Abstract
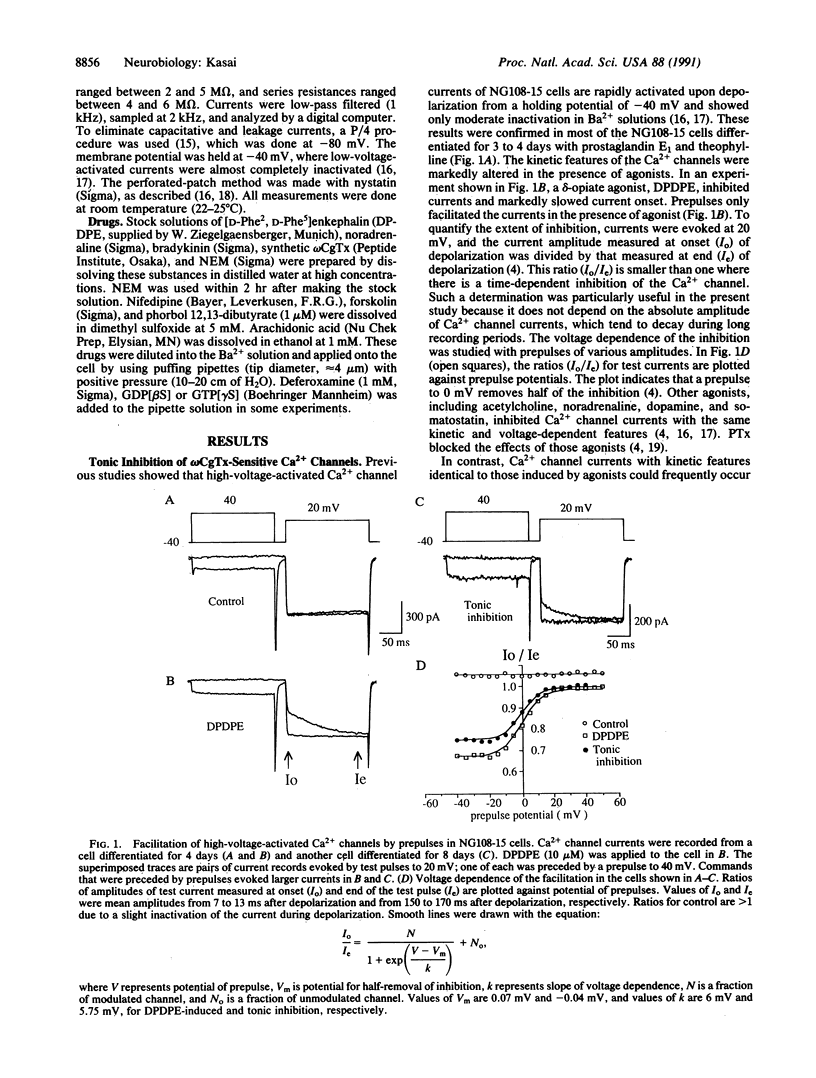
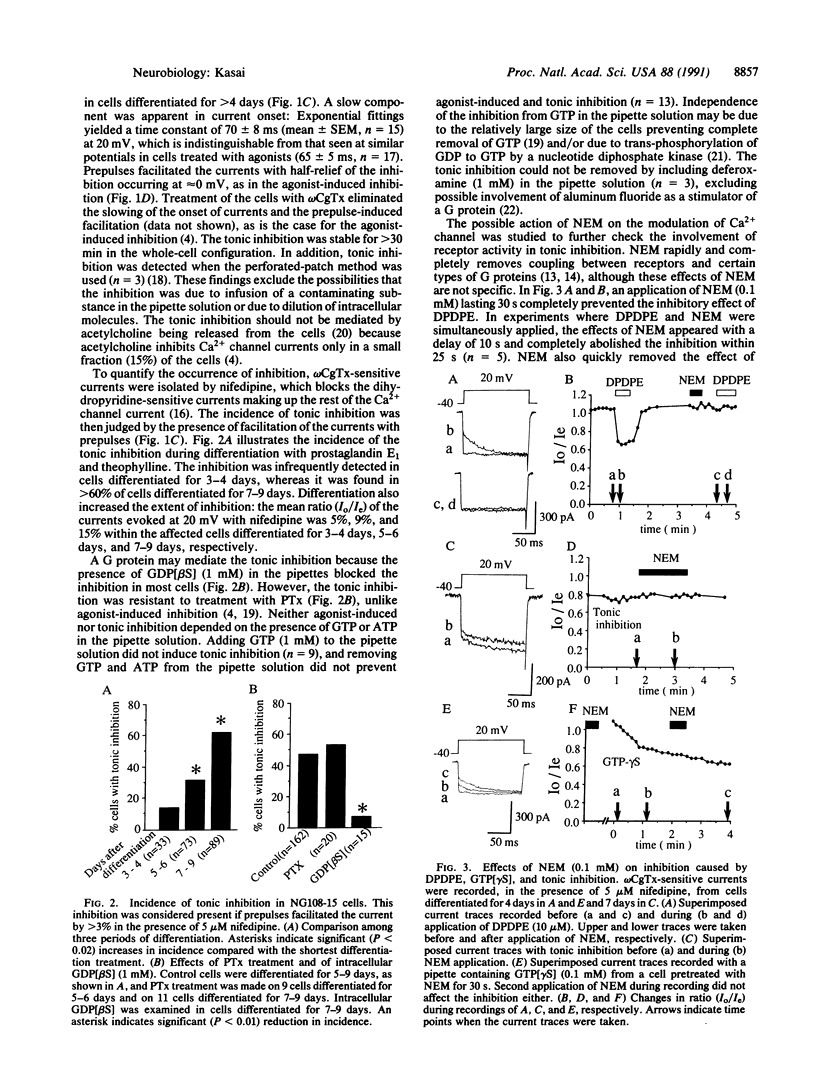
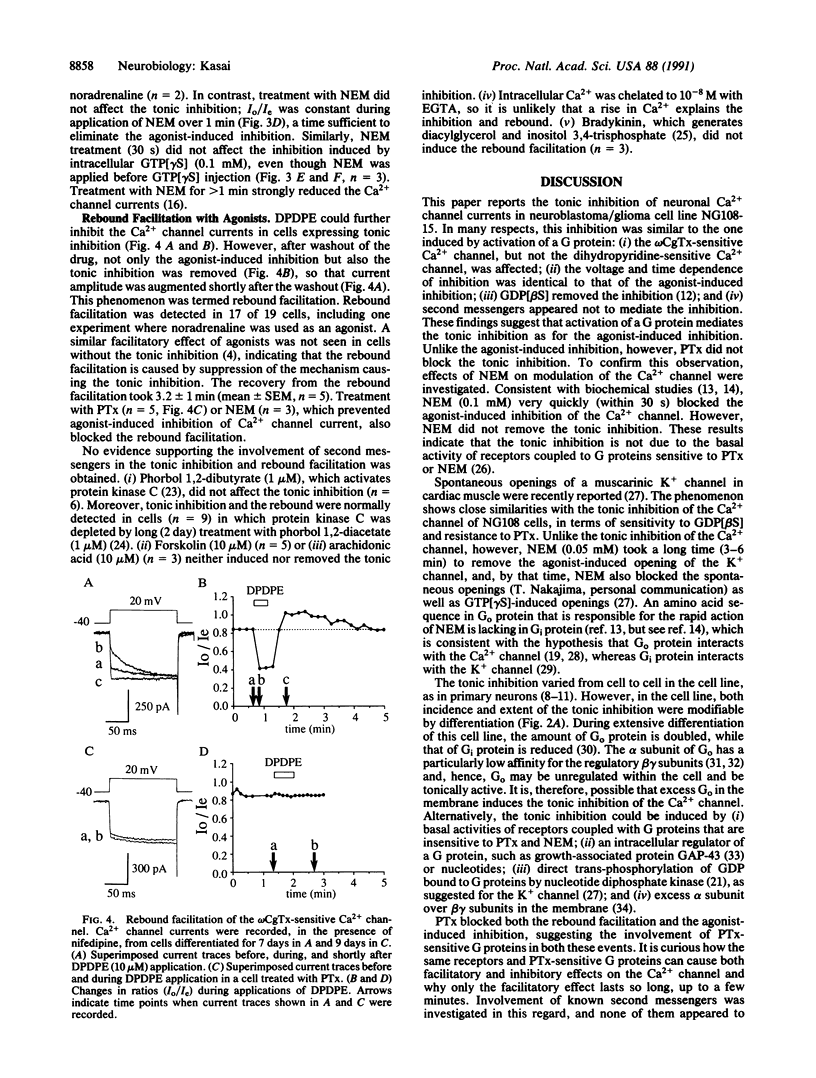
A significant fraction of differentiated NG108-15 neuroblastoma/glioma cells have Ca2+ channel current different from that of undifferentiated cells. In the former cells, the Ca2+ channel sensitive to omega-conotoxin GVIA had slowed activation kinetics and was facilitated by depolarizing prepulses. These kinetic features are identical to those produced by inhibition of the channel by G proteins. Prolonged treatment with prostaglandin E1 and theophylline, agents that cause cellular differentiation, promoted incidence and extent of the tonic inhibition. Intracellular guanosine 5'-[beta-thio]diphosphate removed the tonic inhibition, suggesting sustained activation of a G protein, but pertussis toxin did not block it. A sulfhydryl alkylating agent, N-ethylmaleimide (0.1 mM), rapidly eliminated agonist-induced inhibition, whereas N-ethylmaleimide spared the tonic inhibition and the one induced by intracellular guanosine 5'-[gamma-thio]triphosphate. An agonist could further inhibit the Ca2+ channel that was already tonically inhibited. After washout of an inhibitory agonist, the tonic inhibition was temporarily removed. This "rebound facilitation" gradually faded within a few minutes. Pertussis toxin or N-ethylmaleimide prevented the rebound facilitation, whereas phorbol ester, forskolin, or arachidonic acid induced neither the rebound facilitation nor the tonic inhibition. Whatever its mechanism, the tonic inhibition of Ca2+ channels may serve as the basis for long-term and bidirectional regulation of activity of neuronal Ca2+ channels.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo C. R., Ariano M. A., Perlman R. L., Fox A. P. Activation of facilitation calcium channels in chromaffin cells by D1 dopamine receptors through a cAMP/protein kinase A-dependent mechanism. Nature. 1990 Nov 15;348(6298):239–242. doi: 10.1038/348239a0. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989 Jul 13;340(6229):153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Borner C., Eppenberger U., Wyss R., Fabbro D. Continuous synthesis of two protein-kinase-C-related proteins after down-regulation by phorbol esters. