Abstract
Background
The buccopharyngeal membrane is a thin layer of cells covering the embryonic mouth. The perforation of this structure creates an opening connecting the external and the digestive tube which is essential for oral cavity formation. In humans, persistence of the buccopharyngeal membrane can lead to orofacial defects such as choanal atresia, oral synechiaes and cleft palate. Little is known about the causes of a persistent buccopharyngeal membrane and importantly how this structure ruptures.
Results
We have determined that Xenopus embryos deficient JNK signaling, using antisense and pharmacological approaches, have a persistent buccopharyngeal membrane. JNK deficient embryos have decreased cell division and increased cellular stress and apoptosis. However, altering these processes independently of JNK did not affect buccopharyngeal membrane perforation. JNK deficient embryos also have increased intercellular adhesion and defects in e-cadherin localization. Conversely, embryos with overactive JNK have epidermal fragility, increased E-cadherin internalization and increased membrane localized clathrin. In the buccopharyngeal membrane, clathrin is colocalized with active JNK. Further, inhibition of endocytosis results in a persistent buccopharyngeal membrane, mimicking the JNK deficient phenotype.
Conclusions
The results of this study suggest that JNK has a role in the disassembly adherens junctions via endocytosis that is required during buccopharyngeal membrane perforation.
Keywords: buccopharyngeal membrane, embryonic mouth, JNK, intercellular adhesion
INTRODUCTION
The primary or embryonic mouth is the first connection between the external and the digestive system in the anterior of the embryo (for reviews see (Dickinson and Sive, 2007; Soukup et al., 2013)). This structure undergoes several morphological changes during its formation, the last of such changes is the perforation of a 1–2 cell layer that covers the oral cavity. This covering is called the oral or buccopharyngeal membrane and it must rupture to create an oral cavity that is open to the external environment (Waterman, 1977; Waterman and Schoenwolf, 1980; Dickinson and Sive, 2006; Soukup et al., 2013). Initially, this opening is necessary for ingestion of fluids that is necessary for proper lung and digestive tract development (Greenough, 2000; Wagner et al., 2008). Later the embryonic mouth opening allows for eating and breathing in all vertebrates. In humans, defects in the perforation of the buccopharyngeal membrane causes a condition known as persistent buccopharyngeal membrane (Verma and Geller, 2009). While rare on its own, this condition is also thought to be an underlying cause of other more common orofacial defects such choanal atresia and oral synechiaes (Gartlan et al., 1993; Kwong, 2015). These defects can be isolated or can occur as part of several different syndromes and may also lead to cleft palate (Tomlinson et al., 2006; Turksen et al., 2012; Mascarella et al., 2015). In humans born with defects in the buccopharyngeal membrane, air and/or food cannot freely pass in the oral passages which can in turn cause embryonic distress and feeding problems. In the worst cases, asphyxiation and death of the newborn infant can result from such blockages in the oral cavity. We know very little about why these human defects occur during embryonic mouth development and moreover what regulates buccopharyngeal membrane perforation or rupture. Therefore, we have turned to Xenopus laevis to better understand mechanisms driving this final step of embryonic mouth development.
Previous work (Dickinson and Sive, 2009) and a small screen in the lab (unpublished) identified c-Jun N-terminal kinase (JNK) signaling as a candidate regulator of buccopharyngeal membrane perforation. JNK is a member of the MAPK (mitogen-activated protein kinase) family that regulates a large range of biological processes such as apoptosis, cell division, migration, tissue integrity and homeostasis (for examples see (Davis, 2000; Dong et al., 2001; Karin and Gallagher, 2005; Dush and Nascone-Yoder, 2013)). Further, JNK phosphorylates an ever increasing list of proteins in the cell that range in function from transcriptional regulation to basic cellular dynamics (Bogoyevitch and Kobe, 2006). For example, among the best characterized roles for JNK is that it phosphorylates c-Jun and thereby promotes activator protein-1 (AP-1) mediated transcription (Shaulian, 2010). JNK can also phosphorylate proteins associated with cellular junctions such as beta-catenin and zo-1 as well as focal adhesions such as paxillin (Huang et al., 2003; Huang et al., 2004; Yamauchi et al., 2006; Lee et al., 2009). In the embryo, JNK is known for its role in the Wnt-PCP pathway (Kuhl, 2002; Pandur et al., 2002) (Kuhl, 2002; Pandur et al., 2002). For example, in Xenopus, Wnt/PCP signals mediates convergent extension, kidney development and regeneration through JNK signaling (Yamanaka et al., 2002; Sugiura et al., 2009; Cizelsky et al., 2014).
While JNK activation via Wnt signals has been shown to be important for facial morphogenesis (Geetha-Loganathan et al., 2014; Zhu et al., 2016), a role for this protein has not been specifically associated with the buccopharyngeal membrane in any vertebrate. This may be due in part to the difficulty in viewing the buccopharyngeal membrane in most vertebrates (Dickinson, 2016) as well as the devastating effects of completely knocking out JNK in the embryo (Sabapathy et al., 1999). The embryonic mouth of mice hypomorphic for JNK1 and JNK2 have not, to our knowledge, been examined. However, we do know that these mice have defects in epidermal morphogenesis (Weston et al., 2004). Interestingly, the buccopharyngeal membrane is at least in part composed of cells that are continuous with and resemble the epidermis in Xenopus. For example, the structure is comprised of cells that have typical epidermal adherens junctions, a subset of the cells are epidermal multiciliated cells and the buccopharyngeal membrane cells maintain barrier function as does the epidermis (manuscript in prep). For this reason we have proposed that the buccopharyngeal membrane is essentially an epidermal structure and its perforation relies on similar signals that regulate epidermal morphogenesis. Thus, it is possible that a more general role for JNK in epidermal development may also explain the requirement of this essential signal during buccopharyngeal membrane perforation.
In the present study, we take you through a detective story in our pursuit to uncover how JNK regulates buccopharyngeal membrane perforation. First, we uncovered several roles for JNK signals in the embryo, including cell proliferation and maintaining cell survival and homeostasis. However, these processes did not seem critical in embryonic mouth development. Rather, we determined that JNK signals are also required for changes in intercellular adhesion in the epidermis and this requirement is also important for buccopharyngeal membrane perforation. We propose that JNK remodels the intercellular junctions via endocytosis which is necessary for buccopharyngeal membrane perforation.
RESULTS
1. Decreased JNK function results in embryonic mouth defects
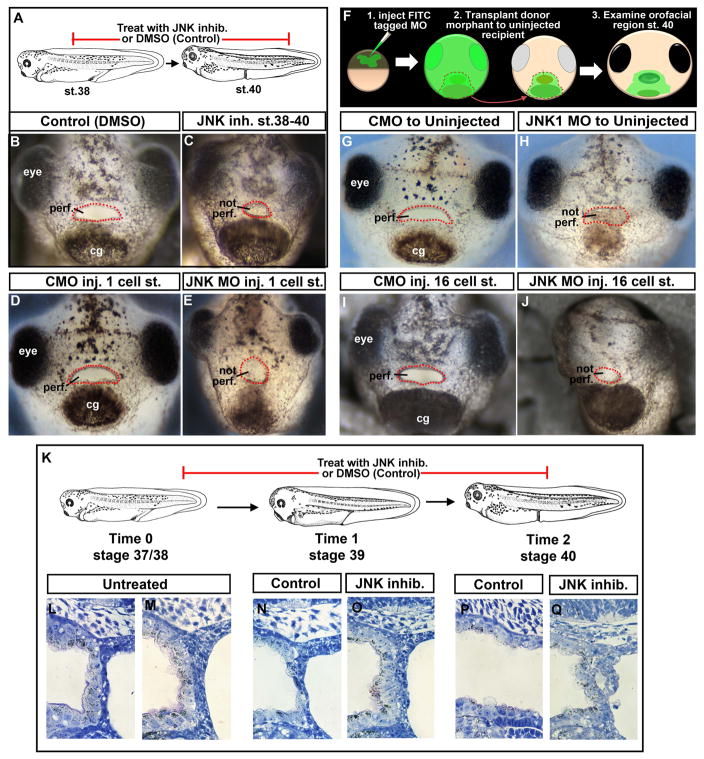
Inhibition of JNK signaling using a well studied pharmacological inhibitor SP600125 (Bennett et al., 2001), resulted in the failure of the embryonic mouth to form. More specifically, treatment with the JNK inhibitor (100 μm) from stage 37/38–40 resulted in a buccopharyngeal membrane that did not perforate at the appropriate stage (stage 40) in 95% of the embryos (n=20, 2 experiments; Fig. 1 A–C). Concentrations ranging from 50–100 μm were effective in causing a persistent buccopharyngeal membrane when the media and inhibitor was refreshed one time at stage 39. Earlier treatments also resulted in embryonic mouth defects but these may be due to JNK’s role in other processes during orofacial development (Jacox et al., 2016) and are not shown.
Figure 1.
A) Schematic showing treatment paradigm. B) Frontal view of a representative Control treated with 1% DMSO. C) Frontal view of a representative embryo treated with JNK inhibitor. D) Frontal view of a representative embryo injected with control morpholino at the 1 cell stage. E) Frontal view of a representative embryo injected with JNK1 morpholino at the 1 cell stage. F) Schematic showing face transplant paradigm. G) Frontal view of a representative control transplant. H) Frontal view of a representative embryo with JNK1 morphant tissue transplanted to its orofacial region. I) Frontal view of a representative embryo with control morpholino injected into D12 blastomere at the 16 cell stage. J) Frontal view of a representative embryo with JNK1 morpholino injected into D12 blastomere at the 16 cell stage. K) A schematic of the JNK inhibitor treatment paradigm and stages examined by histology. L, M) Shows sagittal sections through the buccopharyngeal membrane just prior to JNK inhibitor treatment at stage 37/38 (two representative images). N, O) Shows sagittal sections through the buccopharyngeal membrane at stage 39 in controls (N) and JNK inhibitor treated (O). P, Q) Shows sagittal sections through the buccopharyngeal membrane at stage 40 in controls (N) and JNK inhibitor treated. Abbreviations: perf. =perforated; cg=cement gland, inj. = injected, MO=morpholino, st. = stage. Red dots outline the embryonic mouth.
The JNK inhibitor targets all JNKs, however, we knew from studies in mice, chick and cell lines that JNK1 is a major effector in epidermal development, PCP signals and craniofacial development. Therefore, we focused on a role for JNK1 in our more targeted approaches. We used a JNK1 MO that has been validated and utilized in at least three studies in Xenopus (Yamanaka et al., 2002; Liao et al., 2006; Dush and Nascone-Yoder, 2013). Results revealed 86.2% of the JNK1 morphants (injected at the one cell stage with 40–60 ng of JNK1MO) had a persistent buccopharyngeal membrane. Control morphants injected with 40–60 ng at one cell stage did not display defects in buccopharyngeal membrane perforation (n=65, 3 experiments; Fig. 1 D, E).
