Abstract
The transforming growth factor β-1 (TGFβ-1) signaling pathway plays a central role in the pathogenesis of pulmonary fibrosis. Two TGFβ-1 receptors, TβRI and TβRII, mediate this pathway. TβRI protein stability, as mediated by the ubiquitin/de-ubiquitination system, has been well studied; however, the molecular regulation of TβRII still remains unclear. Here we reveal that a de-ubiquitinating enzyme, USP11, promotes TGFβ-1 signaling through de-ubiquitination and stabilization of TβRII. We elucidate the role that mitoxantrone (MTX), an USP11 inhibitor, has in the attenuation of TGFβ-1 signaling. Inhibition or downregulation of USP11 results in increases in TβRII ubiquitination and reduction of TβRII stability. Subsequently, TGFβ-1 signaling is greatly attenuated, as shown by the decreases in phosphorylation of SMAD2/3 levels as well as that of fibronectin (FN) and smooth muscle actin (SMA). Overexpression of USP11 reduces TβRII ubiquitination and increases TβRII stabilization, thereby elevating phosphorylation of SMAD2/3 and the ultimate expression of FN and SMA. Further, elevated expression of USP11 and TβRII were detected in lung tissues from bleomycin-challenged mice and IPF patients. Therefore, USP11 may contribute to the pathogenesis of pulmonary fibrosis by stabilization of TβRII and promotion of TGFβ-1 signaling. This study provides mechanistic evidence for development of USP11 inhibitors as potential antifibrotic drugs for pulmonary fibrosis.
Transforming growth factor β-1 (TGFβ-1) signaling pathway is initiated by the binding of the TGFβ-1 to TGFβ-1 receptor II (TβRII), which then binds to TβRI and activates TβRI by phosphorylating a characteristic SGSGSG sequence, called the GS domain.1, 2 This phosphorylation signal is then transduced within the cells via SMAD proteins 2 and 3 (SMAD2/3). These SMADS then form a complex with SMAD4, which ultimately translocates to the nucleus where interaction with other transcription factors results in the specific expression of upwards of 500 genes.3, 4, 5 Expression of these genes, including fibronectin (FN) 1 and α-smooth muscle actin (SMA, gene ACTA2), are associated with the myofibroblast phenotype and progression of fibrosis.6, 7 The regulation of TβRI and its role in fibrosis have been well elucidated.8, 9, 10, 11 Comparatively less is known about its counterpart, TβRII.
Regulation of the TGFβ-1 pathway can be achieved in a number of ways, ubiquitination and de-ubiquitination being key regulators. Ubiquitin is an 8-kDa protein that can be attached to a protein's lysine residue via a three-step process: activation, conjugation, and ligation; each of which are carried out by their own enzyme: E1 ubiquitin-activating enzyme, E2-conjugating enzyme, and E3 ubiquitin ligase, respectively.12, 13, 14 Additional ubiquitins can be added to the preceding ubiquitin resulting in a poly-ubiquitinated protein. The number and linkage type of these ubiquitins determine a protein's fate within the cell. The process of ubiquitination can be reversed via a process called de-ubiquitination, which is carried out by de-ubiquitinating enzymes (DUBs).15, 16, 17 This process adds another layer to the protein-regulatory processes carried out by the cells. De-ubiquitination by DUBs promotes target proteins' lifespan and regulates protein localization and biological activity.17, 18, 19
There are numerous studies on DUBs specific for TβRI. It has been revealed that the DUBs, UCH37, USP11, and USP15, de-ubiquitinate and stabilize TβRI.20, 21, 22 However, very little research has been performed to examine the regulation of its co-receptor, TβRII. It is the goal of this study to elucidate the role that DUB USP11 has in the regulation of TβRII stability and how the anticancer drug, mitoxantrone (MTX), an inhibitor of USP11,23 mitigates TGFβ-1-induced signaling through reduction of TβRII stability.
Results
MTX inhibits TGFβ-1-induced phosphorylation of SMAD2/3 and TβRI as well as the expression of FN and SMA in lung fibroblast cells
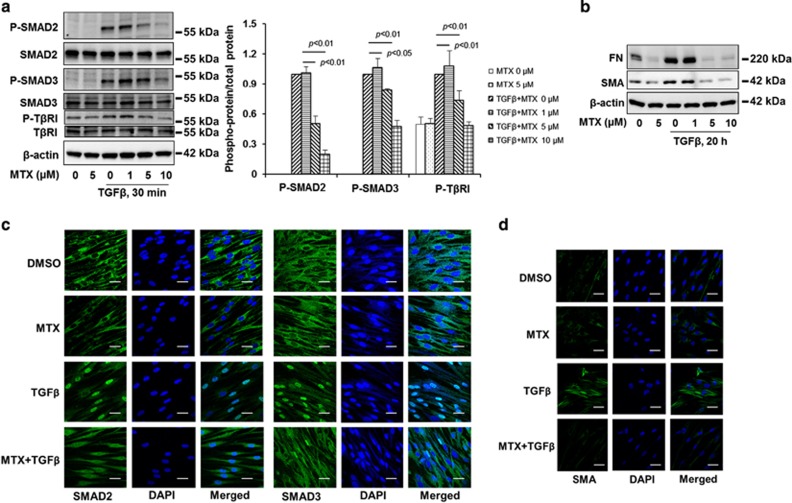
MTX, an anticancer drug, has been known to inhibit TGFβ1-induced COL1A1 expression in human dermal fibroblast cells, suggesting that MTX has an antifibrotic effect.24 To further understand the molecular mechanism by which MTX inhibits TGFβ-1-mediated pro-fibrotic responses, we first examined the effect of MTX on TGFβ-1 signaling. MRC5 cells were treated with TGFβ-1 (2 ng/ml) for 30 min, and as expected, phosphorylation of SMAD2/3 (p-SMAD2/3) and TβRI (p-TβRI) were induced while total SMAD2/3 and TβRI levels remained constant (Figure 1a). Ultimately, the levels of FN and α-SMA were increased in cells treated with 2 ng/ml TGFβ-1 for 20 h (Figure 1b). These results were attenuated, however, when the cells were pretreated with MTX. The levels of p-SMAD2/3, p- TβRI, FN, and SMA were decreased in a dose-dependent manner (Figures 1a and b). Phosphorylation promotes interaction of SMAD2/3 with SMAD4, leading to nuclear translocation and transcription of FN and SMA. We show that MTX attenuated TGFβ-1-induced SMAD2 and SMAD3 nuclear translocation (Figure 1c) and expression of SMA (Figure 1d). These data suggest that MTX exhibits an antifibrotic effect and mitigates TGFβ-1 signaling in the upstream of SMAD2/3 and TβRI.
Figure 1.
