM cells and off target K562 cells were incubated with sgc8c-7 or LIB-7 for 4 h to allow time for specific binding and internalization, then new media was added and the cells were incubated for another 48 h. The capability of cell proliferation was evaluated using an MTS assay. For K562 cells, sgc8c-7 didn’t induce appreciable cytotoxicity. However, for the target CCRF-CEM cells, sgc8c-7 exhibited dose-depedent cytotoxicity with a IC50 = 2.39 ± 1.50 µM (Figure 6). LIB-7 was not toxic to both CEM and K562 cells. This result confirms the goal of combining the cytotoxicity of an NHC-Au complex with the selectivity mediated by the sgc8c aptamer.
Figure 6.
MTS cell proliferation assay results demonstrating the specific cytotoxicity of sgc8c-7 towards CCRF-CEM cells.
In summary, two NHC-Au(I) complexes were conjugated to the CCRF-CEM specific aptamer sgc8c. Using fluorescent dyes tagged to the NHC-Au(I) and aptamer fragments, conclusive evidence of successful conjugation was achieved. Notably, the cytotoxicity of the NHC-Au(I) ion was enhanced upon conjugation to the aptamer by as much as 30×. Furthermore, flow cytometry and confocal microscopy confirms sgc8c-7 is able to selectively recognize and internalized into target cancer cells. In addition, in vitro MTS cell proliferation assays show that sgc8c-7 is relatively non-toxic to off-target K562 cells but is acutely cytotoxic to target CCRF-CEM cancer cells. Providing evidence that the sgc8c DNA sequence is critical to effective delivery, a random library of aptamers conjugated with the NHC-Au(I) complex is non-toxic to both K562 and CCRF-CEM cells at 2.5 µM. Finally, given the diversity of NHC-metal complexes developed over the past decade, it is conceivable that our targeted delivery system can be applied to a wide variety of metal ions with tailored NHC ligands, and therefore opens a new opportunity for future drug development.
Supplementary Material
Acknowledgments
ASV and WT acknowledge UF for support. This work was supported in part by the National Institutes of Health (GM079359 and GM 111386).
References
- 1.Hopkinson MN, Richter C, Schedler M, Glorius F. Nature. 2014;510:485–496. doi: 10.1038/nature13384. [DOI] [PubMed] [Google Scholar]
- 2.Diez-Gonzalez S, Marion N, Nolan SP. Chem. Rev. 2009;109:3612–3676. doi: 10.1021/cr900074m. [DOI] [PubMed] [Google Scholar]; Droege T, Glorius F. Angew. Chem. Int. Ed. 2010;49:6940–6952. doi: 10.1002/anie.201001865. [DOI] [PubMed] [Google Scholar]
- 3.Cisnetti F, Gautier A. Angew. Chem. Int. Ed. 2013;52:11976–11978. doi: 10.1002/anie.201306682. [DOI] [PubMed] [Google Scholar]; Liu W, Gust R. Chem. Soc.Rev. 2013;42:755–773. doi: 10.1039/c2cs35314h. [DOI] [PubMed] [Google Scholar]
- 4.Teyssot M-L, Jarrousse A-S, Manin M, Chevry A, Roche S, Norre F, Beaudoin C, Morel L, Boyer D, Mahiou R, Gautier A. Dalton Trans. 2009:6894–6902. doi: 10.1039/b906308k. [DOI] [PubMed] [Google Scholar]
- 5.Rana BK, Nandy A, Bertolasi V, Bielawski CW, Das Saha K, Dinda J. Organometallics. 2014;33:2544–2548. [Google Scholar]; Raubenheimer HG, Cronje S. Chem. Soc. Rev. 2008;37:1998–2011. doi: 10.1039/b708636a. [DOI] [PubMed] [Google Scholar]; Schuh E, Pflueger C, Citta A, Folda A, Rigobello MP, Bindoli A, Casini A, Mohr F. J Med. Chem. 2012;55:5518–5528. doi: 10.1021/jm300428v. [DOI] [PubMed] [Google Scholar]; Bertrand B, Stefan L, Pirrotta M, Monchaud D, Bodio E, Richard P, Le Gendre P, Warmerdam E, de Jager MH, Groothuis GMM, Picquet M, Casini A. Inorg. Chem. 2014;53:2296–2303. doi: 10.1021/ic403011h. [DOI] [PubMed] [Google Scholar]; Garner ME, Niu W, Chen X, Ghiviriga I, Abboud KA, Tan W, Veige AS. Dalton Trans. 2015;44:1914–1923. doi: 10.1039/c4dt02850c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mkandawire MM, Lakatos M, Springer A, Clemens A, Appelhans D, Krause-Buchholz U, Pompe W, Roedel G, Mkandawire M. Nanoscale. 2015;7:10634–10640. doi: 10.1039/c5nr01483b. [DOI] [PubMed] [Google Scholar]
- 7.Hickey JL, Ruhayel RA, Barnard PJ, Baker MV, Berners-Price SJ, Filipovska A. J Am. Chem. Soc. 2008;130:12570–12571. doi: 10.1021/ja804027j. [DOI] [PubMed] [Google Scholar]
