Abstract
Objective
To study whether reported, but inconsistent, associations between the FMR1 CGG repeat lengths in the intermediate, high normal, or low normal range differentiate women diagnosed with diminished ovarian reserve (DOR) from population controls, and whether associations vary by race-ethnic group.
Design
Case-control study.
Setting
Academic and private fertility clinics.
Patients
DOR cases (n=129; 95 Caucasians, 22 Asian, 12 other) from 5 US fertility clinics were clinically diagnosed, with regular menses and no fragile X syndrome family history. Normal fertility controls (n=803; 386 Caucasians, 219 African-Americans, 102 Japanese, 96 Chinese) from the US-based SWAN Study had ≥1 menstrual period in the 3 months pre-enrollment, ≥1 pregnancy, no history of infertility or hormonal therapy, and menopause ≥46 years. Previously, the SWAN Chinese and Japanese groups had similar FMR1 CGG repeat lengths, thus they were combined.
Intervention
Not applicable.
Main Outcome Measure
FMR1 CGG repeat lengths
Results
Median CGG repeats were nearly identical by case/control group. DOR cases had fewer CGG repeats in the shorter FMR1 allele than controls among Caucasians, but this was not significant among Asians. Caucasian cases had fewer CGG repeats in the shorter allele than Asian cases. No significant differences were found in the high normal/intermediate range between cases and controls, or by race/ethnic group within cases in the longer allele.
Conclusions
This study refutes prior reports of an association between DOR and high normal/intermediate repeats, and confirms an association between DOR and low normal repeats in Caucasians.
Keywords: diminished ovarian reserve; FMR1; race-ethnicity; Asian; Caucasian; ovarian reserve; infertility, female
CAPSULE
Race-ethnic differences were found (shorter FMR1 CGG repeats Caucasian vs. Asian cases, p=0.02), while no significant differences were found in the FMR1 high normal/intermediate range (cases vs. controls, p>0.25).
INTRODUCTION
Research has confirmed an association between premutation level trinucleotide repeat lengths in the FMR1 gene (55–199 CGG) and premature ovarian failure in women (also termed primary ovarian insufficiency). The odds ratio for experiencing premature ovarian failure, which clinically presents as a cessation of menses before age 40 and postmenopausal follicle stimulating hormone levels, among women who carry the premutation was recently estimated to be 5.4 (95% CI 1.7–17.4) (1). The association of the FMR1 gene with other forms of ovarian dysfunction such as pathologic DOR is less clear, as reviewed in 2014 (2). Some reports have suggested that women with <26 CGG repeat lengths (3), <28 CGG repeat lengths (4), 35–44 CGG repeats (5), 45–54 CGG repeats (6), ≥35 CGG repeats (7), and >40 CGG repeats (8) may be associated with diminished ovarian reserve or infertility, while others have reported no association between FMR1 repeat lengths and DOR (9) or infertility in general (10). The lack of consistency in these infertility reports may be due to (a) analytic differences, such using alleles rather than women as the unit of analysis (7, 8), (b) having infertility patients as controls (4, 6, 9), and/or (c) not restricting the case definition DOR (3, 10). It is still an outstanding question whether or not the FMR1 gene is associated with low ovarian reserve, and if it is, which repeat length confers the greatest risk.
Race-ethnic differences in the FMR1 CGG repeat distribution have been reported (11–13). Using 8 general-population studies, Genereux and Laird reported that Asian and non-Asian populations followed similar distributional curves (their analysis was restricted to ≥40 CGG repeat lengths), but the Asian curve was left-shifted and “almost completely non-overlapping” relative to the non-Asian distribution (12). None of the previously cited studies on FMR1 and DOR examined potential race-ethnic group differences, though race-ethnic variation in the lower allele triplet length has been reported in a cohort of 385 fertility clinic patients unrestricted by cause of infertility (14).
Stratifying the analysis by the higher and lower alleles in females is also important, as some researchers have raised the possibility that the lower allele confers increased risk of early ovarian aging (4). Only two of the seven FMR1/diminished ovarian reserve studies referenced above examined each allele individually (4, 6).
The goal of this study was to determine whether the reported associations between the FMR1 CGG repeat lengths in the intermediate, high normal, or low normal range discriminate women diagnosed with DOR from women with normal reproductive histories using a general female population comparison group. The analysis investigated each allele individually and examined race-ethnic group differences. The null hypothesis was that the FMR1 gene distribution below the premutation level (<55 CGGs) would not vary between the women with and without a diagnosis of DOR.
MATERIALS AND METHODS
Case Population Description
Women clinically diagnosed with DOR were enrolled between March 2005 – Feb 2014 from academic reproductive endocrinology and infertility clinics in California (33% of the participants), North Carolina (19%) and Virginia (15%), as well as private fertility clinics in Virginia (30%) and North Carolina (3%).
To be eligible as a case, women were required to have a diagnosis of DOR based on: (1) elevated but not postmenopausal-level FSH timed to her menstrual cycle, (2) low anti-mullerian hormone (AMH) for her age, or (3) fewer than 6 antral follicles sized 2–10 mm on an ovarian ultrasound (AFC), as detailed previously (5, 15). Additionally, women were required to be ≤ 42 years old at diagnosis (age requirement was tightened to ages≤41 in early 2009), and have had regular menstrual cycles for the previous 6 months. Only the Stanford University site, where the high patient volume provided confidence in the consistency of AFC measurement, used the AFC as an entrance criterion. The day 2–5 FSH enrollment criterion was adjusted for the different laboratory machines at each site to ensure consistency in the enrollment criteria across sites, as described previously (5). Approximately 70% of the DOR cases were diagnosed based on elevated FSH, 30% based on low AMH, and 10% based on low AFC, with a subset meeting more than one of those criterion. Women were excluded as a case if there was a known cause of elevated FSH for her age unrelated to fragile X (e.g., surgical removal of either one or both ovaries, chemotherapy or radiation therapy, Turners Syndrome, autoimmune disease), or she had a family history of fragile X syndrome (FXS) or premutation.
