Abstract
Assessment provides the foundation for diagnosis, selection of treatments, and evaluation of treatment effectiveness for pediatric patients with acute, recurrent and chronic pain. Extensive research has resulted in the availability of a number of valid, reliable and recommended tools for assessing children’s pain. Yet, evidence suggests children’s pain is still not optimally assessed or treated. In this article, we provide an overview of pain assessment for premature neonates to adolescents. The difference between pain assessment and measurement will be highlighted; and the key steps in pain assessment identified. Information about self-report and behavioral pain assessment tools appropriate for children will be provided; and factors to be considered when choosing a pain assessment tool will be outlined. Finally, we will preview future approaches to personalized pain medicine in pediatrics that include harnessing the assessment potential of digital health technologies and genomics.
Introduction
Acute, recurrent, and chronic pain is not optimally assessed, evaluated or treated in children. Extensive research has led to the availability of valid, reliable, and recommended tools to assess and measure children’s pain. Efforts to improve pediatric pain management have included strategies to standardize and improve use of validated pain assessment tools and encourage comprehensive evaluation. The disparity between improvements in pain assessment practices and pain management outcomes suggests that the way the evidence for pain assessment and evaluation has been translated in clinical practice may have failed.
Assessing pain does not cause pain! Physiologic, behavioral, and self-report indicators can be used to assess children’s pain; but it is inappropriate to use these assessments to negate children’s pain experiences. Failing to adequately assess, failing to sufficiently evaluate, and failing to appropriately respond to pain assessments has perpetuated poor pediatric pain management and suboptimal treatment of children’s pain.
Simplified approaches to pain assessment, even with valid and reliable tools, may be especially problematic in pediatric settings where children present with a variety of developmental, cognitive, and affective characteristics, posing unique challenges for clinical decision-making. Despite these challenges there is a movement towards personalized medicine using patient and parent reported outcomes through mobile and ehealth technologies. Combined with genetic, sensory and psychological tests, we may be able to anticipate patients’ pain experiences and responses to treatments. In this article, we will provide an overview of valid and reliable pediatric pain assessment tools. We will also explore the future of pediatric pain evaluation with genetic, sensory, psychological, and mobile ehealth assessment strategies.
Pain Definitions and Differentiation
Pain is a multidimensional and complex phenomenon that requires comprehensive and ongoing assessment for effective management. Pain is a biopsychosocial phenomenon that includes sensory, emotional, cognitive, developmental, behavioral, spiritual and cultural components. Defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage; pain is always subjective. Each individual learns the application of the word through experiences related to injury in early life.”1 A more commonly quoted definition, “pain is whatever the experiencing person says it is, existing whenever the experiencing person says it does;” emphasizing the subjective nature of pain.2 However, “the inability to communicate (i.e., infants, young children, those with cognitive impairments) in no way negates the possibility that an individual is experiencing pain and is in need of appropriate pain relieving treatment.”3
Pain may be acute, recurrent, chronic, or a combination of acute on chronic. Acute pain typically serves as a warning of disease or a threat to the body. Acute pain is associated with (1) medical procedures (e.g., injections, electromyogram, surgery), (2) injury, (e.g., bumps, bruises, broken bones), and (3) acute illness (e.g., meningitis, strep throat) and exacerbation of diseases (e.g., sickle cell disease, juvenile idiopathic arthritis, ulcerative colitis).4 Unrelieved acute pain has a number of undesirable physical and psychological consequences which can negatively impact all aspects of quality of life and may lead to chronic pain. Chronic pain is a term used to describe persistent or recurrent pain.
Chronic pain in children and adolescents is commonly defined as any prolonged pain that lasts longer than expected (arbitrarily defined as more than 3 months) or any recurrent pain that occurs at least 3 times throughout a period of 3 months, such as headaches. 5 In a systematic review of recurrent and chronic pain in children, the most common pains were headaches, stomachaches and musculoskeletal pain.6
Pain was found to increase with age and was more common in females. A subgroup of children and adolescents (5–8%) report severe disability and distress associated with chronic pain.7 It is now postulated that acute and persistent or chronic pain are a continuum, rather than separate entities. Pain can be nociceptive (arising from activation of nociceptors due to actual or threatened damage to non-neural tissue), neuropathic (pain caused by a lesion or disease of the somatosensory nervous system), or mixed (both nociceptive and neuropathic). Nociceptive pain is further subdivided as somatic (bone, muscle, joint, skin or connective tissue) or visceral (organs such as stomach and pancreas). Nociceptive pain quality is usually described as sharp or aching sensation, well-localized or diffuse. Neuropathic pain quality is usually described as a burning or shooting sensation, and may be associated with heightened sensitivity to stimuli (allodynia) or abnormal sensations (paresthesia and dysesthesia).
Pain Measurement, Assessment and Evaluation
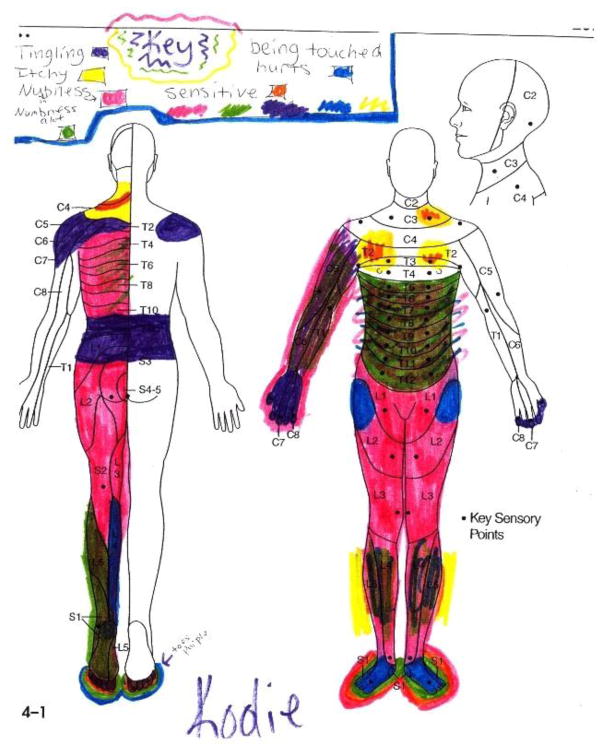
Pain measurement generally describes the quantification of pain intensity. The emphasis is on the quantity, extent or degree of pain.8 Whereas, pain assessment is a broader concept and involves clinical judgment based on observation of the nature, significance and context of the child’s pain experience.8 The majority of pain assessment tools focus on measuring pain intensity. However, a thorough pain assessment provides information critical for evaluating the pain experience, diagnosing the most likely cause of pain, and choosing the most appropriate treatments. Pain assessment emphasizes the multidimensional nature of pain: 1) intensity; 2) location; 3) duration; 4) sensory qualities (e.g. word descriptors); 5) cognitive aspects (e.g. perceived impact on activities of daily life); 6) affective aspects (e.g. pain unpleasantness); and 7) the contextual and situational factors that may influence children’s perceptions of pain (See Figure 1).
