Abstract
Colorectal cancer has become one of the most prevalent malignant diseases for both men and women. Patients with inflammatory bowel diseases or certain inherited cancer syndromes are at extremely high risk of developing colorectal cancer and have naturally the highest need for cancer prevention. In familial adenomatous polyposis (FAP) and Lynch syndrome most of the underlying germline mutations can be detected by DNA sequencing, and medical counselling of affected individuals involves both surveillance tests and chemopreventive measures. However, as the mechanisms leading to colorectal cancer differ in these high risk groups, the molecular action of chemopreventive drugs needs to be adjusted to the certain pathway of carcinogenesis. In the last decades a number of drugs have been tested, including sulindac, aspirin, celecoxib, and mesalazine, but some of them are still controversially discussed. This review summarizes the advances and current standards of colorectal cancer prevention in patients with inflammatory bowel disease, FAP and Lynch syndrome.
Keywords: colorectal cancer, chemoprevention, Lynch syndrome, FAP, inflammatory bowel disease, 5-ASA, aspirin, sulindac, dietary supplements
Introduction
The lifetime risk for the development of colorectal cancer (CRC) in the general population is approximately 2%, in Western countries 3-4% [1]. CRC accounts for 8% of all cancer deaths worldwide [1]. Numerous factors lead to an increased risk of CRC. Inheritable forms of CRC only account for 3-5% of all cases. If left untreated, the lifetime risk to develop CRC increases up to 80% for Lynch syndrome (formerly known as hereditary non-polyposis colorectal cancer or HNPCC) and up to 100% for familial adenomatous polyposis (FAP). Other risk factors include inflammatory bowel disease (IBD) with an 1.7 to 2.4-fold CRC risk increase compared to the general population [2,3], other polyposis syndromes (such as MUTYH-polyposis or juvenile polyposis syndromes), and unknown genetic mutations resulting in a familial aggregation of CRC cases (2-6 fold increase). Besides genetic alterations and chronic intestinal inflammation, Western lifestyle influences CRC development: High intake of alcohol, red meat, cigarette smoking, obesity and lack of regular physical activity are important and potentially avoidable risk factors.
Endoscopic surveillance, early detection and early surgery have become the main strategies to tackle this problem as late stages of CRC are difficult to treat, cost-intensive and lethal. In adjunct to these measures, primary cancer prevention with pharmaceuticals, phytochemicals and/or dietary agents has become standard of care in certain high risk populations. This review will focus on primary chemoprevention in IBD, FAP and Lynch syndrome patients.
Inflammatory bowel disease
It is a well-known fact that patients with ulcerative colitis (UC) and colonic Crohn’s disease (CD) have an increased risk for the development of CRC. Extent, severity, early onset and duration of the disease as well as coexistence of primary sclerosing cholangitis (PSC), and a family history of CRC, are well established risk factors [4,5]. A meta-analysis carried out in 2001, including 116 studies, estimated the prevalence of CRC in any UC patient to be 3.7% (95% CI 3.2-4.2%) with cumulative probabilities of 2%, 8%, and 18% by 10, 20, and 30 years, respectively [6]. A meta-analysis for CD patients, including 6 publications, reported a comparable CRC risk with a standardized incidence ratio (SIR) of 1.9 (95% CI 1.4-2.5) [7]. Only Crohn’s patients with colonic involvement have a 4.5 times greater CRC risk. Crohn’s ileitis does not trigger colonic cancer risk (relative risk 1.1; 95% CI 0.8-1.5), again pointing to the fact that colonic inflammation itself is the main cause in such patients [8]. A recent meta-analysis of population-based cohort studies found a declining risk for CRC in IBD with a SIR of only 1.7 (95% CI 1.2-2.2) [2]. High-risk groups identified were, as also previously found, IBD diagnosis before the age of 30 with a SIR of 7.2 (95% CI 2.9-17.8) and extensive colitis with a SIR of 6.4 (95% CI 2.4-17.5). Another study published a year earlier showed similar results with respect to a decreasing CRC incidence with a SIR of 2.4 (95% CI 2.1-2.7) for UC patients within 14 years of follow-up [3]. Another population-based study from Denmark even found no increased CRC risk in IBD (relative risk 1.07; 95% CI 0.95-1.21) [9], which is an extension to what was published earlier from the same cohort [10]. Only subgroups with onset of UC in childhood or concomitant PSC remained at increased risk. The authors concluded that the decreased risk of CRC in IBD patients might be the result of improved therapies for IBD, including mesalazine (5-aminosalicylic acid; 5-ASA), thiopurines, TNF-α antagonists, and high colectomy rates.
Mesalazine
5-ASA, the first-line therapy for mild-to-moderate UC and for maintaining remission, is an anti-inflammatory agent with proposed chemopreventive properties. Despite the drug causes mucosal healing in the majority of patients, its chemopreventive effect is still under debate after decades of its clinical use. Extensive research on the molecular actions of 5-ASA support both its anti-inflammatory and its anti-neoplastic properties. 5-ASA is a potent scavenger for reactive oxygen species [11], interferes with TNF-α/TGF-β [12]/NF-κB [13] pathways, and has antimicrobial activity [14]. Furthermore, 5-ASA induces apoptosis [15], targets eukaryotic translation initiation factors [16], induces S-phase arrest [17], and improves replication fidelity [18,19], thereby reducing microsatellite instability, which has recently been shown also for an inflammation independent model of CRC [20]. Other functions of 5-ASA include interference with the WNT/β-catenin pathway, the MAPK/ERK pathway and increase in cell adhesion, with Pak1 as a common mediator, which is downstream of Rac1 [21,22]. Several cohort- and case-control studies verifying the chemopreventive properties of 5-ASA have been published with controversial results. A meta-analysis, including 3 cohort and 6 case-control studies published between 1996-2004, showed a protective effect of 5-ASA for CRC with an OR of 0.51 (95% CI 0.37-0.69) [23]. An epidemiological study based on the UK General Practice Research Database found a decreased risk of CRC, for regular 5-ASA users compared with irregular users with an OR of 0.6 (95% CI 0.22-0.96) [24]. A population-based study carried out in Canada failed to show such a protective effect [25]. Another meta-analysis of non-referral populations did not find a protective effect for CRC in IBD as well (adjusted OR 0.95, 95% CI 0.66-1.38) [26]. The same publication showed a protective effect for a meta-analysis of nine clinic-based studies with a pooled OR of 0.58 (95% CI 0.45-0.75). At this point, the chemopreventive effects of 5-ASA in UC is difficult to prove in a prospective placebo-controlled trial, as it is considered unethical to withhold any UC patient such active drug with excellent safety profile.