To show a specific requirement for JNK within the embryonic mouth tissues we performed face transplants. In these transplants a small region of anterior ectoderm (including the cement gland) and underlying endoderm was transplanted from JNK1 morphants (or control morphants) to uninjected siblings at stage 24–26 as described previously (Dickinson and Sive, 2009) (Fig. 1 F). Results showed that 100% of the embryos with JNK1 morphant tissue in the oral region failed to form an embryonic mouth opening (n=9, 2 experiments, Fig. 1 G, H). Control morphant tissue did not affect buccopharyngeal membrane perforation. To complement these transplant experiments we also performed targeted JNK1 MO injections. We injected 10 ng of morpholino into specific cells (D12) that give rise to head epidermis at the 16 cell stage (Moody, 1987). In these embryos, where fluorescent JNK1 MO containing cells covered the face, we saw a persistent buccopharyngeal membrane in 100% of the embryos (n=25, 2 experiments, Fig. 1 I, J). Together, these results indicate that when the tissues that form the buccopharyngeal membrane are deficient in JNK, perforation of the structure does not occur.
We would like to note that in all cases, where the buccopharyngeal membrane did not perforate in the absence of JNK function, the shape of the mouth was more round. However we know that the shape of the mouth does not govern buccopharyngeal membrane breakdown based on two experiments. First, face explants with very small embryonic mouths still form a perforated mouth. Second, when the face of the embryo is constrained so that the mouth shape is altered, breakdown of the buccopharyngeal membrane can also still occur (manuscript in prep). Wnt/PCP signals from the cranial neural crest acting through JNK are required to shape the oral cavity in Xenopus (Jacox et al., 2016) which could explain the embryonic mouth shape changes we observed.
Histological analysis of the buccopharyngeal membrane revealed a reorganization of cells during perforation. Consistent with observations made in previous work (Dickinson and Sive, 2006) the buccopharyngeal membrane is composed of two distinct layers at stage 37/38. The outer layer of cells appears continuous with the epidermis while the inner layer appears continuous with the epithelium lining the oral cavity (Fig. 1K–M). By stage 39, these two layers become integrated and fewer cells are apparent (Fig. 1N). However, in embryos treated with the JNK inhibitor, this reorganization of cells at stage 39 is not observed in 90% of the embryos examined (Fig. 1O, n=10, 2 experiments). When control embryos reached stage 40, the buccopharyngeal membrane has disappeared (Fig. 1P). However in 80% of the embryos with deficient JNK function, we observed abnormal arrangements of cells in the buccopharyngeal membrane (Fig. 1Q, n=10, 2 experiments). These results suggest that JNK is required for cellular reorganization during buccopharyngeal membrane perforation.
In addition to the orofacial problems, decreased JNK function caused numerous other defects in the whole embryo. Such defects included abnormal gut formation (as described in Dush and Nascone-Yoder, 2013), a curved body axis, a smaller head, failure of the tail fin to form, and skin blisters along the body (not shown).
2. Active JNK is present in the buccopharyngeal membrane, epidermis and in actively dividing cells throughout the embryo
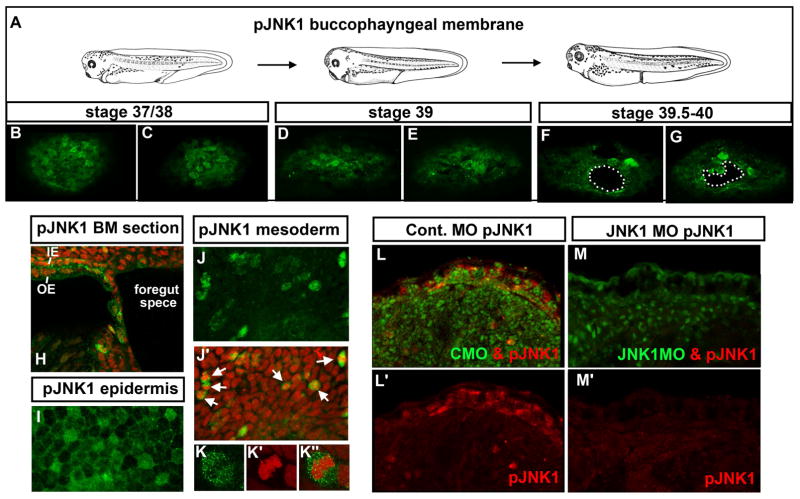
To better understand the role of JNK1 signaling during buccopharyngeal membrane perforation we next examined whether active JNK1 protein was present prior to and during this process (Fig. 1A). The activity of JNKs depend on its phosphorylation state and therefore we used a phospho-JNK (pJNK1) antibody as an indicator of where active JNK1 is localized. At stage 37/38 pJNK appeared enriched in 1–8 cells in the buccopharyngeal membrane (Fig. 2 B, C; two representative images from an n=15, 3 experiments). A few hours later at stage 39, 100% of the embryos examined had 2–10 cells with pJNK enrichment (Fig. 2 D, E; two representative images from n=15, 3 experiments). Finally, as the buccopharyngeal membrane was perforating we noted embryos had 4–10 cells with pJNK enrichment in the buccopharyngeal membrane (Fig. 2 F, G; two representative images from n=15, 3 experiments). In sagittal sections of the head we also observed pJNK enrichment in a subset of cells (Fig. 2 H). In all embryos examined there was no trend in the location of the cells with higher thresholds of pJNK. This is consistent with the variability we observed in the location and timing of the perforation.
Figure 2.
A–G) Frontal views of the buccopharyngeal membrane labeled with pJNK1 antibody (green) at three different stages representing prior to and during perforation. White dots outline “holes” or perforations. H) Sagittal section through the buccopharyngeal membrane and surrounding face showing pJNK (green) and counterstained with propidium iodide. pJNK can also be observed in the two layered epidermis, in the outer epidermis (OE) and inner epidermis (IE). I) Flat mount of the epidermis labeled with pJNK antibody (green). J) The mesoderm labeled with pJNK antibody (green). J′) Corresponding image to J where the pJNK is labeled green and nuclei/DNA labeled with propidium iodide (red). White arrows indicate examples of cells that appear to be undergoing mitosis. K) Magnified image of a mesodermal cell labeled with pJNK antibody (green) K′) corresponding cell from K labeled with propidium iodide to show DNA (red). K″) Cell corresponding to K showing a merge of pJNK (green) and DNA (red). L) Embryos labeled with pJNK antibody (red) and showing the fluorescein tagged control morpholino (green). L′) Same image as L showing only the pJNK labeling (red). M) Embryos labeled with pJNK antibody (red) and showing the fluorescein tagged JNK1 morpholino (green) M′) same image as H showing only the pJNK labeling (red). Abbreviations: pJNK1=phospho JNK1, st. =stage.
In our pJNK labeling experiments we also noticed the presence of this phospho-protein in several other locations in the embryos. Most conspicuously was the presence of pJNK in the epidermis in both superficial and inner ectodermal cells (Fig. 2 H, I). Throughout the embryo, pJNK also appeared in mitotic cells based on its colocalization with condensed chromatin (Fig. 2 J–K″). In double labeling experiments with tubulin we noted that many but not all epidermal multiciliated cells were enriched with pJNK and this protein was also present in many axons in the brain as has been reported ((Hutchins and Szaro, 2013) not shown). pJNK was also observed in the digestive tube consistent with its role in the morphogenesis of this tissue (Dush and Nascone-Yoder, 2013). The specific pJNK labeling pattern was absent in the epidermis of JNK morphants (Fig. 2 L–M′).
Our pJNK localization results suggest that JNK signals are present in the buccopharyngeal membrane prior to and during perforation. Further, JNK is also active in several other tissues in the embryo, particularly the epidermis and dividing cells.
3. Cell division defects are present in the whole embryo with reduced JNK signals, but this is not responsible for the buccopharyngeal membrane defects
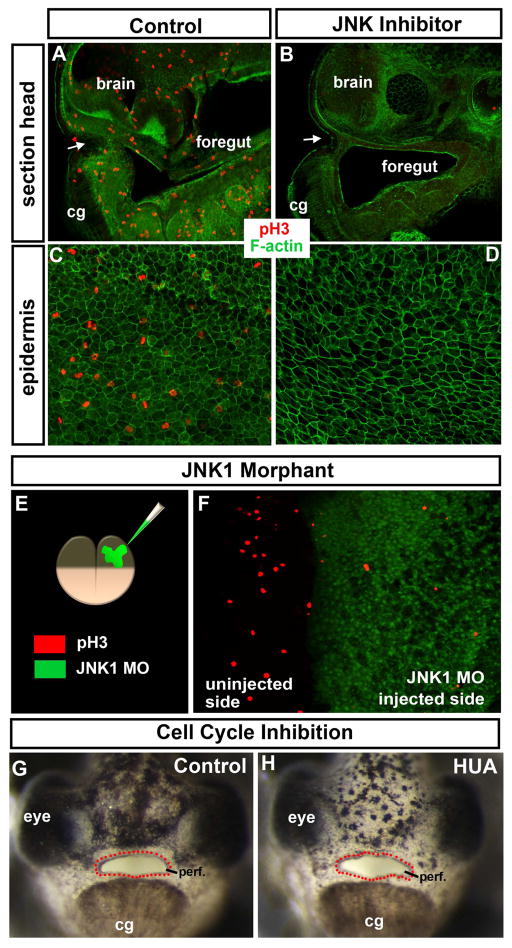
Since we observed active JNK was present in mitotic cells throughout the embryo we first wondered if JNK signals are necessary for cell division and second, whether defects in cell division were the cause of the embryonic mouth defects. To determine if cell division was affected we labeled JNK inhibitor treated and JNK1 morphants with a G2/M phase marker, phospho-histone H3 (ph3). Results indicated that ph3 positive cells were not observed in 100% of the JNK inhibitor treated embryos in the head and embryonic mouth region (Fig 3 A, B; n=30, 3 experiments) and the epidermis (Fig. 3 C, D; n=30, 3 experiments). In half JNK1 morphants, that is embryos injected with JNK1 MO into one cell at the two cell stage, we saw a notable reduction of ph3 positive cells in the morphant half compared to the control half (n=12, 2 experiments, Fig. 3 E,F).
Figure 3.
A–D) Shows phospho-histone H3 (ph3; red) marking mitotic cells and labeling of F-actin with phalloidin (green) is used as a counterstain. White arrows indicate the embryonic mouth. A–B) Sagittal section through the middle of the embryo at stage 37/38 after treatment with DMSO/control (A) or JNK inhibitor (B). C, D) Epidermis at stage 37/38 after treatment overnight with DMSO/control (C) or JNK inhibitor (D). E) Schematic showing the morpholinos injected into one cell at the two cell stage and the key where the morpholino can be seen in green and ph3 is labeled in red. F) Dorsal view of an embryo injected as shown in E. G, H) Frontal views of embryos treated with DMSO (control; G) or cell cycle inhibitors hydroxyurea and aphidicolin (HUA; H). Red dots outline the embryonic mouth. Abbreviations; cg = cement gland, MO = morpholino, ph3= phospho histone H3, perf. =perforated.