After signing an informed consent, women provided a single blood sample for FMR1 trinucleotide assessment and received pretest genetic counseling by one of two experienced certified genetic counselors affiliated with the study. Routine demographic information, reproductive history and family medical history were obtained via self-administered questionnaires and/or review of medical records. This study was approved by the Human Ethics Boards at all academic sites (#11448 at University of Virginia, #11-1535 at University of North Carolina at Chapel Hill, #16182 at Stanford University).
Control Population Description
The comparison data are from the Study of Women’s Health Across the Nation (SWAN), a multi-race, multi-ethnic, multi-site study of the menopausal transition in middle-aged women (www.swanstudy.org). At entry into SWAN, participants were required to be premenopausal, not taking hormones, and between 42–52 years of age at the time of enrollment. For further details on the study design, the reader is referred to Sowers et al (16). From 1996 to 1998, each site recruited a community-based cohort of approximately 450 women. All sites recruited non-Hispanic Caucasian women. Additionally, each site recruited women whose self-identified race-ethnic group was African-American (Boston, MA, Detroit area, MI, Pittsburgh, PA, and Chicago, IL), Chinese (Oakland, CA), Japanese, (Los Angeles, CA), or Hispanic (Hudson County, NJ).
During years 6 and 7 of the study, materials, including buccal cells and whole blood, were collected from a subset of participants to provide a source of DNA (17). Enrollees from the New Jersey site did not participate in the SWAN Genetics Study. DNA samples were available for 1523 subjects for this analysis (http://datawarehouse.swanrepository.com/aboutInventory.php as of March 6, 2014). This study was approved by the Institutional Review Board at each clinical site and all women provided written informed consent.
To be eligible for this FMR1 analysis, women were required to (a) be premenopausal through age 45, (b) have a stored DNA sample, and (c) undergo menopause at the age ≥ 46. Women could have undergone natural or surgical menopause, provided they were still having periods after the age of 45. This definition has been used elsewhere to define a “normal” age at menopause (1). In addition, women were excluded:
if they had ever taken fertility medications,
ever had a period of 12 months when they could not become pregnant despite regular sexual activity without contraception, or
had never been pregnant, or
if the answer to any of these three questions was missing.
There were 805 samples that met these criteria, of which 2 had insufficient DNA volume, leaving 803 controls available for analysis.
FMR1 Assays
Molecular diagnostic testing on DNA from both cases and controls was performed by the University of Virginia (UVA) Molecular Diagnostics Laboratory using capillary electrophoresis with peripheral venous blood samples on an automated ABI 3700 automated DNA sequencer. The PCR reaction contained 2 primer sets, one flanking the CGG repeat area in the 5’ untranslated region of FMR1 and an internal control consisting of primers spanning a highly polymorphic region near the promotor of the androgen receptor gene (18). This assay approach yields highly robust counts of the CGG repeat length on the X chromosomes up to 100 repeats and accuracy of +/− 1 CGG repeat (19). DNA samples from controls that displayed only a single peak were presumed to be homozygous, as opposed to having a full mutation on 1 allele. Cases with single PCR peaks enrolled prior to April 2012 were confirmed as homozygous with Southern Blot testing (62% of cases); cases with single PCR peaks enrolled after that date were presumed to be homozygous. See Pastore et al (20) for further details on the FMR1 lab testing.
Statistical Analysis
A power analysis was performed assuming initial estimates of n=110 cases and n=680 controls, and using published female CGG repeat distributions. The reference distribution was defined as a weighted average of the Streuli (8), Bretherick (21) and Otsuka (22) populations’ FMR1 distributions. These sample size estimates, which were exceeded for this study for both cases and controls, provided 96% power to detect a statistically significant difference (alpha=0.05) in the underlying CGG repeat distributions.
Because women have two X chromosomes, their FMR1 results provide two numbers corresponding to the trinucleotide repeat length in each allele. Consistent with prior reports (4, 23, 24), the allele with fewer CGG repeats was termed “allele 1”, and the allele with the greater number of CGG repeats was termed “allele 2”.
Standard descriptive statistics were calculated for all continuous variables. All FSH values presented have been adjusted to the corresponding cycle day 2–5 value at the primary recruitment site (UVA). No statistical comparisons were performed on the participant characteristics (e.g., demographics) because none of these variables has an influence on the FMR1 trinucleotide repeat length, with the exception of race-ethnic group, which was controlled in the statistical analysis. Discrete categories for CGG repeat length categories were selected a priori to respond to prior reports of high normal (5, 8) and low normal (3) repeats potentially being associated with early ovarian aging. The categorical distributions of allele 1 and allele 2 CGG repeat lengths were compared across race/ethnic groups using a Fisher’s exact test with alpha=0.05. Race-ethnic comparisons were restricted to Caucasian vs. Asian women, because (a) the sample size of African-Americans in the case group was limited, and (b) data to separate Chinese and Japanese ethnicities were unavailable in the case group. In a previous analysis (20) the SWAN Chinese and Japanese groups had similar FMR1 CGG repeat lengths. Quantitative comparisons of CGG repeat length, separate for alleles 1 and 2, were completed by one-way ANOVA, with the allele 2 counts analyzed after log-transformation. A non-parametric Levene’s test for homogeneity of the variance across race-ethnic groups was performed using the aggregate alleles.