Thiopurines
Azathioprine and its metabolites, 6-mercaptopurine and thioguanine, are used for the treatment of IBD, alone or in combination with other drugs. The mechanism of azathioprine within the gut is attributed to its action on CD4-positive T lymphocytes by inhibiting the small GTPase Rac1, thereby inducing apoptosis, mediated by the metabolite 6-thioguanine-triphosphate [27]. This is the same Rac1 which activates PAK1, a known target of 5-ASA (see above). A Dutch IBD cohort study compared thiopurine and 5-ASA users to non-users for the development of advanced colorectal neoplasia, defined by high-grade dysplasia and CRC, resulting in a protective effect for both thiopurine and 5-ASA with adjusted HR of 0.10 (95% CI 0.01-0.75) and 0.56 (95% CI 0.22-1.4), respectively [28]. A recent meta-analysis including 9 case-control and ten cohort studies documented a decreased CRC incidence, in patients treated with thiopurines with a RR of 0.71 (95% CI 0.54-0.017) [29]. CESAME, a large French prospective IBD cohort, was initialized to monitor cancer risk in IBD patients. Among patients with long-standing extensive colitis, thiopurine intake is protective for colorectal high grade dysplasia and CRC with a hazard ratio of 0.28 (95% CI 0.1-0.9) compared to non-users [30]. Despite the anti-inflammatory and potential protective effect of azathioprine on CRC development, the risk for other cancers might be increased, particularly for non-melanoma skin cancer (NMSC) and lymphoma. A publication based on the CESAME cohort reported an increased risk for NMSC for current and past thiopurine exposure with a hazard ratio of 5.9 (95% CI 2.1-16.4) and 3.9 (95% CI 1.3-12.1), respectively [31]. In a retrospective nested-case control study Long et al. showed that recent or persistent thiopurine use was associated with NMSC in CD patients with an OR of 3.56 (95% CI 2.81-4.50) and 4.27 (95% CI 3.08-5.92) [32], a correlation which was not seen in a Dutch patient cohort [33]. A UK population-based case-control study found an association of lymphoma development and azathioprine intake with an OR of 3.22 (95% CI 1.01 – 10.18) [34]. Similarly, in a Danish IBD cohort study the rate ratio for overall cancer risk was 1.41 (95% CI 1.15 – 1.74), comparing non-users to azathioprine users, which was not found for patients who discontinued the use of azathioprine [35]. IBD patients treated with thiopurines, due to the potential increased risk of other cancers, should have a lifelong dermatological and lymphoma screening, appropriate counseling, and should avoid exposure to UV-radiation. Nevertheless, thiopurines have a protective effect for the prevention of CRC specifically in the setting of long-standing UC and Crohn’s colitis.
TNF-α antagonists
Antibodies against TNF-α such as infliximab, adalimumab, and certolizumab are used alone or in combination with standard therapy and have been shown to reduce clinical symptoms and mucosal healing in patients with luminal or penetrating CD and in patients with moderate-to-severe UC which are refractory to other medications [36]. In a Danish population, frequency of severe adverse reactions to infliximab was 3.3%, the risk of cancer (especially lymphomas) was not increased, whereas mortality was significantly higher than expected [37]. In contrast, in a nested-case control study, it has been shown that recent or persistent biologic use among patients with CD was associated with NMSC (OR, 2.07; 95% CI, 1.28 –3.33 and 2.18; 95% CI, 1.07-4.46, respectively) [32]. A more recent meta-analysis compared the effect of adalimumab monotherapy to combination therapy (with thiopurine or methotrexate) in CD patients. The authors found an increasing risk for NMSC and other malignancies with a SIR of 4.59 (95% CI 2.51-7.70) and 3.04 (95% CI 1.66-5.10), respectively [38]. In the context of chemoprevention, it is too early to judge biologic agents in IBD as they have been implemented only 15 years ago. As persistent inflammation is considered the main driver for colitis-associated cancer, any drug that induces and maintains mucosal healing has the potential for reducing the CRC risk.
Ursodeoxycholic acid (UDCA)
PSC is strongly associated with IBD, as is the risk of developing colorectal neoplasia [39]. UDCA, the first line medication for the treatment of PSC, is considered chemopreventive for CRC.
A follow-up study investigating the long-term effect of UDCA in IBD patients with PSC, found a protective effect on colorectal neoplasia development with a relative risk of 0.26 (95% CI 0.07-0.99) with a mean treatment time of 42 months at low dose (<15 mg/kg) [40]. A similar study reported on a decreased prevalence of colonic neoplasia with an OR of 0.18 (95% CI 0.05-0.61) after 3.5-4.5 years at <10 mg/kg UDCA [41]. In contrast, high-dose treatment with UDCA >15 mg/kg did not show a protective effect after 5 years [42]. In line, treatment with even higher doses (~30 mg/kg UDCA) increased the risk of dysplasia or CRC (hazard ratio 4.44; 95% CI 1.3-20.1) [43]. Similarly, a retrospective analysis did not show a significant effect on dysplasia or CRC outcome after a mean intake of 3.4 years [44]. A meta-analysis from 7 publications revealed an overall non-significant risk reduction for patients treated with UDCA, especially at low and medium dosages [45]. In conclusion, current data suggest a trend for CRC risk reduction in PSC/IBD patients treated with UDCA <15mg/kg, whereas higher doses seem to increase the risk. Off topic, UDCA is not effective for polyp regression in FAP patients [46].
Familial adenomatous polyposis
Germline mutations in the adenomatous polyposis coli gene (APC) are inherited in FAP. A second somatic mutation, which is commonly a missense mutation in the somatic mutation cluster region (codons 1,250-1,450), results in the appearance of hundreds and thousands of polyps, mainly found in the large bowel, ultimately progressing into CRC [47]. In addition, duodenal and small intestinal polyps are found in 90% of all FAP patients and with specific FAP mutations also certain other extraintestinal tumors (e.g. desmoid tumors, osteoma; Figure 1). Different variants of FAP exist, which are less common and more benign such as attenuated FAP or MUTYH-associated polyposis. As the disease phenotype is clinically obvious, FAP is a great human model for studying chemoprevention in the colon.
Figure 1. Extracolonic manifestations in FAP.
Lifetime risk (%) of non-colonic tumors and other manifestations in FAP. CHRPE, Congenital hypertrophy of the retinal pigment epithelium; References: 1[87], 2[88] Images were created with SmartDraw Software (San Diego, CA, USA).
Non-steroidal anti-inflammatory drugs (NSAID)
The main action of most NSAIDs is attributed to the inhibition of cyclooxygenases (Cox). Cox-independent mechanisms involve NF-κB inactivation and β-catenin-pathway inhibition [48]. Gastrointestinal side effects of NSAIDs are mostly associated with Cox-1 inhibition. Specific Cox-2 inhibitors such as celecoxib have fewer gastrointestinal side effects, but more cardiovascular events, which led to the market withdrawal of rofecoxib in 2004 [49]. Similar cardiovascular toxicity has later been reported also for NSAIDs (such as diclofenac), which can be considered as non-selective Cox inhibitors.