It is conceivable that the dramatic loss of mitotic cells in the embryo was causing the embryonic mouth defects. To test this we next inhibited cell cycle (independent of JNK) by blocking DNA replication and synthesis using a combination of aphidicolin and hydroxyurea (HUA) (Harris and Hartenstein, 1991). We wondered if HUA treatment from stage 37/38–40 would mimic the JNK inhibitor and result in a delay or failure of buccopharyngeal membrane perforation. Embryos were treated with 20mM hydroxyurea and 150 μm of aphidicolin (concentrations published in (Harris and Hartenstein, 1991)). Our results indicated that the buccopharyngeal membrane perforated normally in 100% of the HUA treated embryos (n=20, 2 experiments, Fig. 3G, H). These embryos did share some similar characteristics with the JNK inhibitor treated embryos such as cardiac edema, smaller eye and abnormal curvature of the body and blisters (not shown). Higher concentrations resulted in death in some embryos and therefore these were not analyzed. Consistent with the HUA treatments is that other mitotic inhibitors (Taxol and nocodazole) applied from stage 34–40 did not prevent the buccopharyngeal membrane from perforating even though there was dramatic effects on embryonic head development and shape of the mouth (not shown). Together, these results indicate that decreased JNK signaling results in major defects in cell division which could explain the smaller size and many other developmental defects in the embryos with decreased JNK. However, this loss of cell proliferation is not likely the cause of the failure in buccopharyngeal membrane perforation in JNK deficient embryos.
4. Decreased JNK signaling causes increased oxidative stress and apoptosis in the epidermis, but this is not the cause buccopharyngeal membrane defects
To help us determine what might be causing the defects in the buccopharyngeal membrane we next turned to a more unbiased approach where we used a microarray expression based analysis of genes misexpressed upon decreased JNK function. However, the buccopharyngeal membrane itself is very small with few cells and it is difficult to isolate. We postulated that since the buccopharyngeal membrane is an epidermal structure, a role for JNK in the buccopharyngeal membrane and surrounding epidermis may be similar. Thus, we broadened our analysis and examined changes in expression of genes in the epidermis after JNK inhibition hoping it would provide insight into the causes of buccopharyngeal membrane defects. To do this we treated embryos from stage 14–24 and then dissected the epidermis of embryos from which RNA was isolated (n=40, 2 experiments). Using Affymetrix gene chips and a bioinformatic analysis from 2 biological replicates we failed to identify genes significantly changed in the JNK inhibitor treated embryos. However, the statistical power may have been lost by the high variability between the two replicates. Regardless, we manually collated a list of genes that appeared both enriched and depleted by at least 2 fold consistently between the two experiments (a subset of these genes are shown in table 1). Several of these genes were validated by RT-PCR in a separate experiment (not shown, see asterisks in table 1). We then used DAVID online software to functionally categorize the genes. From this analysis we found approximately 48 genes which appeared enriched by 2 fold or more upon SP600125 treatment. Of these enriched genes the majority were associated with metabolic pathways and mitochondrial function. Few genes (13) appeared depleted by 2 fold or more, specifically in the epidermis of JNK inhibitor treated embryos. These depleted genes were categorized largely as stress response and nucleotide binding proteins. It is important to note that genes associated with epidermal specification such as (grainyhead-like and EGF genes) were not altered in the epidermis by decreased JNK function. Interestingly, the genes associated with mitochondrial function are often upregulated during cell stress and apoptosis. Since JNK signaling has an established role in these processes we decided to pursue them further.
Table 1.
Select genes enriched or depleted in the epidermis after treatment with the JNK inhibitor.
| # | AFFYID | NAME | ABBREV | UNIGENE | FOLD CHANGE |
|---|---|---|---|---|---|
| Genes enriched | |||||
| 1 | Xl2.18730.1.S1_at | phosphoenolpyruvate carboxykinase 1 | pck1* | Xl.18730 | 14.2 |
| 2 | Xl2.33779.1.A1_at | cytochrome P450 family members | cyp2j2 cyp1a1* cyp2d6 |
Xl.34192 Xl.1220 Xl.26217 |
9.8 |
| 3 | Xl2.5475.1.S1_s_at | glutathione S-transferase pi 1 | gstp1* | Xl.54920 | 4.8 |
| 4 | Xl2.29027.1.S1_a_at | death associated protein-like 1 | dapl1-a | dapl1-a | 2.9 |
| 5 | Xl2.892.1.S1_a_at | arginase 2 | arg2-a | Xl.892 | 2.9 |
| 6 | Xl2.47079.1.S1_at | glutamate-ammonia ligase (glutamine synthase) | glul | Xl.83244 | 2.8 |
| 9 | Xl2.28670.1.S1_at | cysteine dioxygenase type 1 | cdo1 | Xl.34729 | 2.6 |
| 10 | Xl2.11739.1.S1_at | solute carrier family 25 (mitochondrial carrier) | slc25a10 | Xl.11739 | 2.5 |
| 11 | Xl2.28471.1.S1_at | aldolase B, fructose-bisphosphate | aldob | Xl.28471 | 2.4 |
| 12 | Xl2.14135.1.S1_at | aldo-keto reductase family 1, member C2 | akr1c2-b | Xl.14135 | 2.3 |
| 13 | Xl2.12180.1.S1_at | HIG1 hypoxia inducible domain family member | higd1a-a | Xl.12180 | 2.3 |
| 14 | Xl2.1717.1.S1_at | pyruvate dehydrogenase kinase, isozyme 4 | pdk4 | Xl.1717 | 2.2 |
| 15 | Xl2.21131.1.S1_at | ethanolamine-phosphate phospho-lyase | etnppl | Xl.21131 | 2.2 |
| Genes Depleted | |||||
| 1 | Xl2.1055.1.S1_at | ubiquitin conjugating enzyme E2E 1 | ube2e1 | Xl.61475 | 0.20 |
| 2 | Xl2.12198.1.A1_at | small nuclear ribonucleoprotein 70kDa (U1) | snrnp70 | Xl.5366 | 0.42 |
| 3 | Xl2.4515.1.S2_at | cold inducible RNA binding protein | cirbp-a | Xl.4515 | 0.47 |
| 4 | Xl2.24325.1.S1_at | ankyrin repeat and SOCS box containing 12 | asb12.2 | Xl.24325 | 0.48 |
| 5 | Xl2.3664.1.S2_at | heterogeneous nuclear ribonucleoprotein A2/B1 | hnrnpa2b1 | Xl.3664 | 0.50 |
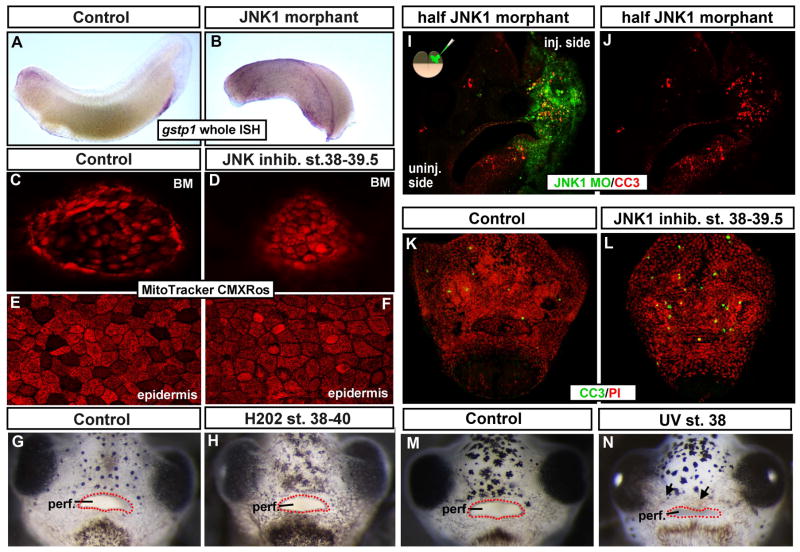
One caveat in our expression analysis is the possibility that our epidermal isolation procedure prior to the collection of RNA is the cause of cellular stress within the tissue. Therefore, we performed in situ hybridizations in the whole embryo for one of the “stress associated genes” isolated in the microarray analysis, gstp1. Results indicated that the expression of this gstp1 did indeed qualitatively increase in 100% of the embryos exposed to JNK inhibitor (n=10, 2 experiments, not shown) and JNK MO (n= 14, 2 experiments, Fig. A, B). Together, our expression analysis indicates an increase in cell stress in the epidermis that is independent of our experimental paradigm.
To further test whether cellular stress was indeed increased within the epidermis as well as the buccopharyngeal membrane of embryos with deficient JNK function we labeled live embryos with MitoTracker Red CMXRos. This reagent fluoresces upon changes in membrane potential of the mitochondria which occurs during cellular stress (Mugoni et al., 2014). Embryos were treated with 100 μm of JNK inhibitor (or DMSO alone) from stage 37/38–39.5 and then incubated in 0.1 μm of the MitoTracker Red CMXRos and imaged live. Results showed that in control embryos a non-homogenous pattern of fluorescent signal was observed in both the buccopharyngeal membrane and flank epidermis. Specifically, in control embryos there was a moderate level of fluorescence in many cells and an absence in other cells. However, in the JNK inhibitor treated embryos there was no longer an absence of signal in some cells and other cells appeared to have a higher intensity of fluorescence in both the buccopharyngeal membrane and flank epidermis (n=20, 2 experiments, Fig. 4C–F). This experiment corroborates our expression analysis and suggests a change in cellular stress in at least some of the epidermal cells along the body as well as the buccopharyngeal membrane when JNK signals are perturbed.
Figure 4.
A,B) Lateral view of representative controls (A) and JNK1 morphants (B) where gst1 mRNA (purple) was detected by in situ hybridization. C–F) Live Mitotracker CMXRos labeling (red) after embryos were treated with DMSO (control; C, E) or JNK inhibitor. C, D) Shows the buccopharyngeal membrane. E, F) Shows the epidermis. G, H) Frontal views of the face of representative embryos untreated (G) or treated with hydrogen peroxide (H202; H). Red dots outline the embryonic mouth. I, J) Transverse sections through the face of a representative embryo injected with JNK1 morpholino into one cell at the two cell stage. Tissue containing the fluorescein tagged JNK1 morpholino is green and cleaved caspase-3 labeling (apoptotic marker) is in red. K, L) Frontal views of whole representative faces from embryos treated with DMSO (K) or JNK inhibitor (L). Cleaved caspase-3 labeling is in green and propidium iodide is used a counterstain (red). M, N) Representative images of frontal views of faces of embryos exposed to UV. M) Control (not irradiated) and N) Irradiated at stage 37/38. Red dots outline the embryonic mouth. Abbreviations; perf. =perforated, MO= morpholino, BM = buccopharyngeal membrane, CC3=cleaved caspase 3, PI= propidium iodide.
While our results thus far point to an increase in cellular stress in the epidermis and buccopharyngeal membrane upon loss of JNK, we wanted to next test whether this was the cause of the embryonic mouth defects. We hypothesized that if the increase in cellular stress caused the defect in buccopharyngeal membrane perforation then increasing cellular stress independently should mimic the effect of inhibiting JNK. First, we treated embryos with hydrogen peroxide, since it is a commonly used inducer of oxidative stress in many systems (for example see (Mugoni et al., 2014)). Our results showed that treatment of embryos with a 0.0015% H202 from stage 37/38–40 resulted in smaller and sick appearing embryos consistent with previous reports (Vismara et al., 2006). That is the heart rate seemed slower and the embryos were less responsive to touch. However this treatment did not affect buccopharyngeal membrane perforation (n=40, 4 experiments, Fig. 4 G, H). Higher concentrations of H202 often caused death of the embryo (not shown) and therefore was not assessed. We also treated embryos with xenobiotic compounds shown to cause oxidative stress in frog and fish larvae. Aroclor 1254, a mixture of polychlorinated biphenyl alcohols, that has been shown to specifically increase Gstp1 protein levels in Xenopus (Gillardin et al., 2009) and Bisphenol A, a specific endocrine disruptor (Xu et al., 2013). Similar to the H202 treatments, the embryos treated with either compound appeared sick but the embryonic mouth formed normally (not shown). Therefore, while increasing cellular stress alone could cause a generally “sick” embryo, it did not cause major orofacial defects over stage 37/38–40.