Figure 1.
Drawing of pain location and sensations from adolescent girl with transverse myelitis.
To treat pain effectively, ongoing assessment of the presence and severity of pain and the child’s response to treatment is essential.9 Pain assessment poses many challenges in infants and children because of: (a) the subjective and complex nature of pain; (b) developmental and language limitations that preclude comprehension and self-report; (c) dependence on others to infer pain from behavioral and physiologic indicators; and (d) the social context of pain (e.g., differences in pain perception and expression depending on age, sex, race and ethnicity). When assessing pain in children there are three key steps (See Box 1).
Box 1. Key Steps to Assessing Children’s Pain.
Step 1) Obtain a pain history;
Step 2) Assess the child’s pain using a developmentally appropriate pain assessment tool
Step 3) Reassess the child’s pain having allowed time for pain-relieving interventions to work
Standardized pain history forms have been developed to facilitate obtaining thorough histories of acute pain experiences from children and parents.10–12 For children with chronic pain, more detailed histories of pain experiences are required (See Box 2). Given the disability associated with chronic and recurrent pain, these critical components of pain assessment should be included when considering referral to pediatric neurologists or pain management specialists.
Box 2. Components of a detailed chronic pain history.
Details of chronic pain histories should include:
description of pain experience
associated symptoms
temporal or seasonal variations
impact on activities of daily living (e.g. physical including sleep, emotional, role, and social functioning); and
pain-relieving efforts and interventions used
The three approaches to measuring pain are: 1) self-report (what the child says), 2) behavioral (how the child behaves), and 3) physiological indicators (how the child’s body reacts). These three approaches may be used separately but have also been combined in several pain assessment tools that are available for clinical use. Self-report tools should be used with children who are: (a) old enough to understand and use a self-report scale, (b) not overtly distressed, and (c) not cognitively impaired. With infants, toddlers, and preverbal, cognitively impaired and sedated children, behavioral pain assessment tools should be used. 13 If the child is overtly distressed (e.g. due to pain, anxiety or some other stressor), no meaningful self-report can be obtained; and efforts should focus on lessening the child’s distress (e.g. administering analgesics, coaching child to use coping skills, modifying the child’s environment).
Self-report pain tools
Of 30 available pediatric self-report pain intensity measures, only 6 have well-established psychometric properties, including evidence of reliability, validity, clinical utility and feasibility for use with children and adolescents with acute and chronic pain (Table 1).14 No single pain intensity scale is reliable and valid across all pediatric age groups or types of pain.
Table 1.
Pediatric self-report pain intensity measures and pain assessment tools
| Tool (Acronym) | Reference (Year) | Age Range | Type of Pain | Comments |
|---|---|---|---|---|
| Adolescent Pediatric Pain Tool (APPT) | Savedra, et al. (1989) Jacob, et al. (2014) |
8+ | Acute, Procedural, Post-op, Disease-related, Chronic | Validated to assess pain intensity, pattern or timing, location (using a body drawing), and quality of pain is reported by the patient indicating or circling sensory, affective, evaluative and temporal words. Available in English and Spanish. |
| Bath Adolescent Pain Questionnaire (BAPQ) | Eccleston, et al. (2005) | 11–18 | Chronic | Validated to assess the impact of chronic pain |
| Faces Pain Scale-Revised (FPS-R) | Hicks et al. (2001) Bieri et al. (1990) | 4–12 years | Acute, Procedural, Post-op, Disease-related | Highly feasible. Neutral anchors. Recommended by PediIMMPACT |
| Numeric Rating Scale (NRS) | 8+ | Acute, Procedural, Post-op, Disease-related, Chronic | Highly feasible and therefore preferred by clinicians but not children, even older children when asked prefer one of the faces scales. | |
| Oucher | Beyer & Aradine (1986) | 3+ | Acute, Procedural, Post-op, Disease-related | Available with photographs of different races/ethnicities to facilitate cultural competency. Color copies needed making feasibility moderate. |
| Pediatric Pain Assessment Tool (PPAT) | Abu-Saad, Kroonen & Halfens (1990) | 5+ | Post-op, Disease-related, Chronic | Validated to assess pain intensity and quality of pain by circling words in the sensory, affective, evaluative and temporal domains of pain. |
| Pediatric Pain Questionnaire (PPQ) | Varni & Thompson (1985) | 5+ | Disease-related, Chronic | Validated to assess chronic pain intensity, location, sensory, evaluative and affective qualities of pain. Available in 7 languages |
| Visual Analog Scale (VAS) | Scott et al. (1977) | 8+ | Acute, Procedural, Post-op, Disease-related, Chronic | Moderate feasibility due to need to mark across 10 cm. line and then measure from 0 to mark. This also makes it more difficult to clinically track over time. Often used in research |
| Wong–Baker FACES Pain Scale (WBPRS) | Wong & Baker (1988) | 3+ | Acute, Procedural, Post-op, Disease-related | Validated with 0–5 & 0–10. Anchors. Anchor faces are smiling and crying tears which may confuse measurement of intensity and affect. |
The numeric rating scale (NRS) allows patients an opportunity to quantify their pain, ranking pain severity on a scale of 0–10 or 0–5, with the 0 anchor representing “no pain” and 5 or 10 representing the “worst possible pain.” This self-report scale is valid for use with children 8 years of age and older who can understand numeric rank and order.15 The scale is easy to use and scores can be tracked over time. Strong correlations have been shown with the NRS, visual analogue scale (VAS) and the Faces Pain Scale-Revised (FPS-R); although children have a tendency to provide a higher rating with the NRS.15–17 To use the VAS, the patient is asked to rate the unpleasantness of pain experience by placing a mark across a 10-cm line at a point that corresponds to the level of pain intensity. The distance in centimeters from the low anchor of the VAS to the patient’s mark is used as a numeric index of pain severity.18
Pictorial adaptations of the VAS have been validated for children as young as 3 years of age. When asked, school-aged children 6 to 16 years of age prefer the FPS-R to the NRS for reporting their pain intensity.17, 19 Of 14 faces scales reviewed, only 4 scales were evaluated as valid and reliable based on psychometric data (Faces Pain Scale (FPS) scored 0–6; FPS-R scored 0–10; Wong-Baker Faces Pain Rating Scale (WBFPRS), and Oucher pain scale).14, 20 Reviewers expressed concerns that use of smiling and crying anchor faces in the WBFPRS may confound pain intensity with affect.14, 20 Yet, emotional response to pain, negative affect or distress secondary to pain is considered an important domain of measurement in clinical trials addressing either acute or chronic pediatric pain therapies 4
A commonly used criterion for evaluating a change in pain as clinically significant has been a 50% reduction in pain intensity. However, in studies of both acute and chronic pain, adults report subjective evaluation of ‘‘Very much’’ and ‘‘Much improved’’ for 30–33% reductions in pain on a NRS.21, 22 The minimum clinically significant difference in pain scores associated with pain relief in school-aged children is only 1 number change on the 11 point NRS.23, 24 Variability in scores in relation to other clinically meaningful outcomes, like need for pain medication and satisfaction with pain management, suggests the use of pre-determined cut-points for pain treatment decisions is inappropriate. There is no research linking analgesic dose to pain relief at specific patient-reported pain intensity numbers; and this practice puts patients at risk for oversedation, respiratory depression, and poorly managed pain.25
Behavioral tools and Tools for Assessing Pre-verbal and Non-verbal Pediatric Patients
Pain evaluation and assessment is challenging in pre-verbal and non-verbal patients. A hierarchy of pain assessment techniques is recommended, which errs on obtaining self-report whenever possible (See Box 3).26 The majority of individuals with intellectual disabilities are verbal and can self-report their pain experience when provided with developmentally appropriate self-report pain assessment tools.
