Abstract
Background
Numerous clinical trials have demonstrated that oncolytic viruses can elicit antitumor responses when they are administered directly into localized cancers. However, the treatment of metastatic disease with oncolytic viruses has been challenging due to the inactivation of viruses by components of human blood and/or to inadequate tumor selectivity.
Methods
We determined the cytolytic potential and selectivity of Seneca Valley Virus-001 (SVV-001), a newly discovered native picornavirus, in neuroendocrine and pediatric tumor cell lines and normal cells. Suitability of the virus for intravenous delivery in humans was assessed by blood inactivation assays. Safety was evaluated in vivo using an immune-competent mouse model, and efficacy was evaluated in vivo in athymic mice bearing tumors derived from human small-cell lung cancer and retinoblastoma cell lines.
Results
Cell lines derived from small-cell lung cancers and solid pediatric cancers were at least 10000-fold more sensitive to the cytolytic activity of SVV-001 than were any of the adult normal human cells tested. Viral infectivity was not inhibited by human blood components. Intravenous doses up to 1 × 1014 virus particles (vp) per kg were well tolerated, and no dose-limiting toxicity was observed in immune-competent mice. A single intravenous dose of 1 × 108 vp per kg into athymic mice bearing preestablished small-cell lung or retinoblastoma tumors resulted in complete, durable responses in ten of ten and five of eight mice, respectively.
Conclusions
SVV-001 has potent cytolytic activity and high selectivity for tumor cell lines having neuroendocrine properties versus adult normal cells. Systemically administered SVV-001 has potential for the treatment of metastatic neuroendocrine cancers.
Oncolytic or cancer-killing viruses are one of several new treatments for cancer that are currently being developed and evaluated. In this approach, replication-competent tumor-selective viruses are delivered to cancer cells via direct injection into a visible tumor mass or via systemic administration. Tumor cells are selectively infected and produce virus progeny that infect and kill neighboring tumor cells. Oncolytic viruses can kill cancer cells directly, by cell lysis, or indirectly, by using the cells’ machinery to express cytotoxic proteins or to induce an inflammatory or immune response; in addition, they can act synergistically with chemotherapeutic agents and radiation therapy (1).
Most clinical trials of oncolytic viruses have focused on controlling the growth of a local tumor via intratumoral administration; only a few have been evaluated as systemically deliverable agents. One example is reovirus, which preferentially replicates in tumor cells that have an activated Ras pathway; it is currently being explored in cancer patients in phase I/II trials in Canada and the United Kingdom (2). Likewise, the oncolytic activity of a systemically administered attenuated strain of Newcastle disease virus was evaluated in a phase I clinical trial in patients with solid tumors, and objective responses were obtained at the higher dose levels tested (3). An attenuated strain of measles virus is currently being evaluated in phase I trials for cutaneous T-cell lymphoma following intratumoral injection and ovarian cancer following intraperitoneal injection (4). In addition, vesicular stomatitis virus (5), Sindbis virus (6), poliovirus (7–8), coxsackievirus A21 (9), and several other RNA viruses (10–14) are at various stages of preclinical development. Antitumor efficacy was reported in clinical trials with intratumoral administration of coxsackievirus A21 and engineered strains of adenovirus and herpes viruses (15). However, for some of these viruses, limited efficacy was reported following systemic delivery and for others, the results of clinical trials are pending (15). The limited efficacy observed may be due to attenuated potency, toxicity to normal tissues, and/or the presence of preexisting immune responses in treated subjects.
In this article, we describe the discovery and development of a newly discovered member of the Picornaviridae family, Seneca Valley Virus-001 (SVV-001), as an oncolytic virus. In vitro cytotoxicity and virus production assays were performed on several tumor cell lines to determine the relationship between cytotoxicity and virus replication. To determine the suitability of SVV-001 for systemic injection, human sera were screened for neutralizing antibodies to SVV-001, whole blood was screened for viral inactivation, and virus was tested for hemagglutination of red blood cells. In vivo assessment of intravenously delivered SVV-001 included a toxicology study in immune-competent A/J mice and antitumor efficacy studies using xenograft tumor models in athymic mice. Infectivity assays and immunohistochemical analysis of tumor sections were carried out to detect virus and viral proteins in the tumor. The ultimate goal was to develop SVV-001 as an oncolytic virus for the treatment of metastatic cancers that have neuroendocrine properties.
Materials and Methods
Cells and Virus
For plaque purification, PER.C6 transformed fetal retinoblast cells [(16); obtained through a license from Crucell, The Netherlands] were plated at 2 × 106 cells per well in a 6-well tissue culture dish in Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (BioWhitaker, Walkersville, MD), 10 mM magnesium chloride (Sigma, St Louis, MO), and 1× penicillin/streptomycin (Invitrogen). Two days later, cells were infected with SVV-001 for 1 hour and overlaid with 1% SeaPlaque agarose (Invitrogen). After 2 more days, clearly isolated plaques were picked. Plaque-purified virus was amplified in PER. C6 cells cultured in NUNC cell factories (Denmark). The infected cells were harvested for virus purification when a complete cytopathic effect, which is characterized by rounding and detachment of cells from the dish, was observed. The harvested cells were lysed by subjecting to three cycles of freeze (− 70 °C) and thaw (37 °C). The virus was purified by two rounds of cesium chloride (CsCl) gradients: step gradient (density of CsCl = 1.24 g/mL and 1.4 g/mL) followed by continuous gradient centrifugation (density of CsCl = 1.33 g/mL). The purified virus (1–3 mL) was dialyzed against 1 L of cold dialysis buffer (200 mM Tris–HCl, 50 mM HEPES, pH 8.0, 10% glycerol [vol/vol]) using a Slide-A-Lyzer dialysis cassette (Pierce, Rockford, IL) for total of 4 hours with an hourly change of buffer. Small aliquots of the virus were stored in sterile 0.5-mL screw cap tubes at − 70 °C until use. The concentration of the purified virus was determined spectrophotometrically, assuming that 1.0 optical density unit at A260 nm is equivalent to 9.5 × 1012 particles (17). The virus titers were determined by standard plaque and tissue culture infective dose 50 (TCID50) assays using PER.C6 cells, as described below. A total of six different virus preparations, each purified by two rounds of CsCl gradient centrifugations and tested for both particle count and infectivity titers, were used for the studies described here. The mean ± standard deviation of the ratio of particle to TCID50 of the six lots was 62.5 ± 37.5. The infectivity titers of these six lots varied by less than fivefold, which is an acceptable limit of variation for any viral titer assay in our laboratory. Therefore, all six virus lots were considered to be similar for the interpretation of study results.
Cytotoxicity Assays
Cell lines were obtained from the American Type Tissue Collection (Manassas, VA), PromoCell (Heidelberg, Germany), Cambrex (Rutherford, NJ), and other vendors (Supplementary Table 1, available online) and were cultured in the media and under the conditions recommended by the supplier. Cytotoxicity of SVV-001 on tumor and normal cells (Supplementary Table 1, available online) was determined by seeding 1 × 104 PER.C6 cells in 180 µL of growth media per well into 96-well tissue culture dishes. The next day, SVV-001 was serially diluted in serum-free RPMI 1640 medium (Invitrogen) and 20 µL of each dilution was added to the wells. Cells were incubated for 3 days, after which the 3-(4,5-dimethylthiazol-2-yl)-5-(3–carboxymethoxyphenyl)-2-(4–sulfophenyl)-2H-tetrazolium) (MTS) reagent (CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay, Promega, Madison, WI) was added and absorbance values were recorded. The absorbance values were expressed as a percentage of that in uninfected cells (control) and plotted versus virus dose. A sigmoidal dose–response curve was fit to the data, and an effective concentration (EC50) value was calculated for each replicate using Prism software, version 3.0 (GraphPad, San Diego, CA). The EC50 value is the dose of virus in particles per cell (ppc) that reduces the maximal light absorbance capacity of an exposed cell culture by 50% and is proportional to the percentage of cells killed by SVV-001. In addition, the NCI-60 cell line panel (Developmental Therapeutics Program of the National Cancer Institute [NCI]) was screened for susceptibility to SVV-001. Cytotoxicity assays for NCI-60 cell lines were performed using 2,3-bis(2–methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide sodium salt at the NCI (Frederick, MD) as previously described (18). All cytotoxicity assays were performed in triplicate. Cell lines showing any SVV-001–mediated killing were tested a minimum of two times to confirm results.
