Abstract
We recently identified a population of 10% of infants who respond with sub-protective antibody levels to most routine primary pediatric vaccinations due to altered innate and adaptive immune responses. We term these infants as low vaccine responders (LVRs). Here we report new data showing that TLR7/8 agonist - R848 stimulation of PBMCs of LVR infants elicit significantly lower IFN-α, IL-12p70 and IL-1β, while inducing higher levels of CCL5 (RANTES) compared to normal vaccine responder (NVR) infants.
Keywords: infant innate immunity, low vaccine response, IRF7, IFN-α, IL12p70, RANTES
Introduction
We have recently identified a population of about 10% of infants who fail to respond or respond with sub-optimal immunity to routine primary pediatric vaccination. We call these infants low vaccine responders (LVRs) [1]. Ten percent translates to 400,000–500,000 children/year in the United States alone. In the initial immune cell characterization of LVR infants we found lower percentages of antigen specific memory B cells, lower levels of T cell activation upon polyclonal stimulation, and lower basal MHC II expression by professional antigen presenting cells (APCs: monocytes, conventional DCs; cDCs, and plasmacytoid DCs; pDCs) [1]. Innate immunity instructs adaptive immunity to subsequently generate antibodies and memory response against infectious diseases. Poor innate immunity in LVR infants may be contributing to their poor adaptive immune response. Further, the suboptimal T and B cell responses in LVR infants[1] makes them rely on a strong innate immune response to protect against viral and bacterial infections. Therefore, we sought to determine the innate cytokine response from LVR infants compared to normal vaccine responder (NVR) infants.
Human conventional DCs (cDCs) and plasmacytoid DCs (pDCs) are the major producers of IL-12p70 and IFN- α/β respectively [2]. IL-12 plays a major role in co-stimulating Th1 immunity and IFN- α/β plays a crucial role in anti-viral immunity [3, 4]. IL-12 also mediates DC directed T cell differentiation in to T follicular helper cells (Tfh) which in turn facilitates generation of long term B cell memory responses [5]. Since the yield of PBMCs from infants is limited, we used a single TLR agonist which can simultaneously activate all 3 APC subsets. Thus we used a synthetic imidazoquinoline TLR7/8 agonist, resiquimod (R848) because human pDCs express TLR7 whereas monocytes and cDCs express TLR8 [6] and enabled us to study APC specific cytokine responses in peripheral blood. R848 is known to induce IL-12 and IFN-α from monocytes/cDCs and pDCs, respectively [2]. By 1 year of age infants are capable of producing adult level IFN-α and moderate levels of IL-12 [7]. Therefore, we stimulated the infant peripheral blood APCs with R848 to study the innate cytokine responses in LVR infants to determine if they were altered/suboptimal and potentially contributory to the previously observed defective T cell function, B cell memory and reduced vaccine response [1].
Methods
Subjects
Healthy, full term birth infants were enrolled from a middle class, suburban socio-demographic population in Rochester NY in a prospective, longitudinal study as previously described [1]. All vaccines were administered according to the recommended schedule of the CDC ACIP. Children were enrolled at age 6 months upon completion of their primary series of vaccinations from a private pediatric practice (Legacy Pediatrics, Rochester, NY). The blood samples analyzed in the current study for cytokines were from 6–9 months old age-matched LVR-NVR infants during healthy routine visits. Written informed consent was obtained from parents of the children in association with a protocol approved by the Rochester General Hospital Institutional Review Board.
LVR Group Definition
LVR children as a group has been defined previously [1]. Briefly, we performed a preliminary survey of antibody levels in 107 children against 13 vaccine antigens: DT, TT, PT, FHA, PRN, PRP, Hepatitis B, Streptococcus pneumoniae (Spn) capsular polysaccharides 6B, 14 and 23F and polio serotypes 1, 2 and 3 at 9–12 months of age. We defined Low Vaccine Responders (LVRs) as those infants who had sub-protective antibody responses to >50% of tested vaccine antigens; all others we defined as Normal Vaccine Responders (NVRs). Statistical analysis of these responses showed that a sub-protective response to 3 out of 6 antigens (among DT, TT, PT, FHA, PRN and PRP) could be used to distinguish LVRs from NVRs in a manner that was not significantly different from analysis of all 13 antigens. Therefore, antibody responses to these 6 antigens were measured in a cohort of 499 children.