Box 3. Hierarchy of pain assessment techniques for pre-verbal and non-verbal patients.
-
1)
Obtain self-report using developmentally appropriate valid and reliable pain assessment tool
When self-report is not possible and pain is suspected:
-
2)
Search for possible causes of pain
-
3)
Assess the pre-verbal or non-verbal patient’s behaviors
-
4)
Attempt and evaluate response to an analgesic trial.
There are 14 commonly used, valid and reliable proxy scales for assessing pain of pre-verbal and non-verbal pediatric patients who cannot self-report their pain experience (Table 2). These pain assessment tools are actually indirect measures of pain; they don’t indicate the intensity of pain but rather the intensity of pain-related distress and pain reactivity. These scales are influenced by contextual factors and are most reliable for procedural pain, rather than ongoing chronic pain assessments.
Table 2.
Tools for assessing pain of pre-verbal and non-verbal pediatric patients
| Tool (Acronym) | Reference (Year) | Age Range | Type of Pain | Parameters |
|---|---|---|---|---|
| Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS) | McGrath et al. (1985) Suraseranivongse et al., (2001) |
4 months to 17 years | Procedural, Post-op | Sum of 6 parameters (cry, facial, verbal, torso, touch, legs) for total observation score from 4 to 13. |
| Children’s and Infants’ Postoperative Pain Scale (CHIPPS) | Bringuier et al., (2009); Buttner & Finke, (2000) | Birth to 5 years | Acute, Post-op | Sum of 5 parameters (cry, facial, trunk poster, leg posture, motor/restlessness) for total observation score from 0 to 10. |
| COMFORT Behavior Scale | de Jong et al., (2010) van Dijk et al., (2000) van Dijk, Peters, van Deventer& Tibboel (2005) | Neonates to 3 years | Acute, Intensive Care Post-op | Used to assess distress, sedation and pain. Sum of 8 parameters (alertness, calmness, respiratory distress, physical movement, muscle tone, facial tension, blood pressure, heart rate) for total observation score from 8 to 40. Also valid without physiologic parameters (COMFORT B). |
| Crying, Requires oxygen, Increased vital signs, Expression, and Sleeplessness (CRIES) | Ahn & Jun, (2007); Krechel & Bildner, 1995 | Neonates | Acute, Intensive Care Procedural, Post-op | Sum of 5 parameters (Crying, Requires oxygen –oxygenation, Increased vital signs, Expression, and Sleeplessness) for total observation score from 0 to 10. |
| Distress Scale for Ventilated Newborn Infants (DSVNI) | Sparshott (1996) | Ventilated Neonates & Infants | Acute, Intensive Care Procedural | Sum of 4 physiologic (heart rate, blood pressure, oxygen saturation, and temperature differential), and 3 behavioral (facial expressions and body movements) |
| Faces, Legs, Activity, Cry, and Consolability Observational Tool (FLACC) | Ahn & Jun (2007); Manworren & Hynan, (2003); Merkel, et al., (1997); Voepel-Lewis et al., (2002); Voepel-Lewis, et al., (2010); Willis, et al, (2003) | 0 to 18 years | Acute, Procedural, Post-op, Disease-related | Sum of 5 parameters (Facial, Legs, Activity, Cry, and Consolability) for total observation score from 0 to 10. |
| Revised Faces, Legs, Activity, Cry, and Consolability Observational Tool (rFLACC) | Malviya, Voepel-Lewis, Burke, Merkel, & Tait, (2006); Voepel-Lewis et al. (2002); Voepel-Lewis, Malviya, Merkel, & Tait, (2003); Voepel-Lewis, Malviya, & Tait, (2005) | 4–19 years with mild to severe intellectual disabilities | Acute, Post-op | Observations and scoring are similar to the FLACC with descriptions to parameters to characterize behaviors of cognitively impaired children in pain and allows the addition of individual patient’s pain behaviors |
| Individualized Numeric Rating Scale (INRS) | Solodiuk & Curley, (2003); Solodiuk et al., (2010) | 6–18 years with severe intellectual disabilities in acute care settings | Post-op | A personalized pain assessment tool for nonverbal children with intellectual disability based on the parent's knowledge of the child. Parents describe and then rank order their child's usual and pain indicators. |
| Neonatal Infant Pain Scale (NIPS) | Lawrence et al. (1993) | Pre-term and term infants | Sum of five behavioral (facial expression, crying, movement of arms and/or legs, state of arousal) and one physiologic (breathing pattern) parameter for a total of 0 to 7. | |
| Neonatal Pain, Agitation, and Sedation Scale (N-PASS) | Hummel, Puchalski, Creech, & Weiss, 2008; Hummel, Lawlor-Klean, & Weiss, 2010 | Pre-mature neonates 23–40 weeks gestation; | Procedural & Postop during mechanical ventilation in Neonatal intensive care unit | Used to assess sedation and pain. Sum of cry, behavior, facial expression, extremity tone, and vital signs in the context of gestational age for total observation score from −2 to +2 for each parameter. |
| Non-communicating Children’s Pain Checklist (Acute care NCCPC) | Breau, et al., (2000, 2001, 2002, 2004); Breau, (2003); Breau & Camfield, (2011); Burkitt, Breau, & Zabalia, (2011); Lotan et al., (2009) | 3 to 18 yrs with intellectual disabilities in hospital, rehab & home/re sidential settings | Post-op, Chronic | Caregivers of children with severe cognitive impairments recorded their observations of their children The NCCPC-PV (post-operative version) has 8 parameters scored 0 to 3 each (vocal, social, facial, activity, body/limbs, physiologic) |
| Paediatric Pain Profile | Hunt et al., 2004 | 1–18 years of age with severe physical and neurologic impairments | Acute Disease-related Chronic | Sum of 20 unique behaviors that may indicate pain in children unable to communicate through speech or augmentative communication devices. Each item is scored from “not at all” to “a great deal” (0 to 3) for a score of 0 to 60. |
| Premature Infant Pain Profile (PIPP) and Premature Infant Pain Profile –Revised (PIPP-R) | Ahn & Jun, (2007); Stevens et al.,(1996); Stevens, Johnston, Taddio, Gibbins, & Yamada, (2010); Gibbins, Stevens, Yamada, et al. (PIPP-R, 2014) | Pre-mature & term neonates | Procedural & Postop in Neonatal intensive care unit | Sum of facial actions, such as brow bulge, eyes squeeze, and nasolabial furrow, heart rate and oxygen saturation, in the context of gestational age and behavioral state for total observation score from 0 to 21. |
| Toddler-Preschooler Postoperative Pain Measure (TPPPM) | Suraseranivongse et al., (2001); Tarbell, Cohen, & Marsh, (1992) | 1 to 5 | Acute, Post-op | Sum of 7 items from 3 pain parameters: (1) Vocal pain expression; (2) Facial pain expression; and (3) Bodily pain expression. |
