Abstract
Enterotoxigenic Escherichia coli (ETEC) strains are among the most common causes of children’s diarrhea and travelers’ diarrhea. Developing effective vaccines against ETEC associated diarrhea becomes a top priority. ETEC heat-labile toxin (LT) and heat-stable toxin (STa) toxoid fusion 3xSTaN12S-dmLT was demonstrated recently to induce neutralizing antitoxin antibodies in intraperitoneally or subcutaneously immunized mice. However, whether antibodies derived from this toxoid fusion are protective against ETEC diarrhea has not been examined. In this study, we intramuscularly immunized pregnant gilts with toxoid fusion 3xSTaN12S-dmLT, challenged suckling piglets with a STa-positive ETEC strain, and assessed protective efficacy of passive acquire antitoxin antibodies against ETEC diarrhea. Data showed all three immunized gilts developed anti-STa IgG and IgA antibodies, and piglets born to the immunized dams acquired anti-STa and anti-LT antibodies. When challenged with a STa+ ETEC strain, none of the piglets born to the immunized dams developed watery diarrhea, with 20 piglets remained normal and the other 8 piglets developed mild diarrhea indicated with stained butt. In contrast, the control dams and born piglets had no anti-STa or anti-LT antibodies detected, and 26 out 32 piglets developed watery diarrhea after challenge of the STa+ ETEC strain. These results indicated that passive acquired anti-STa antibodies are protective against ETEC diarrhea, and suggested potential application of toxoid fusion 3xSTaN12S-dmLT in ETEC vaccine development.
Keywords: toxoid fusion, 3xSTaN12S-dmLT, enterotoxigenic Escherichia coli (ETEC), diarrhea, vaccine, pig challenge model
Introduction
Enterotoxigenic Escherichia coli (ETEC) strains producing heat-stable toxin (STa) and/or heat-labile toxin (LT) continue to be the leading bacterial cause of diarrhea to children under 5 years in development countries and to children and adults of developed countries traveling to developing countries [1–5]. Enterotoxins STa and LT produced by ETEC bacteria elevate intracellular cyclic GMP or AMP levels and disrupt fluid homeostasis in host small intestinal epithelial cells, leading to fluid hyper-secretion and watery diarrhea. Currently, there is no vaccine available against ETEC associated children’s diarrhea or travelers’ diarrhea [6–8].
One major challenge in developing effective ETEC vaccines is inability of having safe antigens to induce protective antibodies against enterotoxicity of STa toxin. STa, a peptide of 19 amino acids, is potently toxic and poorly immunogenic. Recently, we applied LT and STa toxoid and genetic fusion strategies, and demonstrated that nontoxic LT-STa toxoid fusions were able to induce neutralizing anti-STa antibodies [9–12]. More recently, we identified toxoid fusion 3xSTaN12S-dmLT, a toxoid fusion carrying three copies of STa toxoid STaN12S and a monomeric double mutant LT toxoid LTR192G/L211A, potentially an optimal immunogen inducing antitoxin antibodies to neutralize both LT and STa toxins. Toxoid fusion 3xSTaN12S-dmLT, when was used to intraperitoneally [13, 14] or subcutaneously [15] immunize mice, induced neutralizing antibodies against both toxins.
However, antitoxin antibodies derived from this toxoid fusion have yet to be demonstrated for protection against STa enterotoxicity in vivo or more importantly against ETEC diarrhea. In this study, we intramuscularly immunized pregnant pigs and challenged suckling piglets with a STa-producing ETEC strain to determine if passive acquired antitoxin antibodies protect against STa+ ETEC diarrhea, further evaluating the potential application of toxoid fusion 3xSTaN12S-dmLT in ETEC vaccine development.
Materials and Methods
Toxoid fusion antigen, adjuvant and STa+ ETEC challenge strain
Toxoid fusion protein 3xSTaN12S-dmLT was expressed in recombinant E. coli strain 9331, extracted with bacterial protein extraction reagent (B-PER), and refolded using a protein refolding kit (Novagen, Madison, WI) as described previously [13]. Holotoxin-structured double mutant LT (dmLT, LTR192G/L211A) provided by Walter Reed Army Institute of Research (Silver Spring, MD) was used as adjuvant in pig intramuscular immunization.