From the results presented here we concluded that cellular stress is not causing the defect in buccopharyngeal membrane perforation seen in JNK inhibitor treated embryos and thus may be a consequence of other problems. One such problem could be an increase in apoptosis. Certainly JNK has been shown to regulate apoptosis in many tissues and contexts (reviewed in (Davis, 2000)). Further we identified a death associated gene upregulated in our microarray screen upon JNK inhibition (see table 1). To further test whether decreased JNK causes an increase in cell death we examined an established marker of apoptosis, cleaved caspase 3 (CC3) by immunohistochemistry. In JNK morphants that were injected in one cell at the two cell stage, there was a considerable increase in CC3 positive cells in the half of the face where JNK MO was present (Fig. 4 I, J, n=15, 3 experiments). Embryos treated with the JNK inhibitor from stage 37/38–40 also had a qualitatively obvious increase in CC3 positive cells in the face. The less dramatic increase (compared to the JNK morphants) may be due to a shorter period of JNK deficiency (Fig. 4 K, L, n=30, 3 experiments). These results indicate that indeed decreased JNK results in an increase in apoptosis in the face.
Our next goal was to determine if the increase in cell death in the face, as a result of decreased JNK, causes the defect in buccopharyngeal membrane perforation. Here again our rationale is that if increased cell death is the cause of the embryonic mouth defects then inducing cell death independently should mimic the inhibition of JNK with respect to buccopharyngeal membrane perforation. Therefore, we induced cell death by exposing embryos with UV or chemical agents known to increase apoptosis and necrosis in Xenopus. First, the faces of embryos were irradiated for 10 minutes under a short wavelength UV transilluminator. Controls were treated in the same way with the transilluminator turned off. Results showed that the UV exposed embryos developed accumulations of necrotic cells in the orofacial region (Fig. M,N arrows), and this treatment significantly increased the number of CC3 positive cells (not shown). Regardless of such severe deformations in the face, the buccopharyngeal membrane was observed to perforate in 100% of the embryos similar to the controls (Fig. 4 M, N, n=30, 3 experiments). Longer exposure times often lead to severe effects where tissues separated from the face (n=10, 1 experiment, not shown) and therefore were not assessed. We observed similar results when embryos were treated with chemicals shown to induce cell death in Xenopus (2% ethanol (Kot-Leibovich and Fainsod, 2009) and methotrexate; (Wahl et al., 2015); not shown). These results suggest that increased apoptosis is not the reason the buccopharyngeal membrane is failing to perforate in embryos deficient in JNK signals.
Together, the results in this section indicate that decreasing JNK function results in increased cellular stress and apoptosis throughout the embryo. Increasing these processes may be the cause of some defects in the embryo that we see with JNK inhibition but are not the likely cause of the failure of the buccopharyngeal membrane to perforate.
5. Decreased JNK function results in maintained adhesion between cells and this could cause a persistent buccopharyngeal membrane
In addition to its role in transcriptional regulation, JNK is also widely known to phosphorylate cytoskeletal and junctional proteins to facilitate cell movement and adhesion (reviewed in (You et al., 2013)). Therefore, we next wondered whether JNK was acting in the epidermis and the buccopharyngeal membrane to modulate cellular dynamics that may be necessary for the perforation of the structure. Again we turned our analysis to the epidermis postulating that a better understanding of the role of JNK generally in this tissue could lead us to understanding how this signal might be required in the embryonic mouth.
In our work with embryos with deficient JNK we noted that the epidermis did not break apart during microdissection. For this reason the epidermis could be peeled from the underlying tissues easily (not shown). On the other hand the epidermis of control embryos broke apart more easily and thus peeled away in smaller fragments (not shown). We asked whether this effect was due to a problem with the attachment of the cells to the extracellular matrix. Therefore, we examined laminin and integrin after embryos were treated with the JNK inhibitor and these two proteins were indeed present underlying the epidermis (not shown). Therefore, we decided then to more thoroughly test whether decreased JNK function alters intercellular adhesion within the epidermis.
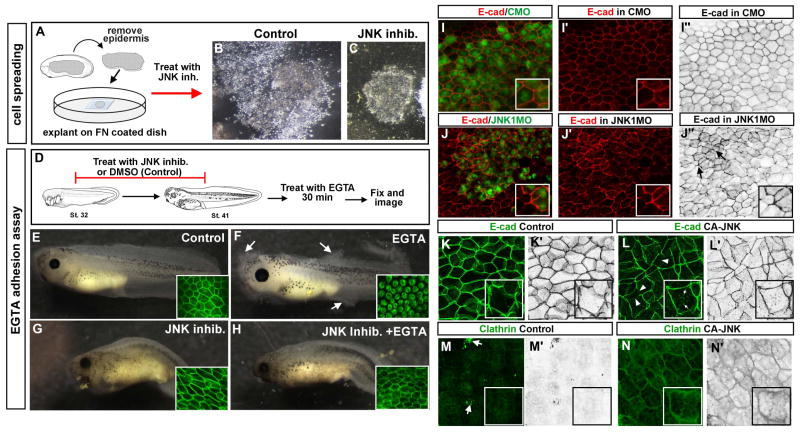
To test whether JNK inhibition results in maintained adhesion we used an explant spreading assay whereby epidermis is explanted onto a fibronectin coated coverslip so that we could observe the behavior of the epidermal cells (Fig. 5A). In 100% of the untreated control epidermal explants the cells were observed to move away from the explant and spread out (n=10, 2 experiments; Fig. 5 B). Conversely, 100% of the explants treated with the JNK inhibitor did not perform this behavior and rather remained tightly grouped together (n=10, 2 experiments; Fig. 5 C). While these results corroborate the idea that inhibition of JNK results in maintained adhesion in the epidermis, one caveat in this cell spreading assay is that JNK signals are also known to be necessary for cells to migrate. Thus, the failed spreading of the epidermis may in fact be an inability of the cells to move rather than maintained cell adhesion. Therefore, we performed an EGTA mediated loss of adhesion assay that more specifically tests intercellular adhesion. Embryos were treated with the JNK inhibitor or DMSO and followed by a brief EGTA treatment (Fig. 5 D–H). The EGTA chelates calcium and reduces cell adhesion. Results revealed that the epidermis of control embryos (treated with EGTA) began to dissociate from the rest of the body after an average of 27 minutes (Fig. 5 F). On the other hand, embryos exposed to the JNK inhibitor and EGTA took on average 63 minutes longer to begin to dissociate (n=16, 2 experiments, Fig. 5 H; average time to begin dissociation = 90min.). To determine whether such dissociation of the cells correlated with changes in adherens junctions we next examined E-cadherin in these EGTA adhesion assay experiments. Results revealed that in embryos treated with the JNK inhibitor and EGTA, the E-cadherin was maintained at the membrane similar to controls that were untreated or treated with SP600125 alone (n=16, 2 experiments, Fig. 5 E–H see insets). However, in the embryos that only received EGTA, the E-cadherin appeared to be internalized (n=16, 2 experiments, Fig. 5F see inset). These results indicate that JNK inhibition can protect the cells from the anti-adhesive effects of EGTA.
Figure 5.
A) Schematic showing the steps in the cell spreading assay. B) 24 hours after explanting epidermis and treating it with DMSO (control). C) 24 hours after explanting epidermis and treating it with the JNK inhibitor. D) Schematic showing the treatment paradigm for the EGTA adhesion assay. E) Control embryo treated with DMSO only. Inset shows E-cadherin labeling confined to edges of cells. F) Representative embryo treated with DMSO followed by EGTA. White arrows point toward regions where the tissue is dissociating. Inset shows internalized E-cadherin. G) Embryo treated with JNK inhibitor alone. Inset shows E-cadherin labeling confined to cell boundaries. H) Embryo treated with JNK inhibitor followed by EGTA. Inset shows E-cadherin largely confined to the cell boundaries. I) The epidermis of a representative embryo showing the cells containing fluorescein labeled Control MO (green) and E-cadherin labeled in red. I′) Corresponding image from I showing only the E-cadherin labeling in red. I″) Corresponding image from I showing only the E-cadherin labeling in black and white. Insets show representative 1–2 cells at 2X magnification. J) The epidermis of a representative embryo showing the cells containing fluorescein labeled JNK1 MO (green) and E-cadherin labeled in red. J′) Corresponding image from J showing only the E-cadherin labeling in red. J″) Corresponding image from J showing only the E-cadherin labeling in black and white. Insets show representative 1–2 cells at 2X magnification. Black arrows indicate groups of cells with higher levels of E-cadherin at the membrane. K–L′) E-cadherin labeling of epidermis from representative embryos (stage 24–26) that were uninjected (K, K′) or injected with CA-JNK (L, L′). Prime letters are the identical pictures in black and white. Insets show a 2 fold magnified image of a cell from the images. White arrows show what appears as puncta of E-cadherin positive bodies within the cytoplasm and arrowheads point to membranes with reduced E-cadherin. Insets show representative 1–2 cells at 2X magnification. M–N′) Clathrin labeling of epidermis from representative embryos (stage 24–26) that were uninjected (M, M′) or injected with CA-JNK (N, N′). Prime letters are the identical pictures in black and white. Insets show representative 1–2 cells at 2X magnification. White arrows indicate non-specific staining where fluorescence is observed on top of the cell.
Our experiments thus far indicate that decreased JNK could enhance intercellular adhesion. One mechanism by which this could be happening is that JNK is required for junctional remodeling. To test whether decreased JNK did indeed affect such remodeling we examined E-cadherin. The FITC tagged morpholinos were injected into one cell at the two cell stage to allow comparison within a single embryo. In this way the uninjected cells from one half of the embryo serve as an internal control. Results revealed that in 80% of embryos examined, we noted abnormal patches of cells that had an increase in E-cadherin at the membrane (n=15, 3 experiments) that were never observed in the uninjected side of the morphant embryos or in cells containing control morpholino (Fig. 5 I–J″). Thus, these results reveal that JNK deficiency results in abnormal accumulations of E-cadherin in the epidermis.
We next asked whether increasing active JNK would have the converse effect and result in a decrease in cell adhesion and E-cadherin. To test this, we expressed a constitutively active JNK (CA-JNK) in embryos. This construct was previously shown to increase active JNK specifically in Xenopus (Yamanaka et al., 2002; Liao et al., 2006). We injected CA-JNK mRNA in one cell at either the one cell stage or 8 cell stage. Embryos expressing the CA-JNK in the head region often developed smaller heads and missing cement glands typical of a ventralized embryo. This is consistent with previously reported effects of this CA-JNK construct on Xenopus development (Liao et al., 2006). In the epidermis, the CA-JNK expression resulted in fragility and pigment abnormalities (not shown). Further, in this tissue E-cadherin localization was abnormal in at least some patches of cells in 100% of the embryos expressing the CA-JNK compared to controls (n=12, 2 experiments). Specifically, in control embryos, the E-cadherin was localized in a smooth line along the membrane (Fig. 5 K, K′ see inset). However, in cells expressing the CA-JNK, the E-cadherin appeared rough and less tight against the membrane but also small puncta were observed in the cytoplasm (Fig. 5 L, L′ see inset and arrows). This change in localization is consistent with a possible increase in the internalization of the E-cadherin via endocytosis. We therefore, next examined clathrin, a protein necessary for cadherin mediated endocytosis (Royle, 2006). In the control epidermis, clathrin was observed faintly at the membrane and within the cytoplasm (Fig. 5M,M′). In embryos expressing CA-JNK in the epidermis there was abundant clathrin at the membranes that seemed to partly surround large vesicles (Fig. 5 N,N′). These results suggest that increased JNK results in an increase in clathrin recruitment at the membrane of epidermal cells.
