Abstract
We evaluated the cross-sectional associations of N-terminal pro-B-type natriuretic peptide (NT-proBNP) with cardiac structural and functional abnormalities in a cohort of chronic kidney disease (CKD) patients without clinical heart failure (HF), the Chronic Renal Insufficiency Cohort (n=3,232). Associations of NT-proBNP with echocardiographically determined left ventricular (LV) mass and LV systolic and diastolic function were evaluated by multivariable logistic and linear regression models. Reclassification of participants’ predicted risk of LV hypertrophy (LVH), systolic and diastolic dysfunction was performed using a category-free net reclassification improvement (NRI) index that compared a clinical model with and without NT-proBNP. The median (interquartile range) NT-proBNP was 126.6 pg/ml (55.5–303.7). The highest quartile of NT-proBNP was associated with nearly three-fold odds of LVH (odds ratio (OR) 2.7, 95% confidence interval (CI) 1.8–4.0) and LV systolic dysfunction (2.7, 1.7–4.5) and two-fold odds of diastolic dysfunction (2.0, 1.3–2.9) in the fully adjusted models. When evaluated alone as a screening test, NT-proBNP functioned modestly for the detection of LVH (area under the curve, AUC 0.66) and LV systolic dysfunction (AUC 0.62), and poorly for the detection of diastolic dysfunction (AUC 0.51). However, when added to the clinical model, NT-proBNP significantly reclassified participants’ likelihood of having LVH (NRI 0.14, 95% CI 0.13–0.15; p<0.001) and LV systolic dysfunction (0.28, 0.27–0.30; p<0.001), but not diastolic dysfunction (0.10, 0.10–0.11; p=0.07). In conclusion, in this large CKD cohort without HF, NT-proBNP had strong associations with prevalent LVH and LV systolic dysfunction.
Keywords: NT-proBNP, left ventricular structure, chronic kidney disease
Introduction
B-type natriuretic peptide (BNP) and its inactive fragment, N-terminal pro-BNP (NT-proBNP), are co-secreted in equimolar amounts from cardiac myocytes into the circulation in response to myocardial stretch.1 In the general population, elevated NT-proBNP levels predict poor outcomes in both asymptomatic and dyspneic individuals, irrespective of renal function.2,3 There is a high prevalence of elevated levels of NT-pro-BNP in asymptomatic patients with CKD.4 This is likely due to some combination of extracellular volume expansion, concomitant heart disease and, possibly, reduced renal clearance,5 but the clinical implications are not clear. In prior studies of asymptomatic patients with CKD, NT-proBNP was associated with prevalent ischemic heart disease and LVH, but not left ventricular (LV) systolic or diastolic dysfunction.4,6 However, controversy remains regarding the extent to which renal dysfunction confounds the association between NT-proBNP levels and LVH in CKD.7 In addition, the diagnostic role of NT-pro-BNP for the detection of cardiac structural and functional abnormalities has not been defined in a large cohort of patients with CKD. If NT-proBNP were to be highly predictive of pathologic cardiac abnormalities, then it could be used to select patients with CKD who might benefit from further evaluation with echocardiography, and targeted care to prevent HF. To understand the clinical significance of elevations in NT-pro-BNP levels and to define better the diagnostic role of NT-proBNP, we examined the associations of circulating NT-proBNP with LV structure and function in a large, diverse population of ambulatory patients without HF at various stages of CKD.