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2110–2114. doi: 10.1073/pnas.85.7.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Birnbaumer L. Ionic channels and their regulation by G protein subunits. Annu Rev Physiol. 1990;52:197–213. doi: 10.1146/annurev.ph.52.030190.001213. [DOI] [PubMed] [Google Scholar]
- Costa T., Herz A. Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7321–7325. doi: 10.1073/pnas.86.19.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C. G protein modulation of calcium currents in neurons. Annu Rev Physiol. 1990;52:243–255. doi: 10.1146/annurev.ph.52.030190.001331. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Scott R. H. Calcium channel currents and their inhibition by (-)-baclofen in rat sensory neurones: modulation by guanine nucleotides. J Physiol. 1987 May;386:1–17. doi: 10.1113/jphysiol.1987.sp016518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Grassi F., Lux H. D. Voltage-dependent GABA-induced modulation of calcium currents in chick sensory neurons. Neurosci Lett. 1989 Oct 23;105(1-2):113–119. doi: 10.1016/0304-3940(89)90021-9. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino S., Kikkawa S., Takahashi K., Itoh H., Kaziro Y., Kawasaki H., Suzuki K., Katada T., Ui M. Identification of sites for alkylation by N-ethylmaleimide and pertussis toxin-catalyzed ADP-ribosylation on GTP-binding proteins. FEBS Lett. 1990 Dec 10;276(1-2):227–231. doi: 10.1016/0014-5793(90)80548-w. [DOI] [PubMed] [Google Scholar]
- Ikeda S. R. Double-pulse calcium channel current facilitation in adult rat sympathetic neurones. J Physiol. 1991 Aug;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W., Marks T. N. Calcium currents in bullfrog sympathetic neurons. II. Inactivation. J Gen Physiol. 1989 Jul;94(1):169–182. doi: 10.1085/jgp.94.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibara M., Nakajima T., Irisawa H., Giles W. Regulation of spontaneous opening of muscarinic K+ channels in rabbit atrium. J Physiol. 