Abstract
Despite much recent interest in music and dementia, music perception has not been widely studied across dementia syndromes using an information processing approach. Here we addressed this issue in a cohort of 30 patients representing major dementia syndromes of typical Alzheimer’s disease (AD, n=16), logopenic aphasia (LPA, an Alzheimer variant syndrome; n=5) and progressive nonfluent aphasia (PNFA; n=9) in relation to 19 healthy age-matched individuals. We designed a novel neuropsychological battery to assess perception of musical patterns in the dimensions of pitch and temporal information (requiring detection of notes that deviated from the established pattern based on local or global sequence features) and musical scene analysis (requiring detection of a familiar tune within polyphonic harmony). Performance on these tests was referenced to generic auditory (timbral) deviance detection and recognition of familiar tunes and adjusted for general auditory working memory performance. Relative to healthy controls, patients with AD and LPA had group-level deficits of global pitch (melody contour) processing while patients with PNFA as a group had deficits of local (interval) as well as global pitch processing. There was substantial individual variation within syndromic groups. No specific deficits of musical temporal processing, timbre processing, musical scene analysis or tune recognition were identified. The findings suggest that particular aspects of music perception such as pitch pattern analysis may open a window on the processing of information streams in major dementia syndromes. The potential selectivity of musical deficits for particular dementia syndromes and particular dimensions of processing warrants further systematic investigation.
Keywords: Alzheimer’s disease, dementia, progressive nonfluent aphasia, logopenic aphasia, music, auditory scene analysis
1. Introduction
Despite much recent interest [1–3], the impact on music processing of Alzheimer’s disease (AD) and other dementias has not been fully defined. Music is first and foremost a complex acoustic phenomenon and the perception of music requires the parsing of a musical stimulus of interest against the acoustic background (musical scene analysis: [4]), representation of the musical source (instrumental or vocal timbre) and tracking of pitch (melody) and temporal (rhythm, metre) information to create a coherent musical ‘object’ [5]. This formulation suggests that music presents the brain with a complex problem of auditory information processing, entailing the decoding of a number of perceptual and cognitive modules [6,7]. On both computational and neuroanatomical grounds, these processes are likely to be vulnerable to the effects of neurodegenerative diseases, most notably AD and primary progressive aphasia syndromes that target peri-Sylvian cortex (progressive nonfluent aphasia (PNFA) and logopenic aphasia (LPA): [8–11]). A substantial body of structural and functional neuroimaging work in the healthy brain and in patients with focal brain lesions has delineated distributed cortico-subcortical networks that analyze the dimensions of music [6,12,13]: these networks closely overlap the networks targeted in canonical dementia syndromes [14,15]. However, to date most studies of music in dementia have focused on the interaction of music and memory [16–18], preserved abilities in trained musicians developing dementia [16,19,20] and potential benefits of music more widely in dementia [21–25].
Aside from its intrinsic interest, music is an attractive candidate paradigm for assessing the processing of complex information streams or patterns in both the healthy and the diseased brain. In the domain of musical pitch, patterns of pitch change can be analyzed at two levels: pitch interval (the magnitude of change between consecutive notes) and pitch change direction (the overall pattern of ‘ups’ and ‘downs’ comprising the contour of the melody: [7,26]). By analogy with the visual domain, pitch interval and melody contour entail the processing of ‘local’ and ‘global’ pitch pattern information, respectively; according to this formulation, pitch intervals can be considered fine-grained musical features while combining these intervals to create a melody contour can be considered an overall (global) ‘gestalt’ of the musical piece. The distinction between these levels is evident in everyday music listening; changing individual pitch intervals is often perceived as a jarring distortion to the musical line, whereas simultaneously changing all pitch intervals but maintaining the relations between them (as in transposition of a melody to another key) retains the same musical gestalt (the tune is still recognisably the same). The concept of local versus global processing levels is fundamental for understanding how percepts are organised and relevant to many sensory domains. Music can be considered a non-visual test case for assessing the generality of effects on sensory information streams and the relative impact on featural (local) versus gestalt (global) perception of clinical disorders such as the dementias.
The local (pitch interval) and global (melody contour) levels of music perception can be differentially affected by focal brain lesions distributed between the cerebral hemispheres [26–30]. Functional neuroimaging studies in the healthy brain have demonstrated separable mechanisms in posterior superior temporal lobe and parietal and prefrontal projection pathways for the processing of pitch interval and melody contour [30,31]. Available evidence suggests that the decoding of musical patterns may be affected by common dementias and may help to stratify dementia syndromes and pathologies without relying on more specialised (and potentially confounding) verbal mechanisms. Elementary pitch discrimination may be retained in AD and impaired in PNFA, consistent with relatively greater involvement of early auditory areas in neurodegenerative processes that target peri-Sylvian cortex [18,32–36]. However, the effects of these diseases on more complex pitch pattern processing have not been resolved. Studies in the visual domain suggest that patients with AD may have disproportionate difficulty in the analysis of global structure with relatively intact analysis of local features: this profile is likely to reflect dysfunction of integrative mechanisms in parietal cortex that are particularly targeted by AD pathology but may be more difficult to interpret in the context of associated executive, verbal or spatial deficits [37–43]. In the musical domain, it follows that AD should produce more severe impairment for processing global (melody contour) than local (pitch interval) patterns; whereas in PNFA, a more pervasive impairment of local and global pitch pattern processing would be anticipated. However, currently available neuropsychological instruments for assessing pitch pattern processing often rely on comparisons between paired musical sequences [44]. Such comparisons are vulnerable to concurrent auditory working memory deficits that accompany AD and the progressive aphasias [17,18,45–47]; moreover, the explicit serial comparison of sequential melodies is seldom required in everyday music listening. Whereas specific musical working memory systems are likely to be integrally linked to the perception of pitch and temporal patterns in music, these are separable from verbal and other working memory systems that might be generically involved in any auditory task [48–50].
Temporal patterns in music can similarly be represented at interval (rhythmic, local) and longer duration stress or accent (metrical, global) levels of analysis [51]. Deficits in these dimensions of musical temporal perception occur with focal lesions involving temporal and parietal cortices [52–55] but frequently dissociate from pitch impairment [26,56] and further dissociate from each other [52,54,57]. In the healthy brain perceptual analysis of rhythm and metre engages cortico-subcortical circuitry jointly involved in preparing motor output [58–61]. While evidence in AD is not conclusive [19,36,62,63], impairments of temporal pattern processing have been described in PNFA associated with involvement of peri-Sylvian cortex [64]: this may be attributable both to loss of dynamic precision mediated by the dominant hemisphere and the high temporal resolution required for accurate processing of speech signals [65,66].
Under most circumstances, the listener must simultaneously decode more than one stream of musical information (whether produced by an ensemble of instruments or a single instrument played polyphonically). Such an analysis is fundamental to the initial parsing of a musical ‘scene’, before more detailed analysis can occur [7]; it is likely to entail an interaction of bottom-up mechanisms for coding perceptual structure with top-down mechanisms for resolving perceptual ambiguities based on stored templates or schemas derived from past experience of music [5,67]. Musical scene analysis has not been widely studied neuropsychologically in clinical populations but is likely to engage posterior superior temporal and parietal lobe regions and their dorsal projections [68–72]. AD has been shown to produce a generic impairment of auditory scene analysis under diverse listening tasks and conditions, including the streaming of sound sequences that bear some similarities to musical melodies; this has been linked to dysfunction of posterior temporo-parietal areas overlapping those involved in music perception [33,35,36,73–75]. On both neuroanatomical and neuropsychological grounds, patients with AD might therefore be anticipated to have difficulties with musical scene analysis; however, this has not been addressed directly in previous work.
In this study we assessed the perceptual components of music processing systematically in a cohort of patients representing major dementia syndromes. Based on the above synthesis of the available literature in both the auditory and visual domains, we anticipated that global versus local levels of musical pitch and temporal information processing and the effect of presenting a melody against a musical background (i.e., processing of musical ‘scenes’) would be the most informative components of music perception to target in the principal neurodegenerative dementias. The framework we addressed in designing the experimental music perception battery is outlined in Figure 1, adapting the modular model of music cognition proposed by Peretz and Coltheart [7]. We studied patients with typical AD in relation to patients with a syndromic diagnosis of primary PNFA and patients with the LPA clinical variant presentation of Alzheimer pathology. Inclusion of these syndromic groups allowed us to assess the effects of disease topography in dominant peri-Sylvian cortex in relation to the predicted underlying molecular pathology (PNFA in relation to LPA). We designed novel neuropsychological tests requiring continuous tracking of musical patterns and detection of deviants from the established pattern in the domains of pitch (interval, melody) and time (rhythm, metre). Our rationale was that detection of a deviant or ‘wrong’ note played during a performance more closely approximates natural music listening than does sequential comparison of melodies or related neuropsychological procedures and also reduces working memory and associated, extraneous executive demands. In addition, we created a test to assess detection of melody patterns within a musical ‘scene’. These dimensions of perceptual pattern processing were assessed in relation to detection of timbral deviants (a measure of sustained auditory attention and executive processing of sound sequences) and recognition of familiar tunes (a widely used index of musical semantic processing). To allow musical perceptual effects to be interpreted without potentially confounding effects from auditory working memory impairment, we controlled for this factor in analysing the musical performance profiles of our patient groups: our concern here was to adjust for generic, task-related auditory working memory capacity rather than any more specifically musical working memory subsystem.
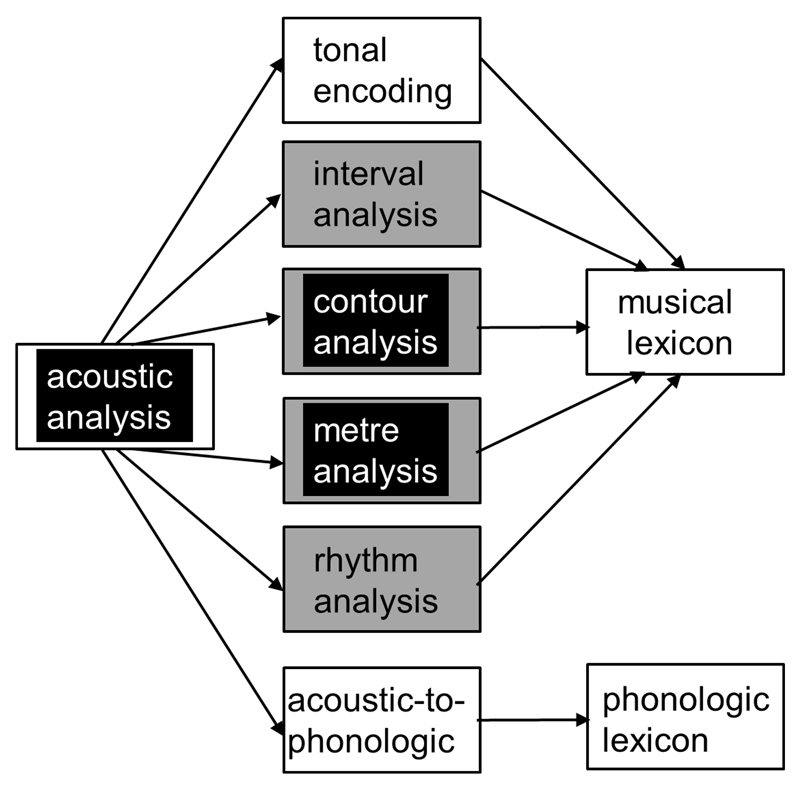
Figure 1. Cognitive framework for the present experiments.