MTX attenuates TGFβ-1 signaling in human lung fibroblast. (a) MRC5 cells were treated with increasing doses of MTX (0, 1, 5, 10 μM) for 1 h, and then cells were treated with TGFβ-1 (2 ng/ml) for 30 min; the phosphorylated and total forms of SMAD2, SMAD3, and TβRI were then analyzed by western blotting. Western blotting images were cropped to improve the conciseness of the data; samples derived from the same experiment and the blots were processed in parallel. Representative of experiments performed at least three independent times. Intensities of blots were measured. The ratio of phosphorylated/total protein was analyzed by the ImageJ software. (b) MRC5 cells were treated with increasing doses of MTX (0, 1, 5, 10 μM) for 1 h, and then cells were treated with TGFβ-1 (2 ng/ml) for 20 h. FN and SMA levels were analyzed by western blotting. Western blotting images were cropped to improve the conciseness of the data; samples derived from the same experiment and the blots were processed in parallel. Representative of experiments performed at least three independent times. (c) MRC5 cells grown on glass-bottom dishes were treated with dimethyl sulfoxide (DMSO) or MTX (5 μM) for 1 h, and then cells were treated with TGFβ-1 (2 ng/ml) for 1 h. Localization of SMAD2 or SMAD3 (green) in the cells were detected by immunostaining with SMAD2 and SMAD3 antibodies. DAPI (4,6-diamidino-2-phenylindole) was used for nuclei staining (blue). Bars, 10 μM. Representative images were shown. (d) MRC5 cells grown on glass-bottom dishes were treated with DMSO or MTX (5 μM) for 1 h, and then cells were treated with TGFβ-1 (2 ng/ml) for 20 h. Localization of SMA (green) in the cells were detected by immunostaining with an SMA antibody. DAPI was used for nuclei staining (blue). Bars, 10 μm. Representative images are shown
USP11 promotes TGFβ-1-induced phosphorylation of SMAD2/3 and the expression of FN and SMA
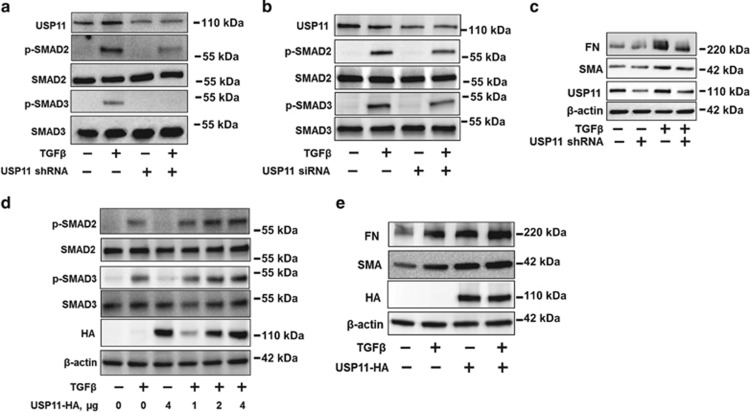
A recent study has revealed that MTX inhibits USP11 activity.23 To investigate the role of USP11 in TGFβ-1 signaling in lung fibroblast cells, MRC5 cells were infected with USP11 shRNA lentivirus or transfected with USP siRNA. Both USP11 shRNA and USP11 siRNA resulted in the reduction of USP11 levels as well as diminishing p-SMAD2/3 levels, while maintaining total SMAD2/3 levels (Figures 2a and b). The downstream effects of TGFβ-1 signaling were also examined by analyzing the expression levels of FN and SMA. As expected, the levels of these proteins were decreased when the cells were infected with USP11 shRNA lentivirus (Figure 2c).
Figure 2.
USP11 regulates TGFβ-1 signaling pathway. (a) MRC5 cells were infected with cont shRNA (−) or USP11 shRNA lentivirus for 3 days as described in the Materials and Methods section, and then cells were treated with TGFβ-1 (2 ng/ml) for 30 min. USP11 and phosphorylated and total SMAD2/3 levels were analyzed by western blotting. (b) MRC5 cells were transfected with cont siRNA (−) or USP11 siRNA for 3 days, and then cells were treated with TGFβ-1 (2 ng/ml) for 30 min. USP11, total, and phosphorylated SMAD2/3 levels were analyzed by western blotting. (c) MRC5 cells were infected with cont shRNA (−) or USP11 shRNA lentivirus for 3 days, and then cells were treated with TGFβ-1 (2 ng/ml) for 20 h. USP11, FN, SMA, and β-actin protein levels were analyzed by western blotting. (d) MRC5 cells were transfected with USP11-HA plasmids (0–4 μg) for 2 days, and then cells were treated with TGFβ-1 (2 ng/ml) for 30 min. USP11-HA, phosphorylated and total SMAD2/3, and β-actin levels were analyzed by western blotting. (e) MRC5 cells were transfected with USP11-HA plasmids (2 μg) for 2 days, and then cells were treated with TGFβ-1 (2 ng/ml) for 20 h. USP11-HA, FN, SMA, and β-actin protein levels were analyzed by western blotting. Western blotting images were cropped to improve the conciseness of the data; samples derived from the same experiment and the blots were processed in parallel. Representative of experiments performed at least three independent times
Next, we determined whether overexpression of USP11 with an HA tag (USP11-HA) in MRC5 cells would enhance the expression and activation of TβRII. In support of a role for USP11 as a regulator of TGFβ signaling, we found that increasing doses of USP11-HA-transfected cells increased TGFβ-1-induced phosphorylation of SMAD2/3 (Figure 2d) and expression of FN and SMA (Figure 2e). Taken together with data from MTX experiment, these results suggest that USP11 regulates TGFβ-1 signaling.