STa+ ETEC challenge strain 8823 (STa/987P) was constructed by transforming a nonpathogenic porcine E. coli isolate G58-1 [16] with plasmid pDMS158 and plasmid p8755 to produce 987P fimbria and STa toxin (NTFYCCELCCNPACAGCY), respectively. The chloramphenicol resistant pDMS158 has the 987P fimbrial gene cassette cloned in vector pACYC184 to express 987P fimbriae [17, 18], and ampicillin resistant plasmid p8755 has porcine-type STa gene (estA) cloned in vector pUC19 to produce STa toxin. In addition, LT+ ETEC recombinant strain 8819 was also included in this study. Strain 8819 (LT/987P) was constructed by transforming E. coli strain G58-1 with pDMS158 and pBR322 expressing porcine-type eltAB genes [19] to produce 987P fimbriae and porcine-type LT toxin.
Pig immunization
Gilts with no record of clinical diarrhea were bred at the university swine unit and used in this immunization study. These pigs received no routine commercial vaccines and were confirmed to lack pre-existing anti-LT or anti-STa antibodies. Three pregnant gilts were IM administered with the toxoid fusion antigen and dmLT adjuvant at the neck behind the ear. Five hundred microgram (µg) toxoid fusion protein (in 500 µl PBS) and 5 µg dmLT (in 5 µl) were mixed and injected to each pregnant gilt at the right side of the neck 6–8 weeks before farrowing. A booster at the same dose of the primary was administered at the left side of the neck 4 weeks later. Three pregnant gilts without immunization were used as the control.
Pigs were transported to the university larger animal research center 10 days prior to the expected farrowing date, surface disinfected, and housed in standard farrowing crates where they farrowed naturally. Blood samples were collected from gilts before the immunization and after farrowing. Colostrum samples were collected from each gilt just before farrowing. Pig serum and colostrum samples were stored at −80°C until use.
Pig serum and colostrum anti-STa and anti-LT IgG and IgA antibody titration
Anti-STa and anti-LT IgG and IgA antibody responses in serum and colostrum samples of each immunized or control dam, and serum of each born piglet were measured in ELISAs as we described previously [9, 20, 21]. Briefly, wells of Costar plates (Corning Inc., Corning, NY) coated with STa-ovalbumin conjugates (10 ng per well, in 100 µl STa ELISA buffer) [22] were incubated with two-fold serially diluted pig serum or colostrum samples (diluted from 1:200 to 1:25600) to detect anti-STa IgG or IgA antibodies; wells of Immulon 2HB microtiter plates (Thermo Scientific, Rochester, NY) coated with LT [List Biological Laboratories, Inc., Campbell, CA; 100 ng per well, in 100 µl coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH9.6)] were incubated with pig serum or colostrum dilutions to measure anti-LT IgG or IgA antibodies. Each serum or colostrum sample was examined in triplicates. Horseradish peroxidase (HRP)-conjugated goat anti-pig IgG (Thermo Fisher Scientific, Rockford, IL; 1:3300) or IgA (Thermo Fisher Scientific; 1:3300) and Microwell peroxidase substrate system (2-C) (KPL, Gaithersburg, MD) were used to measure optical absorbance (OD) at the wavelength of 650 nm. OD650 readings were converted to anti-STa and anti-LT antibody titers, by calculating the highest dilution giving an OD650 of >0.3 after subtraction of background readings (the highest dilution multiplied by the adjusted OD) to a log10 scale, as previously described [9, 20, 21].
Pig serum and colostrum antitoxin antibody neutralization assay
Serum and colostrum samples from the immunized or the control dams, and serum samples of piglets born to the immunized dams or the control dams were examined for in vitro antibody neutralization against STa toxin using a cGMP EIA kit (Enzo Life, Farmingdale, NY) [9, 10, 20, 21]. The serum or colostrum sample (30 µl) from each immunized or control dam, or the serum sample (30 µl) pooled from each litter of piglets born to the immunized dam or the control dam was mixed with 2 ng STa toxin for 30 min at room temperature, and each mixture was brought up to 300 µl with culture medium and was transferred to T-84 cells (ATCC CCL-248; in 700µl culture medium) and incubated in a 37°C CO2 incubator. After 1 h incubation, T-84 cells were gently rinsed with PBS and lysed with HCl (0.1M with 0.5% Triton x-100). T-84 cell lysates were measured for intracellular cGMP (pmole/ml) with the cGMP EIA kit by following the manufacturer’s protocol (Enzo Life).
Intracellular cGMP in T-84 cells incubated with STa (2 ng) alone was to show elevation of cGMP levels by STa enterotoxicity. Intracellular cGMP in T-84 cells incubated with culture medium showed baseline cGMP level.