The diagram is adapted from the modular module of music cognition proposed by Peretz and Coltheart [7]. Oblongs indicate cognitive components and arrows indicate the primary direction of information flow. Shaded oblongs indicate components addressed by the present experiments and about which we had specific hypotheses concerning the profile of deficits in particular dementia syndromes. Based on previous neuropsychological and neuroanatomical evidence, we predicted Alzheimer’s disease (and its language variant, logopenic aphasia; black oblongs) would impair acoustic analysis (here, parsing of a musical scene) and produce more severe deficits of global (melody, metre) than local (interval, rhythm) information processing in the pitch and temporal domains of music; while progressive nonfluent aphasia (grey oblongs) would produce deficits of both local and global musical information processing, more severe in the temporal domain (note that deficits of phonological processing are a feature of both progressive aphasia syndromes but were not directly addressed in the present experiments). The adapted model presented here retains the modular and hierarchical framework proposed by Peretz and Coltheart but in contrast to the original model, makes no strong inferences about the serial dependence of local on global pitch pattern encoding; unlike the situation with focal brain lesions due to stroke (which motivated the original model), neurodegenerative diseases typically damage but do not entirely remove particular perceptual modules so that degraded information flow between modules can continue to occur.
In line with previous evidence including studies of the healthy brain and focal brain damage, we hypothesised that musical deficits would be produced by all three target dementia syndromes, with distinctive profiles of impairment in each syndrome. More specifically, we hypothesised that typical AD would be associated with relatively greater impairment of global than local levels of musical pattern analysis and impaired musical scene analysis, with a similar profile of deficits in LPA; while PNFA would be associated with deficient analysis of both local and global pitch patterns but with more severely impaired analysis of temporal patterns in music.
2. Methods
2.1. Participants
The key inclusion criterion for the study was a clinical diagnosis of one of the target dementia syndromes based on current standard, consensus diagnostic criteria [76,77]. Sixteen patients (six female) fulfilling diagnostic criteria for typical AD (henceforth simply ‘AD’) led by episodic memory decline [76], five patients (two female) with a diagnosis of LPA and eight patients (six female) fulfilling criteria for PNFA [77] were recruited. Nineteen healthy individuals (ten female) matched to the patient cohort for age and musical background, with no history of significant neurological or psychiatric disorders were recruited via our Centre’s research participant database. To provide an index of musical background, patients’ caregivers and healthy control participants completed a questionnaire detailing current musical exposure (estimated hours/week) and years of previous formal musical training. Inability to comply with neuropsychological testing, a clinical history of significant hearing loss or congenital amusia would constitute exclusion criteria for a study of this kind; in the event no individuals were excluded on these grounds.
All participants had audiometric screening of peripheral hearing function and an elementary pitch discrimination screening test (details in Supplementary Material on-line) designed to establish that they could comply with experimental tests involving the processing of pitch sequences. One potential participant with AD and one with PNFA were excluded as they failed to reach the criterion (>80% correct) required to pass screening.
Demographic, clinical and general neuropsychological characteristics of the study cohort are summarised in Table 1. Syndromic diagnoses in the patient groups were corroborated with a comprehensive general neuropsychological assessment (Table 1). Brain MR images (available for 28 patients) revealed a profile of atrophy consistent with the syndromic diagnosis in each case; no brain images showed a significant cerebrovascular burden. Twelve of 12 patients in the AD group and three of four patients in the LPA group for which CSF was available had a protein marker profile suggesting underlying Alzheimer pathology (total CSF tau: beta-amyloid1-42 ratio >1, based on local laboratory reference ranges) and the remaining patient with LPA had a positive Florbetapir PET brain amyloid scan; in contrast, five of six patients with PNFA had a CSF profile that did not suggest underlying AD while the remaining patient had a negative brain amyloid scan. At the time of testing, 13 patients in the AD group were receiving symptomatic treatment with donepezil and two with memantine; in the LPA group, four patients were receiving donepezil and two memantine; while in the PNFA group one patient was receiving donepezil.
Table 1. General demographic, clinical and neuropsychological characteristics of participant groups.
| Characteristic | Healthy controls | AD | LPA | PNFA |
|---|---|---|---|---|
| General | ||||
| No. (m:f) | 9:10 | 10:6 | 3:2 | 2:7 |
| Age (yrs) | 69.7 (4.7) | 68.9 (6.4) | 63.6 (6.2) | 71.9 (7.8) |
| Musical training (yrs) | 5.0 (3.6) | 4.1 (2.9) | 3.2 (4.0) | 2.7 (2.6) |
| Musical listening (hrs/week) | 10.2 (10.1) | 8.8 (11.0) | 5.2 (3.1) | 4.9 (7.2) |
| Education (yrs) | 16.8 (2.0) | 15.3 (2.7) | 14.4 (3.0) | 16.3 (2.6) |
| MMSE (/30) | 29.3 (1.1) | 21 (4.7)* | 16 (9.6)* | 20 (11.2)* |
| Symptom duration (yrs) | - | 6.4 (2.1) | 5.8 (3.1) | 6.8 (3.7) |
|
| ||||
| Neuropsychological | ||||
|
| ||||
| General intellect: IQ | ||||
| WASI verbal IQ | 118 (7) | 98 (14)* | 69 (12)** | 84 (19)* |
| WASI performance IQ | 119 (13) | 91 (20)* | 94 (21) | 100 (20) |
| NART estimated premorbid IQ | 122 (5) | 114 (9)* | 88 (12)** | 106 (16)* |
|
| ||||
| Pitch discrimination | ||||
| screen (/20) | 19.6 (0.7) | 19.1 (1.6) | 19.2 (1.1) | 18.6 (2.1) |
|
| ||||
| Episodic memory | ||||
| RMT words (/50) | 48 (2) | 30 (6)*** | 32 (6)* | 45 (6) |
| RMT faces (/50) | 43 (4) | 31 (6)* | 34 (7) | 36 (6)* |
| Camden PAL (/24) | 20 (3) | 4 (4)*** | 3 (3)*** | 17 (5) |
|
| ||||
| Executive skills | ||||
| WASI Block Design (/71) | 43 (16) | 19 (13)* | 26 (22) | 19 (18)* |
| WASI Matrices (/32) | 25 (4) | 13 (7)* | 17 (9) | 18 (8) |
| WMS-R digit span forward (/12) | 9 (2) | 7 (2) | 3 (3)* | 6 (2) |
| WMS-R digit span reverse (/12) | 8 (2) | 5 (2)* | 2 (1)* | 3 (2)* |
| D-KEFS Stroop colour (s) | 30 (4) | 52 (22)* | 62 (19)* | 67 (21)* |
| D-KEFS Stroop word (s) | 21 (3) | 34 (19) | 35 (13) | 52 (25)* |
| D-KEFS Stroop interference (s) | 60 (17) | 106 (49)* | 115 (17) | 149 (37)* |
| Letter fluency (F: total) | 16 (5) | 11 (5) | 7 (2)* | 4 (3)** |
| Category fluency (animals: total) | 23 (5) | 12 (5)* | 9 (5)* | 10 (3)* |
| Trails A (s) | 33 (10) | 70 (45)* | 84 (39)* | 69 (37)* |
| Trails B (s) | 81 (39) | 199 (75)* | 232 (73)* | 233 (67)* |
| WAIS-R Digit Symbol (total) | 55 (11) | 24 (15)* | 38 (11) | 27 (12)* |
|
| ||||
| Language skills | ||||
| WASI Vocabulary (/80) | 70 (3) | 56 (10)* | 23 (20)** | 35 (21)** |
| WASI Similarities (/48) | 38 (5) | 26 (11)* | 13 (7)* | 25 (12)* |
| GNT (/30) | 26 (2) | 15 (7)* | 7 (8)* | 15 (9)* |
| BPVS (/150) | 148 (2) | 145 (3)* | 141 (7) | 139 (13)* |
| NART (/50) | 43 (4) | 36 (7)* | 17 (11)** | 30 (13)* |
| Single word repetition (/45) | - | - | 40 (4) | 33 (15) |
| Sentence repetition (/10) | - | - | 7 (3) | 6 (4) |
|
| ||||
| Other skills | ||||
| GDA (/24) | 15 (5) | 5 (6)* | 4 (5)* | 4 (4)* |
| VOSP Object Decision (/20) | 19 (1) | 16 (3)* | 18 (2) | 16 (5) |
Mean (standard deviation) values are shown unless otherwise indicated; results in bold indicate mean score <5th percentile for age norms (not available for BPVS, letter fluency, word repetition, sentence repetition); *significantly different from healthy control group **significantly different from healthy control and AD group ***significantly different from healthy control and PNFA group. Reduced numbers of participants completing each of the tests (by group) were as follows: D-KEFS Stroop, 15 AD, four LPA, five PNFA; fluency (letter, category), five PNFA; GDA, eight PNFA; GNT, eight PNFA; NART, six PNFA; RMT (words, faces), 18 controls, 15 AD; Trails, 14 AD, four LPA; VOSP Object Decision, eight PNFA; WAIS-R Digit Symbol, 13 AD, seven PNFA; WASI (Block Design, Matrices, Similarities, Vocabulary), four LPA; WMS-R digit span reverse, four LPA, eight PNFA. AD, Alzheimer’s disease; BPVS, British Picture Vocabulary Scale [99]; D-KEFS, Delis Kaplan Executive System [100]; GDA, Graded Difficulty Arithmetic [101]; GNT, Graded Naming Test [102]; LPA, logopenic aphasia; MMSE, Mini-Mental State Examination score [103]; NART, National Adult Reading Test [104]; PAL, Paired Associate Learning; PNFA, progressive nonfluent aphasia; RMT, Recognition Memory Test [105]; VOSP, Visual Object and Spatial Perception Battery [106]; WAIS-R, Wechsler Adult Intelligence Scale Revised [107]; WASI, Wechsler Abbreviated Scale of Intelligence [108]; WMS-R, Wechsler Memory Scale Revised [109].