The results in this section suggest the possibility that JNK activity is necessary for the endocytosis of proteins associated with the adherens junction and this could in part explain the changes in intercellular adhesion in the epidermis.
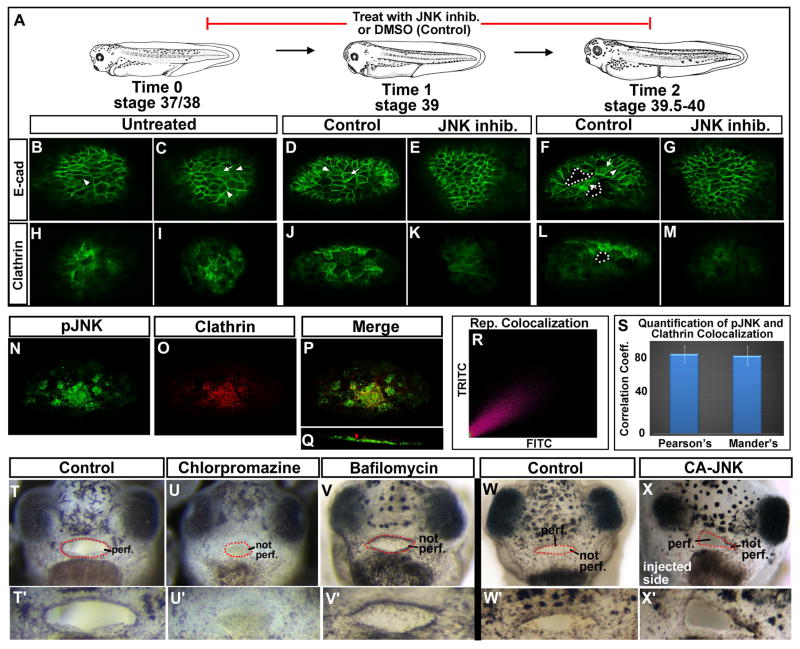
Next we investigated whether decreased JNK could also affect cell junctions in the buccopharyngeal membrane as it does in the epidermis. Based on our observations in the epidermis, we predicted that inhibition of JNK would lead to maintained adhesion in the buccopharyngeal membrane and prevent or delay its perforation. To extend this we postulated that the buccopharyngeal membrane cells need to disassemble their adhesions in order to reorganize and break apart from each other during perforation. To test these predictions we first examined changes in the localization of E-cadherin during buccopharyngeal membrane perforation. We observed that just before and during perforation, E-cadherin appeared to be unequally distributed in cells. Specifically, in some cells it was enriched and conversely in others it was depleted at the membrane. Further, we noted that in some cells, E-cadherin seemed enriched within the cytoplasm during perforation. Such a dramatic dichotomy in localization was not as prevalent at any point in the buccopharyngeal membrane when embryos were treated with the JNK inhibitor (n=30, 4 experiments, Fig. 6 A–G). Importantly, we did not observe high levels of E-cadherin within the cytoplasm in these JNK deficient embryos. To further test our prediction that JNK is required for endocytosis of E-cadherin during buccopharyngeal membrane perforation we examined the endocytosis regulator, clathrin. Results indicated that clathrin was enriched in 4–10 cells of the buccopharyngeal membrane at stage 37/38 and 39 of normal embryos. Such an enrichment was not observed in embryos treated with the JNK inhibitor prior to and during perforation. In fact 80% of the embryos examined had three or less cells where clathrin could be observed and these fewer cells were always less intensely labeled than cells in the controls (n=10, 2 experiments, Fig. 6 H–M). Together, this data supports the idea that endocytosis of adherens junction proteins could be one mechanism by which the buccopharyngeal membrane cells decrease intercellular adhesion during perforation.
Figure 6.
A) Schematic of the stages examined during buccopharyngeal perforation. B–G) Frontal views of the buccopharyngeal membrane labeled with E-cadherin in control and JNK inhibitor treated embryos. White arrows point to examples of cells with an increase in cytoplasmic labeling and white arrow heads indicate lower E-cadherin at the membrane. White dots outline the holes or perforation. H–M) Frontal views of the buccopharyngeal membrane labeled with clathrin in control and JNK inhibitor treated embryos. White dots outline the holes or perforation. N–S) Colocalization experiment with phospho-JNK (green) and clathrin (red). N) pJNK1 in green. O) Clathrin in red. P) Merge of pJNK1 and clathrin. Q) Volume rendered image of pJNK and clathrin co-labeling rotated 45 degrees. R) Scatterplot of pixels labeled in green vs red in the image shown in P. S) Bar graphs of the average correlation coefficients for Pearson’s and Mander’s colocalization tests. T–X′) Frontal views of the entire faces (regular letters) or only the buccopharyngeal membrane at 2 fold magnification (prime letters) from representative embryos at stage 40–41. T,T′) Representative control embryo treated with DMSO. U, U′) Representative embryo treated with chlorpromazine. V, V′). Representative embryo treated with Bafilomycin. W, W′). Representative uninjected embryo. X, X′) Embryo injected with CA-JNK. Abbreviations: perf. =perforated; cg=cement gland, CA-JNK= constitutively active JNK. Red dots outline the embryonic mouth.
To determine if JNK could be necessary for changes for endocytosis during buccopharyngeal membrane perforation we next examined whether active JNK (pJNK) localization correlated with clathrin protein of normal embryos. Results revealed that in all embryos at stage 39–40 both higher levels of pJNK and clathrin appeared to overlap in at least a subset of cells (n=10, 2 experiments, Fig. 6 N–P). Colocalization analysis of pJNK and clathrin labeling showed a positive correlation (Fig. 6R) and that on average there was a 0.82 Pearson’s and a 0.80 Mander’s correlation coefficient (n=10, 2 experiments, Fig. 6S). In embryos that showed a lower correlation we noted non-specific labeling of clathrin outside the cells in the volume rendered images (Fig. 6Q). Taken together, these results support the possibility that JNK signals may be required for endocytosis of E-cadherin during buccopharyngeal membrane perforation.
Our data so far points toward a hypothesis whereby inhibition of JNK increases cell adhesion in the buccopharyngeal membrane by preventing E-cadherin internalization via endocytosis. Therefore, our next goal was to determine if preventing endocytosis of E-cadherin can cause the same persistent buccopharyngeal membrane. Our rationale again was if changes in E-cadherin disassembly are the cause of the embryonic mouth defects when JNK is deficient, then inhibiting endocytosis independently would mimic the inhibition of JNK, with respect to buccopharyngeal membrane perforation. To test this we treated embryos from stages 37/38–40 with two inhibitors of this process, chlorpromazine and bafilomycin (Harada et al., 1997; Feng et al., 2002; Rejman et al., 2005). Treatment with 25uM chlorpromazine, which can inhibit clathrin mediated endocytosis, results in failure of buccopharyngeal perforation (Fig. 6 T–U′, 100%, n=30, 3 experiments). Bafilomycin, is a specific inhibitor of endocytic acidification, which is necessary for endocytic processing also caused defects in buccopharyngeal membrane perforation. 90% of the embryos treated with 200 nm of this inhibitor had an intact buccopharyngeal membrane (Fig. 6 V,V′, n=30, 3 experiments). Together, these experiments indicate that endocytosis is indeed required for buccopharyngeal membrane perforation. Thus, our results support the idea that junctional remodeling via endocytosis is required for buccopharyngeal membrane perforation and this remodeling may be mediated by JNK signals.
Finally, we asked whether expressing CA-JNK in the buccopharyngeal membrane would alter its development. When CA-JNK was injected into one dorsally fated blastomere at the 8 cell stage the active protein was expressed in one half of the face. In 100% of these embryos we noted severe malformations on one side of the embryonic head, including a smaller eye. Further, on the malformed side, of a subset of these embryos (33.3%), we also observed that the embryonic mouth perforated before the uninjected side of the face (Fig. 6 W–X′, n=12, 2 experiments). These results further support the hypothesis that JNK signals regulate adhesion during buccopharyngeal membrane perforation.
In summary, in this section we have shown a role for JNK signals in mediating cell adhesion during epidermal development and that this role is also required for buccopharyngeal membrane rupture.
DISCUSSION
In the present study we have added to the vast list of developmental processes regulated by c-Jun N-terminal Kinase (JNK) signals, that is buccopharyngeal membrane perforation. However, when we began this work we did not know which cellular process, (homeostasis, cell death, cell division, migration or intercellular adhesion), this signal might regulate during this last step in embryonic mouth formation. Therefore, we took an elimination approach where our rationale was that if JNK’s role in a process is important for buccopharyngeal membrane perforation then eliminating that process independently would result in the same defect in the embryonic mouth. We systematically examined JNK’s involvement in proliferation, apoptosis, cell stress and cell adhesion to finally uncover that the latter, cell-cell adhesion was most important for buccopharyngeal membrane perforation. Our goal was to narrow the possible list of roles for JNK and provide us with a foundation on which to further study the role of JNK signals in embryonic mouth development.
Deficient JNK results in changes in cell proliferation, death and cellular stress that don’t affect buccopharyngeal membrane perforation
In our experiments, we determined that deficient JNK results in a multitude of effects in basic processes necessary for tissue growth and homeostasis. First, we observed an increase in apoptosis throughout the face in the JNK morphants and JNK inhibitor treated embryos. Indeed, JNK is well known for its context dependent role in promoting or inhibiting cell death (reviewed in in (Davis, 2000)). Further, JNK signals are often thought to be necessary in maintaining homeostasis (Karpac and Jasper, 2009) and therefore it is certainly plausible that without JNK the cell has difficulty surviving. We also found that active JNK is enriched in cells that appear to be in the midst of mitosis. Moreover, JNK deficiency results in a significant loss of mitotically positive cells. In mammalian cells, JNK has been shown important in the assembly and recruitment of spindle pole proteins (Lim et al., 2015) and to directly phosphorylate cell cycle regulators (Leppa and Bohmann, 1999; Gutierrez et al., 2010a; Gutierrez et al., 2010b). Consistent with these findings is that Weston et. al (Weston et al., 2004) showed that cell cycle markers were decreased in the epidermis of JNK deficient mice. Both a decrease in cell proliferation and especially an increase in cell death would be expected to result in significant stress to the growing embryo. Certainly, we found that several genes associated with cellular stress were up-regulated in the epidermis of JNK inhibitor treated embryos. JNK is well known as a stress activated protein kinase, and upon ROS accumulation it is normally activated under many contexts (Shen and Liu, 2006; Son et al., 2013). Such activation has been shown important for regulating processes that protect the cell or organism (Goberdhan and Wilson, 1998; Wang et al., 2003). It is therefore possible that without JNK activity the cell is not able to modulate oxidative stress responses which could lead to developmental defects.