Challenge of suckling piglet with a STa+ ETEC strain
A total of 36 piglets were born to three immunized dams and 40 piglets were born to three control dams. Among them, 28 piglets born to the immunized mothers and 32 piglets born to the control mothers were randomly selected and used in STa+ ETEC challenge study.
After 24 h suckling, each piglet was orally inoculated with 5×109 CFUs (in 3 ml PBS) of the STa+ ETEC strain 8823 (G58/987P+/STa+). Challenge bacteria were harvested from overnight-grown agar plates and gently suspended in PBS. Bacteria CFUs were calculated based on the pre-established plot of OD600 and CFUs of this strain. Challenged piglets were observed for clinical signs including vomiting, stained butt, watery diarrhea, dehydration and lethargy every 2 to 4 hours during 24 h post-inoculation as we described previously [23]. Protection against watery diarrhea or any diarrhea was measured as [(% of diarrhea in the control group − % of diarrhea in the immunized group)/% of diarrhea in the control group]×100. Piglets were weighed before and after the challenge to assess daily weight gain rates. All piglets were anaesthetized and euthanized 24 h after challenge. Blood samples were collected from each piglet at necropsy. Pig immunization and challenge studies complied with the Guide for the Care and Use of Agricultural Animals in Research and Teaching (FASS, 3rd Ed., 2010) were approved by the Kansas State University IACUC, and were supervised by a staff veterinarian.
Statistical analysis
Sampling size calculations were based on two sample comparison of mean probabilities with repeated measures and after adjusting for clustering of pigs within litters, using Stata 12. Sample sizes to detect differences in the mean probability of animals experiencing diarrhea (main outcome of interest) between immunized and control pigs, considering one measurement before challenge and up to 10 measurements (every 2–4 h for 24 h for pigs) after challenge were calculated. Preliminary data indicated that 14.3% (SD = 35.00%) of immunized and 100% (SD = 0.11%) of control pigs experienced diarrhea [24–26]. Assumptions for sample size determinations included: differences in mean probabilities of 0.4 to 0.9 and SD of 0.1 to 0.5 between immunized and control pigs, average litter size of 10, correlation of 0.7, power > 0.80 and alpha of 0.05. This led to determination of a minimum of 2–6 litters and 9–28 pigs per treatment group would be needed. Results were presented as means ± standard deviations. Student’s t-test was used to compare different treatment groups. Calculated p values of less than 0.05 were considered as significant following pairwise comparison of treatment groups using two-tailed distribution and two-sample equal or unequal variance.
Results
Adult pigs intramuscularly immunized with toxoid fusion 3xSTaN12S-dmLT developed anti-STa and anti-LT antibody responses
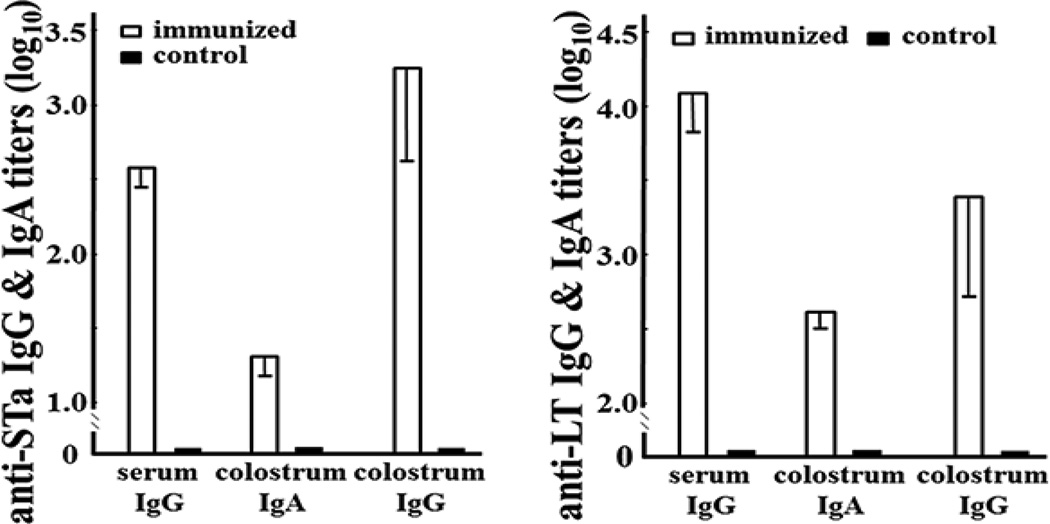
Anti-STa and anti-LT IgG antibodies in the serum samples and IgG and IgA antibodies in the colostrum samples were detected from three pregnant gilts IM immunized with 3xSTaN12S-dmLT (Fig. 1). No anti-STa or anti-LT IgG or IgA antibodies were detected from the serum or colostrum samples of the control gilts, nor the serum samples collected from gilts prior to the immunization.