Sulindac
This non-selective Cox inhibitor, which is metabolized into its active compound sulindac sulfide and sulfone has been extensively studied in FAP with positive results. In 1991, the first randomized, placebo controlled study demonstrated a protective effect of sulindac on rectal polyp formation. After 4 month of sulindac (300 mg/d) a complete polyp regression was observed in 6/9 patients and none in the placebo group [50]. In 1993, three additional clinical trials showed similar results: Twenty-two FAP patients received 150 mg sulindac twice daily or placebo for 9 months. The mean number of polyps and the mean diameter regressed to 44% and 35%, respectively. 3 months after sulindac treatment, some polyps rose again, but remained fewer than at base line [51]. Similarly, in 24 colectomized FAP patients a trend towards reduced duodenal polyps was found, and rectal polyps decreased [52]. Another study reported on polyp regression after 60 days of 200 mg/d sulindac [53].
Local, low-dose rectal therapy in colectomized patients achieved complete polyp disappearance in 13 of 15 patients without relapse for over two years [54,55]. Interesting, in 41 FAP children before the primary polyp appearance, sulindac did not show a protective effect on the primary occurrence of adenomas [56]. Few data exist on long-term effects of sulindac: A prospective study at moderate doses found a decrease in polyp numbers after 12 months as well as after a mean of 5 years though rectal mucosal erosions were reported at the ileorectal anastomosis [57]. A retrospective study in patients with ileorectal anastomosis pointed out a non-significant effect after 4 years, although data at 6 months were significant [58]. Compliance is definitely an issue with long-term therapy. No association exists between the location of the APC mutation and the response to sulindac [59,60]. More distinct endoscopic approaches with chromoendoscopy revealed a decrease in polyp protrusions [60]. These morphological changes from protruding to flat lesions were also described by others [58,61]. Such lesions might serve as precursors for CRC development, if undetected. Long-term surveillance data for CRC development in FAP patients are missing. Sulindac is not available in many countries and thus its clinical use is limited.
Aspirin
Epidemiological data from large populations have pointed to the chemopreventive effects of aspirin [62]. The Concerted Action Polyp Prevention 1 (CAPP1), a randomized, placebo-controlled trial investigated the effect of 600 mg aspirin and/or 30 g resistant starch for a minimum of 1 year in 200 FAP carriers. Aspirin, but not resistant starch, decreased the size of the largest polyp, but not polyp numbers [63]. In a Japanese FAP cohort a similar effect on size reduction was observed with 100 mg for 6-10 month [64].
Celecoxib
Compared to sulindac, aspirin or other NSAIDs, Cox-2 inhibitors have the advantage of reduced gastrointestinal side effects. In a randomized, double-blinded, placebo-controlled trial the effect of adenoma regression upon treatment with 100 or 400 mg celecoxib twice a day for 6 months was investigated in 77 adult FAP patients [65]. A dose-dependent effect of celecoxib on adenoma regression was found, whereas only the high dose group showed a significant reduction in both the mean polyp number and polyp burden. A similar study assessing the effect on duodenal polyposis reported an effect at 400 mg twice daily [66]. In line, a dose-escalation study in children found a dose-dependent decrease of polyps after 3 month at up to 16 mg/kg [67]. A clinical trial with 61 FAP patients treated with 150-200 mg of Tiracoxib, another Cox-2 inhibitor, did not show any effect, which underlines the importance of appropriate dosing [68]. At this point celecoxib is the standard of care for patients before and after colectomy.
Lynch syndrome
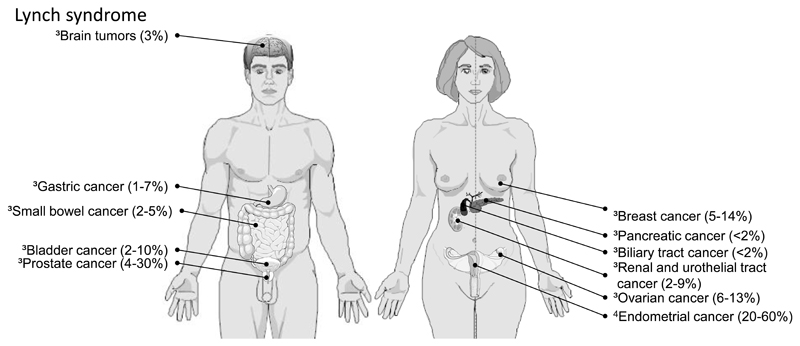
This hereditary colorectal cancer syndrome, formerly known as hereditary non-polyposis colorectal cancer or HNPCC, has an estimated incidence of 1-4% among all patients with CRC [69,70]. It is caused by a germline mutation in one of four DNA mismatch repair genes, MSH2, MLH1, PMS2, or MSH6 leading to a hypermutable condition that fosters cancer development. One of the key features in Lynch syndrome tumors is microsatellite instability (MSI), which describes frameshift mutations in repetitive DNA sequences. Depending on the genetic defect, Lynch syndrome cancers may occur before the age of 50 (with mutations in MSH2 and MLH1) or even beyond 60 to 70 years of age (for MSH6 and PMS2 mutation carriers) [71]. Other typical feature are synchronous and metachronous tumors, right colonic location, extracolonic cancer (e.g. endometrial, gastric, urogenital/bladder; Figure 2), and an autosomal dominant pattern of inheritance [72]. Despite this syndrome is far more common than FAP, mutation carriers are rarely identified as the disease phenotype is less obvious than in FAP.
Figure 2. Extracolonic cancer in Lynch syndrome.
Lifetime risk (%) of non-colonic tumors in Lynch syndrome. References: 3[69], 4[89]. Images were created with SmartDraw Software (San Diego, CA, USA).
The CAPP2 study was the first prospective, randomized, placebo-controlled, double-blind, multicenter, interventional trial to evaluate the effect of aspirin (600 mg) and/or resistant starch (30 g) on colorectal neoplasia development in Lynch syndrome gene carriers. Neither aspirin nor resistant starch or a combination of both had a preventive effect on intestinal neoplasia after a mean intake of 29 months [73]. A secondary analysis conducted 4 years after completion of the trial indicated a possible late effect of aspirin on cancer incidence [74]. Current goals are to replicate this post-hoc analysis in a randomized, double-blind non-inferiority, dose-finding study, CAPP3 [75]. Based on its chemopreventive effects in UC, its molecular improvement of replication fidelity and recent data in a mouse model of Lynch syndrome, another group of investigators just received funding from the European Union to evaluate mesalazine in Lynch syndrome carriers (MesaCAPP or CAPP4) [18,20,23,76].