The increase in cell stress and cell death as well as the reduction in cell proliferation in embryos with deficient JNK would conceivably have dramatic consequences on the developing embryo. Certainly we found that JNK morphants or embryos treated with a JNK inhibitor shared several defects with embryos where cell death was induced or cell proliferation was inhibited independently. For example, embryos treated with the JNK inhibitor or cell cycle inhibitors and cell death inducers were similarly smaller with a curved axis and had heart edema and skin blisters. However, we also found that even in embryos close to death from these treatments still formed a perforated buccopharyngeal membrane. Thus, taken together our results suggest that while an increase in apoptosis and cellular stress as well as decreased cell division has profound effects on various other aspects of development these processes do not affect buccopharyngeal membrane perforation.
JNK modulates intercellular adhesion in the epidermis
We uncovered a role for JNK in the regulation of intercellular adhesion in the embryonic mouth by first investigating its role in the epidermis. Specifically, through a series of experiments we noted that decreased JNK resulted in an increase in intercellular adhesion. For example, inhibition of JNK restricted epidermal cell spreading in vitro and also prevented epidermal cells from dissociating in the presence of a calcium chelator. To corroborate these observations, we also observed an accumulation of E-cadherin at the membrane in epidermal cells of JNK morphants. Further, constitutively active JNK appeared to increase internalized E-cadherin and recruit the endocytosis modulator, clathrin to the membrane. A role for JNK in mediating cell adhesion is not new and has been shown in cultured mammalian keratinocytes, intestinal epithelia and Drosophila (reviewed in in (You et al., 2013)). For example, Lee and colleagues (Lee et al., 2009) showed that JNK modulates cell-cell adhesion by actively phosphorylating both beta-catenin and alpha-catenin in keratinocytes. They also found that JNK inhibition results in the translocation of E-cadherin and beta-catenin to the adherens junction and this correlates with the formation of compact more adherent colonies in culture (Lee et al., 2009). Naydenov and colleagues (Naydenov et al., 2009), also showed that decreased JNK could prevent low calcium induced internalization of E-cadherin remarkably similar to our observations in the frog epidermis. In Drosophila, decreased JNK signaling results in dorsal closure defects and genes encoding proteins involved in cell adhesion are misexpressed (Ip and Davis, 1998; Jasper et al., 2001). Interestingly, JNK has also been shown to be required for endocytosis. For example, elevated JNK activation is associated with increased endosomal proteins such as Rab11 in the immune system (Lizundia et al., 2007) and the JNK scaffold protein JIP can associate with endosomal tubules (Marchesin et al., 2015). Thus, our work and others supports a general role for JNK in modulating cell-cell adhesion via endocytosis which would be essential for many developmental and cellular processes such as cell migration, intercalation and cell sorting.
JNK signals are necessary for modulating intercellular adhesion during buccopharyngeal membrane perforation
Our results provide the first evidence that junctional remodeling is regulated, at least in part, by JNK signals during buccopharyngeal membrane perforation. Specifically, enhanced JNK activity results in decreased adhesion and the buccopharyngeal membrane perforates earlier while deficient function of JNK enhances adhesion and a persistent buccopharyngeal membrane results. Our evidence supports the idea that like in the epidermis, JNK is necessary for decreasing E-cadherin at the membrane via endocytosis. Certainly, the endocytic modulator, clathrin, is colocalized with pJNK in the buccopharyngeal membrane of embryos and was decreased in embryos with deficient JNK function. Further, we found that blocking endocytosis results in a persistent buccopharyngeal membrane. The precise mechanism by which JNK regulates changes in adhesion during buccopharyngeal membrane perforation is still unknown from our work. However, we could speculate that, based on other studies in epidermal cells, JNK phosphorylates alpha and or beta-catenin (You et al., 2013) which leads to adherens junction disassembly and the recruitment of endocytic proteins.
The insight gained from our findings presented here could be helpful in understanding how persistent buccopharyngeal membrane occurs in humans. Failure of the buccopharyngeal membrane to rupture can result in the blockage of the embryonic airway that in turn can lead to oral atresias and synechiaes that prevent proper fluid and air flow in the fetus and newborn (Gartlan et al., 1993; Kwong, 2015). It would be interesting to test whether the tissues associated with such oral blockages have dysfunctional JNK signals and increased adhesion proteins. Certainly, atresias associated with the bile duct, ovary and esophagus are abnormally enriched in adhesion proteins when compared to their surroundings (Makrigiannakis et al., 2000; Sasaki et al., 2001a; Sasaki et al., 2001b; Tugay et al., 2003). These results point to the possibility that defects in cell-cell adhesion could be a common cause of birth defects associated with the blockages of tubes during development.
What is upstream of JNK in buccopharyngeal membrane perforation?
JNK is a member of the MAPK family and is a widely utilized effector protein for numerous signals in the cell (Davis, 2000; Dong et al., 2001). For example, JNK signals are activated by cytokines, growth factors, G protein coupled receptors and non-canonical Wnts. In particular, specific Wnts can activate JNK during planar cell polarity signaling (PCP) which drives a variety processes such as collective cell migrations, epithelial sheet movements and wound healing (Yamanaka et al., 2002; Lee et al., 2012; Geetha-Loganathan et al., 2014). PCP signals are also integrated with adherens junction remodeling to allow for such coordinated cell movements (Ravni et al., 2009; Singh and Mlodzik, 2012; Nagaoka et al., 2014). Thus, it is possible that JNK’s role in modulating adhesion in the buccopharyngeal membrane is also integrated into PCP signaling. Certainly, Wnt/PCP regulates cellular reorganizations during oral cavity formation (Jacox et al., 2016). In addition to more classical activation by non-canonical Wnts and other signals, JNK can also be activated by biomechanical forces. For example, shear stress induces both stem cell migration and endothelial cell alignment via JNK signals (Mengistu et al., 2011; Yuan et al., 2012). Pereira and colleagues showed elegantly using a FRET sensor that JNK is activated upon mechanical stretch in drosophila (Pereira et al., 2011). Interestingly our lab has preliminary evidence that biomechanical forces have a role in buccopharyngeal membrane rupture. We hope that future studies will lead to a more complete view of the regulation of JNK signaling during this understudied process.
Conclusion and Model
The present study has lead us to hypothesize that JNK is necessary generally for clathrin mediated endocytosis of E-cadherin. JNK’s role is most likely an indirect one, via phosphorylation of alpha and/or beta catenin and/or recruiting clathrin to the membrane. Internalization of E-cadherin would then facilitate the remodeling of the adherens junctions necessary for cellular rearrangements. In the epidermis, such remodeling might be necessary during various events in epidermal morphogenesis that shape the embryo. In the buccopharyngeal membrane, such remodeling of the adherens junctions has a unique outcome; it allows for the rearrangement of cells necessary for perforation of the structure. This is possible because the buccopharyngeal membrane is a structure that is without mesoderm or neural crest derived cells and it is backed by the foregut space. Therefore, when cellular movements and rearrangements occur, in concert with other processes such as apoptosis, an opening to the foregut space can be achieved. We believe that this is not a patterned process since the perforation begins in slightly different locations in different embryos and there is no clear pattern of pJNK and clathrin labeling prior to and during perforation. However future work is necessary to confirm this hypothesis. In the absence of JNK function, the cell adhesions are not remodeled, thus the necessary cellular rearrangements cannot occur and a persistent buccopharyngeal membrane results.
In conclusion the present study provides the first glimpse into the molecular regulation of buccopharyngeal membrane rupture. We hope that this work will lead to a better understanding as to why defects in this structure could occur in humans.
Experimental Procedures
Embryos and Chemical Treatments
Xenopus laevis embryos were obtained and cultured using standard methods (Sive et al., 2000)(Sive et al., 2000) and this is approved by an IACUC protocol to A. Dickinson. Embryos were staged according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1967). Embryos were treated with various chemicals including SP600125 (Sigma, S5567; 100mM in DMSO), hydrogen peroxide (3%, from local pharmacy), ethanol (2%), Aroclor 1254 (48586, Supelco), combined Aphidicolin (A0781, Sigma, 100mM DMSO) and Hydroxyurea (H8627, Sigma, 10mM DMSO), Bafilomycin A1 (B1793, Sigma, 100uM in DMSO) and chlorpromazine (C8138, Sigma, 100mM in DMSO). Inhibitor treatments were set up as described (Kennedy and Dickinson, 2014) where 6–10 embryos were placed in 12 well culture dishes in 2 ml of frog embryo media (0.1X Modified Barth’s Saline (MBS)). Stock solutions were added directly to each well, gently swirled and then the dish was placed in the dark until the appropriate stage for further analysis. SP600125 treatments were refreshed (since we noted significant precipitation) after 5–6 hours. The faces of embryos were imaged by removing the body below the heart and placing embryos into a custom made hole in clay as described (Kennedy and Dickinson, 2014).
JNK Morpholinos and Constitutively Active JNK (CA-JNK)
Antisense JNK1 morpholino (TGCTGTCACGCTTGCTTCGGCTCAT, (Liao et al., 2006)) and photo-JNK1 morpholino (AGCCGAAGCPAGCGTGACAG) were purchased from Gene Tools. A standard control morpholino provided by Gene Tools was used as a control. Microinjections were carried out using an Eppendorf microinjector and a Zeiss stereoscope. The plasmid containing the MKK7-JNK1A1 (pcDNA3 flagMKK7B2Jnk1a1) fusion protein (constitutively active JNK (CA-JNK)) was obtained from Addgene (ID: 19726) (Lei et al., 2002) and cloned into the pCS2+ expression vector. mRNA was created using the SP6 mMessage machine kit.
UV exposure
Embryos at stage 37/38–39 were placed face up in deep custom sized holes created in a clay lined petri dish surrounded with cooled 0.1X MBS and 0.1% tricaine. The tops of petri dishes were removed and a fan was used to create increased airflow aimed to reduce localized heating. To induce cell death a short wavelength (312nm) transilluminator (TC-312E Spectroline Select series UV transilluminator (Spectronics Corp)) was placed 15 inches above the petri dish.
Immunochemistry, phalloidin and mitotracker
Embryos were fixed in 4% paraformaldehyde (PFA) and then labeled whole or after vibratome sectioning. To perform vibratome sectioning, fixed embryos were embedded in 5% low-melt agarose and sectioned using a 5000 Series Vibratome at 100–200μm. Antibodies utilized included: anti-phospho JNK-1 (Thr183/Tyr185,Thr221, Tyr223) antibody (07175, Millipore 1:200), cleaved caspase 3 (Cell Signaling Technology, 9661, 1:1000), anti-phospho-Histone H3 (Ser10) (Millipore, 06–570, 1:1000), Clathrin (Abcam, ab2731, 1:200) and E-cadherin, (DSHB, 5D3, 1:50). Appropriate secondary antibody (goat-anti-rabbit Alexa or goat-anti-mouse Alexa Fluors 488 or 568 (Invitrogen, 1:500). F-actin was labeled with Phalloidin (R415 or A12379, Life Technologies, 1:100). In some cases propidium iodide (1:1000 for 1 hour at room temperature) was used as a counterstain as Mitotracker Red CMXRos (ThermoFisher, M7512) was used in live embryos. Briefly, embryos were incubated in at 0.05 μm Mitotracker for 1 hour and then washed 3X10min in 0.1X MBS. The faces of embryos were imaged by removing the body below the heart and placing embryos into a custom made hole in 1% agarose that filled a well in a thick glass slide. All fluorescent images were captured on a Nikon C1 or C2 confocal and processed using Nikon Elements software and Photoshop in the VCU Biology Microscopy facility. All images were adjusted for brightness and color balance in the same way to every pixel in the image. Controls and experimental pictures were also always adjusted in the same way and represent exactly what we could see during imaging. Colocalization analysis was performed in Nikon Elements software where pJNK and clathrin was labeled. Pearson’s and Mander’s colocalization coefficients were determined for each image in the z-stack and then averaged.