Secondary analysis compared the proportion of low normal alleles in the DOR cases to the SWAN controls with Fisher’s exact tests. Two dichotomous definitions of low normal were used based on the literature (≤25 CGG on both alleles (3) and ≤23 CGG on both alleles (25)); This secondary analysis was restricted to Caucasian subjects due to sample size limitations in the other race-ethnic groups. The statistical analysis was conducted using SAS v9.3 (Cary, NC), and the graphs were created with Stata v13 (StataCorp, Texas) and Excel.
RESULTS
Participant characteristics for the two sample populations are displayed in Table 1. As would be expected, the DOR cases had a mean age in the upper thirties (mean 38 years) and the controls were older than 45 at enrollment into the SWAN Study (mean 47 years). As reflective of the eligibility criteria, few cases had given birth (mean parity 0.28), while all the controls had been pregnant at least once and the mean parity was 2.29. Corresponding data by race-ethnic group are in Supplement Table 1. There was no evidence of a difference in the severity of DOR between Caucasian and Asian cases (mean FSH and mean AMH were not clinically different between the race-ethnic groups; data not shown).
TABLE 1.
Participant characteristics of the DOR case cohort and SWAN comparison cohort
| Variable | DOR (n=129) | SWAN (n=803) |
|---|---|---|
| Age at enrollment | ||
| mean (SD), | 37.9 (4.27) | 47.2 (2.57) |
| median, | 38.6 | 47.2 |
| range | 26.6–49.2 | 42.0–52.9 |
| Marital Status (%) | ||
| Single/never married | 9 (9.2%) | 64 (8.2%) |
| Married/partnered | 87 (88.8%) | 544 (70.0%) |
| Separated | 0 (0%) | 25 (3.2%) |
| Widowed | 2 (2.0%) | 24 (3.1%) |
| Divorced | 0 (0%) | 120 (15.4%) |
| Missing (n=31 cases) | ||
| Ever Smoked (%) | 18 (14.0%) | 331 (41.4%) |
| Age at Diagnosis of DOR | ||
| mean (SD), | 36.6 (4.0) | NA |
| median, | 37.8 | |
| range (n) | 25.8–42.6 | |
| Race/Ethnic Group | ||
| Caucasian | 95 (73.6%) | 386 (48.1%) |
| African American | 5 (3.9%) | 219 (27.3%) |
| Asian | 22 (17.1%) | 198 (24.7%) |
| Other | 7 (5.4%) | 0 (0.0%) |
| Parity | ||
| mean (SD), | 0.28 (0.56) | 2.29 (1.28) |
| median, | 0.00 | 2.00 |
| range | 0 – 2 | 0 – 9 |
| Gravidity | ||
| mean (SD), | 1.19 (1.27) | 3.09 (1.63) |
| median, | 1.00 | 3.00 |
| range | 0 – 5 | 1 – 12 |
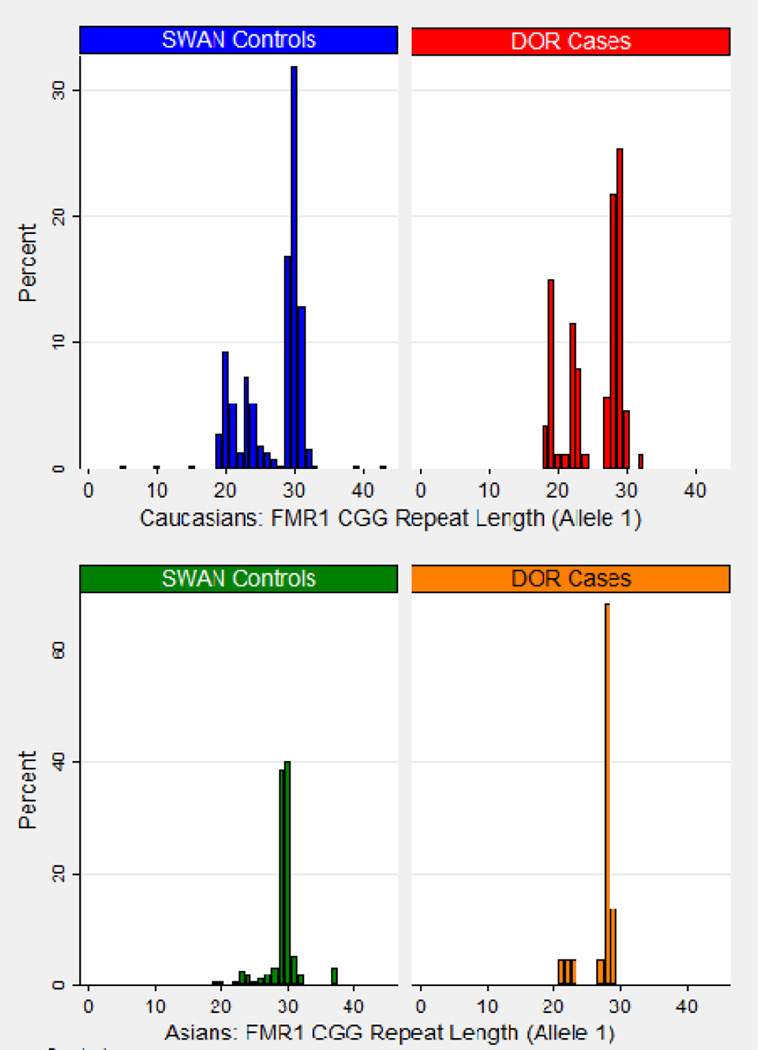
Graphs of the FMR1 trinucleotide repeat length stratified by Caucasian vs. Asian race and by case/control population are displayed in Figure 1a (allele 1) and 1b (allele 2). For Caucasian women, Figure 1a (allele 1) shows that 29 CGG and 30 CGG are the most common trinucleotide repeat lengths among controls, and 28 CGG and 29 CGG were the most common among the DOR cases. A shoulder is apparent around 19–25 CGG in both the case and control groups. For Asian women, more than 60% of the DOR cases had 28 CGG on allele 1, while 29 CGG and 30 CGG were the most common allele 1 repeat lengths among normal fertility controls. Thus, the modal CGG repeat lengths for cases are within 1 repeat of the modal lengths for controls even after stratification by the two race-ethnic groups. Comparing Caucasian vs Asian DOR cases for allele 1, Figure 1a indicates that alleles shorter than 26 CGG were more common in Caucasian than Asian women, which is also observed for the SWAN controls, as previously reported (20).