Unidimensional scales validated to assess pain in pre-verbal and non-verbal pediatric patients generally rely on behaviors associated with acute pain (Box 4.) Behavioral responses are attenuated by severity of illness, gestational age, and development. Therefore, pain assessment tools validated in neonates include adjustments for gestational age. But since older infants, toddlers and non-verbal children may voluntarily alter behaviors when anticipating or experiencing pain; their parents and healthcare providers must consider pain assessments scores obtained using even these validated behavioral tools as proxy pain measures to be interpreted based on the child’s expected or previously experienced pain from similar procedures and conditions. Multidimensional scales may also include cry characteristics, physiologic measures, and methods to communicate children’s individual differences for responding to pain.
Box 4. Behaviors associated with acute pain in pre-verbal and non-verbal pediatric patients.
Vocalizations (e.g. crying);
Facial expressions (e.g. quivering chin, nasolabial furrowing);
Large body movements (e.g. withdrawal of the affected limb, touching the affected area, and movement or tensing of limbs and torso);
Changes in social behavior or appetite;
Changes in sleep/wake state or cognitive functions; and
Behavioral responses to interventions
Multidimensional pain tools
Although pain intensity is the most commonly recorded measure of a painful episode, a more comprehensive pain assessment is often necessary. For example, assessing factors such as pain triggers, pain quality, and how pain interferes with aspects of everyday life are critical for evaluating children with chronic pain. Four self-report pain tools that have been shown to be reliable and valid multidimensional pain measures: Adolescent Pediatric Pain Tool (APPT), 27 Pediatric Pain Assessment Tool (PPAT), 28 Pediatric Pain Questionnaire (PPQ),29 and the Bath Adolescent Pain Questionnaire (BAPQ) 30 (Table 1).
PROMIS® (Patient-Reported Outcomes Measurement Information System) offers psychometrically sound, validated, person-centered measures designed to enhance communication between clinicians and patients to help evaluate and monitor physical, social, and emotional health. 31 PROMIS® was created to be relevant to the general population and across all chronic conditions to assess symptoms and functions. Self-report measures are available for children 8–17 years of age; and parent-proxy measures are available for children 5–17 years of age. PROMIS® is publicly available without license or fee in English and Spanish as short forms, computer adaptive tests, and profiles. Available measures appropriate for assessing children with pain include: global health, emotional distress (anger, anxiety, depressive symptoms), life satisfaction, meaning and purpose, positive affect, psychological stress experiences, fatigue, pain behaviour, pain interference, pain quality, physical activity, physical function (mobility & upper extremity), physical stress experience, strength impact, family relationships, and peer relationships.
Tools for Assessing Other Components of Pain
Emotional response or emotional functioning includes the affective component of pain as well as anxiety, depression, fear, distress, dysphoria, or unhappiness. Behaviorally these may be expressed by avoidance, withdrawal, or active resistance. The Procedure Behavior Check List (PBCL) 32 and the Procedure Behavioral Rating Scale Revised (PBRS-R) 33 are observational measures for assessing behavioral distress during painful procedures, like venipuncture. These measures are validated for assessing the behavioral response to pain with patients 1 year of age and older.
The only single-item tool that specifically measures the affective component of pain is the Facial Affective Scale. 34 This scale consists of 9 faces that vary sequentially in depiction of overt levels of distress. This self-report measure is used with young children; but children less than 8 to 10 years of age may be unable to differentiate the affective component of pain from pain intensity.
Anxiety and depression are commonly elevated in children with acute, chronic and recurrent pain; 35 but most children with pain do not have clinical levels of anxiety or depression.36 There are many validated measures of depression and anxiety for children. However, almost all were validated for use in children with chronic illnesses, including chronic pain, and mental health problems. The Children’s Depression Inventory is a well-established psychometrically sound tool to measure depression for children 7 to 17 years of age.37 It is has been used in many pediatric chronic pain studies. The Revised Child Anxiety and Depression Scale (RCADS) is also frequently used in pediatric chronic pain studies.38 This validated measure of anxiety and depression is a measure of negative affect when the two scales are combined.
Sleep disruption is common in acute, chronic, and recurrent pain.39, 40 Sleep disturbance and fatigue are strongly associated with increased pain and decreased quality of life.41 Self-report questionnaires from samples of adolescents with headache, juvenile idiopathic arthritis, and sickle cell disease pain indicate depressive mood is predictive of sleep problems.42 While the gold standard for measurement of sleep is night time polysomnography, this is an unrealistic measure of sleep for widespread clinical use and ongoing treatment. Actigraphy, another validated strategy for measuring sleep with a movement sensor, is less intrusive and expensive.40 When compared against actigraphy, sleep diaries may be valid for use with healthy adolescents.43 The parent or child records bedtime, time of sleep, and time awakening in sleep diaries. Another tool that may be useful for assessing sleep with school aged children is the Sleep Habits Questionnaire.44
Physical recovery is assessed for acute pain; and physical function, such as activities of daily living like sitting, walking, or even participation in vigorous activities and sports, is assessed for chronic pain. Pain significantly impacts daily functioning, productivity, social interaction, and quality of life.45, 46 Migraine, for example, is ranked as the 8th leading cause of disability. 47
The Functional Disability Inventory is a valid tool for assessing school-aged children and adolescents self-report of ability to function in everyday physical activities. 48–51 Whereas, the PedsQL is a valid tool for assessing the physical functioning of younger children (less than 7 years of age).52 The PedsQL is validated for use with pediatric patients 2 to 18 years of age; and includes parent and child report versions. This multidimensional scale also measures emotional, social, and school function.