SVV-001–mediated cytotoxicity in primary human hepatocytes (In Vitro Technologies, Inc, Baltimore, MD) was determined by using the lactate dehydrogenase (LDH) release assay (CytoTox 96 Non-Radioactive Cytotoxicity Assay, Promega). Primary human hepatocytes (2.5 × 105 cells per well plated in collagen-coated 12-well plates; Cambrex Inc, Walkersville, MD) were infected with SVV-001 at 1, 10, 100, and 1000 ppc in serum-free OptiMem (Invitrogen). After 3 hours, the infection medium was replaced with 2 mL of human hepatocyte maintenance medium (Cambrex Inc), and the cells were cultured for 3 days. The amounts of cell-associated LDH and of LDH in the conditioned media were assessed by determining the absorbance at 490 nm with a Tecan GENios plate reader (Tecan US, Research Triangle Park, NC). The percent of LDH release from the cells was determined using the following formula: LDH release = (absorbance of the supernatant)/(absorbance of the supernatant and cell lysate) × 100%. Each multiplicity of infection was tested in duplicate wells.
Replication Kinetics of Seneca Valley Virus-001 in Permissive and Nonpermissive Tumor Cell Lines
Two tumor cell lines with EC50 values less than 1 (H446 and H69AR), two tumor cell lines with EC50 values greater than 1 and less than 10 (H1299 and M059K), and two tumor cell lines resistant to SVV-001–mediated cytotoxicity with EC50 values greater than 10000 (Hep3B and H460) were plated at 10000 tumor cells per well and infected with SVV-001 at 1 ppc. After 1 hour, unattached virus was washed off, a crude viral lysate was prepared, and the virus titer was determined using PER.C6 cells as described above. Uninfected PER.C6 cells served as a background control in the cytotoxicity assay. Crude viral lysates were also made from additional wells containing tumor cells that had been incubated with virus for 1, 3, 5, and 7 days. The virus titer in these lysates also was determined using PER.C6 cells. The amount of virus produced at each time point (TCID50 /mL) was plotted as a function of time. This experiment was carried out twice in duplicate wells, and virus titers in crude viral lysate were determined in triplicate wells for each dilution tested.
Serum Neutralization Assays
Neutralization assays were performed on randomly collected normal human blood serum samples from 70 individual donors (Bioheme, Salt Lake City, UT, and Bioreclamation, Hicksville, NY). Blood serum samples from an Amish population (n = 50) were provided by Dr Alan Shuldiner (University of Maryland, College Park, MD). Aliquots of serum samples were stored at − 20 °C until tested and were heated to 56 °C for 30 minutes to inactivate complement before use in the serum neutralization assay. Each serum sample was serially diluted (from 1:2 to 1:256) in cell culture medium. The diluted serum samples (40 µL) were each mixed with an equal volume of virus (100 TCID50). As a negative control, fetal bovine serum was also serially diluted and combined with 100 TCID50 of SVV-001. The virus–serum mixtures were incubated for 1 hour at 37 °C, and 40 µL of each mixture was then added to PER.C6 cells (1 × 104) that had been seeded in 96-well plates the day before, and the plates were further incubated for 3 days at 37 °C. The SVV-001–mediated cytotoxicity was then measured using the MTS assay described above. Absorbance at 492 nm was determined using a Tecan GENios plate reader (Tecan US). The absorbance data were then converted to percentage of viable cells, using uninfected cells as the positive control. The neutralization titer was defined as the highest dilution of human serum that completely neutralized 100 TCID50 of SVV-001. Each serum sample was tested twice, and each serum dilution was tested in duplicate wells.
Hemagglutination and Whole-Blood Inactivation Assays
Purified SVV-001 was subjected to serial twofold dilutions starting with 1.25 × 1010 virus particles (vp) per 25 µL per well in 96-well U-bottom plates using phosphate-buffered saline (PBS, 0.7% NaCl, 0.1 M phosphate buffer, pH 7.2) as the diluent. Blood samples used for the isolation of erythrocytes were obtained from eight healthy volunteers of different blood groups (two each of A, B, AB, and O; Lampire Biological Laboratories, Pipersville, PA) with heparin or sodium citrate as an anticoagulant. Erythrocytes were prepared by washing the cells three times with cold PBS. At each washing step, a majority of white blood cells were removed by discarding the white buffy coat. After the final wash, erythrocytes were suspended in PBS (1% vol/vol). An equal volume of 1% erythrocyte suspension was added to each well containing serially diluted SVV-001, and the plates were incubated at room temperature for 1 hour. Each well was then scored for the presence or absence of hemagglutination. In performing this assay, the number of virus particles per erythrocyte ranged from 105 to 106. Hemagglutination of rat erythrocytes (Lampire Biological Laboratories) by human adenovirus 5 (American Type Tissue Collection) was used as positive control. To monitor direct inactivation of SVV-001 by blood components, virus particles (2 × 108) were added to 1 mL of human whole-blood samples. After a 30-minute incubation at room temperature, the plasma was separated from the blood by centrifugation. Cytotoxicity of SVV-001 on PER.C6 cells was assayed as described above to determine the virus titer. This experiment was carried out in duplicate wells and was repeated two times on the same set of blood samples.
Preclinical Toxicology in Mice
A toxicity study was conducted in 10- to 12-week-old male and female A/J mice (n = 100 per sex) purchased from The Jackson Laboratory (Bar Harbor, ME). This particular mouse strain supported replication of the virus when administered intravenously at 109 vp per kg (data not shown) and was thus used as a relevant model for preclinical safety assessment of SVV-001. All procedures on mice were performed in accordance with the Animal Welfare Act and Neotropix institutional guidelines and procedures for the humane treatment of laboratory animals. Dulbecco’s PBS or purified SVV-001 was injected intravenously into mice at 1 × 109, 3 × 1011, and/or 1 × 1014 vp per kg (n = 25 per dose per sex). Body weight was recorded, and clinical observations were made weekly. Change in body weight from that recorded before injection was calculated. Hematology and serum chemistry parameters were evaluated at 24 hours and 1, 3, 6, and 12 weeks after injection, and organ weights, gross pathology, and microscopic pathology were evaluated at 1, 3, 6, and 12 weeks. Hematology analyses included leukocyte (total and differential), erythrocyte and neutrophil counts, and hemoglobin, hematocrit, mean corpuscular hemoglobin, and mean corpuscular volume determinations (AniLytics, Inc, Gaithersburg, MD). Serum chemistry parameters included sodium, potassium, chloride, calcium, phosphorous, glucose, blood urea nitrogen, creatinine, total bilirubin, gamma glutamyltransferase, alanine aminotransferase, aspartate aminotransferase, albumin, globulin, and total protein (AniLytics, Inc). Microscopic pathology was performed by BioReliance Corporation (Rockville, MD) and included all organ systems. Viral infectivity of dosing solutions was confirmed by titration on PER.C6 cells as described above in the methods for cytotoxicity assays.