FIGURE 1.
a. FMR1 Allele 1 Discrete Distribution by Diminished Ovarian Reserve Cases and Normal Fertility Controls
Allele 1 has the shorter FMR1 repeat length.
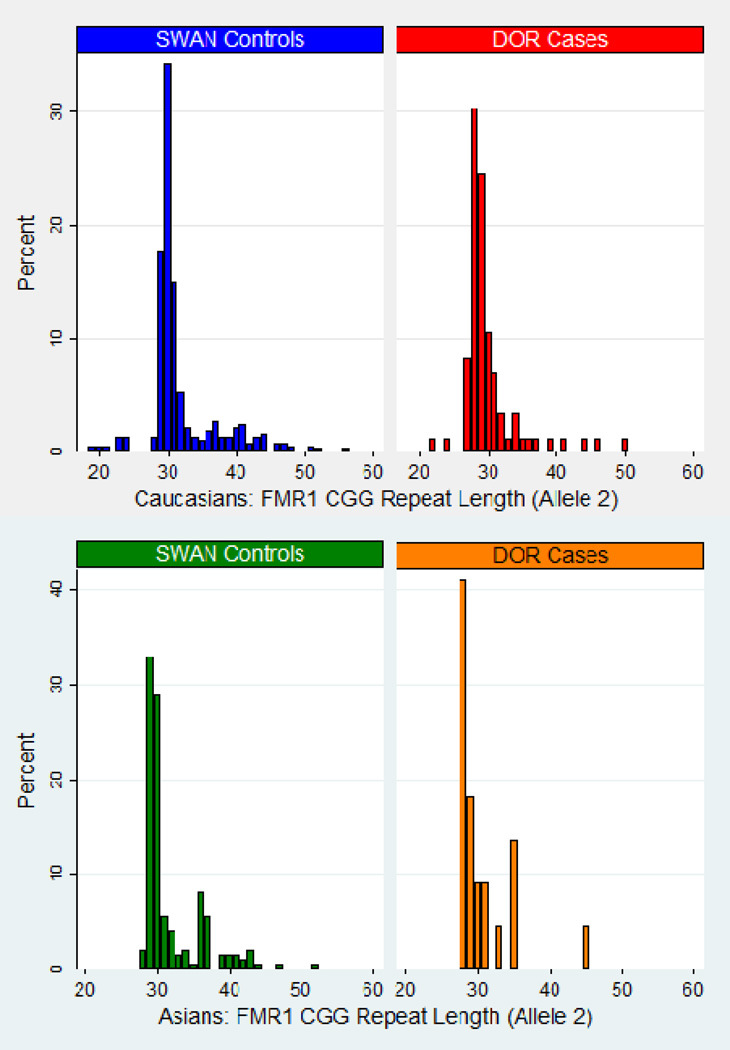
b. FMR1 Allele 2 Discrete Distribution by Diminished Ovarian Reserve Cases and Normal Fertility Controls (excluding premutation carriers)
Allele 2 has the longer FMR1 repeat length. The graphs exclude n=1 Caucasian DOR case with 77 CGG repeats.
The graphs in Figure 1b (allele 2) for Caucasian women overall appear similar between cases and controls. Among Asian women, the graphs show that there are no cases with CGG repeat lengths between 36–44, which may be due to the sample size. Comparing the histograms of Caucasian and Asian cases, the overall pattern is similar, though the Asian case distribution may have a shoulder around 35 CGG; this observation also holds true for controls. For both cases and controls, note the existence of Caucasians with fewer than 26 CGG repeats on allele 2, but no Asian women with allele 2 repeat lengths that short.
Summary statistics (mean, standard deviation, median, and range) of the FMR1 CGG repeat length are shown in Table 2 for all race-ethnic groups. All medians were within 1 CGG repeat when comparing DOR cases to normal fertility controls, for all races in total and within each race-ethnic group (28 or 29 CGG repeats for allele 1, 29 or 30 CGG repeats for allele 2). The spread of the distribution for allele 1 is greater among Caucasian than Asian women within the DOR cases (p=0.0021) and within the controls (p≤0.0001); no comparable differences were found for allele 2 (p=0.687 among cases and p=0.397 among controls).
TABLE 2.