Dietary supplements and phytochemicals
Reliable data on dietary supplementations or phytochemicals in patients at high risk for colorectal cancer are scarce. Fatty acids, calcium, folic acid, wheat fiber, vitamin C/E and curcumin/quercetin have been studied in FAP patients. Several studies are ongoing with regards to curcumin in FAP or IBD patients [77].
ω-3 long-chain polyunsaturated fatty acids (PUFAs) as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are found at high concentrations in fish oil. An inverse relation exists between fish intake and CRC risk [78] and data on the anti-carcinogenic effect of ω-3 PUFAs accumulate [79]. A phase III randomized, double-blind, placebo-controlled trial in FAP patients with 2 g EPA demonstrated both a reduction in polyp number and polyp burden [79].
Small studies evaluating the effect of calcium or vitamin supplementation in FAP patients have been published decades ago with some promising effects. Treatment of 5 FAP patients with 3 g/d ascorbic acid between 4-13 month, led to a total and partial regression of rectal polyps in 2 patients each, whereas in one patient polyps increased [80]. A randomized placebo-controlled study in 36 FAP patients found a significant reduction in polyp area in patients receiving 3 g/d ascorbic acid [81]. The intake of grain fiber, vitamin C and vitamin E for a period of 4 years was evaluated in another 58 FAP patients. A trend towards polyp reduction in the high fiber group was found, but not for vitamin intake [82]. Also calcium carbonate over 6 months in FAP patients had no significant effect on reduction of polyp number or disease progression [83]. Supplementation with folic acid in UC patients reduced neoplasia development with a relative risk reduction of 0.72 (95% CI 0.28-1.83), and was more effective at higher concentrations [84].
Combination therapy
The combination of several single compounds should optimize the chemopreventive properties while reducing drug concentrations and adverse events. Both the CAPP1 and CAPP2 studies were actually designed to also test potential combinatorial effects of aspirin and resistant starch [63,73]. To date only a few other studies exist in FAP cohorts. A small series tested the combination of nutritional supplements, curcumin and quercetin, for their efficacy in adenoma regression. Treatment lowered the number and size of polyps [85]. The combination of celecoxib (400 mg twice daily) and high-dose UDCA was used for duodenal polyp regression. Single celecoxib treatment decreased duodenal polyp density, while combination therapy even reversed such effect [86]. Also UDCA per se had no effect on duodenal polyp regression in separate study [46]. To date, the number of studies on combinatorial treatment is limited. In IBD, combination therapies, such as mesalazine and thiopurines, are widely used in medical practice without proper knowledge of such effects and potential drug interactions [5].
Conclusions
Various drugs have been investigated for its chemopreventive capabilities in high risk populations, some of them showing protective effects on long-term application (Table 1). The incidence of colitis-associated CRC has diminished in the last decades. This decrease might be attributed to active drugs, probably mostly by decreasing inflammation and increasing mucosal healing. Although controversially discussed, treatment with 5-ASA or thiopurines as CRC chemopreventive drugs is advisable in patients with long-standing UC and Crohn’s colitis, respectively. Such drug effects may even help reduce the necessity for annual surveillance colonoscopy as intervals could be stretched [Joel Rubenstein, personal communication]. In FAP, celecoxib reduces polyp formation and subsequent CRC development, but still, prophylactic surgery seems unavoidable. The effects of celecoxib are clinically more relevant in attenuated FAP, when patients desire to keep their colons. The biggest medical need is in Lynch syndrome, when patients can choose between questionable effects of 600 mg aspirin which come with significant gastrointestinal adverse events, or a watch and wait strategy with annual colonoscopy. Mesalazine seems to be the proper candidate for these patients, but even more important, the unknown gene carriers need to be identified at least for the sake of cancer screening programs. A proper identification of unknown mutation carriers can only be achieved when index cases are tested for MSI and expression of mismatch repair proteins in the tumor samples. Such pathological tests are recommended for all CRC tumor samples from patients under 70 years [69]. This approach needs collaboration of surgeons, gastroenterologists, pathologists and oncologists. It is time to implement such strategy at broad.
Table 1. Adenoma/CRC chemoprevention based on treatment and disease type.
| Treatment | Ulcerative colitis | Crohn’s disease | FAP | Lynch syndrome |
|---|---|---|---|---|
| 5-ASA |  |
 |
 |
|
| Thiopurine |  |
 |
||
| TNF-antagonist |  |
 |
||
| UDCA |  |
 |
||
| Sulindac |  |
|||
| Aspirin |  |
 |
||
| Celecoxib |  |
|||
| EPA |  |
|||
| Folic acid |  |
|||
| Vitamin C |  |
|||
| Calcuim |  |
|||
| Grain fiber |  |
|||
| Resistant starch |  |
 |
||
| Curcumin/quercetin |  |
 Effective for adenoma/CRC chemoprevention
Effective for adenoma/CRC chemoprevention
 Potentially effective for adenoma/CRC chemoprevention
Potentially effective for adenoma/CRC chemoprevention
 Chemopreventive effect needs to be determined
Chemopreventive effect needs to be determined
 Not effective for the prevention of adenoma/CRC
Not effective for the prevention of adenoma/CRC
Abbreviations
- 5-ASA
5-aminosalicylic acid
- APC
adenomatous polyposis coli
- CD
Crohn’s disease
- Cox
cyclooxygenase
- CRC
colorectal cancer
- IBD
inflammatory bowel disease
- EPA
eicosapentaenoic acid
- ERK
extracellular-signal regulated kinases
- FAP
familial adenomatous polyposis
- HNPCC
hereditary non-polyposis colorectal cancer
- MAPK
mitogen activated protein kinases
- MSI
microsatellite instability
- NF-κB
nuclear factor-kappa B
- NMSC
non-melanoma skin cancer
- NSAID
non-steroidal anti-inflammatory drug
- OR
odds ratio
- PAK1
p-21 activated kinase 1
- PSC
primary sclerosing cholangitis
- PUFA
polyunsaturated fatty acids
- RR
relative risk
- SIR
standardized incidence ratio
- TGF-β