Pain intensity variability throughout a day is common in patients with chronic pain. Disease severity predicts high pain variability, and higher pain variability predicts lower quality of life. 53 Acute, chronic, and recurrent pain can significantly interfere with children’s roles, like student, friend, employee, and family member. Children and adolescents with chronic pain have fewer friends, are subjected to more peer victimization, and viewed as more isolated and less likeable than healthy peers.54 A variety of measures have been used to examine peer relationships. Measures specifically designed to assess peer relationships, especially the quality of peer relationships of children and adolescents with pain, are needed. PedMIDAS is a measure of role functioning in children 6 to 18 years of age; and has been validated for use with children experiencing persistent headache.55, 56 School attendance is also a simple measure of school-aged children’s role function.
Families of children with chronic pain generally have poorer family functioning than families of healthy children and adolescents.57 Pain-related disability is more consistently related to family functioning than pain intensity. There is significant variation in family functioning measures. These differences must be considered when selecting a tool for clinical use, since family-function may be an important target for intervention.
Prospective and mobile ehealth assessment strategies
There are currently no validated tools that measure aggravating and alleviating factors of pediatric pain. Yet, this is often the key assessment information needed to develop an effective individualized pain management plan. Research has shown that pediatric migraines are precipitated by stress and more specifically, stress related to school examinations.58, 59 School stressors such as harassment by peers, schoolwork pressure, and classroom disturbances are associated with both frequent headaches and abdominal pain.60 Other factors and predictors for headaches have included: parental headaches, family stress and fewer friends, parental divorce, relationships with parents, housing conditions, socio-economic status, and the presence of emotional– behavioral problems.61–66 We may be missing other potentially relevant triggers such as physical activity, mood, fatigue, and more specific stressors at home and school settings and during leisure time, simply by not asking about these potential triggers. About 50% of 10-to 17-year olds report knowing the cause of their headaches.67 However, our understanding of headache triggers in children and adolescents is hampered by study designs.60–67
Prospective diary recordings in community populations are needed to elicit more accurate and reliable information to thoroughly evaluate headache prevalence and characteristics in children and adolescents and to conduct statistical analyses of temporal and casual relationships. Pain diaries are another way to track pain in children with recurrent or chronic pain. While paper-based diaries have been used in clinical and research practice for decades, they are prone to recall biases and poor compliance. More recently, real-time data collection methods using electronic hand-held diaries have been developed for children with recurrent and chronic pain.68–73
Electronic pain diaries have the advantage over paper headache diaries for maximizing participant use, and may be valuable for tracking treatment outcomes.73–77 Electronic pain diaries are now readily available; however availability should not be confused with quality. In a systematic review of 21 headache e-diaries, only 5 reported on their development, components, features, psychometric properties, and feasibility.78 In another review of 279 pain management apps, less than 10% had a healthcare professional on their development team and only 1 app had been evaluated for effectiveness.79 Healthcare professionals must evaluate e-diaries and pain apps as rigorously for reliability, validity, and efficacy as any other pain assessment tool or treatment.
Quantitative Sensory Testing (QST) and Genetics
Parents and healthcare providers have recognize that some children and adolescents are more sensitive to pain than others. Quantitative Sensory Testing (QST) is feasible and valid for children over 5 years of age. Like adults, there are gender and body site differences of somatosensory functions; for example, the face is more sensitive than the foot.80 Younger children (6 to 8 year olds) are generally less sensitive to thermal and mechanical stimuli; but more sensitive to pain stimuli than older children (>9 year olds). Girls are more sensitive to thermal stimuli; but not mechanical stimuli. Reference values differ from adults. While QST has been used extensively in research, and may provide insight into the etiology of pain, it is not currently applicable for routine pediatric clinical diagnostics.81
Family history of chronic pain conditions suggests a genetic predisposition to pain sensitivity and pain disorders. Catechol-O-methyltransferase (COMT) is the most studied and well characterized gene with a role in pain sensitivity.82 COMT has been implicated in migraine and neuropathic pain. On the other hand, individuals homozygous for a rare GTP cyclohydrolase 1 (GCH1) allele experience less pain even after nerve injury. Phenotypic associations with specific allele variances may eventually explain individual variability in pain experiences from similar painful stimuli.
Children and adolescents with chronic pain, who report analgesic ineffectiveness or adverse analgesic effects, may benefit from pharmacogenetic testing as part of their clinical evaluation. In our case series of 19 patients who reported ineffective or adverse analgesic effects from a mean of 12 analgesics, significant genetic variances of CYP2D6 and CYP2C19 were identified in all but one patient.83 Further research is needed to translate pharmacogenetic test results into clinical guidance for pediatric analgesic pharmacotherapy.
Genomics, metabolomics, and proteinomics may provide the objective biomarkers of pain necessary to optimize pain assessment sensitivity and specificity; and treatment for even the most vulnerable non-verbal patients experiencing pain. Precision medicine may allow us to better assess individual variability of pain sensitivity and advance a new paradigm for treating children with pain.
Conclusion
Assessing pain does not cause pain; but not assessing children for pain leads to under-recognition and unrelieved pain. Children’s self-reports of pain are important and should be sought whenever possible. Physiologic and behavioral indicators do not negate children’s self-reported pain experiences. Children who are non-verbal or who have cognitive impairments are more vulnerable for pain and for having their pain underestimated. Valid and reliable pain assessment tools are available for children of all ages and developmental levels. Pain intensity measures (pain scores) alone are insufficient for assessing pain; and therefore should not be used in isolation to guide pain treatment decisions. Additional valid and reliable tools should be used to assess the impact of pain on children’s quality of life (sleep, activities, social, school, etc.) and develop holistic pain management plans.
Pain assessment is vital for effective pain management. The first step in assessing pain is recording a pain history. The second step is assessing the child’s pain using a developmentally appropriate pain assessment tool. The third step is evaluating the effectiveness of the pain-relieving interventions. Pain should be assessed regularly to detect the presence of pain and to evaluate the effectiveness of treatments. In the future, use of genomics, patient and parent reported outcome measures, and digital health technologies may help tailor treatments to individual patients based on their unique pain profiles and preferences.
Table 3.
Recommended Resources for acute and chronic pain in children
| Online Resources |
|
| Books for Children |
|
| Books for Parents |
|
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Renee CB Manworren, Posy and Fred Love Chair in Nursing Research, Advanced Practice Nurse, Acute Pain Management Program, Ann & Robert H. Lurie Children’s Hospital of Chicago, Associate Professor, Department of Pediatrics, Feinberg School of Medicine, Northwestern University, Chicago, IL, Principal Investigator, UCONN Center for Advancing Management of Pain, NIH Center of Excellence in Pain Education.