Summary statistics for the FMRI CGG repeat length by allele, race/ethnic group, and case/control group
| Variable | DOR cases (n=129) Mean (SD) Median, Range |
SWAN controls (n=803) Mean (SD) Median, Range |
|---|---|---|
| Allele 1 CGG repeat length | ||
| Total cohort | 25.78 (3.96) | 27.82 (3.95) |
| 28, 17–35 | 29, 5–43 | |
| Caucasian: | 25.28 (4.11) | 27.08 (4.46) |
| 28, 18 –32 | 29, 5 –43 | |
| Asian: | 27.27 (2.21) | 29.23 (2.32) |
| 28, 21 –29 | 29, 19 –37 | |
| Black: | 26.40 (5.27) | 27.87 (3.82) |
| 29, 17 –29 | 29, 8 –38 | |
| Other: | 27.29 (4.46) | NA |
| 28, 20 –35 | ||
| Allele 2 CGG repeat length | ||
| Total cohort | 30.68 (6.00) | 31.73 (4.75) |
| 29, 22–77 | 30, 19–63 | |
| Caucasian: | 30.62 (6.49) | 31.90 (5.12) |
| 29, 22 –77 | 30, 19 –56 | |
| Asian: | 30.59 (4.04) | 31.89 (4.15) |
| 29, 28 –45 | 30, 28 –52 | |
| Black: | 29.60 (3.71) | 31.29 (4.57) |
| 29, 25 –35 | 30, 20 –63 | |
| Other: | 32.57 (6.00) | NA |
| 29, 28 –43 |
Allele 1 has the shorter CGG repeat length, and Allele 2 has the longer CGG repeat length.
The FMR1 CGG repeat lengths on allele 1, separated by Caucasians versus Asians, and stratified by cases versus controls, are displayed in the top portion of Table 3. There was a significant difference in the CGG repeat lengths of allele 1 between the DOR cases and the SWAN controls among Caucasians (p<0.0001), but not among Asians (p=0.239). This difference was predominantly seen in the proportion of women with very few repeats among the cases, e.g., 17.9% of Caucasian cases and 3.6% of Caucasian controls had fewer than 20 CGG repeats. There was a significant difference in the CGG repeat lengths by race/ethnic group within the DOR cases (p=0.023) and within the normal fertility controls (p<0.0001).
TABLE 3.
The FMR1 CGG repeat length by allele, case/control group, and race/ethnic group
| Caucasian | Asian | |||
|---|---|---|---|---|
| Allele 1 CGG Repeat Length |
DOR (n=95) |
SWAN (n=386) |
DOR (n=22) |
SWAN (n=198) |
| <20 | 17 (17.9%) | 14 (3.6%) | 0 (0.0%) | 1 (0.5%) |
| 20–24 | 22 (23.2%) | 110 (28.5%) | 3 (13.6%) | 11 (5.6%) |
| >24 | 56 (59.0%) | 262 (67.9%) | 19 (86.4%) | 186 (93.9%) |
|
Allele 2 CGG Repeat Length |
||||
| <35 | 84 (88.4%) | 313 (81.1%) | 18 (81.8%) | 151 (76.3%) |
| 35–39 | 5 (5.3%) | 31 (8.0%) | 3 (13.6%) | 33 (16.7%) |
| 40–44 | 3 (3.2%) | 30 (7.8%) | 0 (0.0%) | 12 (6.1%) |
| 45–54 | 2 (2.1%) | 11 (2.9%) | 1 (4.6%) | 2 (1.0%) |
| >54 | 1 (1.1%) | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) |
Fisher exact tests for Allele 1: Case comparison by Caucasian v Asian (p=0.023); Control comparison by Caucasian v Asian (p<0.0001); Caucasian Cases v Caucasian Controls (p<0.0001); Asian Cases v Asian Controls (p=0.239)
Fisher Exact tests for Allele 2: Case comparison by Caucasian v Asian (p=0.441); Control comparison by Caucasian v Asian (p=0.013); Caucasian Cases v Caucasian Controls (p=0.253); Asian Cases v Asian Controls (p=0.372)
The FMR1 CGG repeat lengths on allele 2, separated by Caucasians versus Asians, and stratified by cases versus controls, are displayed in the bottom half of Table 3. No differences were observed on allele 2 between the DOR cases and the SWAN controls among Caucasians (p=0.253) or Asians (p=0.372). No race-ethnic differences were observed among the DOR cases on allele 2 (p=0.441). Race-ethnic differences in the CGG repeat length on allele 2 were observed among the controls and have been reported previously (20).
The proportion of cases compared with controls who had both alleles in the “low normal” range (using two definitions from the literature (3, 25)) was not significantly different among Caucasians in this study. Restricted to Caucasians, 4.1% (16/386) of the normal fertility controls had ≤25 CGG repeats on both alleles vs 3.2% (3/95) of cases (p=1.00). Using the second definition of low normal, there were 2.8% (11/386) of the controls with ≤23 CGG repeats on both alleles vs 2.1% (2/95) of cases (p=1.00).
DISCUSSION
In this study we found significantly fewer CGG trinucleotide repeats in the lower of the two FMR1 alleles (allele 1) in Caucasian women diagnosed with DOR compared with women who have a normal reproductive history. (A larger sample of Asian women may have observed the same finding, but our sample was not large enough to detect this with statistical significance.) Caucasian women diagnosed with DOR were also statistically likely to have fewer CGG repeats in the FMR1 allele 1 compared with Asian DOR cases. No statistically significant differences were found in the CGG repeat length distribution of the higher FMR1 allele (allele 2) among the DOR cases either in comparison with the SWAN Study control population or by race/ethnic group.