Methods
This is a cross-sectional analysis from the Chronic Renal Insufficiency Cohort (CRIC) Study, which was established by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) in 2001 as an observational study to evaluate the determinants of progression to end-stage renal disease (ESRD) and CVD among persons with CKD.8 Participants were recruited from seven clinical centers between July 2003 and March 2007. Inclusion criteria were an estimated glomerular filtration rate (eGFR) between 20–70 ml/min/1.73m2 for persons aged 21–44, 20–60 ml/min/1.73m2 for persons aged 45–64, and 20–50 ml/min/1.73m2 for those aged 65–74. Exclusion criteria included prior transplantation, polycystic kidney disease, multiple myeloma, use of immunosuppression, and severe comorbid illnesses such as cirrhosis, HIV disease, and severe HF. For this analysis, we excluded participants who reported prevalent HF on the CRIC medical history questionnaire (n=443), or who had more than mild mitral regurgitation or significant aortic valve disease based on transthoracic echocardiography (TTE; n=196 after exclusion of participants with chronic HF). There were 3,232 participants with NT-proBNP measurements after exclusion.
The primary predictor for this study was NT-proBNP, measured using the Elecsys 2010 analyzer (Roche Diagnostics, Indianapolis, IN) at the University of Maryland. The coefficient of variation for the NT-proBNP assay was 3.5% during the testing period, and the analytical measurement range for NT-proBNP was 5 to 35,000 pg/mL. For adjusted models, we chose covariates that would be available clinically; these included demographic characteristics (age, sex, race and clinical site); clinical characteristics (body mass index, systolic and diastolic blood pressure, hypertension, diabetes, hypercholesterolemia, current smoking, alcohol and illicit drug use, coronary artery disease (prior myocardial infarction or revascularization), and peripheral vascular disease); hemoglobin level, high-sensitivity C-reactive protein (hsCRP), and eGFR using cystatin C (eGFR_CysC).9
TTE was performed in all CRIC participants at year 1 of follow-up according to American Society of Echocardiography guidelines,10 and the data were sent to a core echocardiography laboratory for measurement and analysis (University of Pennsylvania). For this analysis, we excluded participants with more than mild mitral regurgitation or significant aortic valve disease based on TTE because these valvular abnormalities could potentially confound the relationship between NT-proBNP and cardiac structural and functional abnormalities in CKD.
LV mass was calculated using the area-length method and indexed to height2.7.10 LVH was defined as LV mass/height2.7≥47g/m2.7 in women and ≥50g/m2.7 in men.11 LV end-diastolic and end-systolic volumes (EDV and ESV, respectively) were calculated using the modified biplane method and ejection fraction (EF) was calculated as: (EDV – ESV)/EDV. LV systolic dysfunction was defined as an EF <0.45.12,13 Mitral inflow E-and A-wave velocities, E-wave deceleration time and pulmonary venous reverse A-wave duration were used to categorize LV diastolic function into: normal, mildly, moderately or severely abnormal.14 Since one center was unable to evaluate diastolic function, these measures were unavailable in 564 participants.
We first depicted the distribution of NT-proBNP in this cohort of participants with CKD. We then categorized NT-proBNP into quartiles to allow an unbiased portrayal of levels. Demographic, laboratory and echocardiographic values were compared across categories of NT-proBNP using analysis of variance (ANOVA) or Kruskal-Wallis tests for continuous variables and chi-square test for categorical variables.
NT-proBNP was modeled as a continuous variable after log-transformation because of its skewed distribution. The association of NT-proBNP with LV mass/height2.7 was assessed by multivariable linear regression. Demographic and laboratory covariates were entered into the multivariable-adjusted models based on the strength of their bivariate association with the outcome (P<0.05). We dichotomized LVH, systolic and diastolic dysfunction and used multivariable logistic regression for these analyses. Diastolic dysfunction was dichotomized with normal and mildly abnormal function as the referent category.