Jennifer Stinson, Mary Jo Haddad Nursing Chair in Child Health, Peter Lougheed CIHR New Investigator, Scientist, Child Health Evaluative Sciences, Nurse Practitioner, Chronic Pain Program, The Hospital for Sick Children, Associate Professor, Lawrence S. Bloomberg, Faculty of Nursing, University of Toronto.
References
- 1.International Association for the Study of Pain. Pain terms: A list with definitions and notes on usage. Pain. 1979;6:249–52. [PubMed] [Google Scholar]
- 2.McCaffery M. Nursing Management of the Patient in Pain. Philadelphia: Lippincott; 1972. [Google Scholar]
- 3.International Association for the Study of Pain. IASP definition of pain. IASP Newsletter. 2001;2:2. [Google Scholar]
- 4.McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain. 2008;9(9):771–783. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 5.American Pain Society. Assessment and management of children with chronic pain: A position statement from the American pain society. Available at: http://americanpainsociety.org/uploads/get-involved/pediatric-chronic-pain-statement.pdf.
- 6.King S, Chambers C, Huguet A, MacNevin RC, McGrath PJ, Parker L, et al. The epidemiology of chronic pain in children and adolescents revisited: A systematic review. Pain. 2011;152:2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Miró J, Huguet A, Nieto R. Predictive factors of chronic pediatric pain and disability: A Delphi poll. J Pain. 2007;8(10):774–92. doi: 10.1016/j.jpain.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Johnston C. Psychometric issues in the measurement of pain. In: Finley GA, McGrath PJ, editors. Measurement of Pain in Infants and Children, Progress in Pain Research Management. Vol. 10. Seattle: IASP Press; 1998. pp. 5–20. [Google Scholar]
- 9.American Medical Association. [Accessed January 2013];Pain management: Pediatric pain management. Available at: www.ama-cmeonline.com/pain_mgmt/printversion/ama_painmgmt_m6.pdf.
- 10.Hester NO, Barcus CS. Assessment and management of pain in children. Pediatrics Nursing Update. 1986;1(14):1–8. [Google Scholar]
- 11.Ball J, Bindler R. Pain assessment and management. In: Ball J, Bindler R, editors. Pediatric nursing: Caring for children. Norwalk: Appleton & Lange; 1995. [Google Scholar]
- 12.Hester NO, Foster RL, Jordan-Marsh M, Ely E, Vojir CP, Miller KL. Putting pain measurement into clinical practice. In: Finley GA, McGrath PJ, editors. Measurement of Pain in Infants and Children, Progress in Pain Research Management. Vol. 10. Seattle: IASP Press; 1998. pp. 179–98. [Google Scholar]
- 13.von Baeyer CL, Spagrud LJ. Systematic review of observational (behavioral) measures for children and adolescents aged 3 to 18 years. Pain. 2007;127:140–50. doi: 10.1016/j.pain.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Stinson J, Yamada J, Kavanagh T, Gill N, Stevens B. Systematic review of the psychometric properties and feasibility of self-report pain measures for use in clinical trials in children and adolescents. Pain. 2006;125(1–2):143–157. doi: 10.1016/j.pain.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 15.von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children's self-reports of pain intensity. Pain. 2009;143(3):223–7. doi: 10.1016/j.pain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Connelly M, Neville K. Comparative prospective evaluation of the responsiveness of single-item pediatric pain-intensity self-report scales and their uniqueness from negative affect in a hospital setting. J Pain. 2010;11(12):1451–60. doi: 10.1016/j.jpain.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Pagé MG, Katz J, Stinson J, Isaac L, Martin-Pichora AL, Campbell F. Validation of the numerical rating scale for pain intensity and unpleasantness in pediatric acute postoperative pain: Sensitivity to change over time. J Pain. 2012;13(4):359–69. doi: 10.1016/j.jpain.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Fowler-Kerry S, Lander JR. Assessment of sex differences in children’s and adolescents’ self-reported pain from venipuncture. J Pediatr Psychol. 1991;16(6):783–793. doi: 10.1093/jpepsy/16.6.783. [DOI] [PubMed] [Google Scholar]
- 19.Miró J, Castarlenas E, Huguet A. Evidence for the use of a numerical rating scale to assess the intensity of pediatric pain. Eur J Pain. 2009;13(10):1089–95. doi: 10.1016/j.ejpain.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Tomlinson D, von Baeyer CL, Stinson JN, Sung L. A systematic review of faces scales for the self-report of pain intensity in children. Pediatrics. 2010;126(5):e1168–98. doi: 10.1542/peds.2010-1609. [DOI] [PubMed] [Google Scholar]
- 21.Farrar JT, Berlin JA, Strom BL. Clinically important changes in acute pain outcome measures: A validation study. J Pain Symptom Manage. 2003;25(5):406–411. doi: 10.1016/s0885-3924(03)00162-3. [DOI] [PubMed] [Google Scholar]
- 22.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole MR. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 23.Voepel-Lewis T, Burke CN, Jeffreys N, Malviya S, Tait AR. Do 0-10 numeric rating scores translate into clinically meaningful pain measures for children? Anesth Analg. 2011;112(2):415–21. doi: 10.1213/ANE.0b013e318203f495. [DOI] [PubMed] [Google Scholar]
- 24.Bailey B, Daoust R, Doyon-Trottier E, Dauphin-Pierre S, Gravel J. Validation and properties of the verbal numeric scale in children with acute pain. Pain. 2010;149(2):216–21. doi: 10.1016/j.pain.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Pasero C, Quinlan-Colwell A, Rae D, Broglio K, Drew D. American society for pain management nursing position statement: Prescribing and administering opioid doses based solely on pain intensity. Pain Manag Nurs. 2016;17(3):170–80. doi: 10.1016/j.pmn.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Herr K, Coyne PJ, Manworren RCB, McCaffery M, Merkel S. Pain assessment in the patients unable to self-report: Position statement update. Pain Manag Nurs. 2011;12(4):230. doi: 10.1016/j.pmn.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Savedra MC, Tesler MD, Holzemer WL, Ward JA. Adolescent Pediatric Pain Tool (APPT): Preliminary User’s Manual. University of California, San Francisco, School of Nursing; 1989. [Google Scholar]
- 28.Abu-Saad HH, Kroonen E, Halfens R. On the development of a multidimensional Dutch pain assessment tool for children. Pain. 1990;43(2):249–56. doi: 10.1016/0304-3959(90)91079-X. [DOI] [PubMed] [Google Scholar]
- 29.Varni JW, Thompson KL, Hanson V. The Varni/Thompson pediatric pain questionnaire: I. Chronic musculoskeletal pain in juvenile rheumatoid arthritis. Pain. 1987;28:27–38. doi: 10.1016/0304-3959(87)91056-6. [DOI] [PubMed] [Google Scholar]
- 30.Eccleston C, Jordan A, McCracken LM, Sleed M, Connell H, Clinch J. The Bath Adolescent Pain Questionnaire (BAPQ): development and preliminary psychometric evaluation of an instrument to assess the impact of chronic pain on adolescents. Pain. 2005;118(1–2):263–270. doi: 10.1016/j.pain.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 31.Northwestern University. PROMIS® (Patient-Reported Outcomes Measurement Information System), National Institutes of Health grant U2C CA186878 01, © 2016. http://www.healthmeasures.net/resource-center/about-us.