Figure 1.
Anti-STa and anti-LT IgG and IgA antibody titers (in log10) in the serum and colostrum samples of the immunized (□) or the control (■) dams. Left: anti-STa IgG antibody titers in the serum samples, and anti-STa IgG and IgA in the colostrum samples. Right: anti-LT IgG antibody titers in the serum samples, and anti-LT IgG and IgA in the colostrum samples. Boxes and bars are antibody titer means and standard deviations.
The serum and colostrum samples of the immunized dams showed neutralization activity against STa toxin
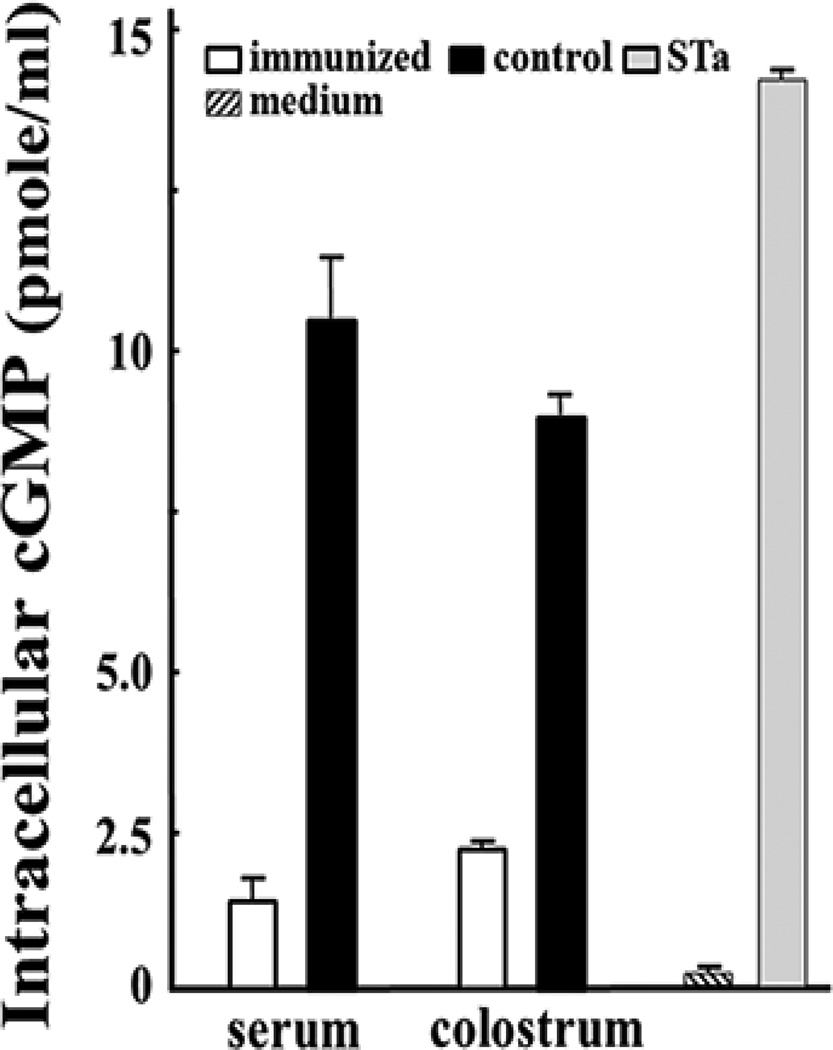
The serum and colostrum samples from three IM immunized dams showed in vitro neutralization activity against STa toxin (Fig. 2). The cGMP levels in the T-84 cells incubated with STa and the serum or colostrum samples from the immunized dams were significantly lower than those in cells incubated with STa and the serum or colostrum samples from the control dams (p<0.001).
Figure 2.
Pig antibody in vitro neutralization activity against STa toxin, measured by preventing STa toxin from stimulation of cGMP in T-84 cells with an intracellular cyclic GMP EIA kit. The serum or colostrum samples (30 µl) of the immunized dams (□) or the control (■) dams were incubated with STa toxin (2 ng) and then added to T-84 cells. Intracellular cGMP levels (pmole/ml) in the incubated cells were measured based on manufacturer’s protocol (Enzo Life). T-84 cells incubated with STa toxin or cell culture medium alone were used to show stimulation of cGMP by STa enterotoxicity or baseline cGMP levels. Boxes and bars are cGMP means and standard deviations.