PBMC sample processing and in vitro stimulation
PBMC processing was accomplished as previously described [1]. For in vitro stimulations, after thawing and washing, PBMCs were counted and rested overnight (200 μl/well at 2 X 106 cells/ml) in 96 well round bottom plates at 37°C in 5% CO2 incubator. After resting, total PBMCs were incubated for an additional 24 hours at 37°C in 5% CO2 incubator with or without R848 (1 μg/ml). After 24 hour culture, cells were spun and supernatants were collected and stored at -80°C until Luminex analysis.
Cytokine measurement
Supernatants stored at -80°C were thawed at room temperature and cytokine and chemokine levels of IL-1β, IL-6, IL-8, IL-10, IL12p70, IFN-γ, CXCL10 (IP-10), CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES) and TNF-α were measured from undiluted samples using Bio-Plex Pro Human Cytokine Group I 12-plex assay kit (Bio-Rad Laboratories, Hercules, CA) according to manufacturer’s instructions. IFN-α levels were analyzed separately by human IFN-α multi-subtype ELISA kit (Life Technologies). Prepared samples were run on a Bio-Plex 200 system with Luminex xMAP technology (Bio-Rad).
Statistics
All data were analyzed using Graph Pad Prism Software version 6.04(GraphPad Software, La Jolla, CA). Luminex and ELISA results were analyzed by nonparametric Mann-Whitney U test using background subtracted R848 stimulated individual cytokine values. p<0.05 was considered significant.
Results and Discussion
LVR infants induce higher baseline RANTES
We analyzed innate-associated cytokines/chemokines from PBMCs (number of infants per cohort included in figure legends) with and without R848 stimulation. Basal levels from un-stimulated PBMCs of most cytokines/chemokines tested were similar between LVR and NVR infants. However, LVR infants had significantly (p=0.03) higher basal RANTES (CCL5) levels compared to NVR infants (Fig. 1). R848 induced RANTES levels from LVR PBMCs were also high (p=0.06) compared to NVRs (Fig. 1). Multiple studies [8, 9] have shown that viral URI especially with influenza, rhinovirus and RSV result in elevated serum and nasopharyngeal levels of RANTES (CCL5), IP-10 (CXCL10) and IL-8. RANTES has been shown to induce T cell migration and its expression can be induced by respiratory viral infections [8]. Prospectively collected findings from children in our study cohort and our prior publications shows that LVR children are significantly more prone to influenza and respiratory bacterial infections compared to NVR children ([1] and unpublished observations). Further, RANTES is a ‘late’ T cell expressed cytokine. Therefore, we speculate that enhanced infection proneness in LVR children might be causing enhanced RANTES levels as a compensatory T cell recruitment mechanism. Fortunately due to herd immunity, LVR children have not shown an identifiable increase in vaccine preventable infections, except for influenza (unpublished observations). The PBMCs used for cytokine measurements in our studies were from LVR infants during “healthy” visits; however, LVRs have a significantly increased frequency of viral upper respiratory infections (URIs) so it is probable that we more often took their samples just prior to or in recovery from viral URIs thereby allowing us to capture high levels of RANTES. In our study, we also saw higher average values of IP-10 and IL-8 in LVR infants emphasizing the higher proinflammatory status of LVR compared to NVR infants (data not shown).
Figure 1.
Baseline and R848 induced RANTES (pg/ml) secretion from peripheral blood supernatants of LVR (N=17) and NVR (N=15) infants. Statistical significance was assessed by Mann-Whitney U test and data shows the geometric mean with 95% confidence interval.