Microarray Expression
Embryos were treated with SP600125 from stage 14 to 24 (this time point was used because the epidermis is more easily dissected at this stage). The epidermis was removed from the whole embryos using two glass needles created from pulled out glass capillary tubes. RNA was isolated from pooled epidermal dissections from approximately 20 embryos for each treatment and control using Trizol extraction and lithium chloride precipitation as described (Dickinson and Sive, 2009). Microarray analysis was performed by the genome core at University of Virginia using the Xenopus laevis Genome 2.0 Array GeneChip (# 901214, Affymetrix). All microarray data preprocessing and analysis was done using R. Affy CEL files were imported using the Affy package. Expression intensities were summarized, normalized, and transformed using Robust Multiarray Average algorithm (Irizarry et al., 2003). Probe sets were annotated using the GEO query package, and for each gene a linear model was fit with empirical-Bayes moderated standard errors using the limma package in R. The files generated by this analysis have been uploaded to GEO (GSE83672).
In situ hybridization
In situ hybridizations were performed as described (Sive et al., 2000), omitting the proteinase K treatment. The plasmid used to generate the gstp1 probe was purchased from GE Healthcare Dharmacon (clone: ID 3399306, XB-GENE-5846294).
Histology
Histology was performed as described (Dickinson and Sive, 2006) with some modifications. Briefly, embryos were fixed in 2% PFA and 2% glutaraldehyde in PBT buffer for 24 hours and then embedded in plastic resin (JB-4 Plus) and sectioned at 5μm using a tungsten carbide knife. Sections were stained with Giemsa at 1:20 for 1 hour followed by 10 second 0.05% acetic acid differentiation wash. Slides were dried and covered with Permount and imaged on a Nikon compound microscope fitted with a digital camera (VCU Biology microscopy core).
Cell spreading assays
To assess cell spreading capability, the epidermis was removed (as described above) and explanted onto glass coverslips coated with fibronectin (fibronectin-bovine, ThermoFisher, 33010018, diluted to 50μg/mL in water) and held in place with a small piece of coverslip for 20–30min. The coverslip was removed and then the explants were treated with 100uM SP600125 or DMSO for 24–48 hours at 15C.
EGTA induced cell dissociation
Embryos were treated at 15C in 100 μm SP600125 from stage 32–41. This was followed by a low calcium MBS wash (5 minutes) and 4mM EGTA treatment. When embryos visibly appeared to have epidermis dissociating from the body the time was recorded. In another experiment all embryos were fixed after 30 minutes and labeled for E-cadherin and imaged as described above.
Key Findings.
Decreased JNK signals cause a persistent buccopharyngeal membrane
Decreased JNK signals cause decreased cell proliferation and increased cell death and oxidative stress but these problems do not cause a persistent buccopharyngeal membrane.
JNK signals regulate intercellular adhesion in the epidermis and the buccopharyngeal membrane.
Acknowledgments
This work is supported by an NSF Career award (IOS1349668) and NIAMS R03 (RAR065583A) to A. Dickinson. This work used Microarray services in the DNA Sciences Core which is supported by the University Of Virginia School Of Medicine. This work also used services of Stephen Turner at the Bioinformatics Core which is supported by the University Of Virginia School Of Medicine. We would like to thank the undergraduate class of VCU BIOZ 491 2014 for analyzing mitotic inhibitors on embryonic mouth development. We would especially like to thank our lab manager and animal technician Deborah Howton for helping with many aspects of this work. Finally, we thank colleagues Santiago Lima, Sarah Rothschild and Greg Walsh for helpful discussions surrounding this work.
Footnotes
Author contributions
AD Conceived and wrote the manuscript, performed experiments concerning the JNK inhibitor and JNK MO analysis of the buccopharyngeal membrane and epidermis, JNK MO transplants, all mouth analysis after treatment with inhibitors, labeling the mouth with pJNK, mitotracker, ph3, CC3, clathrin, e-cadherin and actin labeling. NH performed CA-JNK analysis, cell spreading assay, microarray in collaboration with UVA core, gstp1 in situs, ph3 and CC3 labeling in the epidermis. NB subcloned CA-JNK into pCS2+ and EGTA experiments. ST performed bioinformatics analysis of microarray data.
References
- Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70:1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizelsky W, Tata A, Kuhl M, Kuhl SJ. The Wnt/JNK signaling target gene alcam is required for embryonic kidney development. Development. 2014;141:2064–2074. doi: 10.1242/dev.107938. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Sive H. Positioning the extreme anterior in Xenopus: cement gland, primary mouth and anterior pituitary. Semin Cell Dev Biol. 2007;18:525–533. doi: 10.1016/j.semcdb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Dickinson AJ. Using frogs faces to dissect the mechanisms underlying human orofacial defects. Semin Cell Dev Biol. 2016;51:54–63. doi: 10.1016/j.semcdb.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson AJ, Sive H. Development of the primary mouth in Xenopus laevis. Dev Biol. 2006;295:700–713. doi: 10.1016/j.ydbio.2006.03.054. [DOI] [PubMed] [Google Scholar]
- Dickinson AJ, Sive HL. The Wnt antagonists Frzb-1 and Crescent locally regulate basement membrane dissolution in the developing primary mouth. Development. 2009;136:1071–1081. doi: 10.1242/dev.032912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Davis RJ, Flavell RA. Signaling by the JNK group of MAP kinases. c-jun N-terminal Kinase. J Clin Immunol. 2001;21:253–257. doi: 10.1023/a:1010975124110. [DOI] [PubMed] [Google Scholar]
- Dush MK, Nascone-Yoder NM. Jun N-terminal kinase maintains tissue integrity during cell rearrangement in the gut. Development. 2013;140:1457–1466. doi: 10.1242/dev.086850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Schwarz H, Jesuthasan S. Furrow-specific endocytosis during cytokinesis of zebrafish blastomeres. Exp Cell Res. 2002;279:14–20. doi: 10.1006/excr.2002.5579. [DOI] [PubMed] [Google Scholar]
- Gartlan MG, Davies J, Smith RJ. Congenital oral synechiae. Ann Otol Rhinol Laryngol. 1993;102:186–197. doi: 10.1177/000348949310200305. [DOI] [PubMed] [Google Scholar]
- Geetha-Loganathan P, Nimmagadda S, Fu K, Richman JM. Avian facial morphogenesis is regulated by c-Jun N-terminal kinase/planar cell polarity (JNK/PCP) wingless-related (WNT) signaling. J Biol Chem. 2014;289:24153–24167. doi: 10.1074/jbc.M113.522003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillardin V, Silvestre F, Divoy C, Thome JP, Kestemont P. Effects of Aroclor 1254 on oxidative stress in developing Xenopus laevis tadpoles. Ecotoxicol Environ Saf. 2009;72:546–551. doi: 10.1016/j.ecoenv.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Goberdhan DC, Wilson C. JNK, cytoskeletal regulator and stress response kinase? A Drosophila perspective. Bioessays. 1998;20:1009–1019. doi: 10.1002/(SICI)1521-1878(199812)20:12<1009::AID-BIES7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Greenough A. Factors adversely affecting lung growth. Paediatr Respir Rev. 2000;1:314–320. doi: 10.1053/prrv.2000.0070. [DOI] [PubMed] [Google Scholar]
- Gutierrez GJ, Tsuji T, Chen M, Jiang W, Ronai ZA. Interplay between Cdh1 and JNK activity during the cell cycle. Nat Cell Biol. 2010a;12:686–695. doi: 10.1038/ncb2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez GJ, Tsuji T, Cross JV, Davis RJ, Templeton DJ, Jiang W, Ronai ZA. JNK-mediated phosphorylation of Cdc25C regulates cell cycle entry and G(2)/M DNA damage checkpoint. J Biol Chem. 2010b;285:14217–14228. doi: 10.1074/jbc.M110.121848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Shakado S, Sakisaka S, Tamaki S, Ohishi M, Sasatomi K, Koga H, Sata M, Tanikawa K. Bafilomycin A1, a specific inhibitor of V-type H+-ATPases, inhibits the acidification of endocytic structures and inhibits horseradish peroxidase uptake in isolated rat sinusoidal endothelial cells. Liver. 1997;17:244–250. doi: 10.1111/j.1600-0676.1997.tb01025.x. [DOI] [PubMed] [Google Scholar]
- Harris WA, Hartenstein V. Neuronal determination without cell division in Xenopus embryos. Neuron. 1991;6:499–515. doi: 10.1016/0896-6273(91)90053-3. [DOI] [PubMed] [Google Scholar]
- Huang C, Jacobson K, Schaller MD. A role for JNK-paxillin signaling in cell migration. Cell Cycle. 2004;3:4–6. [PubMed] [Google Scholar]
- Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003;424:219–223. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- Hutchins EJ, Szaro BG. c-Jun N-terminal kinase phosphorylation of heterogeneous nuclear ribonucleoprotein K regulates vertebrate axon outgrowth via a posttranscriptional mechanism. J Neurosci. 2013;33:14666–14680. doi: 10.1523/JNEUROSCI.4821-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)--from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacox L, Chen J, Rothman A, Lathrop-Marshall H, Sive H. Formation of a “Pre-mouth Array” from the Extreme Anterior Domain Is Directed by Neural Crest and Wnt/PCP Signaling. Cell Rep. 2016;16:1445–1455. doi: 10.1016/j.celrep.2016.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper H, Benes V, Schwager C, Sauer S, Clauder-Munster S, Ansorge W, Bohmann D. The genomic response of the Drosophila embryo to JNK signaling. Dev Cell. 2001;1:579–586. doi: 10.1016/s1534-5807(01)00045-4. [DOI] [PubMed] [Google Scholar]
- Karin M, Gallagher E. From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57:283–295. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- Karpac J, Jasper H. Insulin and JNK: optimizing metabolic homeostasis and lifespan. Trends Endocrinol Metab. 2009;20:100–106. doi: 10.1016/j.tem.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AE, Dickinson AJ. Quantification of orofacial phenotypes in Xenopus. J Vis Exp. 2014:e52062. doi: 10.3791/52062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kot-Leibovich H, Fainsod A. Ethanol induces embryonic malformations by competing for retinaldehyde dehydrogenase activity during vertebrate gastrulation. Dis Model Mech. 2009;2:295–305. doi: 10.1242/dmm.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl M. Non-canonical Wnt signaling in Xenopus: regulation of axis formation and gastrulation. Semin Cell Dev Biol. 2002;13:243–249. doi: 10.1016/s1084-9521(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Kwong KM. Current Updates on Choanal Atresia. Front Pediatr. 2015;3:52. doi: 10.3389/fped.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Koria P, Qu J, Andreadis ST. JNK phosphorylates beta-catenin and regulates adherens junctions. FASEB J. 2009;23:3874–3883. doi: 10.1096/fj.08-117804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Wysocki A, Warburton D, Tuan TL. Wound healing in development. Birth Defects Res C Embryo Today. 2012;96:213–222. doi: 10.1002/bdrc.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Thompson CB, Bar-Sagi D, Davis RJ. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Mol Cell Biol. 2002;22:4929–4942. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppa S, Bohmann D. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene. 1999;18:6158–6162. doi: 10.1038/sj.onc.1203173. [DOI] [PubMed] [Google Scholar]