- 32.LeBaron L, Zeltzer L. Assessment of acute pain and anxiety in children and adolescents by self-reports, observer reports, and a behavior checklist. J Consult Clin Psychol. 1984;52:729–38. doi: 10.1037//0022-006x.52.5.729. [DOI] [PubMed] [Google Scholar]
- 33.Katz ER, Kellerman J, Siegel SE. Behavioral distress in children with cancer undergoing medical procedures: Developmental considerations. J Consult Clin Psychol. 1980;48:356–65. doi: 10.1037//0022-006x.48.3.356. [DOI] [PubMed] [Google Scholar]
- 34.McGrath PA, Seifert CE, Speechley KN, Booth JC, Stitt L, Gibson MC. A new analogue scale for assessing children’s pain: An initial validation study. Pain. 1996;64:435–43. doi: 10.1016/0304-3959(95)00171-9. [DOI] [PubMed] [Google Scholar]
- 35.Palermo TM. Impact of recurrent and chronic pain on child and family daily functioning: A critical review of the literature. J Dev Behav Pediatr. 2000;21:58–69. doi: 10.1097/00004703-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Larsson BS. Somatic complaints and their relationship to depressive symptoms in Swedish adolescents. J Child Psychol Psychiat. 1991;32:821–32. doi: 10.1111/j.1469-7610.1991.tb01905.x. [DOI] [PubMed] [Google Scholar]
- 37.Kovacs M. Ratings scales to assess depression in school-aged children. Acta Paedopsychiatr. 1981;46:305–15. [PubMed] [Google Scholar]
- 38.Chorpita BF, Yim L, Moffitt CE, Umemoto LA, Francis SE. Assessment of symptoms of DSM IV anxiety and depression in children: A revised child anxiety and depression scale. Behav Res Ther. 2000;38:835–55. doi: 10.1016/s0005-7967(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 39.Walters AS, Williamson GM. The role of activity restrictions in the association between pain and depression: A study of pediatric patients with chronic pain. Child Health Care. 1999;28:33–50. [Google Scholar]
- 40.Kundermann B, Krieg CC, Schreiber W, Lautenbacher S. The effect of sleep deprivation on pain. Pain Res Manag. 2004;9:25–32. doi: 10.1155/2004/949187. [DOI] [PubMed] [Google Scholar]
- 41.Butbul Aviel Y, Stremler R, Benseler SM, Cameron B, Laxer RM, Ota S, et al. Sleep and fatigue and the relationship to pain, disease activity and quality of life in juvenile idiopathic arthritis and juvenile dermatomyositis. Rheumatology (Oxford) 2011;50:2051–60. doi: 10.1093/rheumatology/ker256. [DOI] [PubMed] [Google Scholar]
- 42.Palermo TM, Kiska R. Subjective sleep disturbances in adolescents with chronic pain: Relationship to daily functioning and quality of life. J Pain. 2005;6:201–7. doi: 10.1016/j.jpain.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Gaina A, Sekine M, Chen X, Hamanishi S, Kagamimori S. Validity of child sleep diary questionnaire among junior high school children. J Epidemiol. 2004;14:1–4. doi: 10.2188/jea.14.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–51. [PubMed] [Google Scholar]
- 45.Brattberg G. Do pain problems in young school children persist into early adulthood? A 13-year follow-up. Eur J Pain. 2004;8:187–99. doi: 10.1016/j.ejpain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Siniatchkin M, Jonas A, Baki H, van Baalen A, Gerber WD, Stephani U. Developmental changes of the contingent negative variation in migraine and healthy children. J Headache Pain. 2010;11:105–13. doi: 10.1007/s10194-009-0180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 49.Claar RL, Walker LS. Functional assessment of pediatric pain patients: Psychometric properties of the functional disability inventory. Pain. 2006;121:77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reid GJ, Lang BA, McGrath PJ. Primary juvenile fibromyalgia: Psychological adjustment, family functioning, coping, and functional disability. Arthritis Rheum. 1997;40:752–60. doi: 10.1002/art.1780400423. [DOI] [PubMed] [Google Scholar]
- 51.Vervoort T, Goubert L, Eccleston C, Bijttebier P, Crombez G. Catastrophic thinking about pain is independently associated with pain severity, disability, and somatic complaints in school children and children with chronic pain. J Pediatr Psychol. 2006;31:674–83. doi: 10.1093/jpepsy/jsj059. [DOI] [PubMed] [Google Scholar]
- 52.Varni JW, Seid M, Rode CA. The PedsQL: Measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126–39. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Tupper SM, Rosenberg AM, Pahwa P, Stinson JN. Pain intensity variability and its relationship with quality of life in youths with juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2013;65(4):563–70. doi: 10.1002/acr.21850. [DOI] [PubMed] [Google Scholar]
- 54.Forgeron PA, King S, Stinson JN, McGrath PJ, MacDonald AJ, Chambers CT. Social functioning and peer relationships in children and adolescents with chronic pain: A systematic review. Pain Res Manag. 2010;15(1):27–41. doi: 10.1155/2010/820407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hershey AD, Powers SW, Vockell AL, LeCates S, Kabbouche MA, Maynard MK. PedMIDAS: Development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57(11):2034–9. doi: 10.1212/wnl.57.11.2034. [DOI] [PubMed] [Google Scholar]
- 56.Hershey AD, Powers SW, Vockell AL, LeCates SL, Segers A, Kabbouche MA. Development of a patient-based grading scale for PedMIDAS. Cephalalgia. 2004;24:844–9. doi: 10.1111/j.1468-2982.2004.00757.x. [DOI] [PubMed] [Google Scholar]
- 57.Lewandowski AS, Palermo TM, Stinson J, Handley S, Chambers CT. Systematic review of family functioning in families of children and adolescents with chronic pain. J Pain. 2010;11(11):1027–38. doi: 10.1016/j.jpain.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vahlquist B. Migraine in children. Int Arch Allergy. 1955;7:348–55. doi: 10.1159/000228238. [DOI] [PubMed] [Google Scholar]
- 59.Bille B. Migraine in school children. Acta Paediatrica. 1962;51(Suppl 136):1–151. [PubMed] [Google Scholar]
- 60.Alfvén G, Östberg V, Hjern A. Stressor, perceived stress and recurrent pain in Swedish schoolchildren. J Psychosom Res. 2008;65(4):381–7. doi: 10.1016/j.jpsychores.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 61.Bruni O, Fabrizi P, Ottaviano S, Cortesi F, Gianotti F, Guidetti V. Prevalence of sleep disorders in childhood and adolescence with headache: A case-control study. Cephalalgia. 1997;17:492–8. doi: 10.1046/j.1468-2982.1997.1704492.x. [DOI] [PubMed] [Google Scholar]