Suckling piglets born to the dams immunized with toxoid fusion 3xSTaN12S-dmLT acquired anti-STa antibodies
Anti-STa and anti-LT IgG antibody responses were detected in each piglet born to the immunized dams. Piglet serum anti-STa and anti-LT IgG titers were detected at 2.87±0.04 and 3.6±0.29 (log10), respectively. No anti-STa or anti-LT IgG antibodies were detected in serum samples of the piglets born to the control dams.
The serum samples of the piglets born to the immunized mothers showed neutralizing activity against STa toxin
Serum samples of piglets born to the immunized dams also showed neutralizing STa toxin activity. The cGMP levels in T-84 cells incubated with STa toxin and the serum of piglets born to the immunized dams were 0.67±0.64 pmole/ml, whereas the cGMP in T-84 cells incubated with STa toxin and the serum of piglets born to the control dams were 10.4±2.7 pmole/ml.
Suckling piglets born to the immunized dams were protected against STa+ ETEC challenge
After being verified for STa expression and enterotoxicity in STa competitive ELISA and cGMP EIA ELISA, STa recombinant strain 8823 was used to challenge piglets after 24 h suckling. None of the 28 piglets born to three immunized dams developed watery diarrhea in the 24 hour observation period. Twenty piglets remained healthy, and other 8 piglets (28.8%) showed stained butt which indicated mild diarrhea. These outcomes were significantly different compared to those in piglets born to the control dams (p≤0.01). For piglets born to the control dams, 26 out 32 (81.3%) developed watery diarrhea after inoculation of 8823. Protection against watery diarrhea was 100% [(81.3% − 0%)/81.3% × 100], and protection against any diarrhea was 65% [(81.3% − 28.8%)/81.3% × 100].
Daily weight gain rate (%) during 24 h post-inoculation for the piglets born to the immunized dams was 8.7±2.8. That was significantly different compared to the rate of the piglets born to the control dams (−0.2±11.1; p<0.01).
Additionally, 8 piglets born to two immunized dams and 8 piglets born to two control dams were included in a pilot LT+ ETEC challenge study. During 24 h post inoculation with LT+ ETEC recombinant strain 8819, only 1 out 8 piglets born to the immunized dams developed watery diarrhea (12.5%). In contrast, 7 out 8 piglets (87.5%) born to the control dams developed watery diarrhea after challenged with this LT+ strain, indicating a protection value of 86% [(87.5% − 12.5%)/87.5% × 100].
Discussion
Results from this study showed that suckling piglets born to the dams IM immunized with toxoid fusion 3xSTaN12S-dmLT with native dmLT adjuvant acquired anti-STa (and anti-LT) antibodies and did not develop watery diarrhea after challenge of STa+ ETEC strain 8823. These results for the first time demonstrated the anti-STa antibodies derived from the human-type STa toxoid antigen protected against STa+ ETEC diarrhea in vivo. It was reported previously that piglets born to the sow IM immunized with a toxoid fusion were protected against STa+ ETEC challenge, but the STa toxoid in that STa-LT toxoid fusion was porcine ETEC origin (pSTa, not hSTa) [9]. Despite human ETEC origin STa toxoids, when genetically fused to a LT toxoid, were shown to induce neutralizing antibodies against STa toxin in vitro [10, 12, 13, 15], anti-STa antibodies derived from these toxoid fusions were never examined for protection against STa+ ETEC diarrhea.
Effective ETEC vaccines need to induce protective anti-STa antibodies [8, 27]. STa+ ETEC strains are among the top three causes of moderate-to-severe diarrhea to children under 5 years in developing countries [3, 28]. While anti-LT antibodies do not cross protect against STa toxin, only ETEC vaccines that carry safe STa antigens and induce protective anti-STa antibodies would provide effective protection against ETEC diarrhea. However, unlike the strongly immunogenic LT, STa is a poorly immunogenic small peptide. Natural infection with STa+ ETEC strains or experimental administration of STa antigens does not induce anti-STa antibody responses. Inability of induction of protective anti-STa antibodies is indeed one major challenge in ETEC vaccine development. Demonstration of toxoid fusion 3xSTaN12S-dmLT for induction of protective anti-STa antibodies against ETEC diarrhea in this study can lead to acceleration of ETEC vaccine development.