MTX reduces TβRII stability by increasing its poly-ubiquitination
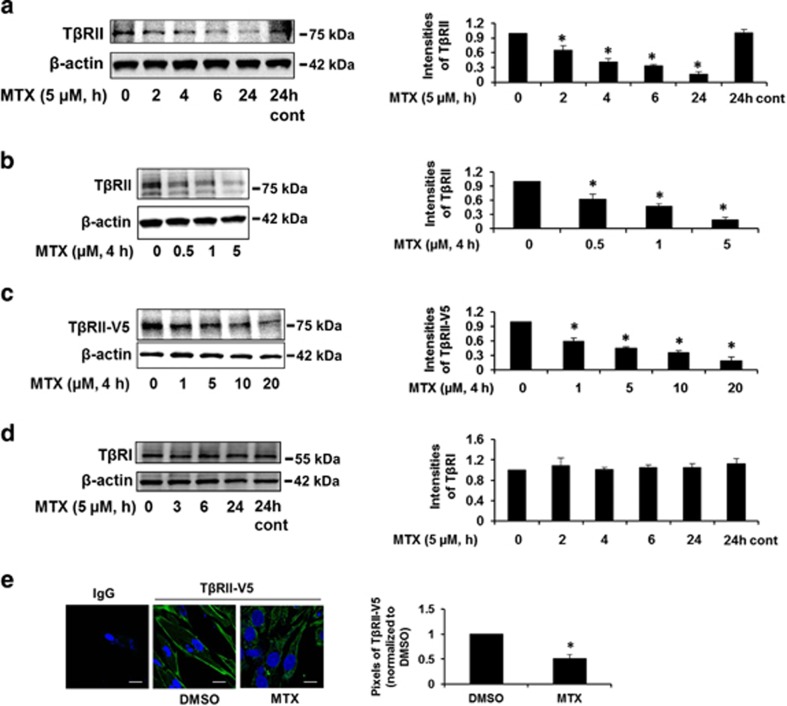
To elucidate the molecular mechanisms by which MTX regulates TGFβ-1 signaling, we examined the protein levels of TβRII. MTX reduced TβRII levels in a time- and dose-dependent manner (Figures 3a and b). Further, we investigated the effect of MTX on overexpressed TβRII. MRC5 cells were transfected with a V5-tagged TβRII (TβRII-V5) plasmid for 48 h. Cells were then treated with MTX for 4 h. As shown in Figure 3c, MTX reduced TβRII-V5 levels in a dose-dependent manner. Consistent with the data shown in Figure 1a, MTX had no effect on the levels of TβRI (Figure 3d). The reduction of TβRII by MTX was confirmed by immunofluorescence staining (Figure 3e), which indicate that MTX reduces TβRII levels in a short time frame (2–4 h).
Figure 3.
MTX reduces TβRII levels in human lung fibroblast cells. (a) MRC5 cells were treated with 5 uM MTX for 0–24 h. TβRII and β-actin levels were analyzed by western blotting. Intensities of TβRII were analyzed by the ImageJ software. *P<0.01 compared with 0 h. (b) MRC5 cells were treated with MTX (0–5 μM) for 4 h. TβRII and β-actin levels were analyzed by western blotting. Intensities of TβRII were analyzed by the ImageJ software. *P<0.01 compared with 0 μM. (c) MRC5 cells were transfected with TβRII-V5 plasmid for 48 h, and then cells were treated with MTX (0–20 μM) for 4 h. TβRII-V5 and β-actin levels were examined by western blotting. Intensities of TβRII-V5 were analyzed by the ImageJ software. *P<0.01 compared with 0 μM. (d) MRC5 cells were treated with 5 μM MTX for 0–24 h. TβRI and β-actin levels were analyzed by western blotting. Intensities of TβRI were analyzed by the ImageJ software. Western blotting images were cropped to improve the conciseness of the data; samples derived from the same experiment and the blots were processed in parallel. Representative of experiments performed at least three independent times. (e) MRC5 cells grown on glass-bottom dishes were transfected with TβRII-V5 plasmid for 48 h, and then cells were treated with MTX (10 μM) for 4 h. Localization of TβRII-V5 (green) in the cells were detected by immunostaining with V5 tag. DAPI (4,6-diamidino-2-phenylindole) was used for nuclei staining (blue). Bars, 50 μm. Representative images were shown. Pixels of TβRII-V5 on the plasma membrane were quantified with the NIS-Elements software. *P<0.01 compared with dimethyl sulfoxide (DMSO)
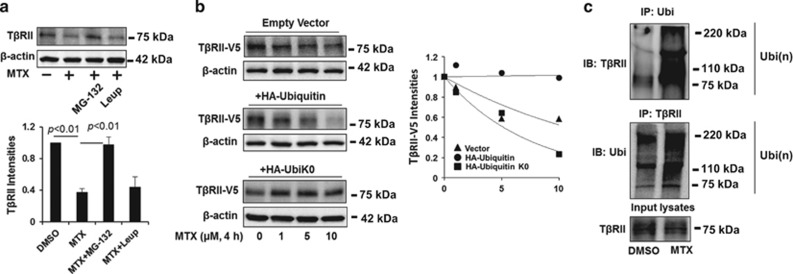
To further investigate the molecular mechanisms by which MTX reduces TβRII levels, we focused on the effect of MTX on TβRII protein stability. Protein degradation occurs primarily through the proteasome or lysosome systems. MTX-induced TβRII degradation was inhibited by the proteasome inhibitor, MG-132, but not the lysosomal inhibitor, leupeptin (Figure 4a). This suggests that TβRII is degraded via the proteasome pathway. Therefore, we next explored the effects of overexpression of HA-tagged ubiquitin on MTX-induced TβRII degradation to reveal whether the TβRII proteasomal degradation is mediated by ubiquitination. As shown in Figure 4b, HA-ubiquitin overexpression promotes MTX-induced TβRII degradation, while overexpression of HA-ubiquitin lacking lysine residues (UbiK0), which losses its ability to form polyubiquitiantion, stabilizes TβRII. These observations support the conclusion that TβRII degradation by MTX is mediated by the ubiquitin–proteasome pathway. Additionally, MTX increased TβRII poly-ubiquitination, as indicated by the in vivo ubiquitination assay in Figure 4c.
Figure 4.