We evaluated the C-statistic, which is equivalent to the area under the receiver operating characteristics (ROC) curve (AUC), for NT-proBNP as the single predictor of each outcome. We then determined the sensitivity, specificity, positive and negative likelihood ratios for NT-proBNP as a predictor of LVH, LV systolic and diastolic dysfunction using cut-points of the 75th and 90th percentiles of the NT-proBNP distribution. We also evaluated the clinical impact of NT-proBNP using the category-free net reclassification improvement (NRI) analysis.15 A baseline multivariable logistic regression model with clinical predictors was used to generate the probability of each participant having LVH, LV systolic dysfunction or LV diastolic dysfunction (P0). Covariates included age, race, sex, body mass index, eGFR_CysC, albuminuria, diabetes, current smoking, any CVD, and hemoglobin. These probabilities were then recalculated for each outcome with the addition of NT-proBNP into the clinical model (P1). If P1>P0, then the person was considered to have an upward reclassification; whereas, if P1<P0, then there was a downward reclassification; and, if P1= P0, then there was no change with the addition of NT-proBNP to the clinical model. The NRI was then calculated using the following formula: 16 Improvement in net reclassification was indicated by an NRI significantly greater than 0.15 STATA version 11 (StataCorp, College Station, TX) was used for the analysis.
Results
Among 3,232 participants included in this analysis, the distribution of the NT-proBNP levels was skewed rightward; the median (interquartile range, IQR) was 126.6 (55.5–303.7) pg/mL, and the 90th percentile was 734.4 pg/mL (Figure 1). The mean (standard deviation) age of the participants was 59 ± 11 years; 45% were women and 43% were non-Hispanic white. Compared with those with the lowest levels of NT-proBNP, participants with the highest level of NT-proBNP were older and more likely to be Hispanic (Table 1). Higher levels of NT-proBNP were also associated with higher prevalences of diabetes, hypertension, hyperlipidemia, tobacco use, cardiovascular and peripheral vascular disease, with higher systolic blood pressure (SBP) and urine albumin/creatinine, and with lower hemoglobin and eGFR_CysC. NT-proBNP and eGFR_CysC were moderately correlated (−0.49, p<0.001).
Figure 1.
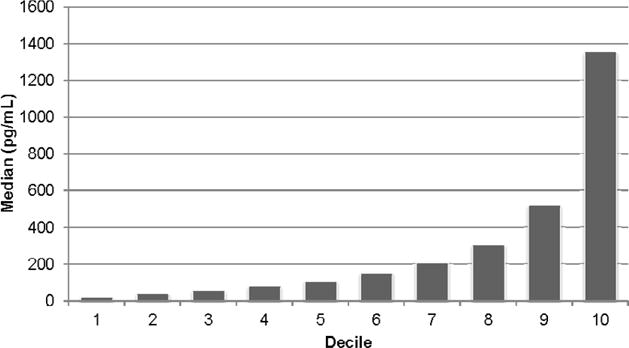
Distribution of NT-proBNP Described by the Median Level in Each Decile
Table 1.
Characteristics of Chronic Renal Insufficiency Cohort participants without chronic heart failure by quartile of N-terminal pro b-type natriuretic peptide (NT-proBNP)*
| Quartile | I (N=808) |
II (N=809) |
III (N=807) |
IV (N=808) |
P Value |
|---|---|---|---|---|---|
| NT-proBNP Cut Points | 2.5–55.4 | 55.5–126.6 | 126.9–303.6 | 303.7–33742.0 | <0.001 |