- 8.Wong E, Giandomenico CM. Chem. Rev. 1999;99:2451–2466. doi: 10.1021/cr980420v. [DOI] [PubMed] [Google Scholar]; Rabik CA, Dolan ME. Cancer Treat. Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; Monneret C. Ann. Pharm. Fr. 2011;69:286–295. doi: 10.1016/j.pharma.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Mirabelli CK, Johnson RK, Sung CM, Faucette L, Muirhead K, Crooke ST. Cancer Res. 1985;45:32–39. [PubMed] [Google Scholar]; Mirabelli CK, Johnson RK, Hill DT, Faucette LF, Girard GR, Kuo GY, Sung CM, Crooke ST. J Med. Chem. 1986;29:218–223. doi: 10.1021/jm00152a009. [DOI] [PubMed] [Google Scholar]
- 10.Chen D, Milacic V, Frezza M, Dou QP. Curr. Pharm. Des. 2009;15:777–791. doi: 10.2174/138161209787582183. [DOI] [PubMed] [Google Scholar]
- 11.Ellington AD, Szostak JW. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]; Shangguan D, Li Y, Tang Z, Cao ZC, Chen HW, Mallikaratchy P, Sefah K, Yang CJ, Tan W. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11838–11843. doi: 10.1073/pnas.0602615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma H, Liu J, Ali MM, I. Mahmood MA, Labanieh L, Lu M, Iqbal SM, Zhang Q, Zhao W, Wan Y. Chem. Soc. Rev. 2015;44:1240–1256. doi: 10.1039/c4cs00357h. [DOI] [PubMed] [Google Scholar]; Oh SS, Lee BF, Leibfarth FA, Eisenstein M, Robb MJ, Lynd NA, Hawker CJ, Soh HT. J Am. Chem. Soc. 2014;136:15010–15015. doi: 10.1021/ja5079464. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shieh Y-A, Yang S-J, Wei M-F, Shieh M-J. ACS Nano. 2010;4:1433–1442. doi: 10.1021/nn901374b. [DOI] [PubMed] [Google Scholar]; Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Proc. Natl. Acad. Sci. 105:17356–17361. doi: 10.1073/pnas.0809154105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D, Jeong YY, Jon S. ACS Nano. 2010;4:3689–3696. doi: 10.1021/nn901877h. [DOI] [PubMed] [Google Scholar]; Bagalkot V, Farokhzad OC, Langer R, Jon S. Angew. Chem. Int. Ed. 2006;45:8149–8152. doi: 10.1002/anie.200602251. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y-F, Shangguan D, Liu H, Phillips JA, Zhang X, Chen Y, Tan W. Chembiochem. 2009;10:862–868. doi: 10.1002/cbic.200800805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang R, Zhu G, Mei L, Xie Y, Ma H, Ye M, Qing F-L, Tan W. J Am. Chem. Soc. 2014;136:2731–2734. doi: 10.1021/ja4117395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed LA, El-Maraghy SA. Biochem. Pharmacol. 2014;92:517–517. [Google Scholar]
- 17.Pestalozzi BC, Sotos GA, Choyke PL, Fisherman JS, Cowan KH, Oshaughnessy JA. Cancer. 1993;71:1797–1800. doi: 10.1002/1097-0142(19930301)71:5<1797::aid-cncr2820710514>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Smith L, Watson MB, O'Kane SL, Drew PJ, Lind MJ, Cawkwell L. Mol. Cancer Ther. 2006;5:2115–2120. doi: 10.1158/1535-7163.MCT-06-0190. [DOI] [PubMed] [Google Scholar]
- 19.Fang X, Tan W. Acc. Chem. Res. 2010;43:48–57. doi: 10.1021/ar900101s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radzicka A, Wolfenden R. J Am. Chem. Soc. 1996;118:6105–6109. [Google Scholar]
- 21.Kalia J, Raines RT. Curr. Org. Chem. 2010;14:138–147. doi: 10.2174/138527210790069839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montalbetti C, Falque V. Tetrahedron. 2005;61:10827–10852. [Google Scholar]; Humphrey JM, Chamberlin AR. Chem. Rev. 1997;97:2243–2266. doi: 10.1021/cr950005s. [DOI] [PubMed] [Google Scholar]
- 23.Lemke J, Metzler-Nolte N. Eur. J. Inorg. Chem. 2008:3359–3366. [Google Scholar]
- 24.Chen T, Shukoor MI, Wang R, Zhao Z, Yuan Q, Bamrungsap S, Xiong X, Tan W. ACS Nano. 2011;5:7866–7873. doi: 10.1021/nn202073m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keefe AD, Pai S, Ellington A. Nat. Rev. Drug Discov. 2010;9:537–550. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J, Rossi JJ. Mol. Ther.-Nuc. Acids. 2014;3 doi: 10.1038/mtna.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao Z, Shangguan D, Cao Z, Fang X, Tan W. Chem. Eur. J. 2008;14:1769–1775. doi: 10.1002/chem.200701330. [DOI] [PubMed] [Google Scholar]
- 28.Mahato R, Tai W, Cheng K. Adv. Drug Del. Rev. 2011;63:659–670. doi: 10.1016/j.addr.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Citta A, Schuh E, Mohr F, Folda A, Massimino ML, Bindoli A, Casini A, Rigobello MP. Metallomics. 2013;5:1006–1015. doi: 10.1039/c3mt20260g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.