Efficacy Studies in Athymic Mice Bearing Xenograft Tumors
Athymic female mice (nu/nu; n = 123), aged 6–7 weeks, were purchased from Harlan Sprague Dawley (Indianapolis, IN) and used in efficacy studies. Mice were injected subcutaneously in the flank with 5 × 106 H446 small-cell lung cancer cells or 1 × 106 Y79 retinoblastoma cells in 50% phenol red–free Matrigel (Becton Dickinson Biosciences, San Jose, CA). Tumors were measured in two dimensions with digital calipers, and volumes were calculated using the formula: π/6 × W × L2, where L = length and W = width of the tumor. To model treatment of small, early-stage tumors, mice bearing H446 (n = 10 per group) or Y79 (n = 7–8 per group) tumors were randomly divided into treatment groups 15 days (when tumor volume reached 73–210 mm3) or 20 days (when tumor volume reached 64–239 mm3) after injection of tumor cells. To model late-stage bulky tumors, mice bearing H446 tumors were randomly divided into treatment groups 48 days (when tumor volume reached 486–1996 mm3) after injection of tumor cells. Mice were injected with SVV-001 into the lateral tail vein at a dose volume of 10 mL/kg. SVV-001 treatment concentrations were 1 × 107, 1 × 108, 1 × 109, 1 × 1010, 1 × 1011, 1 × 1012, and 1 × 1013 vp per kg for small H446 tumors, 1 × 1013 vp per kg for large H446 tumors, and 1 × 108, 1 × 1011, and 1 × 1014 vp per kg for Y79 tumors. A control group of mice in each experiment was injected with an equivalent volume of saline vehicle. Antitumor efficacy was determined by measuring tumor volumes twice weekly following SVV-001 administration. Mice were euthanized by CO2 asphyxiation when their tumors grew to greater than 2000 mm3 or at study end (small H446 tumor study: day 20, Y79 study: day 84, and large H446 tumor study: day 77). Study end for the small H446 study was defined as the first time point at which study mice were euthanized due to tumor burden. Study end for the large H446 and Y79 studies was defined as the first time point at which all mice with tumors were euthanized due to tumor burden or mice were tumor free.
Immunohistochemistry
In a separate experiment from the efficacy studies described above, H446 xenograft tumors of approximately 1000 mm3 from athymic mice injected intravenously with saline (n = 3) or SVV-001 (n = 3) at 109 vp per kg were harvested after euthanasia by CO2 asphyxiation, which occurred 24 hours after injection with vehicle or SVV-001. At least three formalin-fixed, paraffin-embedded sections per tumor were microwaved after deparaffinization in citrate buffer. Immunohistochemistry was performed using the Mouse on Mouse kit from Vector Laboratories (Burlingame, CA), according to the manufacturer’s protocol. The specimens were incubated with a 1:100 dilution of SVV-001 antiserum, which was raised in CD-1 mice at Neotropix. Antiserum was generated by injecting live virus intravenously at 1012 vp per kg on days 0 and 21 and collecting serum on day 35 when mice were killed by CO2 asphyxiation. Reactions were visualized with an avidin–biotin–peroxidase method (Vectastain ABC Kit, Vector Laboratories) using diaminobenzidine as a chromagen (DAB Substrate Kit, Vector Laboratories), according to manufacturer’s protocol. Sections were counterstained with 0.1% (vol/vol) hematoxylin. The slides were viewed at ×320 magnification on a Zeiss Axiovert 2000 microscope.
Statistical Analysis
All statistical tests were performed using Prism software, version 3.0 (GraphPad). Group comparisons in body weight change and white blood cell counts were made using one-way analysis of variance followed by Dunnett’s multiple comparison test. In xenograft tumor studies, tumor volumes were subjected to transformation [Y = √(Y + 3/8)] to correct for unequal variance (19) before comparison using one-way analysis of variance followed by Dunnett’s multiple comparison test. Survival curves were compared using the log-rank test. Fisher’s exact test was used to compare the response rate of complete regression between treatment and control in the large H446 tumor study. All statistical tests were two-sided, and P values of less than .05 were considered to be statistically significant for all tests.
Results
Discovery of SVV-001
SVV-001 had originally been isolated from cultured cells (Hales LM, Knowles NJ, Xu L, Hay C, Reddy PS, Hallenbeck PL: unpublished data) and could have been introduced into cell cultures via fetal bovine serum or porcine trypsin during cultivation of cells in the laboratory. The identified cytolytic agent was plaque-cloned and designated Seneca Valley Virus-001 (SVV-001). Genomic sequencing and analysis showed the virus to be a new member of the family Picornaviridae (GenBank accession #DQ641257; Hales LM, Knowles NJ, Xu L, Hay C, Reddy PS, Hallenbeck PL: unpublished data), and swine are believed to be a natural host (Hales LM, Jones BJ, Knowles NJ, Landgraf JG, Swenson SL, Skele KL, et al.: unpublished data).
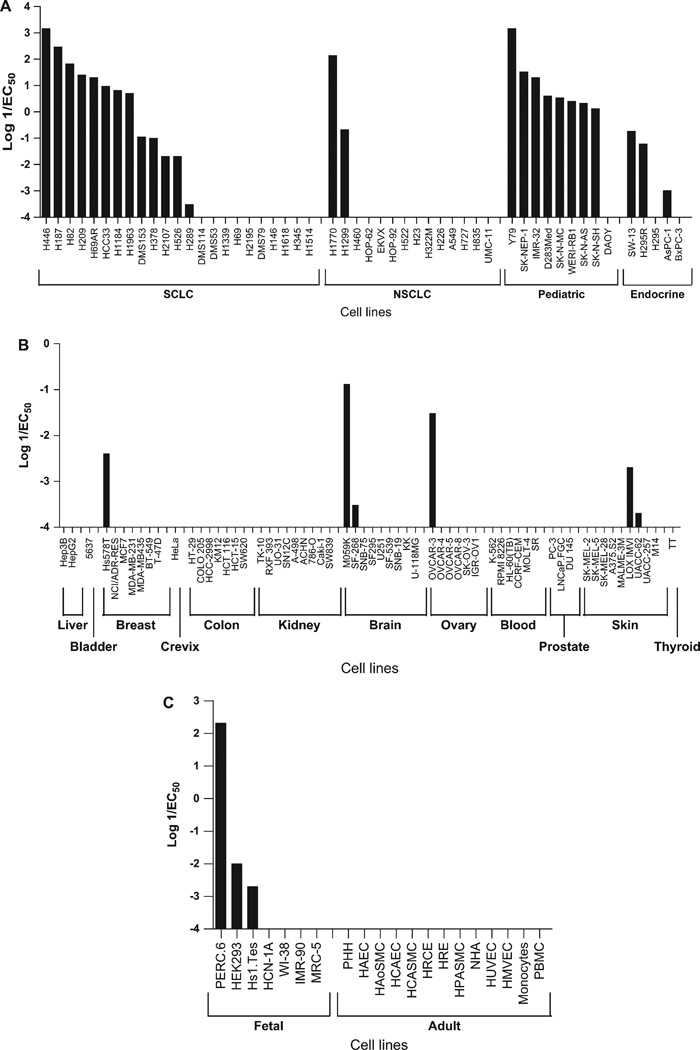
The cytolytic potential of SVV-001 was assessed in tumor cell lines, primary cells, and immortalized lines using cytotoxicity assays (Fig. 1). The EC50 values for SVV-001 cytotoxicity demonstrated a wide range of susceptibility to the virus and ranged from 10−4 to greater than 10000 ppc. Tumor cell lines from cancers possessing neuroendocrine properties, including multidrug-resistant small-cell lung cancer and neuroblastoma cell lines (Fig. 1, A), were sensitive to SVV-001. More than half (13 of 23) of the small-cell lung cancer lines tested were sensitive to SVV-001–mediated killing, i.e., EC50 values were less than 10. The two non–small-cell lung cancer cell lines (H1770 and H1299) and seven of eight neuroendocrine pediatric cancer cell lines tested were also sensitive to SVV-001–mediated killing. Two of three adrenal gland cortical carcinoma cell lines (SW-13 and H295R with 5.6 and 16.5 EC50 values, respectively) were highly sensitive to SVV-001–mediated killing. A majority of the tumor cell lines that were sensitive to SVV-001–mediated killing are known to express one or more known neuroendocrine markers, such as gastrin-releasing peptide receptors, synaptophysin, neuron-specific enolase, and CD56 (20–22). A few immortalized cell lines of embryonic or fetal origin were also sensitive to SVV-001–mediated killing (Fig. 1, B); again, these are also known to have neuroendocrine features. All normal primary human cells tested were resistant to SVV-001 cell killing (Fig. 1, B). The virus did not lyse primary hepatocytes, as determined by an LDH release assay (data not shown). Most other cancer cell lines were resistant to SVV-001–mediated cell killing (Fig. 1, C). A few cell lines tested from other species, including mouse, cow, and pig, were found to be sensitive and supported the use of mice as a relevant model for SVV-001 infection (data not shown).