The lack of association between a diagnosis of DOR and the CGG repeat length on allele 2 refutes four prior papers that reported an association between various CGG repeat lengths and DOR (5–8), but is consistent with two publications (9, 10). Control group definitions are an element that varies across the publications and may contribute to the inconsistent findings. Prior research used the following comparison groups: women referred for genetic testing unrelated to fertility or mental impairment (8), women with no history of infertility (7), women with a natural conception and recent birth (10), and infertility clinic patients with other diagnoses (4, 6, 9). No other reports on this topic had a population-based comparison group as used in this study.
Few reports (only 2) have investigated each individual FMR1 allele among women with DOR. Lower CGG repeat lengths on allele 1 among women with DOR have been reported by Gleicher et al (4), but was not observed by Karimov et al (6) using a case definition that corresponded to a “liberal characterization of diminished ovarian reserve”. Neither of those reports analyzed race-ethnic group. The Gleicher/Barad research team has highlighted CGG repeats ≤26 as being associated with ovarian reserve in multiple articles including Gleicher et al (3), based on interquartile ranges, whereas our analysis was not focused on a single dichotomy for “low repeats”.
The lack of association between a diagnosis of DOR and having both alleles with few CGG repeats might suggest that low repeat lengths have no true association with early ovarian aging. Thus, even though our data contained a statistically significant difference in the distribution of allele 1 in Caucasian DOR cases compared with Caucasian normal fertility controls, the lack of the association of both alleles having few CGG repeats with DOR shows that the dose-response gradient (26) is not evident in this study population. On the other hand, a dose-response relation may not be biologically relevant for this ovarian phenotype, in which case analysis of a single allele would be more informative than examination of the two alleles simultaneously. Without additional data from other DOR cohorts, this causality question remains unanswered. Scientists with existing FMR1 data on females with early ovarian aging are encouraged to re-examine their data with a focus on allele 1, to confirm or refute an association between few CGG repeats and low ovarian reserve. Knowing if low normal repeats have clinical relevance is an important outstanding clinical question, because it is reasonable to extrapolate that FMR1-associated infertility would be both inherited and passed to the subsequent generation of daughters. An association between low CGG repeat lengths and reductions in AMH over time have been found in oocyte donors (27), thus providing a possible biologic connection between ovarian reserve markers and FMR1 outside of DOR studies.
This study found race-ethnic differences in the repeat length distribution in allele 1 but not allele 2 between Asian and Caucasian female populations diagnosed with DOR. Race-ethnic variation in the allele 1 triplet length has been reported previously from a large New York City fertility clinic using a cohort of patients unrestricted by cause of infertility (14). In that New York study, Asian female patients, who were predominantly of Chinese heritage, were less likely to have an allele with 25 or fewer CGG repeats than other race-ethnicities (p≤0.03), and no race-ethnic differences were found in the prevalence of repeats >33 CGG. Our findings concur with the Gleicher et al publication that Asian women with a diagnosis of DOR are unlikely to have very short CGG repeat lengths.
The primary limitation of this study is the lack of African-American DOR cases, hence we cannot comment on the FMR1 trinucleotide distribution in that race-ethnic group. Our DOR case sample size is not as large as some prior reports (4, 6, 9), but is larger than other papers (5, 7, 8). Our comparison population potentially includes women with a family history of FXS because those data were not collected by the SWAN Study, but the controls would by definition exclude women with premature ovarian failure. The FMR1 trinucleotide distributions were combined for the Chinese and Japanese women in this analysis. For the controls alone, the distribution of CGG repeat counts were previously examined and reported separately for each of those Asian groups (20). That previous analysis indicated that the FMR1 CGG repeat lengths among Chinese and Japanese women are more similar to one another than they are to other race-ethnic groups. Given that the further breakdown of race/ethnicity is not available for the DOR cases, our results are not likely to be biased from the decision to summarize the Asian populations.
The primary strength of this study is having a control population drawn from the general female population rather than infertility clinic patients. Relative to prior research on this topic, having a normal reproductive history in the controls is a strength, as discussed previously. An additional key strength is the ability to examine race/ethnic genetic differences due to the targeted enrichment for race-ethnic groups by the SWAN initial study design. This allowed for analysis of race-ethnic group data separate for alleles 1 and 2 in women diagnosed with DOR, which has not previously been published to our knowledge. The similarity of the proportion of premutation (0.3%) and intermediate (2.3%) length alleles in the controls to the literature (28, 29) provide support for the adequacy of the comparison population. Our population is well-matched to clinical definitions of poor ovarian reserve by requiring regular menstrual periods and has the advantage of representing multiple practices in multiple geographic states. These findings can serve as a reference for researchers.
As people are increasingly screened for genetic conditions, the predictive value of the FMR1 repeat size will become more significant. Universal FMR1 screening of pregnant women (30, 31) and preconception patients (30) has been recommended, though not yet adopted. If universal screening is implemented, data from which to interpret those results will be critical. This report does not support universal screening for FMR1 repeats in populations of women diagnosed with DOR, unless future studies provide evidence of an association between few CGG repeats and DOR. The uncertainty about clinical outcomes with a given size of repeat expansion makes counseling of patients difficult and an increasing amount of testing by clinicians makes these questions more common. Potential associations between infertility and genes are personally relevant to women in their family planning and for the family planning of future generations, especially in consideration of the trend toward delayed childbearing in women of higher educational levels (32). A clear association between the trinucleotide repeat length and ovarian phenotypes, or a lack of an association, will need to be demonstrated to allow these individuals, clinicians, and genetic counselors to correctly interpret FMR1 test results and make informed reproductive decisions. Previous authors who reported FMR1 results for only allele 2 are encouraged to re-examine their data on allele 1, to confirm or refute these findings. Consideration of race-ethnic group in future FMR1 study designs and analyses is warranted based on our study findings.