1991 Feb;433:589–613. doi: 10.1113/jphysiol.1991.sp018445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Kasai H., Aosaki T. Modulation of Ca-channel current by an adenosine analog mediated by a GTP-binding protein in chick sensory neurons. Pflugers Arch. 1989 Jun;414(2):145–149. doi: 10.1007/BF00580956. [DOI] [PubMed] [Google Scholar]
- Katada T., Oinuma M., Ui M. Two guanine nucleotide-binding proteins in rat brain serving as the specific substrate of islet-activating protein, pertussis toxin. Interaction of the alpha-subunits with beta gamma-subunits in development of their biological activities. J Biol Chem. 1986 Jun 25;261(18):8182–8191. [PubMed] [Google Scholar]
- Kikkawa S., Takahashi K., Takahashi K., Shimada N., Ui M., Kimura N., Katada T. Conversion of GDP into GTP by nucleoside diphosphate kinase on the GTP-binding proteins. J Biol Chem. 1990 Dec 15;265(35):21536–21540. [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. Short-term desensitization of muscarinic K+ channel current in isolated atrial myocytes and possible role of GTP-binding proteins. Pflugers Arch. 1987 Oct;410(3):227–233. doi: 10.1007/BF00580270. [DOI] [PubMed] [Google Scholar]
- McFadzean I., Mullaney I., Brown D. A., Milligan G. Antibodies to the GTP binding protein, Go, antagonize noradrenaline-induced calcium current inhibition in NG108-15 hybrid cells. Neuron. 1989 Aug;3(2):177–182. doi: 10.1016/0896-6273(89)90030-5. [DOI] [PubMed] [Google Scholar]
- Mullaney I., Magee A. I., Unson C. G., Milligan G. Differential regulation of amounts of the guanine-nucleotide-binding proteins Gi and Go in neuroblastoma x glioma hybrid cells in response to dibutyryl cyclic AMP. Biochem J. 1988 Dec 1;256(2):649–656. doi: 10.1042/bj2560649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg M., Wilson S., Higashida H., Rotter A., Krueger K., Busis N., Ray R., Kenimer J. G., Adler M. Modulation of synapse formation by cyclic adenosine monophosphate. Science. 1983 Nov 18;222(4625):794–799. doi: 10.1126/science.6314503. [DOI] [PubMed] [Google Scholar]