The study was approved by the local institutional ethics committee and all participants gave informed consent in accordance with the guidelines of the Declaration of Helsinki.
2.2. Experimental music perception battery
2.2.1. General structure
The overall structure of the music perception battery is schematised in Figure 2; examples of the stimuli are provided in Supplementary Material on-line.
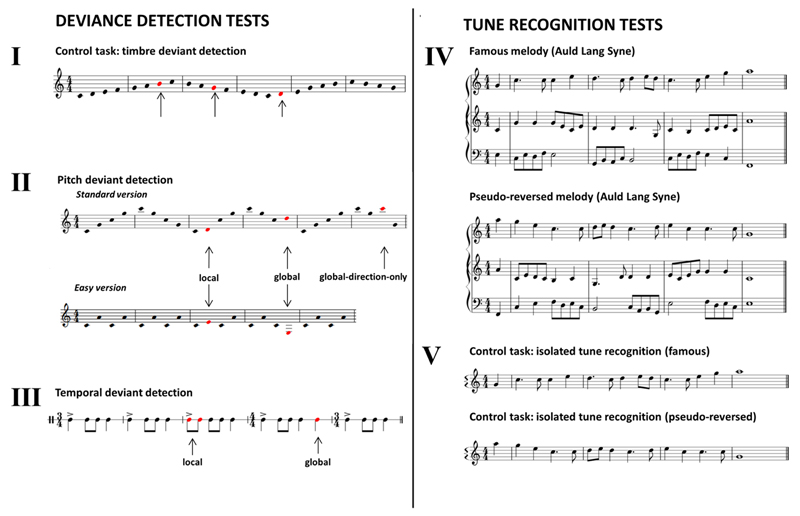
Figure 2. Schematic representation of the experimental music battery.
Examples of stimuli used for all tests in the music experimental battery. Roman numerals I to V code the presentation order of tests comprising the battery. For all deviance detection tasks, deviant notes are shown in red; for the timbral deviant detection task, the red notes signify a change in spectral envelope. For illustrative purposes, local and global deviants are shown here within the same trial; however, the experimental stimuli as presented contained only a single deviant type (condition) per trial. The tune recognition tests comprised a test of musical scene analysis (decision on familiarity of tunes presented with polyphonic harmony; target shown on top stave for each example) and a baseline test of tune recognition (decision on familiarity of tunes presented in isolation, acting as a control for the tune streaming task); examples represent Auld Lang Syne in natural and pseudo-reversed forms (see section 2.2 and supplementary material for details).
Procedures were adapted from previously described tests of musical deviance detection [78,79]. Detection of deviant notes has been employed in previous music psychology paradigms that sought to capture on-line analysis of musical information in pitch and temporal domains under conditions that resemble natural musical listening; such paradigms establish a continuous musical context, allow precise programming of incongruent events that violate musical expectancies, capture moment-to-moment tracking of musical structure [78] and allow estimation of processing latencies [79] while at the same time avoiding any explicit requirement to make delayed, serial comparisons with episodes held in musical memory (potentially, a particular advantage in patients with dementia). For the present pitch and temporal processing tests, participants were required to listen to a sequence of musical notes that conformed to a basic pattern with randomly presented notes that deviated from the pattern according to the musical parameter of interest; for each subtest, the task on each trial was to press a button as soon as a deviant note occurred. As a control for the attentional and response requirements of these tests, we designed a task that required detection of timbral deviants in note sequences. For the musical scene analysis (‘tune streaming’) test, highly familiar or novel melodies were presented against a harmonic background with similar perceptual characteristics; the task on each trial was to decide whether or not a familiar tune was present. As a baseline test of tune recognition, familiar or novel melodies were presented alone and the task on each trial was to decide whether or not the tune was familiar; this task acted as a control for the tune recognition component of the musical scene analysis test. The order of the experimental tests (fixed for all participants) and approximate times to administer the tests were as follows: timbre deviant task (approximately two minutes); pitch deviant tasks (approximately six minutes); temporal deviant tasks (approximately five minutes); tune streaming (approximately five minutes); tune recognition (five to ten minutes).
Note sequences were synthesised in MATLAB® (pitch, temporal, timbral deviant detection tests) or MuseScore (tune recognition tests). Stimulus parameters were in line with values used in previous work [78,79]. Stimuli were presented from a notebook computer running MATLAB® via headphones (Audio-Technica®) at a comfortable listening level (at least 70 dB) in a quiet room. Participants were first familiarised with each test using visual aids (see examples in Figure S1 in Supplementary Material online) and practice examples to ensure they understood the task instructions and were able to comply reliably. For all tests based on deviance detection, participants were instructed to press the keyboard spacebar as quickly as possible whenever they heard a ‘wrong note’; presses within a pre-specified window (see Supplementary Material for details) after deviant onset were counted as correct detections. Participant responses were recorded for offline analysis. During the tests no feedback was given about performance and no time limits were imposed.
Further details of stimulus parameters in each condition are in Supplementary Material on-line.
2.2.2. Assessment of pitch pattern processing
Stimulus note sequences comprised alternating tonic and dominant pitches (intervals of five or four tones) in one of three keys, spanning two octaves (range F2 to C5) and arranged to form a single simple template melody contour (five ascending – five descending – five ascending – five descending…; see Figure 2). Individual notes lasted either 500 or 400 ms with inter-note interval of 100 or 80 ms, yielding a base tempo for the sequence of either 100 or 125 beats/minute; total sequence duration for a given trial ranged from 33.1 to 41.4 seconds. Each trial contained five deviant notes, each of which diverged from the template pitch pattern in one of three ways: local (interval step altered, global melody contour preserved), global (melody contour direction altered) or global direction-only (melody contour direction altered, using only notes previously heard in the pattern so that only the order of notes was altered). The global direction-only condition was intended to access a ‘pure’ process of melody contour analysis that could not be performed (for example) by detecting the occurrence of novel out-of-pattern notes. The magnitude of a deviant ranged from two to eleven semitones; all deviant notes adhered to the diatonic scale of that trial. Deviant notes occurred with random onsets over the course of the trial such that the complete (unviolated) pattern occurred at least once before any deviants occurred and the interval between deviants was at least 1.5 seconds. Four trials for each deviant type were presented as blocks, yielding 20 deviants for each condition (local, global, direction-only). Responses within 1.5 seconds from deviant onset were counted as correct detections.
If a participant correctly detected fewer than 50% of deviants for any of the condition blocks, they completed half of all subsequent blocks and continued to an easier version of the pitch test (see Figure 2). In this ‘easy’ version of the test, the pitch pattern comprised only two notes; local deviants changed the interval and global deviants the melody contour. Two trials (10 deviants) were presented for each condition. Data on this test were also collected for six healthy control individuals, to provide a performance reference.
2.2.3. Assessment of temporal pattern processing
Stimulus sequences for the temporal test comprised repeated rhythmic patterns, adapted after the stimuli described by Geiser et al. [78] (see Figure 2); a given sequence (trial) established a template rhythm with metre (time signature) fixed at either three or four beats per cycle (3/4 or 4/4 time), emphasising the first note of the cycle (bar) with increased sound intensity. Individual notes had fixed pitch (either D4, Eflat4 or E4) with note duration 200 ms, and a base tempo for the sequence of either 100 or 120 beats/minute; total sequence duration for a given trial ranged from 22.5 to 38.4 seconds. Each trial contained four deviants, each of which diverged from the temporal template pattern in one of two ways: local (rhythm altered by varying inter-note interval by 100 to 600 ms) or global (metre altered by varying the position of a louder note, perceived as an ‘early’ or ‘late’ beat). Deviant notes occurred with random onsets over the course of the trial such that the complete (unviolated) pattern occurred at least three times before any deviants occurred and the interval between deviants was at least 2 seconds. Five trials for each deviant type were presented as blocks, yielding 20 deviants for each condition (rhythm, metre); the same set of temporal templates was used in each condition. Responses within 2 seconds from deviant onset (allowing time to make decisions on the inter-note interval) were counted as correct detections.
2.2.4. Assessment of acoustic deviance detection
In order to assess participants’ performance on acoustic deviance detection beyond the pitch and temporal domains, we designed a test that required detection of timbre deviants presented as elements of a note sequence based on an ascending or descending major scale. Deviants were created by altering the envelope of frequency intensities composing the spectrogram of the tone (its ‘spectral shape’) to produce one of two different timbre variants. Individual notes had duration 600 ms, with base tempo 100 beats/minute and sequence duration 32.4 seconds for each trial. Five timbre deviants were presented randomly during each trial; four trials were presented, yielding 20 timbre deviants in total. Responses within 1.5 seconds from deviant onset were counted as correct detections.
2.2.5. Tune recognition tests
In order to assess the parsing of melodies within complex musical scenes, we designed a test requiring detection (streaming) of tunes against a harmonic background (see Figure 2). Stimuli were created in three part harmony in a major key with a synthetic piano carrier. The top line of the harmony carried the tune for all trials; 10 trials contained very familiar tunes (based on pilot data in older British individuals; the tunes were Auld Lang Syne, Frere Jacques, God Save the Queen, Jingle Bells, London Bridge is Falling Down, Mary had a Little Lamb, Silent Night, Three Blind Mice, Twinkle Twinkle, Little Star, When the Saints Go Marching In) while for the remaining 10 trials, the original tunes were pseudo-reversed (such that the phrase ended on a long tonic or dominant note). Trial duration ranged between 7 seconds and 13 seconds. On each trial, the task was to respond ‘yes’ if a famous tune was present and ‘no’ if not.
To provide a baseline measure of tune recognition, the same 20 famous and pseudo-reversed tunes previously presented in the tune streaming test were presented in isolation, in randomised order. On each trial, the task was to respond ‘yes’ if the tune was famous and ‘no’ if not.