MTX promotes TβRII ubiquitination and degradation in the proteasome. (a) MRC5 cells were treated with MG-132 (20 μM) or leupeptin (100 μM) for 1 h prior to MTX treatment (5 μM, 2 h). TβRII and β-actin levels were analyzed by western blotting. Intensities of TβRII were analyzed by the ImageJ software. (b) MRC5 cells were co-transfected with TβRII-V5 and with either empty vector, HA-ubiquitin, or HA-ubiquitin without lysine (HA-UbiK0) plasmids, cells were then treated with increasing concentrations of MTX (0–10 μM) for 4 h. TβRII-V5 and β-actin levels were analyzed by western blotting. Intensities of TβRII-V5 were analyzed by the ImageJ software and then compared between the three groups. (c) MRC5 cells were treated with 5 μM MTX for 1 h, and then cell lysates were subjected to immunoprecipitation with an ubiquitin antibody or a TβRII antibody, followed by TβRII or ubiquitin immunoblotting. Input lysates were analyzed by TβRII immunoblotting. Western blotting images were cropped to improve the conciseness of the data; samples derived from the same experiment and the blots were processed in parallel. Representative of experiments performed at least three independent times
USP11 de-ubiquitinates and stabilizes TβRII
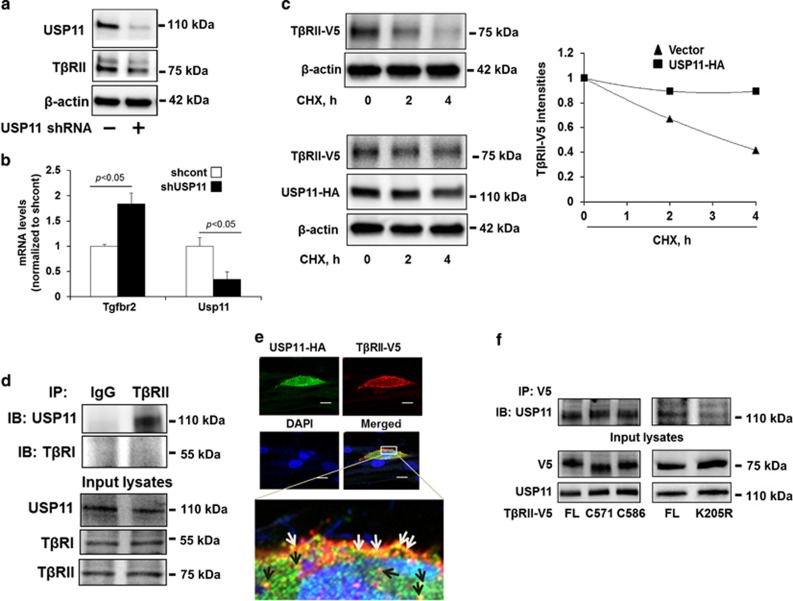
To examine whether USP11 affects TβRII stability, MRC5 cells were infected with USP11 shRNA lentivirus, resulting in the reduction of both USP11 and TβRII protein levels (Figure 5a). To investigate whether this reduction occurred on the RNA or protein level, qPCR was performed to analyze mRNA levels for both USP11 and TβRII; this revealed that knockdown of USP11 has no reduction in the amount of RNA coded for TβRII (Tgfbr2) (Figure 5b). Therefore, the reduction of TβRII observed is on the protein level. Next TβRII protein stability was then examined by cycloheximide (CHX) chase assay, revealing the half-life of TβRII-V5 to be approximately 2 h. Overexpression of USP11-HA enhanced TβRII's lifespan (Figure 5c). Further, co-immunoprecipitation (Co-IP) confirmed that, in untreated lung fibroblast cells, as expected, TβRII does not interact with TβRI, while intriguingly, TβRII is associated with USP11 (Figure 5d). Both TβRII-V5 and USP11-HA are co-localized at the plasma membrane and cytoplasm in MRC5 cells (Figure 5e). To determine the USP11-binding site within TβRII, plasmids encoding two c-terminal deletion mutants (C571 and C586) and a K205R mutant of TβRII were generated. Co-IP experiments show that both C571 and C586 deletion mutants and wild type are associated with USP11, while TβRIIK205R exhibits a reduction of association with USP11 (Figure 5f), indicating that lys205 has a critical role in binding to USP11. Taken together, these results suggest that USP11 interacts and stabilizes TβRII.
Figure 5.
USP11 regulates TβRII stability in human lung fibroblast cells. (a) MRC5 cells were infected with cont shRNA (−) or USP11 shRNA lentivirus for 3 days, and then USP11, TβRII, and β-actin levels were analyzed by western blotting. (b) MRC5 cells were infected with cont shRNA (−) or USP11 shRNA lentivirus for 3 days, and then total RNA was extracted. Tgfbr2 and Usp11 gene expression were examined by RT-real time PCR. (c) MRC5 cells were co-transfected with TβRII-V5 and empty vector or USP11-HA plasmids for 48 h, and then cells were treated with CHX (20 μg/ml) for 0–4 h. TβRII-V5, USP11-HA, and β-actin levels were analyzed by western blotting. Intensities of TβRII-V5 were analyzed by the ImageJ software and then compared between the two groups. (d) MRC5 cell lysates were subjected to immunoprecipitation with IgG or a TβRII antibody, followed by USP11 and TβRI immunoblotting. Input lysates were analyzed by USP11, TβRI, and TβRII immunoblotting. (e) MRC5 cells grown on glass-bottom dishes were co-transfected with USP11-HA and TβRII-V5 plasmids for 48 h. Localization of USP11-HA (green) and TβRII-V5 (red) in MRC5 cells were examined by immunostaining. Nuclei were stained by DAPI (4,6-diamidino-2-phenylindole; blue). USP11-HA and TβRII-V5 co-localization on the plasma membrane are indicated by white arrows; two protein co-localization in the cytoplasm are indicated by black arrows. Bars, 50 μm. Representative images are shown. (f) MRC5 cells were transfected with plasmids encoding TβRII-V5 full-length (FL), C571, C586 deletion mutants, or K205R mutants for 48 h. Cell lysates were subjected to immunoprecipitation with a V5 antibody, followed by USP11 immunoblotting. Input lysates were analyzed by USP11 and V5 immunoblotting. Western blotting images were cropped to improve the conciseness of the data; samples derived from the same experiment and the blots were processed in parallel. Representative of experiments performed at least three independent times
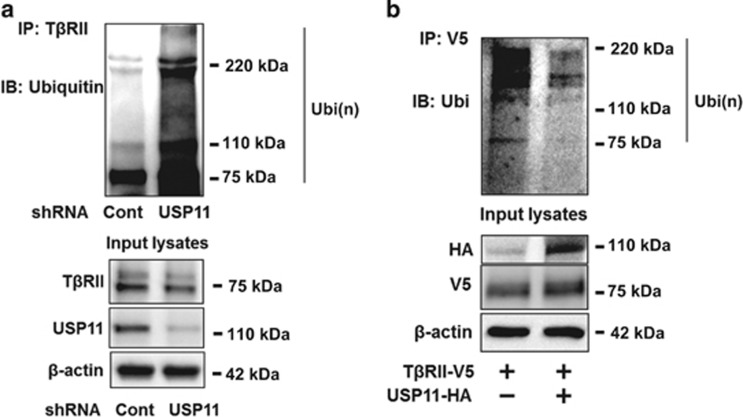
To investigate whether USP11 promotes TβRII stability through de-ubiquitinating TβRII, USP11 was downregulated by USP11 shRNA lentivirus infection prior to in vivo ubiquitination assay. As shown in Figure 6a, USP11 shRNA increased TβRII ubiquitination, whereas the level of ubiquitination was reduced by overexpression of USP11-HA (Figure 6b). Taken together, the data indicate that USP11 stabilizes TβRII through de-ubiquitinating TβRII. Inhibition of USP11 promotes TβRII proteasomal degradation, suggesting that USP11 is a pro-fibrotic DUB.
Figure 6.