Fig. 1.
Cytotoxicity of Seneca Valley Virus-001 (SVV-001) in selected tumor cell lines and normal cells (described in Supplementary Table 1, available online). Cytotoxicity assays were performed as described in the text. Effective concentration 50 (EC50; number of viral particles per cell required to produce 50% cell death) was determined. Log of the inverse EC50 value is plotted for the cell lines listed. A) Small-cell lung cancer (SCLC), non–small-cell lung cancer (NSCLC), pediatric neuroendocrine, and endocrine (adrenal gland and pancreas) cell types. B) Other adult tumor cell types. C) Embryonic cell lines (fetal) and adult normal primary cells (PHH, primary human hepatocytes; HAEC, human aortic endothelial cells; HAoSMC, human aortic smooth muscle cells; HCAEC, human coronary artery endothelial cells; HCASMC, human coronary artery smooth muscle cells; HRCE, human renal cortical epithelial cells; HRE, human renal epithelial cells; HPASMC, human pulmonary artery smooth muscle cells; NHA, normal human astrocytes; HUVEC, human umbilical vein endothelial cells; HMVEC, human microvascular endothelial cells (skin); PBMCs, peripheral blood mononuclear cells). Means from one experiment performed in duplicate are shown.
Kinetic Assays of Replication
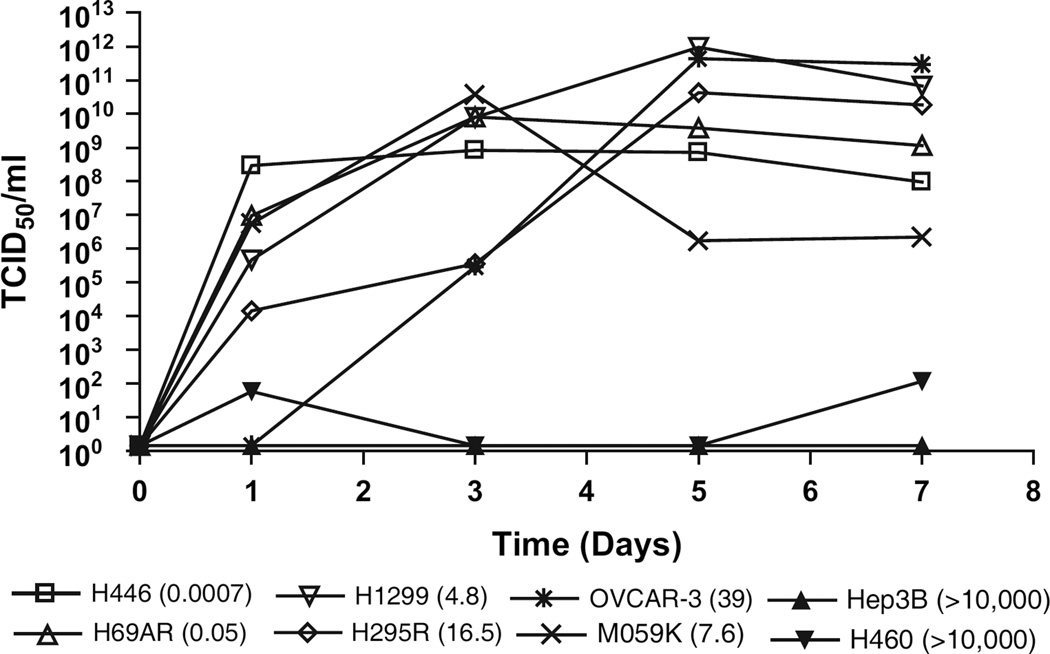
To assess the relationship between the degree of sensitivity of cell lines to SVV-001–mediated cytotoxicity and virus replication, we quantified viral production over time following infection. A high amount of virus (>1 × 106 TCID50/mL) was produced from H446, H69AR, H1299, H295R, OVCAR-3, and M059K cells that were sensitive to SVV-001–mediated killing with EC50 values ranging from 0.0007 to 39 (Fig. 1). In contrast, Hep3B and H460 cells, which were not killed by SVV-001, produced little or no virus (Fig. 2). At 1 and 3 days after infection, the amount of virus produced was inversely proportional to the EC50. Low levels of virus replication were observed until 3 days after infection in H295R and OVCAR-3 cells, with EC50 values of 16.5 and 39, respectively. In these two cell types, virus production peaked 5 days after infection. Together, these data demonstrate that cell lines that are sensitive to the cytolytic activity of SVV-001 support virus replication.
Fig. 2.
Seneca Valley Virus-001 (SVV-001) replication kinetic assay. The cell lines used for the assay had effective concentration 50 (EC50) values (given in parenthesis) that ranged from 0.0007 to greater than 10000 particles per cell (ppc). These included two cell lines with EC50 values less than 1 (H446 and H69AR), two cell lines with EC50 values greater than 1 and less than 10 (H1299 and M059K), and two cell lines resistant to SVV-001–mediated cytotoxicity and with EC50 values greater than 10000 (Hep3B and H460). Cells plated in tissue culture dishes were infected with SVV-001 at 10 virus ppc, and crude viral lysates taken at the indicated time points after infection were titered on PER.C6 cells. The amount of virus produced at each time point expressed as tissue culture infective dose 50 (TCID50 /mL) is plotted as a function of time. Means from one experiment of two performed are shown.
Hemagglutination and Whole-Blood Inactivation Assays
To test the possibility of SVV-001 inactivation by components of human blood, SVV-001 (2 × 108 particles) was incubated with whole-blood samples for 1 hour at room temperature. Plasma separated from the blood was assayed for virus inactivation using PER. C6 cells. No drop in the virus titer was observed. In addition, to determine whether SVV-001 binds to and causes agglutination of human erythrocytes, hemagglutination assays were carried out using human erythrocytes from eight volunteers representing blood groups A, B, AB, and O. No hemagglutination was observed with human erythrocytes (data not shown).
Prevalence of Neutralizing Antibodies in Human Populations
We assessed the exposure of the human population to SVV-001 by screening 70 sera samples from a random human population and 50 samples from an Amish farmer population in a neutralization assay (data not shown). Only one sample had very low levels of neutralizing antibodies (1:8) to SVV-001, the relevance of which is currently unknown. Therefore, normal exposure to SVV-001 is likely not prevalent in the human populations tested, or the virus does not replicate sufficiently in normal humans to produce a humoral immune response.