2.3. Analysis of behavioural data
2.3.1. General characteristics
All behavioural data were analyzed using Stata12®. Most demographic and neuropsychological data violated normality assumptions and groups were therefore compared using a Kruskal-Wallis equality-of-populations rank test followed by pairwise comparisons with Wilcoxon rank sum tests with Bonferroni adjusted p-values to account for the six pairwise comparisons; gender distributions were compared using Fisher’s exact test. Tone detection thresholds on audiometry screening were analyzed using multiple linear regression model adjusted for age, using bias corrected, accelerated confidence intervals calculated from 2000 bootstrap replications. Pairwise comparisons used Bonferroni-adjusted confidence intervals (99.17%) to account for the six pairwise comparisons between experimental groups.
2.3.2. Deviance detection tests
As participants were free to respond at any time, an individual participant’s proportion of correct presses was first adjusted for ‘guesses’ (or indiscriminate responses), as estimated using a Poisson distribution of that participant’s rate of incorrect presses outside the ‘correct’ time window. This can be represented by the following equation:
where S = score; P = proportion correct presses and λ = rate of incorrect presses x correct time window. This transformation resulted in a ‘corrected detection score’ for each participant for each condition; these corrected scores were entered into further analysis. As pitch and temporal deviance detection data did not conform to normality assumptions, data were analyzed using a multiple linear regression model comparing groups using bias corrected, accelerated confidence intervals calculated from 2000 bootstrap replications. Initially we tested for a differential effect of condition for each patient group compared to control by examining the interaction terms between condition and group based on 95% confidence intervals. If these suggested a significant interaction, we then assessed pairwise comparisons between patient groups within condition using Bonferroni-adjusted confidence intervals to account for the six comparisons between experimental groups. An effect was considered significant if the confidence interval did not cross zero, after controlling for general auditory working memory performance as indexed by reverse digit span (a standard measure of verbal auditory working memory) in the regression model.
2.3.3. Processing of familiar tunes
Tune recognition performance was analyzed using multiple linear regression model comparing groups using bias corrected, accelerated confidence intervals calculated from 2000 bootstrap replications and subsequent Bonferroni-corrected pairwise comparisons. A different approach was required for analysis of the tune streaming task: if a participant was unable to correctly identify a famous tune as famous when presented in isolation, this item was excluded from analysis of their responses on the tune streaming test. This resulted in varying numbers of famous and pseudo-reversed (non-famous) items for each participant on this test. A logistic regression model incorporating all participants' binary responses, controlling for reverse digit span performance, was used to model scores on the tune streaming task. To take account of any bias introduced by this imbalance of trial numbers, a framework based on signal detection theory was used to fit a logistic regression model for odds of labelling a tune as famous [80]. The dependent variable was a binary category indicating for each test item whether or not each participant in a group had responded ‘famous’. Accordingly, this model assessed famous tune detection accuracy as odds ratios comparing labelling of famous and non-famous tunes across all participants in each group. Here, an odds ratio of 1 corresponds to chance level performance, i.e., the group had equal likelihood of labelling a famous or non-famous tune as famous; an odds ratio >1 corresponds to increased accuracy discriminating famous from non-famous tunes; and an odds ratio <1 corresponds to over-rejection of famous tunes as non-famous or over-labelling of non-famous tunes as famous. Overall effects of experimental group were therefore assessed through the interaction of group and labelling tunes correctly. The Wald criterion was used to test for any interaction effect or specific group differences, with Bonferroni adjusted P-values to account for the six pairwise comparisons between experimental groups.
2.3.4. Correlates of musical perceptual performance
Where deficits on music processing tasks relative to healthy controls were identified, Spearman’s correlation coefficient was used to assess associations of performance on the relevant musical tasks with background musical training, general disease measures (Mini-Mental State Examination score, symptom duration) and speech encoding measures (word and sentence repetition) in the patient cohort. A threshold p<0.05 was accepted as the criterion for statistical significance for all associations.
3. Results
3.1. General characteristics of participant groups
The analysis of demographic, clinical and background neuropsychological data is summarised in Table 1. Due to time constraints, reduced numbers of participants completed particular assessments (these are detailed in Tables 1 and 2). Patient and healthy control groups were well matched for age (χ2(3) = 6.32, P = 0.10), gender (χ2(3) = 2.23, P = 0.56), education (χ2(3) = 6.41, P = 0.09), musical training (χ2(3) =3.74, P = 0.29) and current music listening (χ2(3) = 2.81, P = 0.42). Patient groups were well matched for Mini-Mental State Examination score (χ2(2) = 1.58, P = 0.45) and symptom duration (χ2(2) = 0.26, P = 0.88). Patient groups showed anticipated profiles of general neuropsychological impairment.
Table 2. Summary of performance of participant groups on music experimental tests.
| Musical attribute | Measure | Healthy controls | AD | LPA | PNFA |
|---|---|---|---|---|---|
| Pitch interval (pitch local) | Mean (SD) | 0.93 (0.10) | 0.74 (0.25) | 0.59 (0.25) | 0.37 (0.43) |
|
| |||||
| Vs controls | -0.14 (-0.43 to 0.09) |
-0.22 (-0.67 to 0.10) |
-0.46
(-0.90 to -0.04) |
||
| Vs AD | -0.08 (-0.49 to 0.16) |
-0.33 (-0.72 to 0.09) |
|||
| Vs LPA | -0.24 (-0.68 to 0.22) |
||||
|
| |||||
| Melody contour: global | Mean (SD) | 0.92 (0.12) | 0.60 (0.32) | 0.37 (0.44) | 0.40 (0.30) |
|
| |||||
| Vs controls |
-0.26
(-0.61 to -0.01) |
-0.42 (-0.96 to 0.11) |
-0.43
(-0.79 to -0.15) |
||
| Vs AD | -0.16 (-0.65 to 0.37) |
-0.17 (-0.48 to 0.16) |
|||
| Vs LPA | -0.01 (-0.54 to 0.47) |
||||
|
| |||||
| Melody contour: global direction-only | Mean (SD) | 0.84 (0.18) | 0.53 (0.29) | 0.30 (0.34) | 0.21 (0.24) |
|
| |||||
| Vs controls |
-0.26
(-0.55 to -0.002) |
-0.42
(-0.96 to -0.08) |
-0.54
(-0.84 to -0.17) |
||
| Vs AD | -0.16 (-0.66 to 0.16) |
-0.28 (-0.59 to 0.05) |
|||
| Vs LPA | -0.12 (-0.46 to 0.33) |
||||
|
| |||||
| Rhythm (temporal local) | Mean (SD) | 0.92 (0.07) | 0.75 (0.15) | 0.51 (0.33) | 0.46 (0.38) |
|
| |||||
| Metre (temporal global) | Mean (SD) | 0.82 (0.16) | 0.59 (0.17) | 0.31 (0.22) | 0.31 (0.30) |
|
| |||||
| Timbre | Mean (SD) | 0.99 (0.01) | 0.98 (0.04) | 0.81 (0.25) | 0.84 (0.36) |
|
| |||||
| Tune detection (tune streaming) | OR | 86 | 10 | 6 | 8 |
|
| |||||
| Tune recognition (in isolation) | Mean (SD) (/20) | 19.7 (0.5) | 19.3 (0.8) | 17.4 (2.3) | 18.4 (3.3) |
Within-group mean (standard deviation) scores on experimental music tests are presented; corrected detection scores are presented for detection of local and global pitch deviants (interval, melody), temporal deviants (rhythm, metre) and timbre deviants timbre control task: see text). Bonferroni-corrected pairwise comparisons are shown: for all group comparisons, mean difference (99% confidence interval) values are presented. Reduced numbers of participants completing each of the tests (by group) were as follows: pitch deviance detection, 19 controls, 13 AD, five LPA, eight PNFA; temporal deviance detection, 19 controls, 13 AD, five LPA, nine PNFA; timbre deviance detection, 19 controls, 14 AD, five LPA, eight PNFA, tune streaming, 18 controls, 15 AD, four LPA, seven PNFA. Significant group differences after adjustment for auditory working memory performance are indicated in bold. AD, Alzheimer’s disease; LPA, logopenic aphasia; PNFA, progressive nonfluent aphasia; OR, odds ratio; SD, standard deviation.
On the screen of peripheral hearing function, relative to healthy controls, the AD and LPA groups showed no significant performance difference but deficits compared to both the healthy control and the AD group were shown by the PNFA group; there was no difference between the LPA and PNFA groups (details summarised in Table S1 in Supplementary Material on-line). A combined audiometry score using the sum of detection thresholds for all frequencies was derived as an overall measure of peripheral hearing function to test for associations with performance on the experimental tasks: no significant associations were found and audiometry scores were therefore not included in further analyses. For the pitch discrimination screening task (Table 1), total scores did not differ significantly between experimental groups (χ2(3) = 2.66, p = 0.45).
3.2. Performance on experimental tests of music processing
Performance profiles for each group on all conditions and mean difference between groups for pairwise comparisons for the experimental music battery are presented in Table 2 with further details in Tables S2 and S3 on-line; individual data are shown in Figure 3.
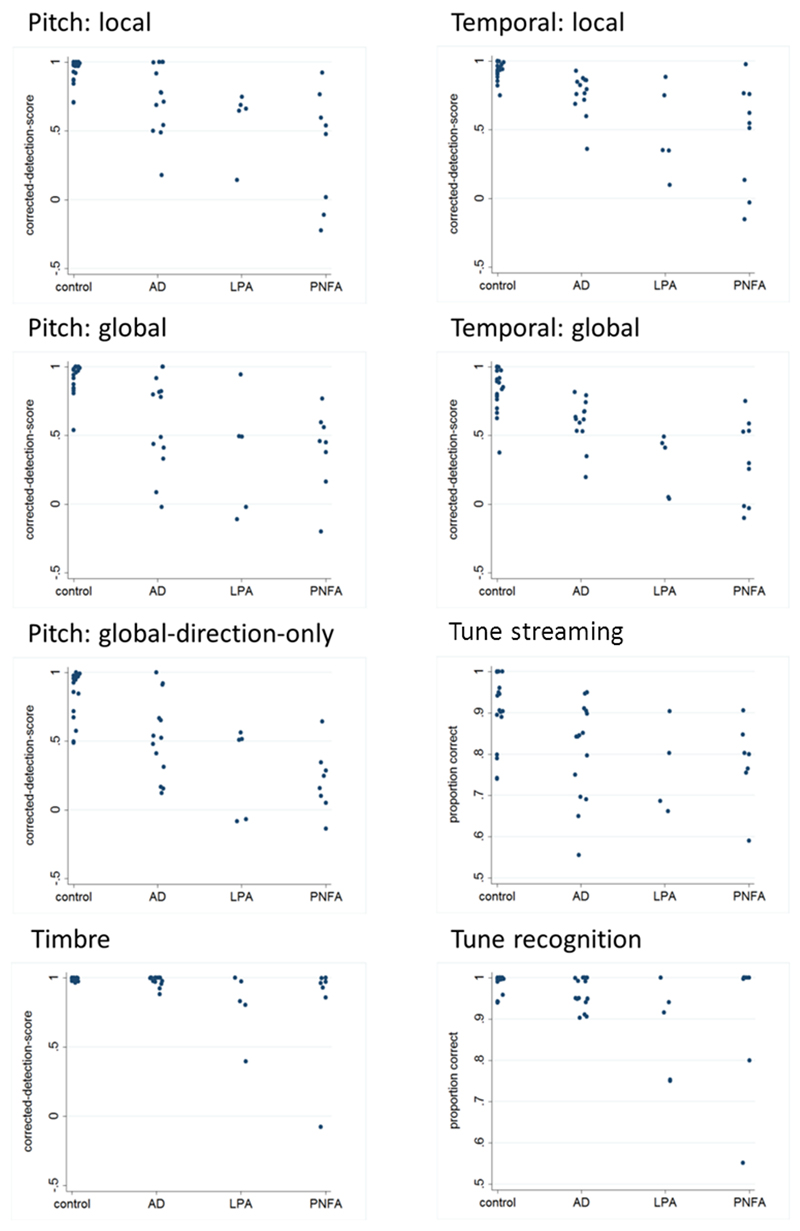
Figure 3. Individual performance data for musical tasks.