Supplementary Material
Acknowledgments
FUNDING
This work was supported by the National Center for Child Health and Human Development at the National Institutes of Health (NIH, Grant R01HD068440 to LMP). The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The SWAN Repository is funded by NIH grant U01AG017719. This publication was also supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
From the University of Virginia, we thank James Bowden and Regina Seaner of the Molecular Diagnostics Lab for their FMR1 processing/reporting of all the SWAN samples, and Logan Karns and Karen Ventura for pretest genetic counseling of all case participants. We also thank the SWAN Study staff at Massachusetts General Hospital and the Repository at the University of Michigan for their assistance with the datasets/questionnaires. We appreciate being able to recruit from private clinics in North Carolina and Virginia (Carolina Conceptions, Reproductive Medicine and Surgery Center of Virginia). We thank the participants in this study, and the clinical research coordinators at all participating clinics: Parchayi Dalal, Hannah Spencer, Amy Brown, Amanda DeSmit, Angie Morey, Rebecca Briggs, and Janetta Phillips.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Murray A, Schoemaker MJ, Bennett CE, Ennis S, Macpherson JN, Jones M, et al. Population-based estimates of the prevalence of FMR1 expansion mutations in women with early menopause and primary ovarian insufficiency. Genetics in medicine : official journal of the American College of Medical Genetics. 2014;16:19–24. doi: 10.1038/gim.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pastore LM, Johnson J. The FMR1 Gene, Infertility and Reproductive Decision-Making: A Review. Frontiers in genetics. 2014:5. doi: 10.3389/fgene.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gleicher N, Weghofer A, Barad D. Ovarian reserve determinations suggest new finction of FMR1 (fragile X gene) in regulating ovarian ageing. Reproductive biomedicine online. 2010;20:768–775. doi: 10.1016/j.rbmo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Gleicher N, Weghofer A, Oktay K, Barad D. Relevance of triple CGG repeats in the FMR1 gene to ovarian reserve. Reproductive biomedicine online. 2009;19:385–390. doi: 10.1016/s1472-6483(10)60173-3. [DOI] [PubMed] [Google Scholar]
- 5.Pastore LM, Young SL, Baker VM, Karns LB, Williams CD, Silverman LM. Elevated Prevalence of 35–44 FMR1 Trinucleotide Repeats in Women with Diminished Ovarian Reserve. Reproductive Sciences. 2012;19:1226–1231. doi: 10.1177/1933719112446074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karimov CB, Moragianni VA, Cronister A, Srouji S, Petrozza J, Racowsky C, et al. Increased frequency of occult fragile X-associated primary ovarian insufficiency in infertile women with evidence of impaired ovarian function. Human reproduction. 2011;26:2077–2083. doi: 10.1093/humrep/der168. [DOI] [PubMed] [Google Scholar]
- 7.Barasoain M, Barrenetxea G, Huerta I, Telez M, Carrillo A, Perez C, et al. Study of FMR1 gene association with ovarian dysfunction in a sample from the Basque Country. Gene. 2013;521:145–149. doi: 10.1016/j.gene.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 8.Streuli I, Fraisse T, Ibecheole V, Moix I, Morris MA, de Ziegler D. Intermediate and premutation FMR1 alleles in women with occult primary ovarian insufficiency. Fertility and sterility. 2009;92:464–470. doi: 10.1016/j.fertnstert.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Schufreider A, McQueen DB, Lee SM, Allon R, Uhler ML, Davie J, et al. Diminished ovarian reserve is not observed in infertility patients with high normal CGG repeats on the fragile X mental retardation 1 (FMR1) gene. Human reproduction. 2015 doi: 10.1093/humrep/dev220. [DOI] [PubMed] [Google Scholar]
- 10.De Geyter C, M'Rabet N, De Geyter J, Zurcher S, Moffat R, Bosch N, et al. Similar prevalence of expanded CGG repeat lengths in the fragile X mental retardation I gene among infertile women and among women with proven fertility: a prospective study. Genetics in medicine : official journal of the American College of Medical Genetics. 2013 doi: 10.1038/gim.2013.146. [DOI] [PubMed] [Google Scholar]
- 11.Weiss K, Orr-Urtreger A, Kaplan Ber I, Naiman T, Shomrat R, Bardugu E, et al. Ethnic effect on FMR1 carrier rate and AGG repeat interruptions among Ashkenazi women. Genetics in medicine : official journal of the American College of Medical Genetics. 2014;16:940–944. doi: 10.1038/gim.2014.64. [DOI] [PubMed] [Google Scholar]
- 12.Genereux DP, Laird CD. Why do fragile X carrier frequencies differ between Asian and non-Asian populations? Genes Genet Syst. 2013;88:211–224. doi: 10.1266/ggs.88.211. [DOI] [PubMed] [Google Scholar]
- 13.Crawford DC, Meadows KL, Newman JL, Taft LF, Scott E, Leslie M, et al. Prevalence of the fragile X syndrome in African-Americans. American journal of medical genetics. 2002;110:226–233. doi: 10.1002/ajmg.10427. [DOI] [PubMed] [Google Scholar]