- Otero A. S., Li Y., Szabo G. Receptor-mediated deactivation of Gk in cardiac myocytes. Pflugers Arch. 1991 Jan;417(5):543–545. doi: 10.1007/BF00370953. [DOI] [PubMed] [Google Scholar]
- Pang I. H., Sternweis P. C. Isolation of the alpha subunits of GTP-binding regulatory proteins by affinity chromatography with immobilized beta gamma subunits. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7814–7818. doi: 10.1073/pnas.86.20.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobon D., Hess P. Novel mechanism of voltage-dependent gating in L-type calcium channels. Nature. 1990 Aug 16;346(6285):651–655. doi: 10.1038/346651a0. [DOI] [PubMed] [Google Scholar]
- Schultz G., Rosenthal W., Hescheler J., Trautwein W. Role of G proteins in calcium channel modulation. Annu Rev Physiol. 1990;52:275–292. doi: 10.1146/annurev.ph.52.030190.001423. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Dolphin A. C. Voltage-dependent modulation of rat sensory neurone calcium channel currents by G protein activation: effect of a dihydropyridine antagonist. Br J Pharmacol. 1990 Apr;99(4):629–630. doi: 10.1111/j.1476-5381.1990.tb12981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Aluminum: a requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter S. M., Valenzuela D., Kennedy T. E., Neer E. J., Fishman M. C. G0 is a major growth cone protein subject to regulation by GAP-43. Nature. 1990 Apr 26;344(6269):836–841. doi: 10.1038/344836a0. [DOI] [PubMed] [Google Scholar]
- Swandulla D., Carbone E., Lux H. D. Do calcium channel classifications account for neuronal calcium channel diversity? Trends Neurosci. 1991 Feb;14(2):46–51. doi: 10.1016/0166-2236(91)90018-p. [DOI] [PubMed] [Google Scholar]
- Tsunoo A., Yoshii M., Narahashi T. Block of calcium channels by enkephalin and somatostatin in neuroblastoma-glioma hybrid NG108-15 cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9832–9836. doi: 10.1073/pnas.83.24.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K., Higashida H., Inoue R., Nozawa Y. Bradykinin-induced rapid breakdown of phosphatidylinositol 4,5-bisphosphate in neuroblastoma X glioma hybrid NG108-15 cells. J Biol Chem. 1984 Aug 25;259(16):10201–10207. [PubMed] [Google Scholar]