Individual corrected detection scores (not adjusted for auditory working memory performance) are plotted for healthy control, Alzheimer’s disease (AD), logopenic aphasia (LPA) and progressive nonfluent aphasia (PNFA) groups for tests of pitch, temporal and timbral deviant detection, tune streaming and baseline tune recognition (see supplementary material for details)
Inspection of the individual performance data prior to adjustment for general auditory working memory performance (Figure 3) suggests that patients in each syndromic group (and most prominently, the progressive aphasia groups) performed substantially worse than the healthy control group across the experimental tests of music processing. However, this was in the context of wide individual variation within each group. An analysis of group performance profiles without adjustment for general auditory working memory effects is summarised in Table S4 in Supplementary Material on-line; the following is based on the main analysis adjusted for this factor.
3.2.1. Pitch pattern processing
For the pitch pattern processing tasks, the PNFA group showed overall (across all three conditions) poorer performance compared to the healthy control (beta = -0.47, 95% CI -0.81 to -0.16) and AD groups (-0.33, 95% CI -0.61 to -0.02); no other significant overall performance differences between groups were found. Examining for effects of condition, poorer performance was found across all groups in the global-direction-only compared to the local condition (beta = -0.09, 95% CI -0.17 to -0.03). Compared to healthy controls, the AD group performed significantly worse in the global and global-direction-only pitch conditions but not the local condition; the LPA group performed significantly worse only in the global-direction-only condition; and the PNFA group performed significantly worse in all pitch conditions (Table 2). No significant performance differences between patient groups were identified. No significant correlations of task performance with prior musical training, general disease measures (Mini-Mental State Examination score, symptom duration) or standard speech encoding measures (word and sentence repetition) were found within the patient cohort.
Thirteen patients (five AD, two LPA, six PNFA) were also administered the ‘easy’ version of the pitch pattern test having detected <50% of deviants in the more difficult test (we ran an additional analysis of this subset of patients; data for all patients were included in the main analysis of the more difficult test). Although raw detection scores (Table S2 in Supplementary Material on-line) suggested impaired performance of the PNFA and LPA groups in each pitch condition relative to the healthy control group, no significant differences between groups were found after taking auditory working memory performance into account.
3.2.2. Temporal pattern processing
For the temporal pattern processing tasks, no significant effects of patient group on performance were found after adjusting for auditory working memory capacity (vs controls: AD beta = -0.02, 95% CI -0.12 to 0.09; LPA beta = -0.07, 95% CI -0.32 to 0.22; PNFA beta = -0.18, 95% CI -0.40 to 0.03). Across all experimental groups, the global condition resulted in poorer performance than the local condition (beta = -0.10, 95% CI -0.18 to -0.04). However, there was no indication of a significant interaction between condition and group (vs controls x condition: AD beta = -0.06, 95% CI -0.15 to 0.04; LPA beta = -0.10, 95% CI -0.29 to 0.08; PNFA beta = -0.05, 95% CI -0.14 to 0.08).
3.2.3. Timbral deviance detection
On the timbre processing (general acoustic deviance detection) task no significant effect of group on performance was found after adjusting for auditory working memory capacity (vs controls: AD beta = 0.05, 99% CI -0.04 to 0.26; LPA beta = -0.03, 99% CI -0.33 to 0.41; PNFA beta = -0.05, 99% CI -0.23 to 0.07).
3.2.4. Tune recognition tasks
No significant interactions were found between group and correctly labelling a tune as ‘famous’ in the tune streaming (musical scene analysis) task (χ2(3) = 3.92, p = 0.27), indicating no effect of patient group on performance on this task. No effect of patient group was found for the baseline tune recognition task (vs controls: AD beta = 0.13, 99% CI -0.09 to 0.74; LPA beta = -0.95, 99% CI -4.04 to 2.60; PNFA beta = -0.34, 99% CI -2.83 to 0.74).
3.2.5. Correlations between dimensions of music processing
Significant pairwise correlations were found between all measures of pitch pattern and temporal pattern processing (all p<0.05). Significant correlations were found for performance on the tune streaming and global pitch pattern processing (direction-only) tasks; and for performance on general acoustic (timbral) deviance detection and global pitch (direction-only) and local temporal processing tasks (all p<0.05; see Table S3). Tune recognition correlated with performance on timbral deviance detection; years of musical training correlated with global temporal processing. Peripheral audiometry detection thresholds did not correlate significantly with any of the experimental measures.
4. Discussion
Here we have shown that canonical dementia syndromes of typical AD, LPA and PNFA may be associated with profiles of impaired music perception relative to healthy older individuals. Deficits exhibited by the present syndromic groups affected the analysis of pitch pattern and were not simply attributable to prior musical expertise, general cognitive, elementary perceptual or task factors. After taking general auditory working memory performance into account, detection of acoustic deviants (indexed by varying note timbre) was comparable to healthy controls in all syndromic groups. Patients’ performance on pitch pattern analysis tasks deteriorated with increasing perceptual difficulty (as indexed by the more versus less difficult versions of the pitch pattern tests), consistent with a true deficit of pitch pattern processing. Patients with typical AD had impaired processing of global pitch (melody contour) information but (after accounting for general auditory working memory capacity) intact processing of local pitch (interval) and temporal pattern, as well as intact tune recognition whether in isolation or within a polyphonic ‘musical scene’. Patients with LPA (a syndrome generally underpinned by AD pathology) showed a similar profile with predominant impairment of global pitch processing, albeit the evidence of impairment was most apparent in the more demanding processing of direction-only contour variation (produced by deviance in the ordering of the same note sequence). In contrast, patients with PNFA exhibited deficits affecting local (interval) as well as global (melody) information in pitch patterns but (again, after controlling for general auditory working memory capacity) performance that did not reach statistical significance when compared to control processing of temporal pattern, tune recognition and musical scene analysis.
These findings are broadly consistent with a modular organisation of music cognition, as previously proposed [7]. More specifically, the relatively greater impairment of global than local pitch pattern analysis in typical AD and the similar profile in LPA corroborate our experimental predictions. Impaired global processing of pitch information in music is in line with other evidence for defective formation of coherent global stimulus representations in AD: this deficit might reflect increased demand for coordinated integrative computations between temporo-parietal association cortices vulnerable to Alzheimer pathology [81–85], though any disadvantage with respect to the coding of local stimulus features is likely to be relative rather than absolute [37–40,86]. While the processing of global stimulus characteristics unfolding over longer time windows requires attentional resources [40,82,86], it is unlikely that the profile of pitch deficits here was entirely underpinned by attentional compromise: as our paradigm required a single response to consecutively presented stimuli, it is unlikely to have taxed divided attention, while demands on sustained attention are likely to have been similar in the timbral deviance detection task, on which the present AD group performed normally. Moreover, pitch pattern deficits in our patient groups were documented after taking auditory working memory capacity into account. On the other hand, the present data suggest any claim that dementia syndromes differentially affect particular components of music cognition must be qualified. Syndromic profiles were documented in the context of wide individual variability (Figure 3). Moreover, across the patient cohort, correlated performance was observed for processing local and global information and pitch and temporal patterns. As the neural mechanisms mediating different components of music perception are likely to be affected together by the spreading neurodegenerative process, the finding of correlation (or absence of differential impairment) in this setting cannot be used to draw inferences about the underlying cognitive architecture.
Our findings provide further evidence that LPA and PNFA have associated phenotypes of nonverbal auditory impairment [32,64,87–89]. The musical phenotype was more severe in the PNFA group here; the involvement of pitch pattern analysis in this syndrome is in line with previous work [32] and suggests a putative mechanism linking generic mechanisms of dynamic auditory encoding with speech production via the dorsal auditory cortical pathway, extending over a range of timescales relevant to processing of individual and sequential speech sounds [9,77,90–92], Marked involvement of musical perceptual mechanisms might be anticipated from the severe and focal involvement of auditory association areas in the progressive aphasias [9,11]. Although we did not demonstrate a correlation of musical measures with standard measures of verbal encoding, pitch processing mechanisms are likely to be more relevant to prosody (a crucial non-linguistic attribute of speech signals) than phonemic sequencing, at least for non-tonal languages. Both perception and production of prosody are abnormal in PNFA [89], raising the possibility of a common mechanism linking musical pitch encoding with the programming of pitch variations in speech.