- 62.Karwautz A, Wöber C, Lang T, Böck A, Wagner-Ennsgraber C, Vesely C. Psychosocial factors in children and adolescents with migraine and tension-type headache: A controlled study and review of the literature. Cephalalgia. 1999;19:32–43. doi: 10.1111/j.1468-2982.1999.1901032.x. [DOI] [PubMed] [Google Scholar]
- 63.Kröner-Herwig B, Morris L, Heinrich M. Biopsychosocial correlates of headache: What predicts pediatric headache occurrence? Headache. 2008;48:529–44. doi: 10.1111/j.1526-4610.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 64.Laurell K, Larsson B, Eeg-Olofsson O. Headache in schoolchildren: Agreement between different sources of information. Cephalalgia. 2003;23:420–8. doi: 10.1046/j.1468-2982.2003.00540.x. [DOI] [PubMed] [Google Scholar]
- 65.Metsähonkala L, Sillanpää M, Tuominen J. Headache diary in the diagnosis of childhood migraine. Headache. 1997;37(4):240–4. doi: 10.1046/j.1526-4610.1997.3704240.x. [DOI] [PubMed] [Google Scholar]
- 66.van den Brink M, Bandell-Hoekstra ENG, Huijer Abu-Saad H. The occurrence of recall bias in pediatric headache: A comparison of questionnaire and diary data. Headache. 2001;41:11–20. doi: 10.1046/j.1526-4610.2001.111006011.x. [DOI] [PubMed] [Google Scholar]
- 67.Passchier J, Orlebeke J. Headaches and stress in schoolchildren: An epidemiological study. Cephalalgia. 1985;5(3):167–76. doi: 10.1046/j.1468-2982.1985.0503167.x. [DOI] [PubMed] [Google Scholar]
- 68.Palermo T, Valenzuela D, Stork P. A randomized trial of electronic versus paper pain diaries in children: Impact on compliance, accuracy, and acceptability. Pain. 2004;107:213–9. doi: 10.1016/j.pain.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 69.Stinson J, Yamada J, Kavanagh T, Gill N, Stevens B. Systematic review of the psychometric properties and feasibility of self-report pain measures for use in clinical trials in children and adolescents. Pain. 2006A;125(1–2):143–57. doi: 10.1016/j.pain.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 70.Stinson J, Petroz G, Tait G, Feldman B, Streiner D, McGrath P, et al. E-Ouch: Usability testing of an electronic chronic pain diary for adolescents with arthritis. Clin J Pain. 2006b;22(3):295–305. doi: 10.1097/01.ajp.0000173371.54579.31. [DOI] [PubMed] [Google Scholar]
- 71.Lewandowski AS, Palermo TM, Kirchner HL, Drotar D. Comparing diary and retrospective reports of pain and activity restriction in children and adolescents with chronic pain conditions. Clin J Pain. 2009;25(4):299–306. doi: 10.1097/AJP.0b013e3181965578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Connelly M, Neville K. Comparative prospective evaluation of the responsiveness of single-item pediatric pain-intensity self-report scales and their uniqueness from negative affect in a hospital setting. J Pain. 2010;11(12):1451–60. doi: 10.1016/j.jpain.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 73.Jacobs E, Stinson J, Duran J, Gupta A, Gerla M, Ann Lewis M, et al. Usability testing of a smartphone for accessing a web-based e-diary for self-monitoring of pain and symptoms in sickle cell disease. J Pediatr Hematol Oncol. 2012;34(5):326–35. doi: 10.1097/MPH.0b013e318257a13c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dale O, Hagen KB. Despite technical problems personal digital assistants outperform pen and paper when collecting patient diary data. J Clin Epidemiol. 2007;60:8–17. doi: 10.1016/j.jclinepi.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 75.Stinson JN. Improving the assessment of pediatric chronic pain: Harnessing the potential of electronic diaries. Pain Res Manag. 2009;14:59–64. doi: 10.1155/2009/915302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Control Clin Trials. 2003;24:182–99. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 77.Walker LS, Smith CA, Garber J. Appraisal and coping with daily stressors by pediatric patients with chronic abdominal pain. J Pediatr Psychol. 2007;32(2):206–16. doi: 10.1093/jpepsy/jsj124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wöber C, Wöber-Bingöl C. Triggers of migraine and tension-type headache. Handb Clin Neurol. 2010;97:161–72. doi: 10.1016/S0072-9752(10)97012-7. [DOI] [PubMed] [Google Scholar]
- 79.Stinson J, Huguet A, McGrath P, Rosenbloom B, Soobiah C, White M, et al. A qualitative review of the psychometric properties and feasibility of electronic headache diaries for children and adults: Where we are and where we need to go. Pain Res Manag. 2013;18(3):142–52. doi: 10.1155/2013/369541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lalloo C, Jibb LA, Rivera J, Agarwal A, Stinson JN. “There’s a Pain App for That”: Review of patient-targeted smartphone applications for pain management. Clin J Pain. 2015;31(6):557–63. doi: 10.1097/AJP.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 81.Blankenburg M, Boekens H, Hechler T, Maier C, Krumova E, Scherens A, et al. Reference values for quantitative sensory testing in children and adolescents: Developmental and gender differences of somatosensory perception. Pain. 2010;149(1):76–88. doi: 10.1016/j.pain.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 82.Vetter TR. Assessment tools in pediatric chronic pain: Reliability and validity. In: McClain BC, Suresh S, editors. Handbook of Pediatric Chronic Pain: Current Science and Integrative Practice. New York: Springer; 2011. pp. 63–85. [Google Scholar]
- 83.Manworren RCB, Ruaño G, Young E, St Marie B, McGrath JM. Translating the human genome to manage pediatric post-operative pain. J Ped Surg Nurs. 2015;4(1):28–39. doi: 10.1097/jps.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manworren RCB, Jeffries L, Pantaleao A, Seip R, Zempsky WT, Ruaño G. Pharmacogenetic testing for analgesic adverse effects: Pediatric case series. Clin J Pain. 2016;32(2):109–15. doi: 10.1097/AJP.0000000000000236. [DOI] [PubMed] [Google Scholar]