|
| |||||
| Age (years) | 54 (12) | 58 (11) | 60 (11) | 62 (10) | <0.001 |
| Female | 35% | 48% | 52% | 47% | <0.001 |
| Non-Hispanic White | 43% | 44% | 47% | 39% | <0.001 |
| Non-Hispanic Black | 46% | 41% | 36% | 36% | <0.001 |
| Hispanic | 7% | 10% | 13% | 21% | <0.001 |
| Other | 4% | 5% | 4% | 3% | <0.001 |
| Diabetes mellitus | 33% | 41% | 49% | 60% | <0.001 |
| Hypertension | 79% | 87% | 90% | 96% | <0.001 |
| High Cholesterol** | 82% | 86% | 85% | 89% | <0.01 |
| Current Smoker | 10% | 9% | 15% | 18% | <0.001 |
| Alcohol intake (DHQ,g) | 6.0 (12.4) | 7.0 (19.7) | 6.4 (31.4) | 6.4 (24.1) | <0.001 |
| Cardiovascular Disease | 14% | 20% | 29% | 47% | <0.001 |
| Peripheral Vascular Disease | 3% | 3% | 6% | 13% | <0.001 |
| Body Mass Index (kg/m2) | 32.0 (7.4) | 32.0 (7.5) | 32.0 (8.4) | 31.8 (8.1) | 0.86 |
| Systolic Blood Pressure (mmHg) | 128 (18) | 131 (18) | 134 (21) | 140 (22) | <0.001 |
| Diastolic Blood Pressure (mmHg) | 75 (12) | 76 (11) | 75 (13) | 74 (13) | 0.07 |
| eGFR CysC (mL/min/1.73m2) | 63.5 (20.5) | 52.9 (18.1) | 46.4 (16.6) | 38.7 (14.1) | <0.001 |
| eGFR_Scr (mL/min/1.73m2) | 50.5 (13.6) | 43.5 (13.5) | 39.4 (13.7) | 33.2 (13.3) | <0.001 |
| Urine Albumin-to-Creatinine Ratio (μg/mg)*** | 17 (5–117) | 30 (7–316) | 48 (9–447) | 240 (29–1329) | <0.001 |
| Hemoglobin (g/dL) | 13.6 (1.7) | 12.9 (1.6) | 12.6 (1.7) | 12.1 (1.8) | <0.001 |
| Highly Sensitive C-Reactive Protein (mg/L) | 4.7 (9.1) | 4.7 (6.7) | 6.1 (11.9) | 6.1 (10.4) | 0.01 |
Note: Mean (Standard Deviation) reported for continuous variables and percentage for categorical variables. P values are obtained using ANOVA or Kruskal-Wallis tests for continuous variables and Chi-square tests for categorical variables.
High cholesterol defined as total cholesterol ≥240 mg/dL, low-density lipoprotein (LDL) ≥160mg/dL, high-density lipoprotein ≤40 mg/dL, triglycerides ≥200mg/dL or self-reported use of cholesterol-lowering drug.
Reported as Median (Interquartile Range)
Abbreviations: N-terminal pro b-type natriuretic peptide (NT-proBNP); Diet history questionnaire (DHQ); Cystatin C-based estimated glomerular filtration rate (eGFR_CysC); Serum creatinine-based estimated glomerular filtration rate (eGFR_Scr)
Across deciles of NT-proBNP, the prevalence of LVH rose incrementally (Figure 2A). In the multivariable-adjusted linear regression model, the highest three quartiles of NT-proBNP had significantly higher LV mass/height2.7 (Quartile II: β 1.7 g/m2.7, p=0.011; Quartile III: β 3.2 g/m2.7, p<0.001; Quartile IV: β 6.4 g/m2.7, p<0.001).
Figure 2.
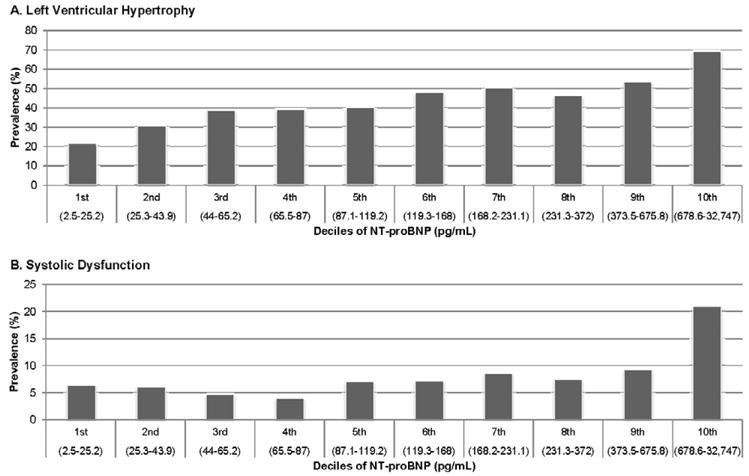
A–B. Prevalence of Left Ventricular Hypertrophy and Systolic Dysfunction by NT-proBNP Deciles*
*Decile range based on participants with systolic dysfunction measurements.