Allowing for the relatively small cohorts here, the present data offer relatively little support for specific musical signatures of particular dementia pathologies: when syndromic groups were directly compared, no measures indicated robust differences. Our findings suggest that certain musical perceptual attributes such as melody (pitch contour) tax neural computational resources across dementia syndromes; the data do not suggest any simple dichotomisation of dementias according to whether they degrade or spare the perception of music. Though the overall profile of pitch pattern deficits suggested some selectivity for particular syndromes (predominantly affecting global pitch characteristics in AD and LPA and more widespread in PNFA), any syndromic effects were relative rather than syndrome-specific. An important theme emerging from this study is that auditory working memory deficits are likely to amplify any purely musical deficits (compare Figure 3 prior to adjustment for this factor and the unadjusted analysis summarised in Table S4 with the adjusted significance attributions in Table 2): patients presenting with impairments of music processing may be comparably impaired on processing of other extended auditory information streams. The extent to which musical deficits reflect music-specific processes might then depend on the nature of the interaction between auditory working memory and the relevant musical characteristic, as suggested by previous work [48–50]. This factor may partly explain the lack of evidence here for specific deficits of temporal pattern processing from music, which we anticipated particularly in the PNFA group [64]. While in principle this could also reflect the small study cohort or failure to sample relevant temporal windows (as temporal characteristics of music are less constrained than pitch variations), temporal analysis of musical sequences may be more intimately reliant on auditory working memory capacity than pitch analysis; moreover, the linkage between temporal analysis and working memory mechanisms may have a neuroanatomical substrate (including insular cortex) that is targeted in PNFA [93]. This is a difficult issue to resolve, as particular subsystems of working memory are likely to be music-specific [48–50]. On the other hand, it has been shown that musical listening tasks also engage domain-general working memory circuits [94]. In this study, we set out to adjust for a general index of (verbal) auditory working memory capacity that might affect performance on auditory tracking tasks; however, the relative effects of music-specific and music-independent buffer systems on the perception of musical structure will only be resolved by assessing indices of musical pitch and temporal short-term memory directly alongside standard working memory measures. Even if underpinned by separable neural substrates, music-specific and music-independent working memory systems may be affected together in neurodegenerative disease.
Perhaps more surprisingly, we found no evidence for a specific deficit of musical scene analysis (as indexed by the processing of polyphonic melodies) in any patient group. This contrasts with previous work characterising a generic impairment of auditory scene analysis in AD [33,35,36,72–75] and may in part reflect the relatively wide variation in healthy control performance on our tune streaming test (Figure 3). However, it is possible that the analysis of musical scenes benefits to a greater degree than other kinds of auditory scenes from the availability of stored templates, here familiar tunes. If (as the present data also suggest) recognition of familiar tunes is relatively preserved in these dementia syndromes, patients may be able to engage ‘top-down’ mechanisms for parsing the musical scene even despite degraded mechanisms of early scene analysis [95,96].
The present findings have certain practical and clinical implications. Deficits of pitch pattern analysis here were demonstrated using stimuli that required tracking of musical information over time. Conventional neuropsychological (including music psychology) procedures that assess discrete stimulus tokens presented in isolation may not fully capture information processing deficits in dementia, particularly earlier in the disease course. Novel neuropsychological instruments that require on-line tracking of information streams could be relevant for assessing the encoding of verbal as well as musical sequences in these diseases. Though conclusions must be qualified pending further detailed investigation, our findings suggest that particular musical attributes (such as rhythm) might be used as a vehicle for designing musical interventions in at least some patients with dementia. At present, formal trials of music therapy in dementia often yield disappointing results despite anecdotal reports of benefit [97]. Targeting of those musical components where the prospect of benefit is greatest would provide a rational basis for music therapy in patients with dementia; moreover, rhythm-based interventions might be more straightforward to deliver and outcomes (for example, patient motor responses) may be easier to code than more complex musical interventions [98].
Considered together, our findings suggest that music perception may be a useful paradigm for assessing neural computational processes that support the analysis of information streams over different time windows and levels of complexity and the impact of dementias on those processes. Impaired encoding of pitch contour may have potential utility as a novel nonverbal and nonvisual biomarker across dementia syndromes while the overall profile of pitch pattern processing may have relative selectivity for particular pathologies such as AD. In addition, potential linkages between musical pitch processing and the processing of speech prosody warrant further investigation particularly in patients with progressive aphasia. This study has several limitations that should direct further work. The numbers of patients recruited here to particular syndromic groups were small and additionally, particular tests were not completed by all patients (Table 2), further underlining the need to study larger patient cohorts to corroborate these findings. Future study cohorts should ideally encompass a wider range of neurodegenerative syndromes and diseases with longitudinal assessments to determine the sensitivity and specificity of particular musical perceptual indices and patterns of evolution over time, ultimately with histopathological and molecular correlation. Combining multi-centre patient cohorts might improve power to detect effects and potentially, to stratify neurodegenerative syndromes and pathologies. Even within the AD spectrum, factors such as age and disease stage (severity) may importantly modify phenotype [39]; moreover the present data underline the need to take into account individual variability, which may be amplified by prior musical competence. Besides the analysis of local and global information per se, dementia syndromes might degrade associated cognitive operations, such as perceptual learning, executive shifting between processing levels or top-down attentional modulation of perceptual mechanisms [39,43]: future musical paradigms should address these possibilities. Structural and functional neuroanatomical studies comparing patient and healthy older cohorts will be required to delineate the alterations in brain mechanisms of music processing produced by these diseases and to more fully understand the musical phenotypes demonstrated here. More broadly, our findings may provide a prima facie case for tackling theoretical and practical issues of sensory information processing in the dementias that go beyond the domains of language and vision.
Supplementary Materials
Acknowledgements
We are grateful to all participants for their involvement. The Dementia Research Centre is supported by Alzheimer's Research UK, the Brain Research Trust and the Wolfson Foundation. This work was funded by the Wellcome Trust, the UK Medical Research Council and the NIHR Queen Square Dementia Biomedical Research Unit. HLG was supported by an Alzheimer Research UK PhD Fellowship. CNC is supported by The National Brain Appeal – Frontotemporal Dementia Research Fund. SJC is supported by an Alzheimer Research UK Senior Research Fellowship and an ESRC/NIHR (Grant no ES/K006711/1). JDW was supported by a Wellcome Trust Senior Clinical Fellowship (Grant No 091673/Z/10/Z).
References
- [1].Baird A, Samson S. Music and dementia. Prog Brain Res. 2015;217:207–235. doi: 10.1016/bs.pbr.2014.11.028. [DOI] [PubMed] [Google Scholar]
- [2].Clark CN, Warren JD. Music, memory and mechanisms in Alzhiemer’s disease. Brain. 2015;138:2114–25. doi: 10.1093/brain/awv148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jacobsen J, Fritz T, Stelzer J, Turner R. Why musical memory can be preserved in advanced Alzheimer ’ s disease. Brain. 2015;138:2438–2450. doi: 10.1093/brain/awv135. [DOI] [PubMed] [Google Scholar]
- [4].Bregman AS. Auditory Scene Analysis: The Perceptual Organization of Sound. MIT Press; 1990. [Google Scholar]
- [5].Griffiths TD, Warren JD. What is an auditory object? Nat Rev Neurosci. 2004;5:887–892. doi: 10.1038/nrn1538. [DOI] [PubMed] [Google Scholar]
- [6].Clark CN, Golden HL, Warren JD. Handbook of clinical neurology: The human auditory system. Elsevier: 2015. Acquired amusia; pp. 607–631. [DOI] [PubMed] [Google Scholar]
- [7].Peretz I, Coltheart M. Modularity of music processing. Nat Neurosci. 2003;6:688–691. doi: 10.1038/nn1083. [DOI] [PubMed] [Google Scholar]
- [8].Chételat G, Desgranges B, Landeau B, Mézenge F, Poline JB, de la Sayette V, Viader F, Eustache F, Baron J-C. Direct voxel-based comparison between grey matter hypometabolism and atrophy in Alzheimer’s disease. Brain. 2008;131:60–71. doi: 10.1093/brain/awm288. [DOI] [PubMed] [Google Scholar]
- [9].Grossman M. The non-fluent/agrammatic variant of primary progressive aphasia. Lancet Neurol. 2012;11:545–555. doi: 10.1016/S1474-4422(12)70099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Herholz K. FDG PET and differential diagnosis of dementia. Alzheimer Dis Assoc Disord. 1995;9:6–16. doi: 10.1097/00002093-199505000-00004. [DOI] [PubMed] [Google Scholar]