Preclinical Studies: Toxicologic Study of SVV-001
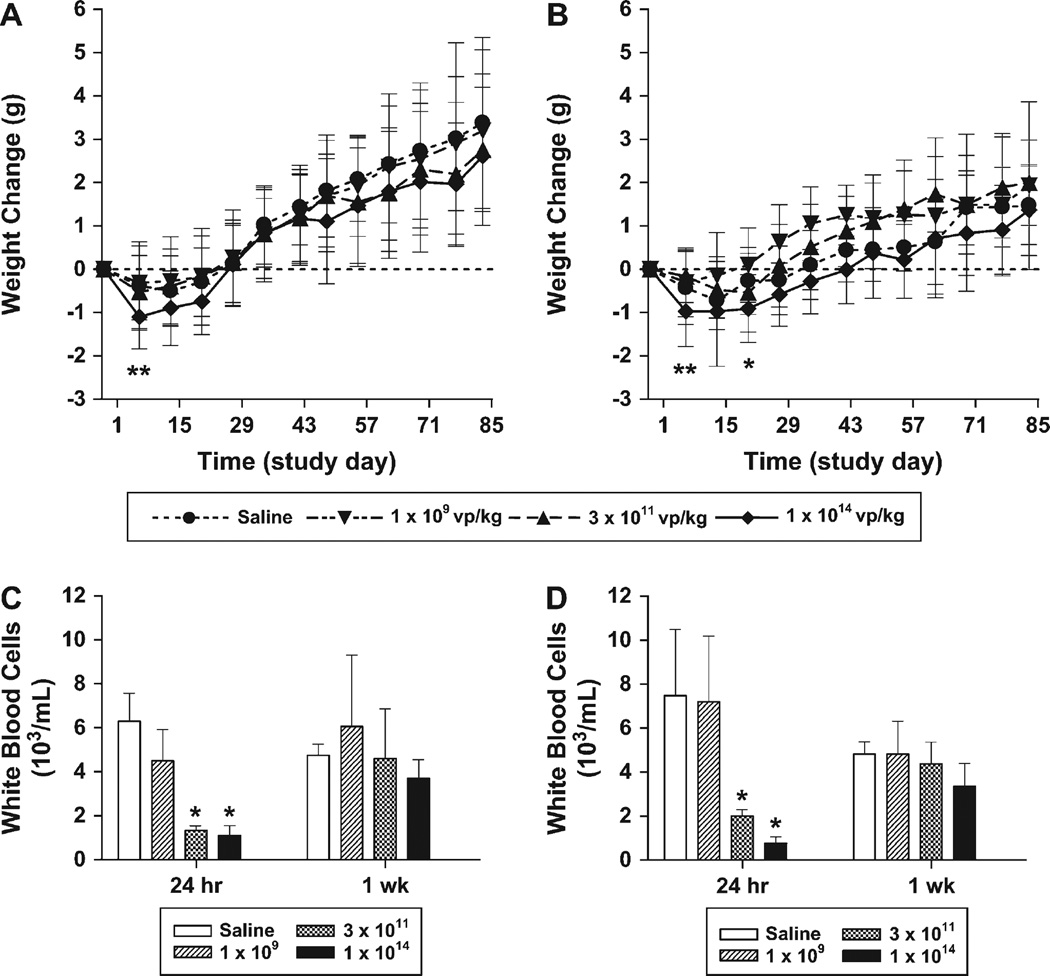
The toxicologic profile of SVV-001 administered as a single intravenous injection was evaluated in male and female A/J mice during a 12-week period. Mice received saline vehicle or SVV-001 at 1 × 109, 3 × 1011, or 1 × 1014 vp per kg in a volume of 10 mL/kg as a single intravenous bolus into the lateral tail vein. A full panel of toxicologic assessments, including clinical observations, body weights, serum chemistry, hematology, and microscopic pathology were then evaluated at 24 hours and 1, 3, 6, and 12 weeks after injection of the virus. Clinical signs of toxic effects occurred at the same frequency in SVV-001–treated groups as saline control groups. Slight decreases in mean body weight were observed in all study groups after injection, including mice injected with saline; however, a transient decrease in net body weight relative to that in control mice was observed in mice that received the highest dose (Fig. 3, A and B). No statistically significant differences in net body weight were observed beyond 1 week in males and 3 weeks in females at any dose, and all study groups gained weight over the 12-week study. A dose-dependent decrease in white blood cells, including monocytes, lymphocytes, and neutrophils, was observed 24 hours after injection in the two highest groups only and was resolved by the 1-week time point (Fig. 3, C and D). No toxicologically significant alterations in serum chemistry parameters, including the liver enzymes aspartate aminotransferase, alanine aminotransferase, and gamma glutamyltransferase, were noted. No gross or microscopic histologic pathologies, which were associated with SVV-001 treatment, were observed in any organ over the 12-week study, including organs with normal neuroendocrine cells, such as brain, adrenal, parathyroid, and thyroid (C cells) glands; carotid body; and pancreas (islet cells) (data not shown). Titration of dosing solutions confirmed that study mice had been injected with live virus at the indicated doses (data not shown).
Fig. 3.
Toxicology parameters in A/J mice following intravenous injection of Seneca Valley Virus (SVV-001). Male and female A/J mice were injected with saline vehicle (n = 25 per sex) or SVV-001 at 1 × 109 (n = 25 per sex), 3 × 1011 (n = 25 per sex), or 1 × 1014 virus particles (vp) per kg (n = 25 per sex) on study day 1. A–B) Body weights (A, male; B, female) were recorded on a weekly basis, and body weight change was calculated from the predose weight. *, P <.05 for 1 × 1014 vp per kg SVV-001 versus vehicle groups; **, P <.01 for 1 × 1014 vp per kg SVV-001 versus vehicle groups. C–D) White blood cell counts from a complete blood count performed on whole blood collected at the indicated time points are shown (C, male; D, female). *, P <.01 for SVV-001 versus vehicle groups. Means and 95% confidence intervals are shown. All P values (two-sided) were calculated using Dunnett’s test.
Preclinical Studies: Antitumor Efficacy of SVV-001
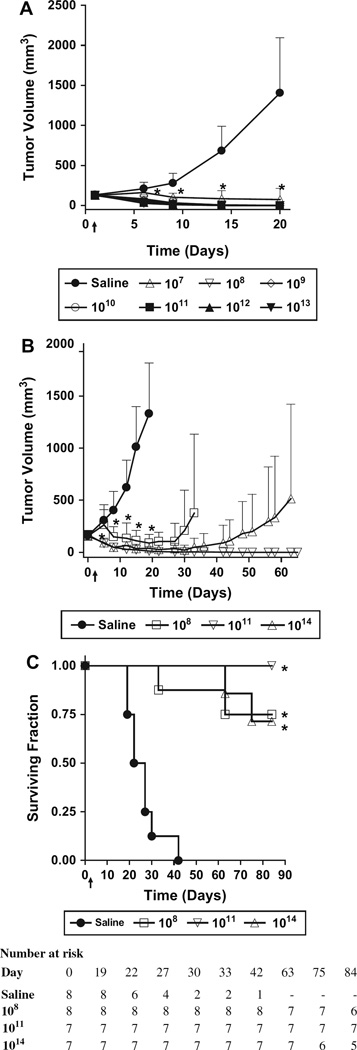
Because small-cell lung cancer is an aggressive, highly metastatic neuroendocrine malignant disease with universally poor prognosis (23, 24), we studied the efficacy of systemically administered SVV-001 in a murine xenograft tumor model using the neuroendocrine small-cell lung cancer line H446. In cytotoxicity assays, SVV-001 mediated efficient killing of H446 cells, with an EC50 of 0.0007 ppc (Fig. 1, A). Doses between 1 × 107 and 1 × 1013 vp of SVV-001 per kg were administered as a single intravenous injection into athymic mice bearing preestablished tumors. H446 tumors from mice that were treated with saline grew rapidly, with mean tumor volume reaching 1405 mm3 by day 20 (Fig. 4, A). In contrast, complete eradication of tumor mass was observed in each of the 60 mice treated with SVV-001 at doses of 1 × 108 vp per kg and higher. In the group of 10 mice receiving 1 × 107 vp per kg, eight mice were tumor free, one mouse had a very large tumor, and another had only a small palpable tumor (25 mm3) on study day 31. The virus cleared from blood rapidly (no virus detected after 6 hours), and high levels of virus (1 × 106 TCID50 per tumor) were observed in H446 tumors (data not shown) following intravenous delivery of 1 × 1012 vp per kg in athymic mice, demonstrating the tropism of the virus toward this neuroendocrine tumor.
Fig. 4.