LVR infants induce low IFN-α, IL-12 and IL-1β
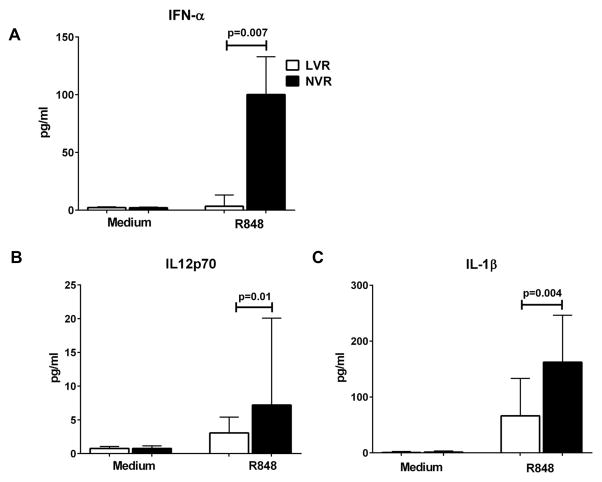
In contrast to higher levels of RANTES, LVR PBMCs stimulated with R848 secreted significantly (p<0.05) less IFN-α and IL-12p70 compared to NVR infants (Fig. 2A–B) suggesting a reduced capacity to respond to viral and bacterial infections. In addition, LVR infant PBMCs secreted significantly (P<0.05) lower pro-inflammatory IL-1β (Fig. 2C) with R848 stimulation compared to NVR infants. IFN-α plays a critical role in anti-viral immunity and IL-12p70 co-stimulates an effective Th1 response to infections [3, 4].
Figure 2.
R848 induced cytokine secretion (pg/ml) from peripheral blood supernatants of LVR and NVR infants. (A) multi-subtype IFN-α (B) IL-12p70 (C) IL-1β. Statistical significance was assessed by Mann-Whitney U test and data shows the median and interquartile range. IFN-α (LVR, N=14; NVR, N=14), IL-12p70 and IL-1β (LVR, N=17; NVR, N=15).
We previously reported low APC-MHC II and IRF7 expression levels indicating altered innate immune response in LVRs [1]. pDCs are one of the main cellular targets of R848 and the substantial IFN-α/β production by pDC is mediated by constitutive expression of IRF7 [10]. Recently, Love et al. [11] demonstrated that in an ex vivo human PBMC experimental model, production of IFN-α occurs through a TLR7 (and TLR9) - IRF7 dependent pathway. Hence, the low IFN-α level in R848 induced PBMCs from LVR infants may be a direct result of suboptimal IRF7 transcription.
The observed lower IL12p70 response in LVR children could also be related to a lower IFN-α level. Both IFN-α and IFN- γ receptors activate a common signaling pathway through STAT-1 phosphorylation and Gautier et al. [12], demonstrated that IL-12p70 production in response to TLR activation was dependent on production of IFN-α/β that regulated IL-12p35 mRNA accumulation. We observed a trend (p<0.08) towards lower fold change in expression of IL-12p35 in LVR infants (data not shown) compared to NVR infants indicating a possible indirect influence of IFN-α on IL-12p70 secretion. IL12p70 levels from 6–9 month old NVR infants showed at least 10 fold increases after stimulation with R848 compared to un-stimulated controls. IL12p70 levels we report here are similar to those described by other groups studying the ontogeny of TLR induced IL12p70 response in infants [7, 13]. Among the type I IFNs, we only tested for IFN-α (all subtypes). It may be of interest to examine other type I IFNs and type III IFNs in future work.
In addition to the TLR mediated effects, R848 has been shown to induce NLRP3 inflammasome-dependent IL-1β secretion from human monocytes and pDCs [7, 14]. The significantly low IL-1β secretion from LVR PBMCs compared to NVRs could be the result of an altered NLRP3 response in LVR infants.
Our study has limitations inherent to human clinical studies involving infants where blood volumes that may be taken are strictly controlled according to human investigation standards and where phlebotomy is extremely challenging. After completing 9 years of prospective enrollment of 499 infants in a 5-year longitudinal study, 56 (11% of total) were identified as LVR [1]. Because immune response changes dramatically with age during the first 2 years of life, it was necessary to use age-matched PBMCs from the infants. Multiple experiments to assess innate and adaptive immune response were undertaken using the samples collected. The limitation of blood volume collected and multiple uses of the PBMCs from infants precluded sorting of APCs from PBMCs to determine the exact source of cytokines. Therefore, we selected R848 stimulation to enable simultaneous stimulation of multiple APC subsets in a controlled fashion to objectively study APC cytokine response in LVR infants. In the future as more infants are identified as LVR and more PBMCs are collected we plan to analyze the APC response to various vaccine antigens and multiple TLR agonists to analyze intracellular signaling pathways in LVR infants.
In conclusion, the suboptimal innate cytokine response from LVR infants might make them unable to effectively regulate the APC antigen presentation and co-stimulatory functions to induce protective CD4 T cell and B cell antibody response as well as to surmount a protective initial response against vaccine-preventable viral and bacterial infections.