A pig challenge model was used in this study to evaluate derived anti-STa antibodies against STa+ ETEC diarrhea. Pigs, especially young pigs are naturally susceptible to ETEC infection, and develop clinical diarrhea similar to human patients after ETEC infection. Neonatal pigs infected with ETEC recombinant strains expressing porcine-origin or human-origin STa (or LT) were shown to develop same clinical diarrhea [22]. Besides, like humans, pigs after infection develop anti-LT or anti-CFA antibody responses which protect against subsequent homologous infection [29]. Moreover, virulence correlation between inoculation of pigs that express specific receptors with ETEC bacteria producing host-specific fimbriae and development of ETEC-associated diarrhea has been well established [30–32]. That makes the pig immunization and challenge model a suitable animal model to unambiguously assess protective efficacy of ETEC antitoxin vaccine candidates.
The current study assessed the efficacy of passively acquired anti-STa antibodies against STa+ ETEC diarrhea. Future studies by directly immunizing neonatal pigs and then challenging them with the STa+ ETEC strain can further assess efficacy of anti-STa antibodies derived from toxoid fusion 3xSTaN12S-dmLT against STa+ ETEC diarrhea, with a different immunization route, for an example, intradermal (ID), and perhaps examining anti-STa antibody cross-reactivity with guanylin and uroguanylin. Although data from this study showed piglets born to the IM immunized dams also acquired anti-LT antibodies and were protected against challenge from LT+ recombinant strain 8819, efficacy against LT+ ETEC diarrhea is only informative due to the small sampling size in the LT+ ETEC challenge study. Future studies with large sampling sizes will be needed to assess better efficacy of derived anti-LT antibodies against LT+ ETEC diarrhea.
Highlight.
Toxoid fusion 3xSTaN12S-dmLT induces neutralizing antibodies in IM immunized pigs.
Passive acquired antibodies protect piglets against STa ETEC diarrhea.
A pig challenge mode is ideal for ETEC antitoxin vaccines.
Acknowledgments
We are grateful to our staff veterinarian Dr. Brooke Bloomberg for her help at pig care and piglet challenge. Financial support for this study was provided by PATH, NIH (AI109209) and research funds from Kansas State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors declare no conflict of interest with this study.
References
- 1.WHO. Future directions for research on enterotoxigenic Escherichia coli vaccines for developing countries. Wkly Epidemiol Rec. 2006;81:97–107. [PubMed] [Google Scholar]
- 2.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–1987. doi: 10.1016/S0140-6736(10)60549-1. PubMed PMID: 20466419. [DOI] [PubMed] [Google Scholar]
- 3.Kotloff KL, Blackwelder WC, Nasrin D, Nataro JP, Farag TH, van Eijk A, et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin Infect Dis. 2012;55(Suppl 4):S232–S245. doi: 10.1093/cid/cis753. PubMed PMID: 23169936; PubMed Central PMCID: PMC3502307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DuPont HL. Systematic review: prevention of travellers' diarrhoea. Aliment Pharmacol Ther. 2008;27(9):741–751. doi: 10.1111/j.1365-2036.2008.03647.x. Epub 2008/02/21. PubMed PMID: 18284650. [DOI] [PubMed] [Google Scholar]
- 5.Hill DRBN. Travelers' diarrhea. Curr Opin Infect Dis. 2010;23(5):481–487. doi: 10.1097/QCO.0b013e32833dfca5. [DOI] [PubMed] [Google Scholar]
- 6.Svennerholm AM. From cholera to enterotoxigenic Escherichia coli (ETEC) vaccine development. Indian J Med Res. 2011;133(2):188–196. Epub 2011/03/19. PubMed PMID: 21415493; PubMed Central PMCID: PMC3089050. [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Sack DA. Current progress in developing subunit vaccines against enterotoxigenic Escherichia coli (ETEC) associated diarrhea. Clin Vaccine Immunol. 2015 doi: 10.1128/CVI.00224-15. PubMed PMID: 26135975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker RI. An assessment of enterotoxigenic Escherichia coli and Shigella vaccine candidates for infants and children. Vaccine. 2015;33(8):954–965. doi: 10.1016/j.vaccine.2014.11.049. PubMed PMID: 25482842. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Zhang C, Francis DH, Fang Y, Knudsen D, Nataro JP, et al. Genetic fusions of heat-labile (LT) and heat-stable (ST) toxoids of porcine enterotoxigenic Escherichia coli elicit neutralizing anti-LT and anti-STa antibodies. Infect Immun. 2010;78(1):316–325. doi: 10.1128/IAI.00497-09. Epub 2009/10/28. PubMed PMID: 19858307; PubMed Central PMCID: PMC2798211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu M, Ruan X, Zhang C, Lawson SR, Knudsen DE, Nataro JP, et al. Heat-labile- and heat-stable-toxoid fusions (LTR1)(9)(2)G-STaP(1)(3)F) of human enterotoxigenic Escherichia coli elicit neutralizing antitoxin antibodies. Infection and immunity. 2011;79(10):4002–4009. doi: 10.1128/IAI.00165-11. PubMed PMID: 21788385; PubMed Central PMCID: PMC3187267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu M, Zhang C, Mateo K, Nataro JP, Robertson DC, Zhang W. Modified Heat-Stable Toxins (hSTa) of Enterotoxigenic Escherichia coli Lose Toxicity but Display Antigenicity after Being Genetically Fused to Heat-Labile Toxoid LT(R192G) Toxins (Basel) 2011;3(9):1146–1162. doi: 10.3390/toxins3091146. Epub 2011/11/10. PubMed PMID: 22069760; PubMed Central PMCID: PMC3202872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C, Knudsen DE, Liu M, Robertson DC, Zhang W the STa Toxoid Vaccine Consortium Group. Toxicity and immunogenicity of enterotoxigenic Escherichia coli heat-labile and heat-stable toxoid fusion 3xSTaA14Q-LTS63K/R192G/L211A in a murine model. PLoS One. 2013;8(10):e77386. doi: 10.1371/journal.pone.0077386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruan X, Robertson DC, Nataro JP, Clements JD, Zhang W the STVCG. Characterization of heat-stable (STa) toxoids of enterotoxigenic Escherichia coli fused to a double mutant heat-labile toxin (dmLT) peptide in inducing neutralizing anti-STa antibodies. Infection and immunity. 2014;82(5):1823–1832. doi: 10.1128/IAI.01394-13. PubMed PMID: 24549325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruan X, Sack DA, Zhang W. Genetic fusions of a CFA/I/II/IV MEFA (multiepitope fusion antigen) and a toxoid fusion of heat-stable toxin (STa) and heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC) retain broad anti-CFA and antitoxin antigenicity. PLoS ONE. 2015 doi: 10.1371/journal.pone.0121623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nandre R, Ruan X, Duan Q, Zhang W. Enterotoxigenic Escherichia coli heat-stable toxin and heat-labile toxin toxoid fusion 3xSTaN12S-dmLT induces neutrlizing anti-STa antibodies in subcutaneously immunized mice. FEMS Microbiol Lett. 2016 doi: 10.1093/femsle/fnw246. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francis DH, Willgohs JA. Evaluation of a live avirulent Escherichia coli vaccine for K88+, LT+ enterotoxigenic colibacillosis in weaned pigs. Am J Vet Res. 1991;52(7):1051–1055. Epub 1991/07/01. PubMed PMID: 1679980. [PubMed] [Google Scholar]
- 17.Schifferli DM, Alrutz MA. Permissive linker insertion sites in the outer membrane protein of 987P fimbriae of Escherichia coli. J Bacteriol. 1994;176(4):1099–1110. doi: 10.1128/jb.176.4.1099-1110.1994. Epub 1994/02/01. PubMed PMID: 7906265; PubMed Central PMCID: PMC205162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi BK, Schifferli DM. Lysine residue 117 of the FasG adhesin of enterotoxigenic Escherichia coli is essential for binding of 987P fimbriae to sulfatide. Infection and immunity. 1999;67(11):5755–5761. doi: 10.1128/iai.67.11.5755-5761.1999. PubMed PMID: 10531225; PubMed Central PMCID: PMCPMC96951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto T, Gojobori T, Yokota T. Evolutionary origin of pathogenic determinants in enterotoxigenic Escherichia coli and Vibrio cholerae O1. J Bacteriol. 1987;169(3):1352–1357. doi: 10.1128/jb.169.3.1352-1357.1987. Epub 1987/03/01. PubMed PMID: 3546273; PubMed Central PMCID: PMC211946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C, Knudsen DE, Liu M, Robertson DC, Zhang W Group STTVC. Toxicity and immunogenicity of Enterotoxigenic Escherichia coli heat-labile and heat-stable toxoid fusion 3xSTa(A14Q)-LT(S63K/R192G/L211A) in a murine model. PLoS One. 2013;8(10):e77386. doi: 10.1371/journal.pone.0077386. PubMed PMID: 24146989; PubMed Central PMCID: PMC3795625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruan X, Robertson DC, Nataro JP, Clements JD, Zhang W the STa toxoid vaccine consortium group. Characterization of heat-stable (STa) toxoids of enterotoxigneic Escherichia coli fused to a double mutant heat-labile toxin (dmLT) peptide in inducing neutralizing anti-STa antibodies. Infection and immunity. 