In vivo efficacy of systemically administered Seneca Valley Virus-001 (SVV-001). A single intravenous injection of saline vehicle or SVV-001 at the indicated doses (virus particles per kg) was given to athymic mice bearing preestablished xenograft tumors on study day 1 (denoted by ↑). A) Human small-cell lung tumor line, H446 (n = 10 per group). B) Human retinoblastoma Y79 (n = 7–8 per group). Mean tumor volumes and 95% confidence intervals are plotted as a function of time. *, P <.01 (two-sided, calculated using Dunnett’s test) compared with saline control for all SVV-001-treated groups with mean values appearing below the asterisk in the figure. C) Survival curves of nude mice bearing xenografts of the human retinoblastoma tumor line Y79. *, P <.001 (two-sided, calculated using the log-rank test) compared with saline control.
We also tested the effect of systemically administered SVV-001 on preestablished tumors of the pediatric neuroendocrine retinoblastoma model Y79 (Fig. 4, B). Complete Y79 tumor regression following a single intravenous injection was observed in six of eight mice treated with 1 × 108 vp per kg, all seven mice treated with 1 × 1011 vp per kg, and five of seven mice treated with 1 × 1014 vp per kg. All mice with eradicated Y79 tumors survived to study end (day 84) without evidence of tumor recurrence or gross toxicity (Fig. 4, C). SVV-001 prolonged survival at each dose tested versus saline vehicle (P<.001; Fig. 4, C).
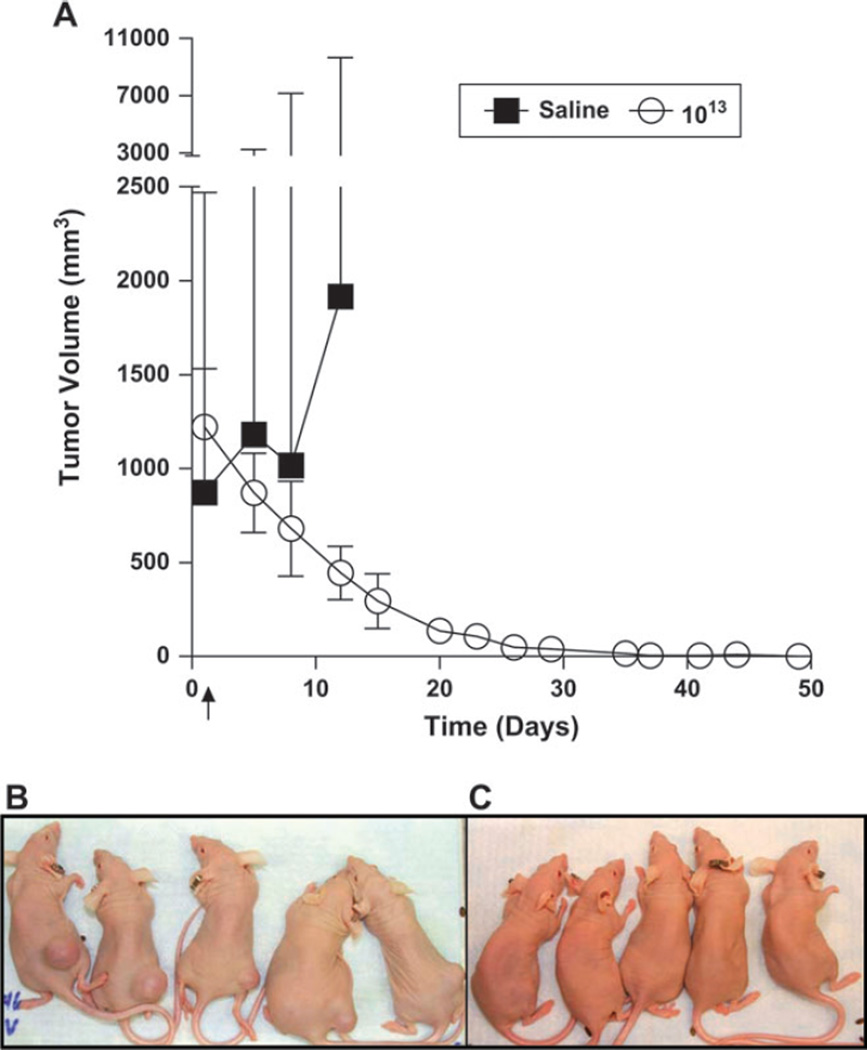
To evaluate the antitumor activity of SVV-001 when administered to mice bearing very large tumors (486–1996 mm3), 10 mice bearing large H446 tumors were injected systemically with a single dose of 1 × 1013 vp per kg. A dramatic regression of tumor volumes was noted, and by study day 49 complete eradication of tumors was observed in all 10 mice that received SVV-001 (Fig. 5). In contrast, all tumors from mice that were injected with saline quickly grew to greater than 2000 mm3.
Fig. 5.
Eradication of large neuroendocrine SCLC H446 xenografts. A single injection of saline vehicle (n = 3) or Seneca Valley Virus-001 (SVV-001) at 1013 virus particles per kg (n = 10) was given to athymic mice bearing large H446 tumors (592–1996 mm3) on study day 1 (denoted by ↑). A) Mean tumor volumes and 95% confidence intervals are plotted as a function of time. B) Tumors on the flanks of mice before treatment. C) Tumors on the flanks of mice 57 days after treatment. Complete tumor regression was observed in all 10 mice treated with SVV-001 but in none of three mice treated with saline (P = .004; twosided, calculated using Fisher’s exact test).
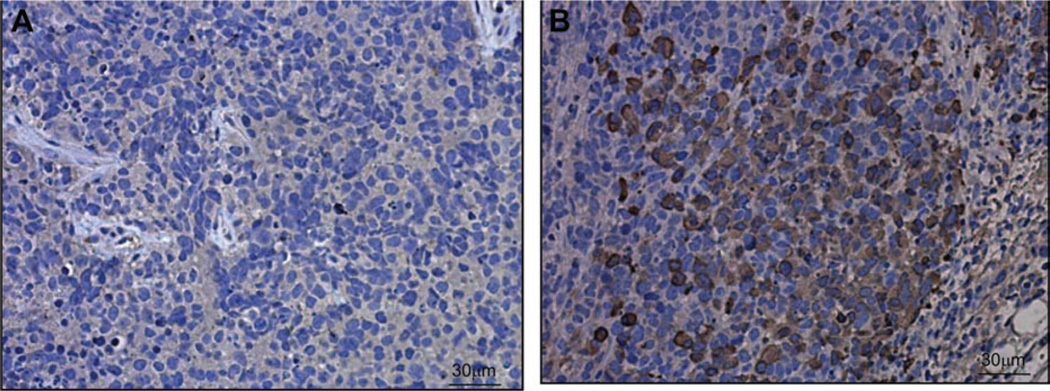
Immunohistochemical analysis of H446 neuroendocrine small-cell lung cancer tumor sections collected 24 hours after injection of SVV-001 at 1 × l09 vp per kg showed that tumor cells stained positive for SVV-001 capsid proteins (Fig. 6). Analysis of corresponding sections from mice that were treated with vehicle showed no viral capsid protein staining, indicating that the staining observed was specific to SVV-001 treatment (Fig. 6). Sections from SVV-001–treated H446 xenograft tumors harvested at later time points showed destruction of tumor architecture with inflammatory infiltrate. The results of immunohistochemical staining for SVV-001 demonstrate the ability of systemically delivered SVV-001 to reach the tumor and undergo replication, leading to a profound eradication of tumor mass in this model.
Fig. 6.
Immunohistochemical analysis of H446 human small-cell lung tumor xenografts from mice that were systemically treated with saline or 109 virus particles (vp) per kg Seneca Valley Virus-001 (SVV-001). Mice were killed 24 hours after injection, and tumor sections were subjected to immunohistochemistry using mouse polyclonal antibody against SVV-001 capsid proteins. Cells that are infected by SVV-001 are indicated by brown intracellular staining. A) Image from a tumor of a mouse injected with saline. B) Image from a tumor of a mouse injected with 109 vp per kg SVV-001. One representative image of three experiments is shown.