USP11 de-ubiquitinates TβRII. (a) MRC5 cells were infected with cont shRNA or USP11 shRNA lentivirus for 3 days. Cell lysates were subjected to immunoprecipitation with a TβRII antibody, followed by ubiquitin immunoblotting. Input lysates were analyzed by TβRII, USP11, and β-actin immunoblotting. (b) MRC5 cells were co-transfected with TβRII-V5 and empty vector or USP11-HA plasmids for 48 h, and then cell lysates were subjected to immunoprecipitation with a V5 antibody, followed by ubiquitin immunoblotting. Input lysates were analyzed by HA, V5, and β-actin immunoblotting. Western blotting images were cropped to improve the conciseness of the data; samples derived from the same experiment and the blots were processed in parallel. Representative of experiments performed at least three independent times
USP11 and TβRII levels are increased in lung tissues from bleomycin-challenged mice and idiopathic pulmonary fibrosis (IPF) patients
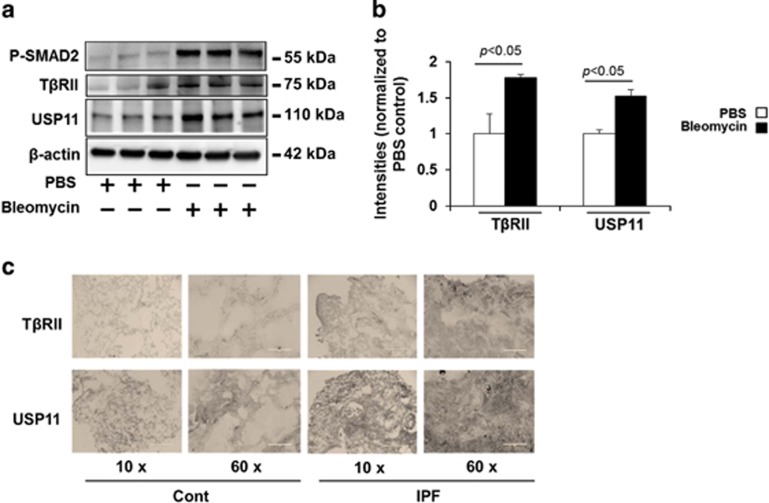
Bleomycin-induced pulmonary fibrosis in mice is the most common experimental study model of human lung fibrosis. Phosphorylation of SMAD2 was detected in 3-week bleomycin-challenged mice, suggesting that TGFβ signaling contributes to the pathogenesis of bleomycin-induced pulmonary fibrosis (Figure 7a). Both USP11 and TβRII were significantly increased in murine lung tissue samples from bleomycin-challenged mice (Figures 7a and b). Further, the expression levels of TβRII and USP11 in the lungs from normal and IPF patients were examined by immunohistochemistry. Both TβRII and USP11 are highly expressed in lung tissues from IPF patients (Figure 7c). It appears that both TβRII and USP11 are upregulated in most lung cells, including epithelial and fibroblast cells. These data suggest that USP11 may contribute to the pathogenesis of lung fibrosis through stabilization of TβRII and enhancement of TGFβ-1 signaling in lung fibroblast cells.
Figure 7.
TβRII and USP11 are increased in the lungs from bleomycin-induced fibrosis model and IPF patients (a) C57BL/6 mice were challenged with intranasal injection of bleomycin for 3 weeks. P-SMAD2, TβRII, USP11, and β-actin levels were analyzed by western blotting. (b) Intensities of TβRII and USP11 were analyzed by the ImageJ software. (c) Human normal and IPF lung tissues were fixed and immunostained with TβRII and USP11 antibodies. Scale bars in × 10 image, 400 μm; scale bars in × 60 images: 50 μm. Representative images (from three per each group) are shown
Discussion
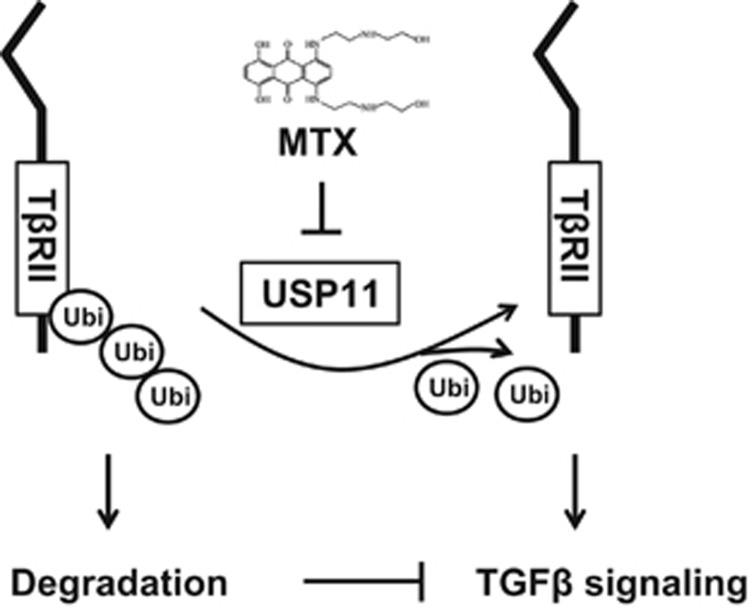
The TGFβ-1 signaling pathway contributes in large part to the pathogenesis of fibrosis through its receptor TβRII/TβRI heterozygous complex.25, 26, 27, 28 Many studies have focused on examining the regulation of TβRI and its role in the progression of fibrosis.8, 9, 10, 11, 20, 21 However, very few studies have explored its co-receptor, TβRII. Similarly, numerous studies have illuminated the roles of DUBs in the regulation of TβRI signaling, including that of the role of USP11 in stabilization of TβRI.21 In the current study, we demonstrate that TβRII degradation is mediated by the ubiquitin–proteasome system. We found that USP11 stabilizes TβRII, not TβRI, in human lung fibroblast cells. Inhibition or knockdown of USP11 increases poly-ubiquitination of TβRII, thereby reducing TβRII stability and impairing TGFβ-1 signaling in lung fibroblast cells (Figure 8). Further, we show that both USP11 and TβRII are highly expressed in murine lung tissues from experimental lung fibrosis and IPF patients. This study is the first to characterize the molecular regulation of TβRII stability by USP11 and suggests that USP11 is a potential target in treating fibrosis.
Figure 8.
USP11 de-ubiquitinates and stabilizes TβRII. TβRII stability is regulated by its poly-ubiquitination. Destabilization of TβRII attenuates TGFβ-1 signaling. USP11 stabilizes TβRII through de-ubiquitination of TβRII. MTX, an inhibitor of USP11, promotes ubiquitination and degradation of TβRII, thus mitigating TGFβ-1 signaling
Ubiquitination is a reversible posttranslational modification process. DUBs counteract ubiquitination by removal of the ubiquitin chain from modified proteins. DUBs have key roles in the regulation of protein stability and signal transduction.15, 16, 17, 19 Several DUBs have been identified to regulate TGFβ-1 signaling through targeting TβRI or SMADs. UCHL37, USP11, and USP15 de-ubiquitinate TβRI,20, 21, 22 while USP15, CYLD, and USP9X target SMAD4 or SMAD7.29, 30, 31, 32 In this study, we show that USP11 has no effect on TβRI stability in human lung fibroblast cells, though Al-Salihi et al.21 showed that USP11 de-ubiquitinates TβRI. This controversial conclusion may be due to use of different cell types. In their study, they investigated the effect of USP11 on TβRI de-ubiquitination in HEK293 cells, while we used human lung fibroblast cells. Consistent with their findings, we also revealed that USP11 promotes TGFβ-1 signaling. In addition, we revealed that lys205 on TβRII is a USP11-binding site. Lys205 is considered one of potential ubiquitin-binding sites in TβRII according to a proteomic analysis.33 It is likely that an ubiquitin-like domain (UBL) in USP11 recognizes the ubiquitin-acceptor site on TβRII. To confirm this hypothesis, a UBL-deletion mutant of USP11 will be generated to test its role in interaction with TβRII. The novel finding in this study is that USP11 de-ubiquitinates and stabilizes TβRII. This is the first study to identify a DUB responsible for de-ubiquitination of TβRII. We demonstrate that knockdown of USP11 increases the ubiquitination of TβRII, whereas overexpression of USP11 greatly decreases ubiquitination of TβRII. This, in concordance with the results of the USP11 inhibitor treatment, proves that USP11 acts as a DUB, stabilizing TβRII and ultimately increasing the expression of profibrotic proteins, such as FN and SMA.