- 14.Gleicher N, Weghofer A, Barad DH. Effects of race/ethnicity on triple CGG counts in the FMR1 gene in infertile women and egg donors. Reproductive biomedicine online. 2010;20:485–491. doi: 10.1016/j.rbmo.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Pastore LM, Antero M, Ventura K, Penberthy JK, Thomas SA, Karns LB. Attitudes towards potentially carrying the FMR1 premutation: before vs after testing of non-carrier females with diminished ovarian reserve. Journal of genetic counseling. 2014;23:968–975. doi: 10.1007/s10897-014-9717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sowers M, Crawford S, Sternfeld B, Morganstein D, Gold EB, Greendale G, et al. SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey JL, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego, CA: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 17.Kardia SR, Chu J, Sowers MR. Characterizing variation in sex steroid hormone pathway genes in women of 4 races/ethnicities: the Study of Women's Health Across the Nation (SWAN) American Journal of Medicine. 2006;119:S3–S15. doi: 10.1016/j.amjmed.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 18.White BJ, Ayad M, Fraser A, Entwistle T, Winkler S, Sbeiti A, et al. A 6-year experience demonstrates the utility of screening for both cytogenetic and FMR-1 abnormalities in patients with mental retardation. Genet Test. 1999;3:291–296. doi: 10.1089/109065799316617. [DOI] [PubMed] [Google Scholar]
- 19.Larsen LA, Grønskov K, Nørgaard-Pedersen B, Brøndum-Nielsen K, Hasholt L, Vuust J. High-throughput analysis of Fragile X (CGG)n alleles in the normal and premutation range by PCR amplification and automated capillary electrophoresis. Hum Genet. 1997;100:564–568. doi: 10.1007/s004390050552. [DOI] [PubMed] [Google Scholar]
- 20.Pastore L, Manichaikhul A, Wang X, Finkelstein J. FMR1 CGG Repeats: Reference Levels and Race–Ethnic Variation in Women With Normal Fertility (Study of Women’s Health Across the Nation) Reproductive Sciences. 2016 Sep;23(9):1225–1233. doi: 10.1177/1933719116632927. in press. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bretherick KL, Fluker MR, Robinson WP. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Human genetics. 2005;117:376–382. doi: 10.1007/s00439-005-1326-8. [DOI] [PubMed] [Google Scholar]
- 22.Otsuka S, Sakamoto Y, Siomi H, Itakura M, Yamamoto K, Matumoto H, et al. Fragile X carrier screening and FMR1 allele distribution in the Japanese population. Brain and Development. 2010;32:110–114. doi: 10.1016/j.braindev.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Voorhuis M, Onland-Moret NC, Janse F, Ploos van Amstel HK, Goverde AJ, Lambalk CB, et al. The significance of fragile X mental retardation gene 1 CGG repeat sizes in the normal and intermediate range in women with primary ovarian insufficiency. Human reproduction. 2014;29:1585–1593. doi: 10.1093/humrep/deu095. [DOI] [PubMed] [Google Scholar]
- 24.Gleicher N, Weghofer A, Barad DH. A pilot study of premature ovarian senescence: I. Correlation of triple CGG repeats on the FMR1 gene to ovarian reserve parameters FSH and anti-Müllerian hormone. Fertility and sterility. 2009;91:1700–1706. doi: 10.1016/j.fertnstert.2008.01.098. [DOI] [PubMed] [Google Scholar]
- 25.Mailick MR, Hong J, Rathouz P, Baker MW, Greenberg JS, Smith L, et al. Low-normal FMR1 CGG repeat length: phenotypic associations. Frontiers in genetics. 2014;5:309. doi: 10.3389/fgene.2014.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill A. The environment and disease: association or causation? Proceedings of the Royal Society of Medicine. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 27.Gleicher N, Yu Y, Himaya E, Barad DH, Weghofer A, Wu YG, et al. Early decline in functional ovarian reserve in young women with low (CGGn < 26) FMR1 gene alleles. Translational research : the journal of laboratory and clinical medicine. 2015;166:502–507. e2. doi: 10.1016/j.trsl.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Cronister A, Teicher J, Rohlfs EM, Donnenfeld A, Hallam S. Prevalence and instability of fragile X alleles: implications for offering fragile X prenatal diagnosis. Obstetrics & Gynecology. 2008;111:596–601. doi: 10.1097/AOG.0b013e318163be0b. [DOI] [PubMed] [Google Scholar]
- 29.Maenner MJ, Baker MW, Broman KW, Tian J, Barnes JK, Atkins A, et al. FMR1 CGG expansions: Prevalence and sex ratios. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2013;162:466–473. doi: 10.1002/ajmg.b.32176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abrams L, Cronister A, Brown WT, Tassone F, Sherman SL, Finucane B, et al. Newborn, Carrier, and Early Childhood Screening Recommendations for Fragile X. Pediatrics. 2012;130:1126–1135. doi: 10.1542/peds.2012-0693. [DOI] [PubMed] [Google Scholar]
- 31.Musci TJ, Caughey AB. Cost-effectiveness analysis of prenatal population-based fragile X carrier screening. American Journal of Obstetrics & Gynecology. 2005;192:1905–1912. doi: 10.1016/j.ajog.2005.02.052. discussion 12-5. [DOI] [PubMed] [Google Scholar]
- 32.te Velde ER, Pearson PL. The variability of female reproductive ageing. Human reproduction update. 2002;8:141–154. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.