- [11].Rohrer JD, Ridgway GR, Crutch SJ, Hailstone J, Goll JC, Clarkson MJ, Mead S, Beck J, Mummery C, Ourselin S, Warrington EK, et al. Progressive logopenic/phonological aphasia: Erosion of the language network. Neuroimage. 2010;49:984–993. doi: 10.1016/j.neuroimage.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Clark CN, Downey LE, Warren JD. Brain disorders and the biological role of music. Soc Cogn Affect Neurosci. 2015;10:444–52. doi: 10.1093/scan/nsu079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zatorre RJ, Salimpoor VN. From perception to pleasure: Music and its neural substrates. Proc Natl Acad Sci. 2013;110:10430–10437. doi: 10.1073/pnas.1301228110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Warren JD, Fletcher PD, Golden HL. The paradox of syndromic diversity in Alzheimer disease. Nat Rev Neurol. 2012;8:451–64. doi: 10.1038/nrneurol.2012.135. [DOI] [PubMed] [Google Scholar]
- [15].Whitwell JL, Josephs Ka. Neuroimaging in frontotemporal lobar degeneration—predicting molecular pathology. Nat Rev Neurol. 2012;8:131–142. doi: 10.1038/nrneurol.2012.7. [DOI] [PubMed] [Google Scholar]
- [16].Cuddy LL, Duffin J. Music, memory, and Alzheimer’s disease: is music recognition spared in dementia, and how can it be assessed? Med Hypotheses. 2005;64:229–35. doi: 10.1016/j.mehy.2004.09.005. [DOI] [PubMed] [Google Scholar]
- [17].Ménard M-C, Belleville S. Musical and verbal memory in Alzheimer’s disease: a study of long-term and short-term memory. Brain Cogn. 2009;71:38–45. doi: 10.1016/j.bandc.2009.03.008. [DOI] [PubMed] [Google Scholar]
- [18].White DA, Murphy CF. Working Memory for Nonverbal Auditory Information in Dementia of the Alzheimer Type. Arch Clin Neuropsychol. 1998;13:339–347. [PubMed] [Google Scholar]
- [19].Beatty WW, Rogers CL, Rogers RL, English S, Testa JA, Orbelo DM, Wilson DA, Ross ED. Piano playing in Alzheimer’s disease: Longitudinal study of a single case. Neurocase. 1999;5:459–469. [Google Scholar]
- [20].Omar R, Hailstone JC, Warren JE, Crutch SJ, Warren JD. The cognitive organization of music knowledge: a clinical analysis. Brain. 2010;133:1200–13. doi: 10.1093/brain/awp345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].El Haj M, Fasotti L, Allain P. The involuntary nature of music-evoked autobiographical memories in Alzheimer’s disease. Conscious Cogn. 2012;21:238–46. doi: 10.1016/j.concog.2011.12.005. [DOI] [PubMed] [Google Scholar]
- [22].Irish M, Cunningham CJ, Walsh JB, Coakley D, Lawlor BA, Robertson IH, Coen RF. Investigating the enhancing effect of music on autobiographical memory in mild Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;22:108–20. doi: 10.1159/000093487. [DOI] [PubMed] [Google Scholar]
- [23].Moussard A, Bigand E, Belleville S, Peretz I. Learning sung lyrics aids retention in normal ageing and Alzheimer’s disease. Neuropsychol Rehabil. 2014;24:894–917. doi: 10.1080/09602011.2014.917982. [DOI] [PubMed] [Google Scholar]
- [24].Simmons-Stern NR, Budson AE, Ally BA. Music as a memory enhancer in patients with Alzheimer’s disease. Neuropsychologia. 2010;48:3164–7. doi: 10.1016/j.neuropsychologia.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Thompson RG, Moulin CJA, Hayre S, Jones RW. Music enhances category fluency in healthy older adults and Alzheimer’s disease patients. Exp Aging Res. 2006;31:91–9. doi: 10.1080/03610730590882819. [DOI] [PubMed] [Google Scholar]
- [26].Peretz I. Processing of local and global musical information by unilateral brain-damaged patients. Brain. 1990;113:1185–1205. doi: 10.1093/brain/113.4.1185. [DOI] [PubMed] [Google Scholar]
- [27].Dowling WJ, Fujitani DS. Contour, interval, and pitch reocgnition in memory for melodies. J Acoust Soc Am. 1970;49:524–531. doi: 10.1121/1.1912382. [DOI] [PubMed] [Google Scholar]
- [28].Liégeois-Chauvel C, Peretz I, Babaï M, Laguitton V, Chauvel P. Contribution of different cortical areas in the temporal lobes to music processing. Brain. 1998;121:1853–1867. doi: 10.1093/brain/121.10.1853. [DOI] [PubMed] [Google Scholar]
- [29].Peretz I, Morais J. Music and modularity. Contemp Music Rev. 1989;4:279–293. [Google Scholar]
- [30].Stewart L, Overath T, Warren JD, Foxton JM, Griffiths TD. fMRI evidence for a cortical hierarchy of pitch pattern processing. PLoS One. 2008;3:e1470. doi: 10.1371/journal.pone.0001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lee Y-S, Janata P, Frost C, Hanke M, Granger R. Investigation of melodic contour processing in the brain using multivariate pattern-based fMRI. Neuroimage. 2011;57:293–300. doi: 10.1016/j.neuroimage.2011.02.006. [DOI] [PubMed] [Google Scholar]
- [32].Goll JC, Kim LG, Hailstone JC, Lehmann M, Buckley A, Crutch SJ, Warren JD. Auditory object cognition in dementia. Neuropsychologia. 2011;49:2755–65. doi: 10.1016/j.neuropsychologia.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Goll JC, Kim LG, Ridgway GR, Hailstone JC, Lehmann M, Buckley AH, Crutch SJ, Warren JD. Impairments of auditory scene analysis in Alzheimer’s disease. Brain. 2012;135:190–200. doi: 10.1093/brain/awr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Johnson JK, Chang C-C, Brambati SM, Migliaccio R, Gorno-Tempini ML, Miller BL, Janata P. Music recognition in frontotemporal lobar degeneration and Alzheimer disease. Cogn Behav Neurol. 2011;24:74–84. doi: 10.1097/WNN.0b013e31821de326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kurylo D, Corkin S, Allard T, Zatorre R, Growdon J. Auditory function in Alzheimer’s disease. Neurology. 1993;43:1893–99. doi: 10.1212/wnl.43.10.1893. [DOI] [PubMed] [Google Scholar]
- [36].Strouse A, Hall JW, Burger MC. Central Auditory Processing in Alzheimer ’s Disease. Ear Hear. 1995;16:230–238. doi: 10.1097/00003446-199504000-00010. [DOI] [PubMed] [Google Scholar]
- [37].Delis DC, Massman PJ, Butters N, Salmon DP, Shear PK, Demadura T, Filoteo JV. Spatial cognition in Alzheimer’s disease: subtypes of global-local impairment. J Clin Exp Neuropsychol. 1992;14:463–77. doi: 10.1080/01688639208402838. [DOI] [PubMed] [Google Scholar]
- [38].Massman PJ, Delis DC, Filoteo JV, Butters N, et al. Mechanisms of spatial impairment in Alzheimer’s disease subgroups: Differential breakdown of directed attention to global-local stimuli. Neuropsychology. 1993;7:172–181. [Google Scholar]
- [39].Matsumoto E, Ohigashi Y, Fujimori M, Mori E. The processing of global and local visual information in Alzheimer’s disease. Behav Neurol. 2000;12:119–125. doi: 10.1155/2000/683156. [DOI] [PubMed] [Google Scholar]
- [40].Slavin MJ, Mattingley JB, Bradshaw JL, Storey E. Local–global processing in Alzheimer’s disease: an examination of interference, inhibition and priming. Neuropsychologia. 2002;40:1173–1186. doi: 10.1016/s0028-3932(01)00225-1. [DOI] [PubMed] [Google Scholar]
- [41].Thaiss L, De Bleser R. Visual agnosia: a case of reduced attentional "spotlight"? Cortex. 1992;28:601–21. doi: 10.1016/s0010-9452(13)80230-4. [DOI] [PubMed] [Google Scholar]
- [42].Stark ME, Grafman J, Fertig E. A restricted 'spotlight' of attention in visual object recognition. Neuropsychologia. 1997;35:1233–49. doi: 10.1016/s0028-3932(97)00049-3. [DOI] [PubMed] [Google Scholar]
- [43].Belleville S, Bherer L, Lepage E, Chertkow H, Gauthier S. Task switching capacities in persons with Alzheimer's disease and mild cognitive impairment. Neuropsychologia. 2008;46:2225–33. doi: 10.1016/j.neuropsychologia.2008.02.012. [DOI] [PubMed] [Google Scholar]
- [44].Peretz I, Champod AS, Hyde K. Varieties of Musical Disorders: The Montreal Battery of Evaluation of Amusia. Ann N Y Acad Sci. 2003;999:58–75. doi: 10.1196/annals.1284.006. [DOI] [PubMed] [Google Scholar]
- [45].Gorno-Tempini M-L, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnsonm JK, Weiner MW, Miller BL. Cognition and Anatomy in Three Variants of Primary Progressive Aphasia. Ann Neurol. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, Perani D, Garibotto V, Cappa SF, Miller BL. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Stopford CL, Thompson JC, Neary D, Richardson AMT, Snowden JS. Working memory, attention, and executive function in Alzheimer’s disease and frontotemporal dementia. Cortex. 2012;48:429–46. doi: 10.1016/j.cortex.2010.12.002. [DOI] [PubMed] [Google Scholar]
- [48].Burunat I, Alluri V, Toiviainen P, Numminen J, Brattico E. Dynamics of brain activity underlying working memory for music in a naturalistic condition. Cortex. 2014;57:254–69. doi: 10.1016/j.cortex.2014.04.012. [DOI] [PubMed] [Google Scholar]
- [49].Teki S, Griffiths TD. Brain Bases of Working Memory for Time Intervals in Rhythmic Sequences. Front Neurosci. 2016;10:239. doi: 10.3389/fnins.2016.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tillmann B, Lévêque Y, Fornoni L, Albouy P, Caclin A. Impaired short-term memory for pitch in congenital amusia. Brain Res. 2016;1640:251–63. doi: 10.1016/j.brainres.2015.10.035. [DOI] [PubMed] [Google Scholar]
- [51].Schuppert M, Mu TF, Wieringa BM, Altenmu E. Receptive amusia : evidence for cross-hemispheric neural networks underlying music processing strategies. Brain. 2000;123:546–559. doi: 10.1093/brain/123.3.546. [DOI] [PubMed] [Google Scholar]
- [52].Di Pietro M, Laganaro M, Leemann B, Schnider A. Receptive amusia: temporal auditory processing deficit in a professional musician following a left temporo-parietal lesion. Neuropsychologia. 2004;42:868–77. doi: 10.1016/j.neuropsychologia.2003.12.004. [DOI] [PubMed] [Google Scholar]
- [53].Robin DA, Tranel D, Damasio H. Auditory perception of temporal and spectral events in patients with focal left and right cerebral lesions. Brain Lang. 1990;39:539–555. doi: 10.1016/0093-934x(90)90161-9. [DOI] [PubMed] [Google Scholar]
- [54].Wilson SJ, Pressing JL, Wales RJ. Modelling rhythmic function in a musician post-stroke. Neuropsychologia. 2002;40:1494–1505. doi: 10.1016/s0028-3932(01)00198-1. [DOI] [PubMed] [Google Scholar]
- [55].Vignolo L. Music agnosia and auditory agnosia. Ann N Y Acad Sci. 2003;999:50–57. doi: 10.1196/annals.1284.005. [DOI] [PubMed] [Google Scholar]
- [56].Peretz I, Kolinsky R. Boundaries of separability between melody and rhythm in music discrimination: a neuropsychological perspective. Q J Exp Psychol A. 1993;46:301–325. doi: 10.1080/14640749308401048. [DOI] [PubMed] [Google Scholar]