Discussion
In vitro cytotoxicity assays clearly showed that SVV-001 has inherent oncolytic activity that is directed to tumor cells with neuroendocrine characteristics, such as small-cell lung cancer and many types of pediatric solid cancers, and had no deleterious effects on normal cells. In tumor cell lines, the virus replication kinetic experiments clearly demonstrated a strong agreement between cytotoxicity and efficiency of virus replication. Data from hemagglutination, whole-blood inactivation, and neutralization assays showed that SVV-001 can be systemically delivered without fear of virus inactivation in the blood.
The mechanism of tumor-selective replication of SVV-001 is currently being investigated. Transfection of genomic RNA into nonpermissive cells resulted in production of virus, indicating that selective tropism of virus replication exists primarily at the level of receptor interaction and/or internalization (Reddy PS, Hales LM, Jones BH, Idamakanti N, Hallenbeck PL: unpublished data).
Systemic delivery of oncolytic viruses will be necessary to treat patients with metastatic cancer. Several oncolytic viruses that are being explored as possible cancer therapy cannot be systemically delivered because they are either inactivated by one or more components of human blood or are too toxic to normal cells (25, 26). However, we found that SVV-001 is not inhibited by any component of human blood. Moreover, preexisting neutralizing antibodies to SVV-001 were very rare in serum from the diverse human populations that were tested. Furthermore, SVV-001 did not bind to erythrocytes — a property of some viruses that can elicit severe toxicity (25). In a toxicity study using A/J mice, a strain that has been demonstrated to support replication of the virus, no dose-limiting toxicity was noted following a single systemic injection of SVV-001 at doses up to 1 × 1014 vp per kg. Measurable changes in response to systemic injection of SVV-001 were limited to a transient decrease in body weight at the 1 × 1014 vp per kg dose and a transient decrease in white blood cells at the 3 × 1011 and 1 × 1014 vp per kg doses. Both of these parameters returned to baseline within 3 weeks and 1 week, respectively. No other toxicity related to SVV-001 was observed in this 12-week study, indicating that systemically delivered SVV-001 was well tolerated.
Antibody development can impede the efficacy of the oncolytic virus when it is administered repeatedly (26). In this study, immune-competent mice that received intravenous injection of SVV-001 developed neutralizing antibodies. However, our preclinical data showed profound efficacy following a single administration of the virus in the human tumor models tested without the need for repeat administrations. In general, preexisting antibodies and antibodies that develop following the first dose of oncolytic viruses impede systemic delivery of repeat injections to treat metastases (26). However, preexisting antibodies to oncolytic viruses may not interfere with intratumoral delivery and associated localized efficacy as has been demonstrated with measles and herpes viruses (27). Moreover, use of immunosuppressants, such as cyclophosphamide, before or during oncolytic virus therapy can minimize development of immune responses (28, 29). In addition, coating of oncolytic viruses with synthetic polymers may also be used to aid in repeat administration (30).
In small-cell lung cancer H446 and neuroendocrine retinoblastoma Y79 xenograft studies, systemically delivered SVV-001 was efficacious even at the lowest dose (107 or 108 vp per kg, respectively) tested. No deaths or toxic effects were noted in mice that received up to 1 × 1014 vp per kg, that is, a therapeutic index of greater than 1000000. Moreover, the virus was very effective, with 100% of large preestablished H446 neuroendocrine tumors being eradicated. Such high efficacy following a single intravenous administration may be due to the efficient entry of the virus into the tumor mass and rapid replication cycle of picornaviruses. The small size of SVV-001 (25–30 nm) and lack of envelope may have helped to rapidly distribute the virus throughout the tumor mass. By contrast, the inability of oncolytic adenovirus, which is 100 nm in diameter, to enter H446 tumors following systemic administration in mice may be related to its large virion size (Paul Hallenbeck, unpublished data). The ability of SVV-001 to enter and replicate rapidly in neuroendocrine cancers following systemic delivery may be useful in the treatment of inaccessible primary tumors and micrometastases that are difficult to detect using current imaging techniques.
In addition, SVV-001 has a variety of unique features that may also enhance its ability to be used as a virotherapeutic agent. SVV-001 is easy to manufacture because the virus can be grown to high titers in PER.C6 cells and is easily purified. Yields of greater than 200000 virus particles per cell can be produced routinely. The life cycle of SVV-001 is very rapid and is completed within 12 hours, thus allowing for rapid spread to neighboring tumor cells and several rounds of virus replication before the development of an immune response. SVV-001 is a simple single-stranded RNA virus and therefore does not require an intermediate DNA step during replication, so there is no possibility for insertion mutagenesis of viral RNA into the host genome. Moreover, the genomes of picornaviruses carry no oncogenes that may induce tumors in animals. Finally, SVV-001 replicates in the mouse, which is a widely accepted relevant model in which to study toxicity and efficacy.
Nonpathogenicity in humans and animal species and stability of the viral genome in vitro and in vivo are two other desirable properties of oncolytic viruses. SVV-001 is not linked to any disease condition in pigs, the natural host of the virus (Hales LM, Jones BJ, Knowles NJ, Landgraf JG, Swenson SL, Skele KL, et al.: unpublished data). We found that systemic administration of the virus into immune-competent and immune-deficient mice was well tolerated and caused no toxicity. Moreover, to evaluate the ability of SVV-001 to adapt to replicate in nonpermissive cells, the virus was passaged intentionally three times in nonpermissive cell lines A549, H460, and Hep3B, and no virus was produced, suggesting that the virus did not change its tropism (data not shown). In addition, no antibody escape mutants of SVV-001 were produced in PER.C6 cells when SVV-001 was grown with media containing anti-SVV mouse hyper immune serum (data not shown). These data suggest that the genome of SVV-001 is stable.
Our study has several potential limitations. Although the in vivo efficacy data reported here were generated using immune-deficient athymic mice, it is unknown whether immune responses in cancer patients would limit the effectiveness of SVV-001 in patients and prevent repeat administration, if it was necessary. In addition, studies were done using subcutaneous tumor models using well-defined cell lines and, as such, may not simulate patients with metastases. Immune-competent and metastasis models are currently being explored to address these limitations.
The data in this report suggest that SVV-001 may overcome many of the challenges faced by traditional therapies and other oncolytic viruses. We demonstrated that SVV-001 exhibits rapid, potent, and selective killing of human cancer cells with neuro endocrine properties, lack of toxicity to normal cells, safety in preclinical mouse models, and profound efficacy in two in vivo xenograft tumor models. Importantly, SVV-001 is not inhibited by any component of human blood and can be administered systemically. These attributes lend promise of this agent for the treatment of small-cell lung cancer and other cancers with neuroendocrine properties. SVV-001 is currently being evaluated in phase I/II dose-escalation clinical trials for safety and efficacy.
Supplementary Material
CONTEXT AND CAVEATS.
Prior knowledge
Oncolytic viruses have potential as cancer therapies, but many are inactivated by components in the blood and/or are toxic to normal cells.
Study design
In vitro cytotoxicity assays in tumor and normal cell lines, inactivation assays with human blood samples, and in vivo mouse models of small-cell lung cancer and retinoblastoma.
Contributions
Seneca Valley Virus-001 (SVV-001) was more cytotoxic to small-cell lung cancer cell lines and solid pediatric cancer cell lines than other tumor cell lines and normal cell lines; this activity was not inhibited by components in human blood. Single intravenous treatment with SVV-001 in mouse models led to complete responses of all mice carrying small-cell lung cancer xenograft tumors and a majority of mice carrying retinoblastoma xenograft tumors. SVV-001 was well tolerated in mice.
Implications
SVV-001 may be useful as a treatment for metastatic tumors with neuroendocrine properties, such as small-cell lung cancer.
Limitations
It is unclear whether these results from immune-deficient mouse models would be similar to those of patients with metastatic cancer. In particular, it is unknown whether the patients ’ immune responses would reduce the effectiveness of SVV-001.
Acknowledgments
Funding
Burroughs Wellcome Fund (1005994 to C. M. R. and D. N. W.).