- Liao G, Tao Q, Kofron M, Chen JS, Schloemer A, Davis RJ, Hsieh JC, Wylie C, Heasman J, Kuan CY. Jun NH2-terminal kinase (JNK) prevents nuclear beta-catenin accumulation and regulates axis formation in Xenopus embryos. Proc Natl Acad Sci U S A. 2006;103:16313–16318. doi: 10.1073/pnas.0602557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim NR, Yeap YY, Zhao TT, Yip YY, Wong SC, Xu D, Ang CS, Williamson NA, Xu Z, Bogoyevitch MA, Ng DC. Opposing roles for JNK and Aurora A in regulating the association of WDR62 with spindle microtubules. J Cell Sci. 2015;128:527–540. doi: 10.1242/jcs.157537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizundia R, Chaussepied M, Naissant B, Masse GX, Quevillon E, Michel F, Monier S, Weitzman JB, Langsley G. The JNK/AP-1 pathway upregulates expression of the recycling endosome rab11a gene in B cells transformed by Theileria. Cell Microbiol. 2007;9:1936–1945. doi: 10.1111/j.1462-5822.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- Makrigiannakis A, Coukos G, Blaschuk O, Coutifaris C. Follicular atresia and luteolysis. Evidence of a role for N-cadherin. Ann N Y Acad Sci. 2000;900:46–55. doi: 10.1111/j.1749-6632.2000.tb06215.x. [DOI] [PubMed] [Google Scholar]
- Marchesin V, Castro-Castro A, Lodillinsky C, Castagnino A, Cyrta J, Bonsang-Kitzis H, Fuhrmann L, Irondelle M, Infante E, Montagnac G, Reyal F, Vincent-Salomon A, Chavrier P. ARF6-JIP3/4 regulate endosomal tubules for MT1-MMP exocytosis in cancer invasion. J Cell Biol. 2015;211:339–358. doi: 10.1083/jcb.201506002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarella MA, Schwartz J, Manoukian JJ. Congenital intra-oral adhesions: a surgical approach to cleft palate lateral synechia syndrome. Int J Pediatr Otorhinolaryngol. 2015;79:769–772. doi: 10.1016/j.ijporl.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Mengistu M, Brotzman H, Ghadiali S, Lowe-Krentz L. Fluid shear stress-induced JNK activity leads to actin remodeling for cell alignment. J Cell Physiol. 2011;226:110–121. doi: 10.1002/jcp.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody SA. Fates of the blastomeres of the 16-cell stage Xenopus embryo. Dev Biol. 1987;119:560–578. doi: 10.1016/0012-1606(87)90059-5. [DOI] [PubMed] [Google Scholar]
- Mugoni V, Camporeale A, Santoro MM. Analysis of oxidative stress in zebrafish embryos. J Vis Exp. 2014 doi: 10.3791/51328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka T, Ohashi R, Inutsuka A, Sakai S, Fujisawa N, Yokoyama M, Huang YH, Igarashi M, Kishi M. The Wnt/planar cell polarity pathway component Vangl2 induces synapse formation through direct control of N-cadherin. Cell Rep. 2014;6:916–927. doi: 10.1016/j.celrep.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Naydenov NG, Hopkins AM, Ivanov AI. c-Jun N-terminal kinase mediates disassembly of apical junctions in model intestinal epithelia. Cell Cycle. 2009;8:2110–2121. doi: 10.4161/cc.8.13.8928. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus Laevis (Daudin) New York: Garland Publishing Inc; 1967. p. 263. [Google Scholar]
- Pandur P, Maurus D, Kuhl M. Increasingly complex: new players enter the Wnt signaling network. Bioessays. 2002;24:881–884. doi: 10.1002/bies.10164. [DOI] [PubMed] [Google Scholar]
- Pereira AM, Tudor C, Kanger JS, Subramaniam V, Martin-Blanco E. Integrin-dependent activation of the JNK signaling pathway by mechanical stress. PLoS One. 2011;6:e26182. doi: 10.1371/journal.pone.0026182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravni A, Qu Y, Goffinet AM, Tissir F. Planar cell polarity cadherin Celsr1 regulates skin hair patterning in the mouse. J Invest Dermatol. 2009;129:2507–2509. doi: 10.1038/jid.2009.84. [DOI] [PubMed] [Google Scholar]
- Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol Ther. 2005;12:468–474. doi: 10.1016/j.ymthe.2005.03.038. [DOI] [PubMed] [Google Scholar]
- Royle SJ. The cellular functions of clathrin. Cell Mol Life Sci. 2006;63:1823–1832. doi: 10.1007/s00018-005-5587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabapathy K, Jochum W, Hochedlinger K, Chang L, Karin M, Wagner EF. Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech Dev. 1999;89:115–124. doi: 10.1016/s0925-4773(99)00213-0. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Nio M, Iwami D, Funaki N, Ohi R, Sasano H. Cytokeratin subtypes in biliary atresia: immunohistochemical study. Pathol Int. 2001a;51:511–518. doi: 10.1046/j.1440-1827.2001.01241.x. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Nio M, Iwami D, Funaki N, Sano N, Ohi R, Sasano H. E-cadherin, alpha-catenin and beta-catenin in biliary atresia: correlation with apoptosis and cell cycle. Pathol Int. 2001b;51:923–932. doi: 10.1046/j.1440-1827.2001.01304.x. [DOI] [PubMed] [Google Scholar]
- Shaulian E. AP-1--The Jun proteins: Oncogenes or tumor suppressors in disguise? Cell Signal. 2010;22:894–899. doi: 10.1016/j.cellsig.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Shen HM, Liu ZG. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;40:928–939. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- Singh J, Mlodzik M. Planar cell polarity signaling: coordination of cellular orientation across tissues. Wiley Interdiscip Rev Dev Biol. 2012;1:479–499. doi: 10.1002/wdev.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive HL, Grainger R, Harlard R. Early development of Xenopus laevis: a laboratory manual. Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Son Y, Kim S, Chung HT, Pae HO. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013;528:27–48. doi: 10.1016/B978-0-12-405881-1.00002-1. [DOI] [PubMed] [Google Scholar]
- Soukup V, Horacek I, Cerny R. Development and evolution of the vertebrate primary mouth. J Anat. 2013;222:79–99. doi: 10.1111/j.1469-7580.2012.01540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Tazaki A, Ueno N, Watanabe K, Mochii M. Xenopus Wnt-5a induces an ectopic larval tail at injured site, suggesting a crucial role for noncanonical Wnt signal in tail regeneration. Mech Dev. 2009;126:56–67. doi: 10.1016/j.mod.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Tomlinson JK, Liem NT, Savarirayan R, Meara JG. Isolated and syndromic syngnathism: management, implications, and genetics. Ann Plast Surg. 2006;57:231–235. doi: 10.1097/01.sap.0000215265.68252.f0. [DOI] [PubMed] [Google Scholar]
- Tugay M, Filiz S, Dalcik H, Guvenc BH, Dalcik C, Korkmaz M, Sozubir S. Expression of cell adhesion molecules in the adriamycin-induced esophageal atresia rat model. Cell Biol Int. 2003;27:929–933. doi: 10.1016/j.cellbi.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Turksen Z, Ozakpinar HR, Tellioglu AT. A case of syngnathia, cleft palate and hypospadias: an isolated case or syndromic syngnathism? J Craniomaxillofac Surg. 2012;40:8–10. doi: 10.1016/j.jcms.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Verma SP, Geller K. Persistent buccopharyngeal membrane: report of a case and review of the literature. Int J Pediatr Otorhinolaryngol. 2009;73:877–880. doi: 10.1016/j.ijporl.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Vismara C, Bacchetta R, Di Muzio A, Mantecca P, Tarca S, Vailati G, Colombo R. H2O2 induces abnormal tail flexure in Xenopus embryos: similarities with Paraquat teratogenic effects. Birth Defects Res B Dev Reprod Toxicol. 2006;77:238–243. doi: 10.1002/bdrb.20080. [DOI] [PubMed] [Google Scholar]
- Wagner CL, Taylor SN, Johnson D. Host factors in amniotic fluid and breast milk that contribute to gut maturation. Clinical Reviews in Allergy & Immunology. 2008;34:191–204. doi: 10.1007/s12016-007-8032-3. [DOI] [PubMed] [Google Scholar]
- Wahl SE, Kennedy AE, Wyatt BH, Moore AD, Pridgen DE, Cherry AM, Mavila CB, Dickinson AJ. The role of folate metabolism in orofacial development and clefting. Dev Biol. 2015;405:108–122. doi: 10.1016/j.ydbio.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev Cell. 2003;5:811–816. doi: 10.1016/s1534-5807(03)00323-x. [DOI] [PubMed] [Google Scholar]
- Waterman RE. Ultrastructure of oral (buccopharyngeal) membrane formation and rupture in the hamster embryo. Dev Biol. 1977;58:219–229. doi: 10.1016/0012-1606(77)90088-4. [DOI] [PubMed] [Google Scholar]
- Waterman RE, Schoenwolf GC. The ultrastructure of oral (buccopharyngeal) membrane formation and rupture in the chick embryo. Anat Rec. 1980;197:441–470. doi: 10.1002/ar.1091970408. [DOI] [PubMed] [Google Scholar]
- Weston CR, Wong A, Hall JP, Goad ME, Flavell RA, Davis RJ. The c-Jun NH2-terminal kinase is essential for epidermal growth factor expression during epidermal morphogenesis. Proc Natl Acad Sci U S A. 2004;101:14114–14119. doi: 10.1073/pnas.0406061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Yang M, Qiu W, Pan C, Wu M. The impact of endocrine-disrupting chemicals on oxidative stress and innate immune response in zebrafish embryos. Environ Toxicol Chem. 2013;32:1793–1799. doi: 10.1002/etc.2245. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi J, Miyamoto Y, Sanbe A, Tanoue A. JNK phosphorylation of paxillin, acting through the Rac1 and Cdc42 signaling cascade, mediates neurite extension in N1E-115 cells. Exp Cell Res. 2006;312:2954–2961. doi: 10.1016/j.yexcr.2006.05.016. [DOI] [PubMed] [Google Scholar]
- You H, Lei P, Andreadis ST. JNK is a novel regulator of intercellular adhesion. Tissue Barriers. 2013;1:e26845. doi: 10.4161/tisb.26845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Sakamoto N, Song G, Sato M. Migration of human mesenchymal stem cells under low shear stress mediated by mitogen-activated protein kinase signaling. Stem Cells Dev. 2012;21:2520–2530. doi: 10.1089/scd.2012.0010. [DOI] [PubMed] [Google Scholar]
- Zhu XJ, Liu Y, Yuan X, Wang M, Zhao W, Yang X, Zhang X, Hsu W, Qiu M, Zhang Z, Zhang Z. Ectodermal Wnt controls nasal pit morphogenesis through modulation of the BMP/FGF/JNK signaling axis. Dev Dyn. 2016;245:414–426. doi: 10.1002/dvdy.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]