Overall, there was a high prevalence of LVH in this cohort, and the prevalence doubled from the lowest to highest quartiles of NT-proBNP (Table 2). The highest quartile of NT-proBNP was associated with nearly four-fold odds of LVH after demographic adjustment, but this was attenuated to approximately three-fold after multivariable adjustment (Table 2). In the clinical model, we repeated this by deciles of NT-proBNP and the multivariable-adjusted associations (odds ratios, [OR], 95% confidence intervals [CI]) of the top three deciles with LVH were 1.9 (1.1–3.4), 2.7 (1.–4.9) and 4.3 (2.3–8.3), compared with the lowest decile.
Table 2.
Association between N-terminal pro b-type natriuretic peptide (NT-proBNP) and left ventricular hypertrophy (LVH), systolic dysfunction, and diastolic dysfunction
| Odds Ratio (95% CI) | Quartile
|
|||
|---|---|---|---|---|
| I | II | III | IV | |
| Left Ventricular Hypertrophy | ||||
| Prevalence (%) | 32 | 44 | 55 | 68 |
| Demographic-Adjusted* | Reference | 1.6 (1.2–2.0) | 2.4 (1.8–3.0) | 3.6 (2.8–4.6) |
| Multivariate-Adjusted** | Reference | 1.3 (0.9–1.8) | 2.1 (1.4–3.0) | 2.7 (1.8–4.0) |
| Systolic Dysfunction | ||||
| Prevalence (%) | 6 | 5 | 7 | 14 |
| Demographic-Adjusted* | Reference | 1.1 (0.7–1.7) | 1.6 (1.0–2.5) | 3.7 (2.5–5.6) |
| Multivariate-Adjusted*** | Reference | 0.9 (0.5–1.5) | 1.2 (0.7–2.0) | 2.7 (1.7–4.5) |
| Diastolic Dysfunction† | ||||
| Prevalence (%) | 8 | 9 | 8 | 10 |
| Demographic-Adjusted* | Reference | 1.3 (0.9–1.9) | 1.2 (0.8–1.8) | 1.9 (1.3–2.8) |
| Multivariate-Adjusted**** | Reference | 1.3 (0.9–1.9) | 1.2 (0.8–1.9) | 2.0 (1.3–2.9) |
Note: Left ventricular hypertrophy is defined as LVM 2D/height2.7≥47 g/m2.7 for females or ≥50 g/m2.7 for males. Diastolic dysfunction is defined as moderately or severely abnormal vs. normal or mildly abnormal. Systolic dysfunction is defined as ejection fraction<0.45.
Adjusted for age, sex, race, and cause of renal disease.
Adjusted for age, sex, race, cause of renal disease, diabetes, hypertension, high cholesterol, prior peripheral vascular disease, any cardiovascular disease, height, weight, body mass index, echo systolic and diastolic blood pressure, hemoglobin, eGFR_CysC, hsCRP.
Adjusted for age, sex, race, cause of renal disease, any CVD, height, weight and echo diastolic blood pressure.
Adjusted for age, sex, race, cause of renal disease, height, and diabetes.
This analysis for diastolic dysfunction does not include the 564 participants without echocardiographic assessment of diastolic function.
Abbreviations: Confidence interval (CI); Left ventricular hypertrophy (LVH); Ejection fraction (EF); Diastolic dysfunction (DD).