Drs Reddy, Burroughs, Hayes, Idamakanti, and Hallenbeck and Mr Jones and Ms Skele are employees of Neotropix, Inc, hold stock in the company, and are performing research for Neotropix, Inc, with the goal of developing SVV-001 as a treatment for cancer. Dr Li and Ms Vasko are former employees of Neotropix, Inc.
We thank Dr Shizuko Sei for leading NCI-60 screen of the Developmental Therapeutics Program, Dr John Minna for select small-cell lung cancer tumor cell lines, Dr Alan Shuldiner for serum samples, Kimberly Schneider for technical assistance, Dr Richard Peluso for critical review of the manuscript, and Janice Ingram for assisting with the manuscript preparation.
References
- 1.Kasuya H, Takeda S, Nomoto S, Nakao A. The potential of oncolytic virus therapy for pancreatic cancer. Cancer Gene Ther. 2005;12:725–736. doi: 10.1038/sj.cgt.7700830. [DOI] [PubMed] [Google Scholar]
- 2.Vidal L, Pandha H, Spicer J, Harrington KJ, Allen S, Leader D, et al. A phase I study of reolysin given intravenously to patients with advanced malignancies. J Clin Oncol. 2006;24:18S. [Google Scholar]
- 3.Pecora AL, Rizvi N, Cohen GI, Meropol NJ, Sterman D, Marshall JL, et al. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J Clin Oncol. 2002;20:2251–2266. doi: 10.1200/JCO.2002.08.042. [DOI] [PubMed] [Google Scholar]
- 4.Heinzerling L, Kunzi V, Oberholzer PA, Kundig T, Naim H, Dummer R, et al. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood. 2005;106:2287–2294. doi: 10.1182/blood-2004-11-4558. [DOI] [PubMed] [Google Scholar]
- 5.Lichty BD, Power AT, Stojdl DF, Bell JC. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol Med. 2004;10:210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Unno Y, Shino Y, Kondo F, Igarashi N, Wang G, Shimura R, et al. Oncolytic viral therapy for cervical and ovarian cancer cells by Sindbis virus AR339 strain. Clin Cancer Res. 2005;11:4553–4560. doi: 10.1158/1078-0432.CCR-04-2610. [DOI] [PubMed] [Google Scholar]
- 7.Gromeier M, Alexander L, Wimmer E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc Natl Acad Sci USA. 1996;93:2370–2375. doi: 10.1073/pnas.93.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toyoda H, Ido M, Hayashi T, Gabazza EC, Suzuki K, Kisenge RR, et al. Experimental treatment of human neuroblastoma using live-attenuated poliovirus. Int J Oncol. 2004;24:49–58. [PubMed] [Google Scholar]
- 9.Shafren DR, Au GG, Nguyen T, Newcombe NG, Haley ES, Beagley L, et al. Systemic therapy of malignant human melanoma tumors by a common cold-producing enterovirus, coxsackievirus A21. Clin Cancer Res. 2004;10:53–60. doi: 10.1158/1078-0432.ccr-0690-3. [DOI] [PubMed] [Google Scholar]
- 10.Russell SJ. RNA viruses as virotherapy agents. Cancer Gene Ther. 2002;12:961–966. doi: 10.1038/sj.cgt.7700535. [DOI] [PubMed] [Google Scholar]
- 11.Smyth M, Symonds A, Brazinova S, Martin J. Bovine enterovirus as an oncolytic virus: foetal calf serum facilitates its infection of human cells. Int J Mol Med. 2002;10:49–53. doi: 10.3892/ijmm.10.1.49. [DOI] [PubMed] [Google Scholar]
- 12.Myers R, Greiner S, Harvey M, Soeffker D, Frenzke M, Abraham K, et al. Oncolytic activities of approved mumps and measles vaccines for therapy of ovarian cancer. Cancer Gene Ther. 2005;12:593–599. doi: 10.1038/sj.cgt.7700823. [DOI] [PubMed] [Google Scholar]
- 13.Shafren DR, Sylvester D, Johansson ES, Campbell IG, Barry RD. Oncolysis of human ovarian cancers by echovirus type 1. Int J Cancer. 2005;115:320–328. doi: 10.1002/ijc.20866. [DOI] [PubMed] [Google Scholar]
- 14.Suter SE, Chein MB, von Messling V, Yip B, Cattaneo R, Vernau W, et al. In vitro canine distemper virus infection of canine lymphoid cells: a prelude to oncolytic therapy for lymphoma. Clin Cancer Res. 2005;11:1579–1587. doi: 10.1158/1078-0432.CCR-04-1944. [DOI] [PubMed] [Google Scholar]
- 15.Liu TC, Kirn D. Systemic efficacy with oncolytic virus therapeutics: clinical proof-of-concept and future directions. Cancer Res. 2007;67:429–432. doi: 10.1158/0008-5472.CAN-06-2871. [DOI] [PubMed] [Google Scholar]
- 16.Fallaux FJ, Bout A, van der Velde I, van den Wollenberg DJ, Hehir KM, Keegan J, et al. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum Gene Ther. 1998;9:1909–1917. doi: 10.1089/hum.1998.9.13-1909. [DOI] [PubMed] [Google Scholar]
- 17.Scraba DG, Palmenberg AC. Cardioviruses (Picornaviridae) In: Webster RG, Granoff A, editors. Encyclopedia of virology, 1999. 2nd. London: Academic Press; 1999. pp. 1–10. [Google Scholar]
- 18.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 19.Zar JH. Biostatistical analysis. 3rd. Upper Saddle River (NJ): Prentice-Hall; 1996. [Google Scholar]
- 20.Giaccone G, Battey J, Gazdar AF, Oie H, Draoui M, Moody TW. Neuromedin B is present in lung cancer cell lines. Cancer Res. 1992;52:2732s–2736s. [PubMed] [Google Scholar]
- 21.Hibi K, Westra WH, Borges M, Goodman S, Sidransky D, Jen J. PGP9.5 as a candidate tumor marker for non-small-cell lung cancer. Am J Pathol. 1999;155:711–715. doi: 10.1016/S0002-9440(10)65169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bepler G, Jaques G, Koehler A, Gropp C, Havemann K. Markers and characteristics of human SCLC cell lines. Neuroendocrine markers, classical tumor markers, and chromosomal characteristics of permanent human small cell lung cancer cell lines. J Cancer Res Clin Oncol. 1987;113:253–259. doi: 10.1007/BF00396382. [DOI] [PubMed] [Google Scholar]
- 23.Murray N, Turrisi AT. A review of first-line treatment for small-cell lung cancer. J Thorac Oncol. 2006;1:270–278. doi: 10.1016/s1556-0864(15)31579-3. [DOI] [PubMed] [Google Scholar]
- 24.Fry WA, Menck HR, Winchester DP. The national cancer data base report on lung cancer. Cancer. 1996;77:1947–1955. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1947::AID-CNCR27>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 25.Morrissey RE, Horvath C, Snyder EA, Patrick J, MacDonald JS. Rodent nonclinical safety evaluation studies of SCH 58500, an adenoviral vector for the p53 gene. Toxicol Sci. 2002;65:266–275. doi: 10.1093/toxsci/65.2.266. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Yu DC, Charlton D, Henderson DR. Pre-existent adenovirus antibody inhibits systemic toxicity and antitumor activity of CN706 in the nude mouse LNCaP xenograft model: implications and proposals for human therapy. Hum Gene Ther. 2000;11:1553–1567. doi: 10.1089/10430340050083289. [DOI] [PubMed] [Google Scholar]
- 27.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumors. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 28.Hirasawa K, Nishikawa SG, Norman KL, Coffey MC, Thompson BG, Yoon CS, et al. Systemic reovirus therapy of metastatic cancer in immune-competent mice. Cancer Res. 2003;63:348–353. [PubMed] [Google Scholar]
- 29.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci USA. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green NK, Hale AB, Mautner V, Harkins R, Hermiston T, Ulbrich K, et al. Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 2004;11:1256–1263. doi: 10.1038/sj.gt.3302295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.