TβRII has been known to be posttranslationally modified by phosphorylation, ubiquitination, and neddylation.34, 35, 36, 37, 38 Autophosphorylation of TβRII is essential for its kinase activity and regulation of downstream signaling;34 however, the effect of phosphorylation of TβRII on its stability has not been revealed. Phosphorylation triggers receptor ubiquitination and internalization,39, 40, 41 therefore, it is possible that the phosphorylation of TβRII has a role in TβRII stability. Further studies will focus on investigating whether phosphorylation of TβRII affects USP11-mediated TβRII de-ubiquitination. Neddylation of TβRII by c-Cbl has been shown to antagonize TβRII ubiquitination and degradation.38 We found that MTX increases TβRII ubiquitination, while it reduces TβRII neddylation (data not shown), suggesting that the balance between ubiquitination and neddylation of TβRII is important for the regulation of TβRII lifespan and its pathophysiological effects. It will be interesting to study whether the phosphorylation determines the balance between ubiquitination and neddylation of TβRII.
As USP11 promotes TGFβ-1 signaling through stabilization of TβRII, we hypothesize that USP11 may have a critical role in the development of lung fibrosis. Here we show that both USP11 and TβRII levels are increased in lung tissues from an experimental lung fibrosis model and IPF patients; however, the molecular regulation of USP11 has not been studied. We found that TGFβ-1 has no effect on the expression of USP11, while it increases tyrosine phosphorylation of USP11 in lung fibroblast cells (data not shown). Taken together, targeting USP11 might be a new strategy to lessen the severity of lung fibrosis. MTX is an FDA-approved anticancer drug; the current study reveals that MTX antagonizes TGFβ-1 signaling in lung fibroblast cells. Therefore, this study provides cellular and molecular evidence to indicate that MTX could be a potential antifibrotic drug to treat pulmonary fibrosis.
Materials and Methods
Cell culture and reagents
Human fetal lung fibroblast (MRC5) cell lines were propagated in EMEM media (Gibco, Rockville, IL, USA) with 10% FBS (Hyclone, Logan, UT, USA), and 1% penicillin/streptomycin antibiotic mix (Lonza, Allendale, NJ, USA). The cells were kept in a 37 °C humidified incubator with 5% carbon dioxide. Immobilized protein A/G beads, FN, α-SMA, TβRI, HA tag, USP11, V5 tag, TβRII, and control IgG antibodies, USP11 siRNA (pools of three to five siRNA), and control siRNA were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Phospho-SMAD2, phospho-SMAD3, total SMAD2, SMAD3, and ubiquitin antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Bleomycin, leupeptin, CHX, MTX, and antibodies against Flag-tag and β-actin were from Sigma-Aldrich (St. Louis, MO, USA). Recombinant TGF-β1 was purchased from Invitrogen (Carlsbad, CA, USA). Proteasome inhibitor MG-132 was from Calbiochem (KGaA, Darmstadt, Germany). DAPI was purchased from ThermoFisher Scientific (Waltham, MA, USA). All materials in highest grades used in the experiments are commercially available.
Cell lysis and immunoblotting
Following the aforementioned cellular treatments, cells were washed with cold PBS. Cells were then collected and lysed in 120 μl of lysis buffer, which consisted of 20 mM Tris HCl (pH 7.4), 150 mM NaCl, 2 mM EGTA, 5 mM beta-glycerophosphate, 1 mM MgCl2, 1% Trton X-100, 1 mM sodium orthovanadate, 10 μg/ml protease inhibitors, 1 μg/ml aprotinin, 1μg/ml leupeptin, and 1μg/ml pepstatin. The cell lysates were then sonicated on ice for 12 s, followed by centrifugation at 4 °C at 5000 r.p.m. for 10 min. Protein concentrations of the samples were then determined with use of a Bio-Rad Protein Assay Kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using BSA as a standard. Samples were all equilibrated to 20 μg and run on a 4–15% SDS-PAGE gel, transferred to a nitrocellulose membrane, and blocked in 5% nonfat biological grade powdered milk dissolved in 25 mM Tris HCl (pH 7.4), 137 mM NaCl, and 0.1% TWEEN20 (TBST) for 30 min. Blots were washed with TBST and incubated with primary antibody in 5% BSA with TBST for 1 h or overnight. The membranes were then washed three times at 10 min intervals with TBST prior to addition of secondary antibody for 1 h. Blots were developed with an Enhanced Chemiluminescence Detection Kit (ThermoFisher Scientific, Waltham, MA, USA) according to the manufacturer's instruction.
Plasmid construction and transfection
C-terminal deletion mutants of TβRII were generated by PCR with a plasmid encoding human TβRII as a template. Primers for TβRIIC571 (amino acid 1–571) and TβRIIC586 (amino acid 1–586) were : TβRII forward: 5′-CACCATGGTCGGGGGCTGCTCAGGGGC-3′ TβRIIC571 reverse: 5′-GAGCCTGTCCAGATGCTCCAGCTC-3′ TβRIIC586 reverse: 5′GCCGTCTTCAGGAATCTTCTC-3′. PCR products were inserted into pcDNA3.1/V5-His-TOPO vector. TβRIIK205R-V5 plasmid was generated using the QuickChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA) based on TβRII-V5 plasmid. The primers are: forward: 5′-GAGCTTCGGCGTCCTGCCGGTTTCCCA-3′ reverse: 5′-TGGGAAACCGGCAGGACGCGGAAGCTC-3′. All the deletion and site-mutation mutants were confirmed by DNA sequencing. HA-Ubiquitin plasmid (Addgene plasmid no. 18712) was a gift from Edward Yeh. HA-Ubiquitin-K0 was a gift from Ted Dawson (Addgene plasmid no. 17603, Cambridge, MA, USA). MRC5 (human lung fibroblast cell line) cells were grown on glass-bottom dishes, six-well plates, D100 and D60 dishes to 60–70% confluence. The cells were then transfected with varying amounts of plasmid using the Polyjet In vitro DNA Transfection Reagent (SignaGen Laboratories, Inc., Rockville, MD, USA) system based on the manufacturer's protocol.