From the entire cohort of 3,232 subjects, 229 participants had LV systolic dysfunction (EF <0.45). Participants in the highest decile of NT-proBNP level had the highest prevalence of LV systolic dysfunction, more than double the prevalence of any other decile (Figure 2B). The median (IQR) NT-proBNP was 209.0 (81.4–680.4) pg/mL in participants with systolic dysfunction compared with 114.4 (52.5–266.2) pg/mL in those with normal systolic function (p<0.001). A nearly three-fold higher adjusted odds of systolic dysfunction was apparent when comparing the highest quartile of NT-proBNP level to the lowest quartile (Table 2). In the clinical model, we repeated this by deciles of NT-proBNP and the multivariable-adjusted associations (OR, 95% CI) of the top three deciles with systolic dysfunction were 1.7 (0.7–4.0), 2.2 (0.9–5.2), and 6.3 (2.7–14.5).
There was no significant difference in the median NT-proBNP levels between participants with moderate or severe diastolic dysfunction (129.1 pg/mL; IQR 59.6–330.4) and those with normal or mildly abnormal diastolic function (123.8 pg/mL; IQR 55.5–286.8; p for comparison =0.83). However, quartiles of NT-proBNP were associated with prevalence of moderate to severe diastolic dysfunction, and the highest quartile was associated with two-fold odds of moderate or severe diastolic dysfunction compared with the lowest quartile in the multivariable-adjusted model (Table 2).
When evaluated as a screening test, NT-proBNP functioned only modestly for the detection of LVH (Table 3). Performance was slightly worse for the detection of LV systolic dysfunction and the combined outcome of LVH, LV systolic or diastolic function, and substantially worse for diastolic dysfunction alone. For each of these structural and functional abnormalities, we identified no optimal threshold value of NT-proBNP. The 90th percentile of NT-proBNP had moderately high positive likelihood ratios for detecting LVH, systolic dysfunction and the combined outcome of LVH, LV systolic or diastolic dysfunction, but negative likelihood ratios were uniformly poor due to the limited sensitivity at each cut-point (Table 3).
Table 3.
N-terminal pro b-type natriuretic peptide (NT-proBNP) as a diagnostic test for left ventricular hypertrophy, diastolic dysfunction and systolic dysfunction
| Variable | ROC | Sensitivity, % | Specificity, % | LR+ | LR− |
|---|---|---|---|---|---|
| Left Ventricular Hypertrophy | 0.66 | ||||
| 75th Percentile NT-proBNP | – | 32 | 86 | 2.22 | 0.80 |
| 90th Percentile NT-proBNP | – | 14 | 96 | 3.92 | 0.89 |
| Diastolic Dysfunction | 0.51 | ||||
| 75th Percentile NT-proBNP | – | 28 | 76 | 1.16 | 0.95 |
| 90th Percentile NT-proBNP | – | 11 | 91 | 1.22 | 0.98 |
| Systolic Dysfunction | 0.62 | ||||
| 75th Percentile NT-proBNP | – | 42 | 78 | 1.90 | 0.74 |
| 90th Percentile NT-proBNP | – | 24 | 92 | 3.18 | 0.82 |
| Any of The Above Outcomes | 0.62 | ||||
| 75th Percentile NT-proBNP | – | 30 | 83 | 1.76 | 0.84 |
| 90th Percentile NT-proBNP | – | 13 | 96 | 2.98 | 0.91 |
Note: Left ventricular hypertrophy is defined as LVM 2D/height2.7≥47 g/m2.7 for females or ≥50 g/m2.7 for males. Diastolic dysfunction is defined as moderately or severely abnormal vs. normal or mildly abnormal. Systolic dysfunction is defined as ejection fraction <0.45.
Abbreviations: Receiver operating characteristic (ROC); Positive likelihood ratio (LR+); Negative likelihood ratio (LR−)
We next evaluated the marginal contribution of adding NT-proBNP to a clinical model that included age, race, sex, history of cardiovascular disease, diabetes, body mass index (BMI), systolic blood pressure, glomerular filtration rate (eGFR_CystC), albuminuria, and hemoglobin level. The addition of NT-proBNP as a linear variable to the clinical model for the prediction of LVH resulted in a small but significant improvement in the AUC (0.822 vs. 0.815; p=0.01). A much larger improvement was attained with the addition of NT-proBNP to the clinical model for systolic dysfunction (0.716 vs. 0.666; p<0.002). The addition of NT-proBNP to the clinical model for diastolic dysfunction had no significant improvement (0.652 vs. 0.640; p=0.11).