transforming growth factor beta
- TNF-α
tumor necrosis factor alpha
- UC
ulcerative colitis
- UDCA
ursodeoxycholic acid
Footnotes
Disclosures: CG has a research collaboration with Shire Pharmaceuticals and received research support, lecturing or consulting honoraria from Ferring, Giuliani, Tillotts, and Dr Falk Pharma.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: Globocan 2008. International journal of cancer Journal international du cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: An updated meta-analysis of population-based cohort studies. Inflammatory bowel diseases. 2013;19:789–799. doi: 10.1097/MIB.0b013e31828029c0. [DOI] [PubMed] [Google Scholar]
- 3.Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: A meta-analysis of population-based cohort studies. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10:639–645. doi: 10.1016/j.cgh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Rubin DT, Cruz-Correa MR, Gasche C, Jass JR, Lichtenstein GR, Montgomery EA, Riddell RH, Rutter MD, Ullman TA, Velayos FS, Itzkowitz S, et al. Colorectal cancer prevention in inflammatory bowel disease and the role of 5-aminosalicylic acid: A clinical review and update. Inflammatory bowel diseases. 2008;14:265–274. doi: 10.1002/ibd.20297. [DOI] [PubMed] [Google Scholar]
- 5.Andrews JM, Travis SP, Gibson PR, Gasche C. Systematic review: Does concurrent therapy with 5-asa and immunomodulators in inflammatory bowel disease improve outcomes? Alimentary pharmacology & therapeutics. 2009;29:459–469. doi: 10.1111/j.1365-2036.2008.03915.x. [DOI] [PubMed] [Google Scholar]
- 6.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jess T, Gamborg M, Matzen P, Munkholm P, Sorensen TI. Increased risk of intestinal cancer in crohn's disease: A meta-analysis of population-based cohort studies. The American journal of gastroenterology. 2005;100:2724–2729. doi: 10.1111/j.1572-0241.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 8.Canavan C, Abrams KR, Mayberry J. Meta-analysis: Colorectal and small bowel cancer risk in patients with crohn's disease. Alimentary pharmacology & therapeutics. 2006;23:1097–1104. doi: 10.1111/j.1365-2036.2006.02854.x. [DOI] [PubMed] [Google Scholar]
- 9.Jess T, Simonsen J, Jorgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143:375–381 e371. doi: 10.1053/j.gastro.2012.04.016. quiz e313-374. [DOI] [PubMed] [Google Scholar]
- 10.Langholz E, Munkholm P, Davidsen M, Binder V. Colorectal cancer risk and mortality in patients with ulcerative colitis. Gastroenterology. 1992;103:1444–1451. doi: 10.1016/0016-5085(92)91163-x. [DOI] [PubMed] [Google Scholar]
- 11.Ahnfelt-Ronne I, Nielsen OH, Christensen A, Langholz E, Binder V, Riis P. Clinical evidence supporting the radical scavenger mechanism of 5-aminosalicylic acid. Gastroenterology. 1990;98:1162–1169. doi: 10.1016/0016-5085(90)90329-y. [DOI] [PubMed] [Google Scholar]
- 12.Koelink PJ, Hawinkels LJ, Wiercinska E, Sier CF, ten Dijke P, Lamers CB, Hommes DW, Verspaget HW. 5-aminosalicylic acid inhibits tgf-beta1 signalling in colorectal cancer cells. Cancer letters. 2010;287:82–90. doi: 10.1016/j.canlet.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 13.Kaiser GC, Yan F, Polk DB. Mesalamine blocks tumor necrosis factor growth inhibition and nuclear factor kappab activation in mouse colonocytes. Gastroenterology. 1999;116:602–609. doi: 10.1016/s0016-5085(99)70182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swidsinski A, Loening-Baucke V, Bengmark S, Lochs H, Dorffel Y. Azathioprine and mesalazine-induced effects on the mucosal flora in patients with ibd colitis. Inflammatory bowel diseases. 2007;13:51–56. doi: 10.1002/ibd.20003. [DOI] [PubMed] [Google Scholar]
- 15.Reinacher-Schick A, Seidensticker F, Petrasch S, Reiser M, Philippou S, Theegarten D, Freitag G, Schmiegel W. Mesalazine changes apoptosis and proliferation in normal mucosa of patients with sporadic polyps of the large bowel. Endoscopy. 2000;32:245–254. doi: 10.1055/s-2000-135. [DOI] [PubMed] [Google Scholar]
- 16.Lyakhovich A, Michlmayr A, Bakulina A, Gerner C, Oehler R, Gasche C. Interaction of mesalasine (5-asa) with translational initiation factors eif4 partially explains 5-asa anti-inflammatory and anti-neoplastic activities. Medicinal chemistry. 2011;7:92–98. doi: 10.2174/157340611794859325. [DOI] [PubMed] [Google Scholar]
- 17.Luciani MG, Campregher C, Fortune JM, Kunkel TA, Gasche C. 5-asa affects cell cycle progression in colorectal cells by reversibly activating a replication checkpoint. Gastroenterology. 2007;132:221–235. doi: 10.1053/j.gastro.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasche C, Goel A, Natarajan L, Boland CR. Mesalazine improves replication fidelity in cultured colorectal cells. Cancer research. 2005;65:3993–3997. doi: 10.1158/0008-5472.CAN-04-3824. [DOI] [PubMed] [Google Scholar]
- 19.Campregher C, Honeder C, Chung H, Carethers JM, Gasche C. Mesalazine reduces mutations in transforming growth factor beta receptor ii and activin type ii receptor by improvement of replication fidelity in mononucleotide repeats. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:1950–1956. doi: 10.1158/1078-0432.CCR-09-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campregher C, Kortüm B, Pinter M, Lang M, Evstatiev R, Khare V, Kucherlapati MH, Edelmann W, Gasche C. Mesalazine decreases microsatellite instability and tumor development in the villin-cre msh2loxp/loxp mouse model of lynch syndrome. Gastroenterology. 2013;144:S-18. [Google Scholar]
- 21.Khare V, Lang M, Dammann K, Campregher C, Lyakhovich A, Gasche C. Modulation of n-glycosylation by mesalamine facilitates membranous e-cadherin expression in colon epithelial cells. Biochemical pharmacology. 2014;87:312–320. doi: 10.1016/j.bcp.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khare V, Lyakhovich A, Dammann K, Lang M, Borgmann M, Tichy B, Pospisilova S, Luciani G, Campregher C, Evstatiev R, Pflueger M, et al. Mesalamine modulates intercellular adhesion through inhibition of p-21 activated kinase-1. Biochemical pharmacology. 2013;85:234–244. doi: 10.1016/j.bcp.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: A systematic review and metaanalysis of observational studies. The American journal of gastroenterology. 2005;100:1345–1353. doi: 10.1111/j.1572-0241.2005.41442.x. [DOI] [PubMed] [Google Scholar]