Co-IP and ubiquitination assay
MRC5 cells were cultured and transfected using the aforementioned protocols and then treated as indicated. The cells were then collected and lysates were prepared. Following the protein assay, lysate protein sample concentrations were equilibrated, input samples were removed, and antibody was added for overnight at 4 °C while rotating. The following morning, agarose beads were added and incubated in the cold on a rotator for at least 2 h. The samples were then centrifuged, supernatant removed, and the beads were washed for three times in PBS. In all, 2 × β-ME dye was added to the beads and heated at 100 °C for 10 min, and then the beads were removed by centrifugation. Samples were loaded on SDS-PAGE gels.
Cells were washed and collected with cold PBS for the ubiquitination assay. The cells in PBS were then centrifuged at 4 °C at 2000 r.p.m. for 5 min, the supernatant was removed, and 1 μl of ubiquitin aldehyde and 1 μl of NEM were added to the pellet. Based on the size of the pellet, 50–80 μl of 2% SDS lysis buffer was added. The cells were then boiled at 100 °C for 10 min following sonication. After boiling, the sample was diluted with between 500 and 800 μl of 1 × TBS. Normal IP procedure was then followed.
Immunofluorescence staining
MRC5 cells were grown to 80–90% confluence on glass-bottom dishes and were treated as indicated. Following treatment, cells were washed with PBS, and fixed with 3.7% formaldehyde for 20 min. Cells were then washed three times with PBS, blocked in 1% BSA in TBST for 30 min, and washed three more times in PBS. Primary antibody was then incubated for 1 h. The cells were washed three times with PBS and incubated for 1 h in the dark with a fluorescent probe conjugated to the secondary antibody. DAPI was used to stain nuclei. Images were taken with a Nikon ECLIPSE TE 300 inverted microscope (Nikon, Tokyo, Japan).
RNA isolation, reverse transcription, and qPCR
Total RNA was isolated from cells using the NucleoSpin RNA Extraction Kit from Clontech Laboratories, Inc (Mountain View, CA, USA). according to the manufacturer's protocol, and the isolated RNA was quantified using spectrophotometry. cDNA was then created using the iScript cDNA Synthesis Kit from Bio-Rad, per their specifications. mRNA expression levels of genes of interest were then analyzed by quantitative PCR using iQ SYBR Green Supermix and the iCycler Real-Time Detection System from Bio-Rad.
Lentivirus preparation and infection
USP11 shRNA lentiviral vector plasmid encoding USP11-specific nucleotide shRNA (CCGGCCCTCCCTTCTAGTCTTTATTCTCGAGAATAAAGACTAGAAGGGAGGGTTTT) was obtained from Sigma-Aldrich. A HEK293T cell line and Lenti-X Lentivirus Packaging System (Clontech Laboratories, Inc.) were used to propagate the lentivirus used in the knockdown experiments. The manufacturer's protocol was followed. For each experiment using USP11 shRNA, 4 μl of lentivirus was mixed with 1 μl of hexadimethrine bromide (10 mg/ml) and added directly to the cells. Cells were then collected 72 h following inoculation.
Bleomycin-induced murine model of pulmonary fibrosis
C57BL/6 mice with body weight of 20–25 g were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Bleomycin (0.045 U) was administered by intranasal injection. Partial right lungs were homogenized in cell lysis buffer. Protein levels were analyzed by western blotting using the indicated antibodies. Immunochemistry stainings were performed by pathology core facility at the University of Pittsburgh, Pittsburgh, PA, USA. All animal procedures in this study were performed in adherence with the National Institute of Health Guidelines on the use of Laboratory Animals and have been approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Statistical analysis
All results were subjected to statistical analysis using Microsoft Excel (Microsoft, Redmond, WA, USA) or ANOVA, and wherever appropriate, the data were analyzed by Student's t-test and expressed as means±S.D. Data were collected from at least three independent experiments, and P<0.05 was considered significant.
Acknowledgments
This study was supported by the US National Institutes of Health (R01 HL112791 and HL131665 to YZ, R01GM115389 to JZ), American Lung Association Biomedical Research Grant RG350146 (to JZ) and American Heart Association GIA award (to YZ).
The authors declare no conflict of interest.
Footnotes
Edited by J Chipuk
References
- Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M et al. TGF beta signals through a heteromeric protein kinase receptor complex. Cell 1992; 71: 1003–1014. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Carcamo J, Attisano L, Cheifetz S, Zentella A, Lopez-Casillas F et al. The type II TGF-beta receptor signals diverse responses in cooperation with the type I receptor. Cold Spring Harb Symp Quant Biol 1992; 57: 81–86. [DOI] [PubMed] [Google Scholar]
- Dennler S, Goumans MJ, ten Dijke P. Transforming growth factor beta signal transduction. J Leukoc Biol 2002; 71: 731–740. [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997; 390: 465–471. [DOI] [PubMed] [Google Scholar]
- Kawabata M, Miyazono K. Signal transduction of the TGF-beta superfamily by Smad proteins. J Biochem 1999; 125: 9–16. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs 2005; 179: 43–55. [DOI] [PubMed] [Google Scholar]
- Lim MJ, Ahn J, Yi JY, Kim MH, Son AR, Lee SL et al. Induction of galectin-1 by TGF-beta1 accelerates fibrosis through enhancing nuclear retention of Smad2. Exp Cell Res 2014; 326: 125–135. [DOI] [PubMed] [Google Scholar]
- Sundar R, Gudey SK, Heldin CH, Landstrom M. TRAF6 promotes TGFbeta-induced invasion and cell-cycle regulation via Lys63-linked polyubiquitination of Lys178 in TGFbeta type I receptor. Cell Cycle 2015; 14: 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wang Q, Du J, Luo S, Xia J, Chen YG. PICK1 promotes caveolin-dependent degradation of TGF-beta type I receptor. Cell Res 2012; 22: 1467–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratomi G, Komuro A, Goto K, Shinozaki M, Miyazawa K, Miyazono K et al. NEDD4-2 (neural precursor cell expressed, developmentally down-regulated 4-2) negatively regulates TGF-beta (transforming growth factor-beta) signalling by inducing ubiquitin-mediated degradation of Smad2 and TGF-beta type I receptor. Biochem J 2005; 386(Pt 3): 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisawa T, Fukuchi M, Murakami G, Chiba T, Tanaka K, Imamura T et al. Smurf1 interacts with transforming growth factor-beta type I receptor through Smad7 and induces receptor degradation. J Biol Chem 2001; 276: 12477–12480. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Suzuki T, Chiba T. The ligation systems for ubiquitin and ubiquitin-like proteins. Mol Cells 1998; 8: 503–512. [PubMed] [Google Scholar]