- [57].Fries W, Swihart AA. Disturbance of Rhythm Sense Following Right Hemisphere Damage. Neuropsychologia. 1990;28:1317–1323. doi: 10.1016/0028-3932(90)90047-r. [DOI] [PubMed] [Google Scholar]
- [58].Chen JL, Penhune VB, Zatorre RJ. Listening to musical rhythms recruits motor regions of the brain. Cereb cortex. 2008;18:2844–54. doi: 10.1093/cercor/bhn042. [DOI] [PubMed] [Google Scholar]
- [59].Grahn JA, Rowe JB. Finding and feeling the musical beat: striatal dissociations between detection and prediction of regularity. Cereb cortex. 2013;23:913–21. doi: 10.1093/cercor/bhs083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Grahn JA. The role of the basal ganglia in beat perception: Neuroimaging and neuropsychological investigations. Ann N Y Acad Sci. 2009;1169:35–45. doi: 10.1111/j.1749-6632.2009.04553.x. [DOI] [PubMed] [Google Scholar]
- [61].Konoike N, Kotozaki Y, Miyachi S, Miyauchi CM, Yomogida Y, Akimoto Y, Kuraoka K, Sugiura M, Kawashima R, Nakamura K. Rhythm information represented in the fronto-parieto-cerebellar motor system. Neuroimage. 2012;63:328–38. doi: 10.1016/j.neuroimage.2012.07.002. [DOI] [PubMed] [Google Scholar]
- [62].Cowles A, Beatty WW, Nixon SJ, Lutz LJ, Paulk J, Paulk K, Ross ED. Musical skill in dementia: a violinist presumed to have Alzheimer’s disease learns to play a new song. Neurocase. 2003;9:493–503. doi: 10.1076/neur.9.6.493.29378. [DOI] [PubMed] [Google Scholar]
- [63].Hellström A, Almkvist O. Tone duration discrimination in demented, memory-impaired, and healthy elderly. Dement Geriatr Cogn Disord. 1997;8:49–54. doi: 10.1159/000106600. [DOI] [PubMed] [Google Scholar]
- [64].Grube M, Bruffaerts R, Schaeverbeke J, Neyens V, De Weer AS, Seghers A, Bergmans B, Dries E, Griffiths TD, Vandenberghe R. Core auditory processing deficits in primary progressive aphasia. Brain. 2016;139:1817–29. doi: 10.1093/brain/aww067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Scott SK, McGettigan C. The neural processing of masked speech. Hear Res. 2013;303:58–66. doi: 10.1016/j.heares.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: music and speech. Trends Cogn Sci. 2002;6:37–46. doi: 10.1016/s1364-6613(00)01816-7. [DOI] [PubMed] [Google Scholar]
- [67].Griffiths TD, Warren JD. The planum temporale as a computational hub. Trends Neurosci. 2002;25:348–353. doi: 10.1016/s0166-2236(02)02191-4. [DOI] [PubMed] [Google Scholar]
- [68].Bey C, Zatorre R. Recognition of Interleaved Melodies: An fMRI study. Ann N Y Acad Sci. 2003;999:152–4. doi: 10.1196/annals.1284.017. [DOI] [PubMed] [Google Scholar]
- [69].Kamourieh S, Braga RM, Leech R, Newbould RD, Malhotra P, Wise RJS. Neural Systems Involved When Attending to a Speaker. Cereb Cortex. 2015;25:4284–4298. doi: 10.1093/cercor/bhu325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mazzoni M, Moretti P, Pardossi L, Vista M, Muratorio a, Puglioli M. A case of music imperception. J Neurol Neurosurg. Psychiatry. 1993;56:322–322. doi: 10.1136/jnnp.56.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].McDonald I. Musical alexia with recovery: A personal account. Brain. 2006;129:2554–2561. doi: 10.1093/brain/awl235. [DOI] [PubMed] [Google Scholar]
- [72].Gates G, Karzon R, Garcia P, Peterein J, Storandt M, Morris J, Miller JP. Auditory dysfunction in aging and senile dementia of the Alzheimer’s type. Arch Neurol. 1995;52:626–634. doi: 10.1001/archneur.1995.00540300108020. [DOI] [PubMed] [Google Scholar]
- [73].Gates GA, Anderson ML, McCurry SM, Feeney MP, Larson EB. Central Auditory Dysfunction as a Harbinger of Alzheimer Dementia. Arch Otolaryngol Neck Surg. 2011;137:390–395. doi: 10.1001/archoto.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Golden HL, Agustus JL, Goll JC, Downey LE, Mummery CJ, Schott JM, Crutch SJ, Warren JD. Functional neuroanatomy of auditory scene analysis in Alzheimer’s disease. NeuroImage Clin. 2015;7:699–708. doi: 10.1016/j.nicl.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Golden HL, Nicholas JM, Yong KXX, Downey LE, Schott JM, Mummery CJ, Crutch SJ, Warren JD. Auditory spatial processing in Alzheimer’s disease. Brain. 2015;138:189–202. doi: 10.1093/brain/awu337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–939. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- [77].Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Geiser E, Ziegler E, Jancke L, Meyer M. Early electrophysiological correlates of meter and rhythm processing in music perception. Cortex. 2009;45:93–102. doi: 10.1016/j.cortex.2007.09.010. [DOI] [PubMed] [Google Scholar]
- [79].Janata P, Birk J, Tillmann B, Bharucha J. Online detection of tonal pop-out in modulating contexts. Music Percept. 2003;20:283–305. [Google Scholar]
- [80].DeCarlo LT. Signal detection theory and generalized linear models. Psychol Methods. 1998;3:186–205. [Google Scholar]
- [81].Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Where in the brain does visual attention select the forest and the trees? Nature. 1996;382:626–628. doi: 10.1038/382626a0. [DOI] [PubMed] [Google Scholar]
- [82].Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Neural mechanisms involved in the processing of global and local aspects of hierarchically organized visual stimuli. Brain. 1997;120:1779–91. doi: 10.1093/brain/120.10.1779. [DOI] [PubMed] [Google Scholar]
- [83].Lamb MR, Robertson LC, Knight RT. Component mechanisms underlying the processing of hierarchically organized patterns: inferences from patients with unilateral cortical lesions. J Exp Psychol Learn Mem Cogn. 1990;16:471–483. doi: 10.1037//0278-7393.16.3.471. [DOI] [PubMed] [Google Scholar]
- [84].Robertson LC, Lamb MR. Neuropsychological contributions to theories of part/whole organization. Cogn Psychol. 1991;23:299–330. doi: 10.1016/0010-0285(91)90012-d. [DOI] [PubMed] [Google Scholar]
- [85].Sanders LD, Poeppel D. Local and global auditory processing: Behavioral and ERP evidence. Neuropsychologia. 2007;45:1172–1186. doi: 10.1016/j.neuropsychologia.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Filoteo J V, Friedrich FJ, Stricker JL. Shifting attention to different levels within global-local stimuli: A study of normal participants and a patient with temporal-parietal lobe damage. Cogn Neuropsychol. 2001;18:227–261. doi: 10.1080/02643290125839. [DOI] [PubMed] [Google Scholar]
- [87].Goll JC, Crutch SJ, Loo JHY, Rohrer JD, Frost C, Bamiou D-E, Warren JD. Non-verbal sound processing in the primary progressive aphasias. Brain. 2010;133:272–85. doi: 10.1093/brain/awp235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hailstone JC, Ridgway GR, Bartlett JW, Goll JC, Crutch SJ, Warren JD. Accent processing in dementia. Neuropsychologia. 2012;50:2233–44. doi: 10.1016/j.neuropsychologia.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Rohrer JD, Sauter D, Scott S, Rossor MN, Warren JD. Receptive prosody in nonfluent primary progressive aphasias. Cortex. 2012;48:308–316. doi: 10.1016/j.cortex.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Libon DJ, Xie SX, Moore P, Farmer J, Antani S, McCawley G, Cross K, Grossman M. Patterns of neuropsychological impairment in frontotemporal dementia. Neurology. 2007;68:369–375. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- [91].Maruta C, Makhmood S, Downey LE, Golden HL, Fletcher PD, Witoonpanich P, Rohrer JD, Warren JD. Delayed auditory feedback simulates features of nonfluent primary progressive aphasia. J Neurol Sci. 2014;347:345–8. doi: 10.1016/j.jns.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Warren JE, Wise RJS, Warren JD. Sounds do-able: auditory-motor transformations and the posterior temporal plane. Trends Neurosci. 2005;28:636–43. doi: 10.1016/j.tins.2005.09.010. [DOI] [PubMed] [Google Scholar]
- [93].Jerde Ta, Childs SK, Handy ST, Nagode JC, Pardo JV. Dissociable systems of working memory for rhythm and melody. Neuroimage. 2011;57:1572–9. doi: 10.1016/j.neuroimage.2011.05.061. [DOI] [PubMed] [Google Scholar]
- [94].Janata P, Tillman B, Bharucha JJ. Listening to polyphonic music recruits domain-general attention and working memory circuits. Cogn Affect Behav Neurosci. 2002;2:121–140. doi: 10.3758/cabn.2.2.121. [DOI] [PubMed] [Google Scholar]
- [95].Caza N, Belleville S. Reduced short-term memory capacity in Alzheimer’s disease: The role of phonological, lexical, and semantic processing. Memory. 2008;16:341–350. doi: 10.1080/09658210701881758. [DOI] [PubMed] [Google Scholar]
- [96].Eustache F, Lambert J, Cassier C, Dary M, Rossa Y, Rioux P, Viader F, Lechevalier B. Disorders of auditory identification in dementia of the Alzheimer type. Cortex. 1995;31:119–27. doi: 10.1016/s0010-9452(13)80110-4. [DOI] [PubMed] [Google Scholar]
- [97].Thornley J, Hirjee H, Vasudev A. Music therapy in patients with dementia and behavioral disturbance on an inpatient psychiatry unit: results from a pilot randomized controlled study. Int Psychogeriatrics. 2015;21:1–3. doi: 10.1017/S1041610215001866. [DOI] [PubMed] [Google Scholar]
- [98].McDermott JH, Wrobleski D, Oxenham AJ. Recovering sound sources from embedded repetition. Proc Natl Acad Sci U S A. 2011;108:1188–93. doi: 10.1073/pnas.1004765108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Dunn LM, Dunn PQ, Whetton C. British Picture Vocabulary Scale. NFER-Nelson; Windsor: 1982. [Google Scholar]
- [100].Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- [101].Jackson M, Warrington EK. Arithmetic skills in patients with unilateral cerebral lesions. Cortex. 1986;22:611–20. doi: 10.1016/s0010-9452(86)80020-x. [DOI] [PubMed] [Google Scholar]
- [102].McKenna P, Warrington E. Graded naming test. NFER-Nelson; Windsor: 1983. [Google Scholar]
- [103].Folstein M, Folstein S, McHugh P. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [104].Nelson HE. National Adult Reading Test. NFER-Nelson; Windsor: 1982. [Google Scholar]
- [105].Warrington EK. Recogntion memory test. NFER-Nelson; Windsor: 1984. [Google Scholar]
- [106].Warrington EK, James M. The visual object and space perception battery. Thames Valley Test Company; Bury St Edmunds: 1991. [Google Scholar]
- [107].Wechsler D. Wechsler Adult Intelligence Scale-Revised. Psychological Corporation; New York: 1981. [Google Scholar]
- [108].Wechsler D. Wechsler abbreviated scale of intelligence: WASI. The Psychological Corporation, Harcourt Brace; San Antonio, TX: 1999. [Google Scholar]
- [109].Wechsler D. Wechsler memory scale: Revised. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.