- 24.van Staa TP, Card T, Logan RF, Leufkens HG. 5-aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: A large epidemiological study. Gut. 2005;54:1573–1578. doi: 10.1136/gut.2005.070896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernstein CN, Nugent Z, Blanchard JF. 5-aminosalicylate is not chemoprophylactic for colorectal cancer in ibd: A population based study. The American journal of gastroenterology. 2011;106:731–736. doi: 10.1038/ajg.2011.50. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen GC, Gulamhusein A, Bernstein CN. 5-aminosalicylic acid is not protective against colorectal cancer in inflammatory bowel disease: A meta-analysis of non-referral populations. The American journal of gastroenterology. 2012;107:1298–1304. doi: 10.1038/ajg.2012.198. quiz 1297, 1305. [DOI] [PubMed] [Google Scholar]
- 27.Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, Lehr HA, Wirtz S, Becker C, Atreya R, Mudter J, et al. Cd28-dependent rac1 activation is the molecular target of azathioprine in primary human cd4+ t lymphocytes. The Journal of clinical investigation. 2003;111:1133–1145. doi: 10.1172/JCI16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Schaik FD, van Oijen MG, Smeets HM, van der Heijden GJ, Siersema PD, Oldenburg B. Thiopurines prevent advanced colorectal neoplasia in patients with inflammatory bowel disease. Gut. 2012;61:235–240. doi: 10.1136/gut.2011.237412. [DOI] [PubMed] [Google Scholar]
- 29.Gong J, Zhu L, Guo Z, Li Y, Zhu W, Li N, Li J. Use of thiopurines and risk of colorectal neoplasia in patients with inflammatory bowel diseases: A meta-analysis. PloS one. 2013;8:e81487. doi: 10.1371/journal.pone.0081487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beaugerie L, Svrcek M, Seksik P, Bouvier AM, Simon T, Allez M, Brixi H, Gornet JM, Altwegg R, Beau P, Duclos B, et al. Risk of colorectal high-grade dysplasia and cancer in a prospective observational cohort of patients with inflammatory bowel disease. Gastroenterology. 2013;145:166–175 e168. doi: 10.1053/j.gastro.2013.03.044. [DOI] [PubMed] [Google Scholar]
- 31.Peyrin-Biroulet L, Khosrotehrani K, Carrat F, Bouvier AM, Chevaux JB, Simon T, Carbonnel F, Colombel JF, Dupas JL, Godeberge P, Hugot JP, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141:1621–1628. e1621–1625. doi: 10.1053/j.gastro.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 32.Long MD, Herfarth HH, Pipkin CA, Porter CQ, Sandler RS, Kappelman MD. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2010;8:268–274. doi: 10.1016/j.cgh.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Schaik FD, van Oijen MG, Smeets HM, van der Heijden GJ, Siersema PD, Oldenburg B. Risk of nonmelanoma skin cancer in patients with inflammatory bowel disease who use thiopurines is not increased. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9:449–450 e441. doi: 10.1016/j.cgh.2011.01.021. author reply 450-441. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong RG, West J, Card TR. Risk of cancer in inflammatory bowel disease treated with azathioprine: A uk population-based case-control study. The American journal of gastroenterology. 2010;105:1604–1609. doi: 10.1038/ajg.2009.745. [DOI] [PubMed] [Google Scholar]
- 35.Pasternak B, Svanstrom H, Schmiegelow K, Jess T, Hviid A. Use of azathioprine and the risk of cancer in inflammatory bowel disease. American journal of epidemiology. 2013;177:1296–1305. doi: 10.1093/aje/kws375. [DOI] [PubMed] [Google Scholar]
- 36.Lv R, Qiao W, Wu Z, Wang Y, Dai S, Liu Q, Zheng X. Tumor necrosis factor alpha blocking agents as treatment for ulcerative colitis intolerant or refractory to conventional medical therapy: A meta-analysis. PloS one. 2014;9:e86692. doi: 10.1371/journal.pone.0086692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caspersen S, Elkjaer M, Riis L, Pedersen N, Mortensen C, Jess T, Sarto P, Hansen TS, Wewer V, Bendtsen F, Moesgaard F, et al. Infliximab for inflammatory bowel disease in denmark 1999-2005: Clinical outcome and follow-up evaluation of malignancy and mortality. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2008;6:1212–1217. doi: 10.1016/j.cgh.2008.05.010. quiz 1176. [DOI] [PubMed] [Google Scholar]
- 38.Osterman MT, Sandborn WJ, Colombel JF, Robinson AM, Lau W, Huang B, Pollack PF, Thakkar RB, Lewis JD. Increased risk of malignancy with adalimumab combination therapy, compared to monotherapy, for crohn's disease. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 39.Brentnall TA, Haggitt RC, Rabinovitch PS, Kimmey MB, Bronner MP, Levine DS, Kowdley KV, Stevens AC, Crispin DA, Emond M, Rubin CE. Risk and natural history of colonic neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis. Gastroenterology. 1996;110:331–338. doi: 10.1053/gast.1996.v110.pm8566577. [DOI] [PubMed] [Google Scholar]
- 40.Pardi DS, Loftus EV, Jr, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889–893. doi: 10.1053/gast.2003.50156. [DOI] [PubMed] [Google Scholar]
- 41.Tung BY, Emond MJ, Haggitt RC, Bronner MP, Kimmey MB, Kowdley KV, Brentnall TA. Ursodiol use is associated with lower prevalence of colonic neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Annals of internal medicine. 2001;134:89–95. doi: 10.7326/0003-4819-134-2-200101160-00008. [DOI] [PubMed] [Google Scholar]
- 42.Lindstrom L, Boberg KM, Wikman O, Friis-Liby I, Hultcrantz R, Prytz H, Sandberg-Gertzen H, Sangfelt P, Rydning A, Folvik G, Gangsoy-Kristiansen M, et al. High dose ursodeoxycholic acid in primary sclerosing cholangitis does not prevent colorectal neoplasia. Alimentary pharmacology & therapeutics. 2012;35:451–457. doi: 10.1111/j.1365-2036.2011.04966.x. [DOI] [PubMed] [Google Scholar]
- 43.Eaton JE, Silveira MG, Pardi DS, Sinakos E, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, Harnois D, Jorgensen R, et al. High-dose ursodeoxycholic acid is associated with the development of colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. The American journal of gastroenterology. 2011;106:1638–1645. doi: 10.1038/ajg.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf JM, Rybicki LA, Lashner BA. The impact of ursodeoxycholic acid on cancer, dysplasia and mortality in ulcerative colitis patients with primary sclerosing cholangitis. Alimentary pharmacology & therapeutics. 2005;22:783–788. doi: 10.1111/j.1365-2036.2005.02650.x. [DOI] [PubMed] [Google Scholar]