2014 doi: 10.1128/IAI.01394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Robertson DC, Zhang C, Bai W, Zhao M, Francis DH. Escherichia coli constructs expressing human or porcine enterotoxins induce identical diarrheal diseases in a piglet infection model. Appl Environ Microbiol. 2008;74(18):5832–5837. doi: 10.1128/AEM.00893-08. Epub 2008/07/29. PubMed PMID: 18658289; PubMed Central PMCID: PMC2547035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Berberov EM, Freeling J, He D, Moxley RA, Francis DH. Significance of heat-stable and heat-labile enterotoxins in porcine colibacillosis in an additive model for pathogenicity studies. Infect Immun. 2006;74(6):3107–3114. doi: 10.1128/IAI.01338-05. Epub 2006/05/23. PubMed PMID: 16714538; PubMed Central PMCID: PMC1479275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruan X, Liu M, Casey TA, Zhang W. A tripartite fusion, FaeG-FedF-LT(192)A2:B, of enterotoxigenic Escherichia coli (ETEC) elicits antibodies that neutralize cholera toxin, inhibit adherence of K88 (F4) and F18 fimbriae, and protect pigs against K88ac/heat-labile toxin infection. Clin Vaccine Immunol. 2011;18(10):1593–1599. doi: 10.1128/CVI.05120-11. Epub 2011/08/05. PubMed PMID: 21813665; PubMed Central PMCID: PMC3187021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruan X, Zhang W. Oral immunization of a live attenuated Escherichia coli strain expressing a holotoxin-structured adhesin-toxoid fusion (1FaeG-FedF-LTA2:5LTB) protected young pigs against enterotoxigenic E. coli (ETEC) infection. Vaccine. 2013;31(2013):1458–1463. doi: 10.1016/j.vaccine.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 26.Rausch D, Ruan X, Nandre R, Duan Q, Hashish E, Casey TA, et al. Antibodies derived from a toxoid MEFA (multiepitope fusion antigen) show neutralizing activities against heat-labile toxin (LT), heat-stable toxins (STa, STb), and Shiga toxin 2e (Stx2e) of porcine enterotoxigenic Escherichia coli (ETEC) Vet Microbiol. 2016 doi: 10.1016/j.vetmic.2016.02.002. PubMed PMID: 26878972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W, Sack DA. Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans. Expert Rev Vaccines. 2012;11(6):677–694. doi: 10.1586/erv.12.37. PubMed PMID: 22873126. [DOI] [PubMed] [Google Scholar]
- 28.Levine MM, Kotloff KL, Nataro JP, Muhsen K. The Global Enteric Multicenter Study (GEMS): impetus, rationale, and genesis. Clin Infect Dis. 2012;55(Suppl 4):S215–S224. doi: 10.1093/cid/cis761. PubMed PMID: 23169934; PubMed Central PMCID: PMC3502311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon HW, Bunn TO. Vaccines for preventing enterotoxigenic Escherichia coli infections in farm animals. Vaccine. 1993;11(2):213-00. doi: 10.1016/0264-410X(93)90020-X. PubMed PMID: 8094931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erickson AK, Willgohs JA, McFarland SY, Benfield DA, Francis DH. Identification of two porcine brush border glycoproteins that bind the K88ac adhesin of Escherichia coli and correlation of these glycoproteins with the adhesive phenotype. Infect Immun. 1992;60(3):983–988. doi: 10.1128/iai.60.3.983-988.1992. Epub 1992/03/01. PubMed PMID: 1347288; PubMed Central PMCID: PMC257584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erickson AK, Baker DR, Bosworth BT, Casey TA, Benfield DA, Francis DH. Characterization of porcine intestinal receptors for the K88ac fimbrial adhesin of Escherichia coli as mucin-type sialoglycoproteins. Infect Immun. 1994;62(12):5404–5410. doi: 10.1128/iai.62.12.5404-5410.1994. Epub 1994/12/01. PubMed PMID: 7960120; PubMed Central PMCID: PMC303281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francis DH, Erickson AK, Grange PA. K88 adhesins of enterotoxigenic Escherichia coli and their porcine enterocyte receptors. Adv Exp Med Biol. 1999;473:147–154. doi: 10.1007/978-1-4615-4143-1_13. Epub 2000/02/05. PubMed PMID: 10659352. [DOI] [PubMed] [Google Scholar]