- Ha BH, Kim EE. Structures of proteases for ubiqutin and ubiquitin-like modifiers. BMB Rep 2008; 41: 435–443. [DOI] [PubMed] [Google Scholar]
- Streich FCJr., Lima CD. Structural and functional insights to ubiquitin-like protein conjugation. Annu Rev Biophys 2014; 43: 357–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboleva TA, Baker RT. Deubiquitinating enzymes: their functions and substrate specificity. Curr Protein Pept Sci 2004; 5: 191–200. [DOI] [PubMed] [Google Scholar]
- Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta 2004; 1695: 189–207. [DOI] [PubMed] [Google Scholar]
- Hanpude P, Bhattacharya S, Dey AK, Maiti TK. Deubiquitinating enzymes in cellular signaling and disease regulation. IUBMB Life 2015; 67: 544–555. [DOI] [PubMed] [Google Scholar]
- Suresh B, Lee J, Kim KS, Ramakrishna S. The importance of ubiquitination and deubiquitination in cellular reprogramming. Stem cells international 2016; 2016: 6705927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober AS, Berra E. DUBs, new members in the hypoxia signaling clUb. Front Oncol 2016; 6: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko YM, Chang CY, Chiou SJ, Hsu FJ, Huang JS, Yang YL et al. Ubiquitin C-terminal hydrolase-L5 is required for high glucose-induced transforming growth factor-beta receptor I expression and hypertrophy in mesangial cells. Arch Biochem Biophys 2013; 535: 177–186. [DOI] [PubMed] [Google Scholar]
- Al-Salihi MA, Herhaus L, Macartney T, Sapkota GP. USP11 augments TGFbeta signalling by deubiquitylating ALK5. Open Biol 2012; 2: 120063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorn PJ, Rodon L, Gonzalez-Junca A, Dirac A, Gili M, Martinez-Saez E et al. USP15 stabilizes TGF-beta receptor I and promotes oncogenesis through the activation of TGF-beta signaling in glioblastoma. Nat Med 2012; 18: 429–435. [DOI] [PubMed] [Google Scholar]
- Burkhart RA, Peng Y, Norris ZA, Tholey RM, Talbott VA, Liang Q et al. Mitoxantrone targets human ubiquitin-specific peptidase 11 (USP11) and is a potent inhibitor of pancreatic cancer cell survival. Mol Cancer Res 2013; 11: 901–911. [DOI] [PubMed] [Google Scholar]
- Gaidarova S, Jimenez SA. Inhibition of basal and transforming growth factor-beta-induced stimulation of COL1A1 transcription by the DNA intercalators, mitoxantrone and WP631, in cultured human dermal fibroblasts. J Biol Chem 2002; 277: 38737–38745. [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Mauviel A. Control of connective tissue gene expression by TGF beta: role of Smad proteins in fibrosis. Curr Rheumatol Rep 2002; 4: 143–149. [DOI] [PubMed] [Google Scholar]
- Sheppard D. Epithelial-mesenchymal interactions in fibrosis and repair. Transforming growth factor-beta activation by epithelial cells and fibroblasts. Ann Am Thorac Soc 2015; 12(Suppl 1): S21–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N, Greenberg AH. The role of TGF-beta in pulmonary fibrosis. Ciba Found Symp 1991; 157: 194–207 discussion 207-111. [DOI] [PubMed] [Google Scholar]
- Kang HR, Lee JY, Lee CG. TGF-beta1 as a therapeutic target for pulmonary fibrosis and COPD. Expert Rev Clin Pharmacol 2008; 1: 547–558. [DOI] [PubMed] [Google Scholar]
- Inui M, Manfrin A, Mamidi A, Martello G, Morsut L, Soligo S et al. USP15 is a deubiquitylating enzyme for receptor-activated SMADs. Nat Cell Biol 2011; 13: 1368–1375. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Thornton AM, Kinney MC, Ma CA, Spinner JJ, Fuss IJ et al. The deubiquitinase CYLD targets Smad7 protein to regulate transforming growth factor beta (TGF-beta) signaling and the development of regulatory T cells. J Biol Chem 2011; 286: 40520–40530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge WL, Xu JF, Hu J. Regulation of oral squamous cell carcinoma proliferation through crosstalk between SMAD7 and CYLD. Cell Physiol Biochem 2016; 38: 1209–1217. [DOI] [PubMed] [Google Scholar]
- Dupont S, Mamidi A, Cordenonsi M, Montagner M, Zacchigna L, Adorno M et al. FAM/USP9x, a deubiquitinating enzyme essential for TGFbeta signaling, controls Smad4 monoubiquitination. Cell 2009; 136: 123–135. [DOI] [PubMed] [Google Scholar]
- Wagner SA, Beli P, Weinert BT, Scholz C, Kelstrup CD, Young C et al. Proteomic analyses reveal divergent ubiquitylation site patterns in murine tissues. Mol Cell Proteomics 2012; 11: 1578–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K, Lodish HF. Positive and negative regulation of type II TGF-beta receptor signal transduction by autophosphorylation on multiple serine residues. EMBO J 1997; 16: 1970–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Esteo M, Sanchez-Elsner T, Letamendia A, Bernabeu C. Extracellular and cytoplasmic domains of endoglin interact with the transforming growth factor-beta receptors I and II. J Biol Chem 2002; 277: 29197–29209. [DOI] [PubMed] [Google Scholar]
- Noh H, Kim HJ, Yu MR, Kim WY, Kim J, Ryu JH et al. Heat shock protein 90 inhibitor attenuates renal fibrosis through degradation of transforming growth factor-beta type II receptor. Lab Invest 2012; 92: 1583–1596. [DOI] [PubMed] [Google Scholar]
- Vi L, Boo S, Sayedyahossein S, Singh RK, McLean S, Di Guglielmo GM et al. Modulation of type II TGF-beta receptor degradation by integrin-linked kinase. J Invest Dermatol 2015; 135: 885–894. [DOI] [PubMed] [Google Scholar]
- Zuo W, Huang F, Chiang YJ, Li M, Du J, Ding Y et al. c-Cbl-mediated neddylation antagonizes ubiquitination and degradation of the TGF-beta type II receptor. Mol Cell 2013; 49: 499–510. [DOI] [PubMed] [Google Scholar]
- Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J Biol Chem 1997; 272: 24735–24738. [DOI] [PubMed] [Google Scholar]
- Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D et al. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev 1995; 9: 1586–1597. [DOI] [PubMed] [Google Scholar]
- Kong SK, Chock PB. Protein ubiquitination is regulated by phosphorylation. An in vitro study. J Biol Chem 1992; 267: 14189–14192. [PubMed] [Google Scholar]