- 45.Hansen JD, Kumar S, Lo WK, Poulsen DM, Halai UA, Tater KC. Ursodiol and colorectal cancer or dysplasia risk in primary sclerosing cholangitis and inflammatory bowel disease: A meta-analysis. Digestive diseases and sciences. 2013;58:3079–3087. doi: 10.1007/s10620-013-2772-0. [DOI] [PubMed] [Google Scholar]
- 46.Parc Y, Desaint B, Flejou JF, Lefevre JH, Serfaty L, Vienne A, Kotti S, Simon T, Tiret E. The effect of ursodesoxycholic acid on duodenal adenomas in familial adenomatous polyposis: A prospective randomized placebo-control trial. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2012;14:854–860. doi: 10.1111/j.1463-1318.2011.02816.x. [DOI] [PubMed] [Google Scholar]
- 47.Lamlum H, Ilyas M, Rowan A, Clark S, Johnson V, Bell J, Frayling I, Efstathiou J, Pack K, Payne S, Roylance R, et al. The type of somatic mutation at apc in familial adenomatous polyposis is determined by the site of the germline mutation: A new facet to knudson's 'two-hit' hypothesis. Nature medicine. 1999;5:1071–1075. doi: 10.1038/12511. [DOI] [PubMed] [Google Scholar]
- 48.Stolfi C, De Simone V, Pallone F, Monteleone G. Mechanisms of action of non-steroidal anti-inflammatory drugs (nsaids) and mesalazine in the chemoprevention of colorectal cancer. International journal of molecular sciences. 2013;14:17972–17985. doi: 10.3390/ijms140917972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. The New England journal of medicine. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 50.Labayle D, Fischer D, Vielh P, Drouhin F, Pariente A, Bories C, Duhamel O, Trousset M, Attali P. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991;101:635–639. doi: 10.1016/0016-5085(91)90519-q. [DOI] [PubMed] [Google Scholar]
- 51.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. The New England journal of medicine. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 52.Nugent KP, Farmer KC, Spigelman AD, Williams CB, Phillips RK. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. The British journal of surgery. 1993;80:1618–1619. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- 53.Spagnesi MT, Tonelli F, Dolara P, Caderni G, Valanzano R, Anastasi A, Bianchini F. Rectal proliferation and polyp occurrence in patients with familial adenomatous polyposis after sulindac treatment. Gastroenterology. 1994;106:362–366. doi: 10.1016/0016-5085(94)90593-2. [DOI] [PubMed] [Google Scholar]
- 54.Winde G, Gumbinger HG, Osswald H, Kemper F, Bunte H. The nsaid sulindac reverses rectal adenomas in colectomized patients with familial adenomatous polyposis: Clinical results of a dose-finding study on rectal sulindac administration. International journal of colorectal disease. 1993;8:13–17. doi: 10.1007/BF00341270. [DOI] [PubMed] [Google Scholar]
- 55.Winde G, Schmid KW, Schlegel W, Fischer R, Osswald H, Bunte H. Complete reversion and prevention of rectal adenomas in colectomized patients with familial adenomatous polyposis by rectal low-dose sulindac maintenance treatment. Advantages of a low-dose nonsteroidal anti-inflammatory drug regimen in reversing adenomas exceeding 33 months. Diseases of the colon and rectum. 1995;38:813–830. doi: 10.1007/BF02049838. [DOI] [PubMed] [Google Scholar]
- 56.Giardiello FM, Yang VW, Hylind LM, Krush AJ, Petersen GM, Trimbath JD, Piantadosi S, Garrett E, Geiman DE, Hubbard W, Offerhaus GJ, et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. The New England journal of medicine. 2002;346:1054–1059. doi: 10.1056/NEJMoa012015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cruz-Correa M, Hylind LM, Romans KE, Booker SV, Giardiello FM. Long-term treatment with sulindac in familial adenomatous polyposis: A prospective cohort study. Gastroenterology. 2002;122:641–645. doi: 10.1053/gast.2002.31890. [DOI] [PubMed] [Google Scholar]
- 58.Tonelli F, Valanzano R, Messerini L, Ficari F. Long-term treatment with sulindac in familial adenomatous polyposis: Is there an actual efficacy in prevention of rectal cancer? Journal of surgical oncology. 2000;74:15–20. doi: 10.1002/1096-9098(200005)74:1<15::aid-jso4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 59.Guldenschuh I, Hurlimann R, Muller A, Ammann R, Mullhaupt B, Dobbie Z, Zala GF, Flury R, Seelentag W, Roth J, Meyenberger C, et al. Relationship between apc genotype, polyp distribution, and oral sulindac treatment in the colon and rectum of patients with familial adenomatous polyposis. Diseases of the colon and rectum. 2001;44:1090–1097. doi: 10.1007/BF02234627. discussion 1097-1099. [DOI] [PubMed] [Google Scholar]
- 60.Matsumoto T, Nakamura S, Esaki M, Yao T, Iida M. Effect of the non-steroidal anti-inflammatory drug sulindac on colorectal adenomas of uncolectomized familial adenomatous polyposis. Journal of gastroenterology and hepatology. 2006;21:251–257. doi: 10.1111/j.1440-1746.2006.04181.x. [DOI] [PubMed] [Google Scholar]
- 61.Lynch HT, Thorson AG, Smyrk T. Rectal cancer after prolonged sulindac chemoprevention. A case report Cancer. 1995;75:936–938. doi: 10.1002/1097-0142(19950215)75:4<936::aid-cncr2820750407>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 62.Shaheen NJ, Straus WL, Sandler RS. Chemoprevention of gastrointestinal malignancies with nonsteroidal antiinflammatory drugs. Cancer. 2002;94:950–963. [PubMed] [Google Scholar]
- 63.Burn J, Bishop DT, Chapman PD, Elliott F, Bertario L, Dunlop MG, Eccles D, Ellis A, Evans DG, Fodde R, Maher ER, et al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer prevention research. 2011;4:655–665. doi: 10.1158/1940-6207.CAPR-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishikawa H, Wakabayashi K, Suzuki S, Mutoh M, Hirata K, Nakamura T, Takeyama I, Kawano A, Gondo N, Abe T, Tokudome S, et al. Preventive effects of low-dose aspirin on colorectal adenoma growth in patients with familial adenomatous polyposis: Double-blind, randomized clinical trial. Cancer medicine. 2013;2:50–56. doi: 10.1002/cam4.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, Su LK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. The New England journal of medicine. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 66.Phillips RK, Wallace MH, Lynch PM, Hawk E, Gordon GB, Saunders BP, Wakabayashi N, Shen Y, Zimmerman S, Godio L, Rodrigues-Bigas M, et al. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut. 2002;50:857–860. doi: 10.1136/gut.50.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lynch PM, Ayers GD, Hawk E, Richmond E, Eagle C, Woloj M, Church J, Hasson H, Patterson S, Half E, Burke CA. The safety and efficacy of celecoxib in children with familial adenomatous polyposis. The American journal of gastroenterology. 2010;105:1437–1443. doi: 10.1038/ajg.2009.758. [DOI] [PubMed] [Google Scholar]
