Abstract
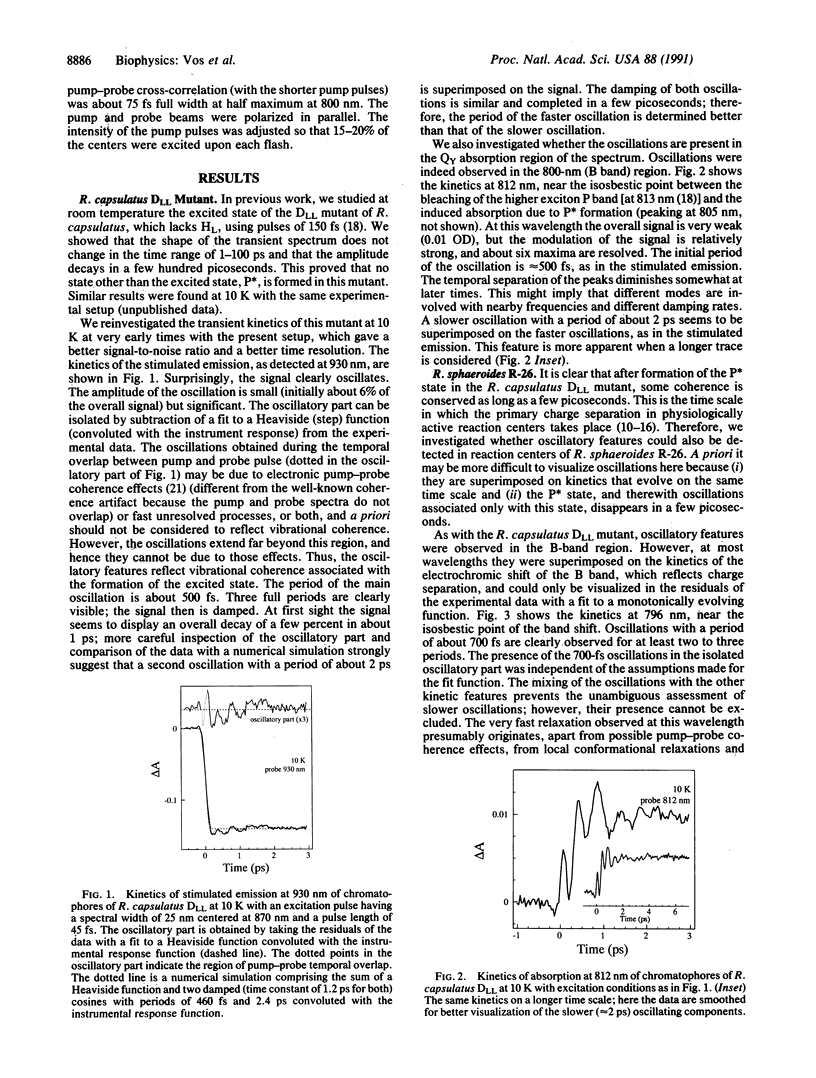
It is shown that vibrational coherence modulates the femtosecond kinetics of stimulated emission and absorption of reaction centers of purple bacteria. In the DLL mutant of Rhodobacter capsulatus, which lacks the bacteriopheophytin electron acceptor, oscillations with periods of approximately 500 fs and possibly also of approximately 2 ps were observed, which are associated with formation of the excited state. The kinetics, which reflect primary processes in Rhodobacter sphaeroides R-26, were modulated by oscillations with a period of approximately 700 fs at 796 nm and approximately 2 ps at 930 nm. In the latter case, at 930 nm, where the stimulated emission of the excited state, P*, is probed, oscillations could only be resolved when a sufficiently narrow (10 nm) and concomitantly long pump pulse was used. This may indicate that the potential energy surface of the excited state is anharmonic or that low-frequency oscillations are masked when higher frequency modes are also coherently excited, or both. The possibility is discussed that the primary charge separation may be a coherent and adiabatic process coupled to low-frequency vibrational modes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G. Crystallization of reaction center from Rhodopseudomonas sphaeroides: preliminary characterization. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4795–4799. doi: 10.1073/pnas.81.15.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnoy J, Mokhtari A. Resonant impulsive-stimulated Raman scattering on malachite green. Phys Rev A Gen Phys. 1988 Oct 1;38(7):3566–3576. doi: 10.1103/physreva.38.3566. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Go N., Noguti T., Nishikawa T. Dynamics of a small globular protein in terms of low-frequency vibrational modes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3696–3700. doi: 10.1073/pnas.80.12.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel W., Finkele U., Kaiser W., Oesterhelt D., Scheer H., Stilz H. U., Zinth W. Initial electron-transfer in the reaction center from Rhodobacter sphaeroides. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5168–5172. doi: 10.1073/pnas.87.13.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmaier C., Holten D. Evidence that a distribution of bacterial reaction centers underlies the temperature and detection-wavelength dependence of the rates of the primary electron-transfer reactions. Proc Natl Acad Sci U S A. 1990 May;87(9):3552–3556. doi: 10.1073/pnas.87.9.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. L., Breton J., Hoff A. J., Migus A., Antonetti A. Femtosecond spectroscopy of electron transfer in the reaction center of the photosynthetic bacterium Rhodopseudomonas sphaeroides R-26: Direct electron transfer from the dimeric bacteriochlorophyll primary donor to the bacteriopheophytin acceptor with a time constant of 2.8 +/- 0.2 psec. Proc Natl Acad Sci U S A. 1986 Feb;83(4):957–961. doi: 10.1073/pnas.83.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles S. J., Breton J., Youvan D. C. Partial symmetrization of the photosynthetic reaction center. Science. 1990 Jun 15;248(4961):1402–1405. doi: 10.1126/science.2192455. [DOI] [PubMed] [Google Scholar]
- Woodbury N. W., Becker M., Middendorf D., Parson W. W. Picosecond kinetics of the initial photochemical electron-transfer reaction in bacterial photosynthetic reaction centers. Biochemistry. 1985 Dec 17;24(26):7516–7521. doi: 10.1021/bi00347a002. [DOI] [PubMed] [Google Scholar]