Acknowledgments
Funding statement: This work was supported by the National Institutes of Health (NIH) [National Institute on Deafness and Other Communications Disorders (NIDCD)] grant R01 08671. NS is supported by NIH NIAID R03 AI11700-01.
We thank Karin Pryharski and Jareth Wischmeyer for technical help and Drs. Robert Zagursky and Matt Morris for comments on the manuscript.
Abbreviations
- APC
antigen presenting cell
- cDC
conventional DC
- DT
diphtheria toxoid
- FBS
fetal bovine serum
- FHA
filamentous haemagglutinin
- IRF
IFN response factors
- LVR
low vaccine responders
- MHC
major histocompatibility complex
- MyD88
myeloid differentiation factor 88
- NVR
normal vaccine responders
- PBMC
peripheral blood mononuclear cells
- PRN
pertactin
- PT
pertussis toxoid
- PBS
phosphate-buffered saline
- pDC
plasmacytoid DC
- PRP
polyribosyl-ribitol-phosphate
- RANTES
regulated on activation, normal T expressed and secreted
- TT
tetanus toxoid
- TLR
toll-like receptor
- TRIF also TICAM 1
toll/IL-1 receptor domain-containing adaptor inducing IFN-β
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pichichero ME, Casey JR, Almudevar A, Basha S, Surendran N, Kaur R, et al. Functional Immune Cell Differences Associated With Low Vaccine Responses in Infants. The Journal of infectious diseases. 2016;213:2014–9. doi: 10.1093/infdis/jiw053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. European journal of immunology. 2009;39:26–35. doi: 10.1002/eji.200838391. [DOI] [PubMed] [Google Scholar]
- 3.Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, et al. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. European journal of immunology. 1996;26:659–68. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 4.Trinchieri G, Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. The Journal of experimental medicine. 1978;147:1314–33. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, et al. Early commitment of naive human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunology and cell biology. 2009;87:590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- 6.Philbin VJ, Levy O. Developmental biology of the innate immune response: implications for neonatal and infant vaccine development. Pediatric research. 2009;65:98R–105R. doi: 10.1203/PDR.0b013e31819f195d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbett NP, Blimkie D, Ho KC, Cai B, Sutherland DP, Kallos A, et al. Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PloS one. 2010;5:e15041. doi: 10.1371/journal.pone.0015041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito T, Deskin RW, Casola A, Haeberle H, Olszewska B, Ernst PB, et al. Respiratory syncytial virus induces selective production of the chemokine RANTES by upper airway epithelial cells. The Journal of infectious diseases. 1997;175:497–504. doi: 10.1093/infdis/175.3.497. [DOI] [PubMed] [Google Scholar]
- 9.Tekkanat KK, Maassab H, Miller A, Berlin AA, Kunkel SL, Lukacs NW. RANTES (CCL5) production during primary respiratory syncytial virus infection exacerbates airway disease. European journal of immunology. 2002;32:3276–84. doi: 10.1002/1521-4141(200211)32:11<3276::AID-IMMU3276>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 10.Izaguirre A, Barnes BJ, Amrute S, Yeow WS, Megjugorac N, Dai J, et al. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. Journal of leukocyte biology. 2003;74:1125–38. doi: 10.1189/jlb.0603255. [DOI] [PubMed] [Google Scholar]
- 11.Love AC, Schwartz I, Petzke MM. Borrelia burgdorferi RNA induces type I and III interferons via Toll-like receptor 7 and contributes to production of NF-kappaB-dependent cytokines. Infection and immunity. 2014;82:2405–16. doi: 10.1128/IAI.01617-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, et al. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. The Journal of experimental medicine. 2005;201:1435–46. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Wit D, Tonon S, Olislagers V, Goriely S, Boutriaux M, Goldman M, et al. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J Autoimmun. 2003;21:277–81. doi: 10.1016/j.jaut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Hurst J, Prinz N, Lorenz M, Bauer S, Chapman J, Lackner KJ, et al. TLR7 and TLR8 ligands and antiphospholipid antibodies show synergistic effects on the induction of IL-1beta and caspase-1 in monocytes and dendritic cells. Immunobiology. 2009;214:683–91. doi: 10.1016/j.imbio.2008.12.003. [DOI] [PubMed] [Google Scholar]