- 68.Iwama T, Akasu T, Utsunomiya J, Muto T. Does a selective cyclooxygenase-2 inhibitor (tiracoxib) induce clinically sufficient suppression of adenomas in patients with familial adenomatous polyposis? A randomized double-blind placebo-controlled clinical trial. International journal of clinical oncology. 2006;11:133–139. doi: 10.1007/s10147-005-0548-z. [DOI] [PubMed] [Google Scholar]
- 69.Vasen HF, Blanco I, Aktan-Collan K, Gopie JP, Alonso A, Aretz S, Bernstein I, Bertario L, Burn J, Capella G, Colas C, et al. Revised guidelines for the clinical management of lynch syndrome (hnpcc): Recommendations by a group of european experts. Gut. 2013;62:812–823. doi: 10.1136/gutjnl-2012-304356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mecklin JP. Frequency of hereditary colorectal carcinoma. Gastroenterology. 1987;93:1021–1025. doi: 10.1016/0016-5085(87)90565-8. [DOI] [PubMed] [Google Scholar]
- 71.De Jong AE, Morreau H, Van Puijenbroek M, Eilers PH, Wijnen J, Nagengast FM, Griffioen G, Cats A, Menko FH, Kleibeuker JH, Vasen HF. The role of mismatch repair gene defects in the development of adenomas in patients with hnpcc. Gastroenterology. 2004;126:42–48. doi: 10.1053/j.gastro.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 72.Lynch HT, Lanspa S, Smyrk T, Boman B, Watson P, Lynch J. Hereditary nonpolyposis colorectal cancer (lynch syndromes i & ii). Genetics, pathology, natural history, and cancer control, part i. Cancer genetics and cytogenetics. 1991;53:143–160. doi: 10.1016/0165-4608(91)90093-a. [DOI] [PubMed] [Google Scholar]
- 73.Burn J, Bishop DT, Mecklin JP, Macrae F, Moslein G, Olschwang S, Bisgaard ML, Ramesar R, Eccles D, Maher ER, Bertario L, et al. Effect of aspirin or resistant starch on colorectal neoplasia in the lynch syndrome. The New England journal of medicine. 2008;359:2567–2578. doi: 10.1056/NEJMoa0801297. [DOI] [PubMed] [Google Scholar]
- 74.Mathers JC, Movahedi M, Macrae F, Mecklin JP, Moeslein G, Olschwang S, Eccles D, Evans G, Maher ER, Bertario L, Bisgaard ML, et al. Long-term effect of resistant starch on cancer risk in carriers of hereditary colorectal cancer: An analysis from the capp2 randomised controlled trial. The lancet oncology. 2012;13:1242–1249. doi: 10.1016/S1470-2045(12)70475-8. [DOI] [PubMed] [Google Scholar]
- 75.Burn J, Mathers JC, Bishop DT. Chemoprevention in lynch syndrome. Familial cancer. 2013;12:707–718. doi: 10.1007/s10689-013-9650-y. [DOI] [PubMed] [Google Scholar]
- 76.Gasche C, Möslein G, Vasen HF, Lubinski J, Karner-Hanusch J, Niv Y. Mesacapp: Mesalamine for colorectal cancer prevention program in lynch syndrome. 2013 http://wwwtranscanfp7eu/transcan/filephp/1/Calls/JTC-2012/Selected_projects/CANCER12-MesaCapppdf.
- 77.Irving GR, Karmokar A, Berry DP, Brown K, Steward WP. Curcumin: The potential for efficacy in gastrointestinal diseases. Best practice & research Clinical gastroenterology. 2011;25:519–534. doi: 10.1016/j.bpg.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 78.Hull MA. Omega-3 polyunsaturated fatty acids. Best practice & research Clinical gastroenterology. 2011;25:547–554. doi: 10.1016/j.bpg.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 79.West NJ, Clark SK, Phillips RK, Hutchinson JM, Leicester RJ, Belluzzi A, Hull MA. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59:918–925. doi: 10.1136/gut.2009.200642. [DOI] [PubMed] [Google Scholar]
- 80.DeCosse JJ, Adams MB, Kuzma JF, LoGerfo P, Condon RE. Effect of ascorbic acid on rectal polyps of patients with familial polyposis. Surgery. 1975;78:608–612. [PubMed] [Google Scholar]
- 81.Bussey HJ, DeCosse JJ, Deschner EE, Eyers AA, Lesser ML, Morson BC, Ritchie SM, Thomson JP, Wadsworth J. A randomized trial of ascorbic acid in polyposis coli. Cancer. 1982;50:1434–1439. doi: 10.1002/1097-0142(19821001)50:7<1434::aid-cncr2820500733>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 82.DeCosse JJ, Miller HH, Lesser ML. Effect of wheat fiber and vitamins c and e on rectal polyps in patients with familial adenomatous polyposis. Journal of the National Cancer Institute. 1989;81:1290–1297. doi: 10.1093/jnci/81.17.1290. [DOI] [PubMed] [Google Scholar]
- 83.Thomas MG, Thomson JP, Williamson RC. Oral calcium inhibits rectal epithelial proliferation in familial adenomatous polyposis. The British journal of surgery. 1993;80:499–501. doi: 10.1002/bjs.1800800432. [DOI] [PubMed] [Google Scholar]
- 84.Lashner BA, Provencher KS, Seidner DL, Knesebeck A, Brzezinski A. The effect of folic acid supplementation on the risk for cancer or dysplasia in ulcerative colitis. Gastroenterology. 1997;112:29–32. doi: 10.1016/s0016-5085(97)70215-4. [DOI] [PubMed] [Google Scholar]
- 85.Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, Giardiello FM. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2006;4:1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 86.van Heumen BW, Roelofs HM, Vink-Borger ME, Dekker E, Mathus-Vliegen EM, Dees J, Koornstra JJ, Langers AM, Nagtegaal ID, Kampman E, Peters WH, et al. Ursodeoxycholic acid counteracts celecoxib in reduction of duodenal polyps in patients with familial adenomatous polyposis: A multicentre, randomized controlled trial. Orphanet journal of rare diseases. 2013;8:118. doi: 10.1186/1750-1172-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Groen EJ, Roos A, Muntinghe FL, Enting RH, de Vries J, Kleibeuker JH, Witjes MJ, Links TP, van Beek AP. Extra-intestinal manifestations of familial adenomatous polyposis. Annals of surgical oncology. 2008;15:2439–2450. doi: 10.1245/s10434-008-9981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet journal of rare diseases. 2009;4:22. doi: 10.1186/1750-1172-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anaya DA, Chang GJ, Rodriguez-Bigas MA. Extracolonic manifestations of hereditary colorectal cancer syndromes. Clinics in colon and rectal surgery. 2008;21:263–272. doi: 10.1055/s-0028-1089941. [DOI] [PMC free article] [PubMed] [Google Scholar]