In contrast, improvements in the category-free NRI were moderate to strong for systolic dysfunction (0.31, 95% CI 0.11–0.61), more modest for LVH (0.14, 0.08–0.39), and statistically insignificant for diastolic dysfunction (0.10, −0.18–0.29). For LVH prediction, this improvement was driven equally by reclassifying persons with LVH (0.06) and those without LVH (0.08). For systolic dysfunction, the improvement was driven mainly by reclassifying persons without systolic dysfunction (0.32) and not by reclassifying persons with systolic dysfunction (−0.01).
Discussion
Given the high prevalence of elevated NT-pro-BNP in CKD and its association with poor prognosis in ESRD,17,18 we evaluated the associations between NT-pro-BNP and prognostically meaningful cardiac structural and functional abnormalities across a wide range of renal function. In this study of a large, diverse population of ambulatory patients at various stages of CKD without HF, our principal findings were: 1) NT-proBNP levels were strongly associated with LVH and LV systolic dysfunction but more modestly with diastolic dysfunction; 2) As a screening test, NT-proBNP was not effective at distinguishing persons with and without cardiac abnormalities on echocardiography; 3) NT-proBNP did improve the ability of clinical models to predict LVH and LV systolic dysfunction, but not LV diastolic dysfunction.
Elevated circulating levels of NT-proBNP in CKD are determined by increased cardiac production secondary to structural heart disease and possibly by reduced renal clearance.19,20 While the renal clearance of NT-proBNP has not been fully characterized, small amounts of the peptide may be recovered in urine.21 Furthermore, levels of NT-proBNP are markedly increased in patients requiring hemodialysis.21,22 Therefore, it is likely that renal clearance is at least partly responsible for the elimination of NT-proBNP from the circulation. However, in a study of hypertensive patients, most of whom had a GFR ≥30mL/min/1.73m2, the serum concentration of NT-proBNP was shown to be determined more by cardiac production than renal clearance.23 In addition, NT-proBNP is associated with prevalence and incidence of HF across a wide spectrum of renal function.2,4 While we found a significant negative correlation between NT-proBNP levels and eGFR, adjustment for eGFR only partially attenuated the strong associations of NT-proBNP and LVH. Previous smaller studies have also reported a strong association between NT-proBNP and LVH.4,6,7 Our results support the finding from prior studies that both structural and functional heart disease and diminished renal function are responsible for elevations in circulating NT-proBNP levels in CKD.
Though an LVEF <0.45 was present in only 229 participants, we found a robust association between NT-proBNP levels and systolic dysfunction that was also independent of renal function. While some prior smaller studies of participants with CKD have reported an association between NT-proBNP levels and systolic dysfunction, others have not found such an association.4,7,18 To our knowledge, this is the largest study to date to investigate the relationship between NT-proBNP levels and LV systolic function and our findings are concordant with the strong association between NT-proBNP and HF in the general population.2 Since increased LV volumes and pressures are pathophysiologically linked to LVH, LV systolic dysfunction and HF, and induce myocardial stretch that stimulates NT-proBNP secretion, it is not surprising that NT-proBNP is strongly associated with LVH, LV systolic dysfunction and HF.24,25
LV diastolic dysfunction is a common finding in CKD.26 However, there are scant data on the association between NT-proBNP levels and diastolic dysfunction in CKD. DeFilippi et al. did not find a significant association between NT-proBNP level and diastolic dysfunction in 99 patients with CKD.4 Only the highest quartile of NT-proBNP was associated with two-fold odds of moderate or severe diastolic dysfunction while the lower three quartiles of NT-proBNP levels had no gradient of association. These findings are consistent with NT-proBNP representing elevated LV diastolic filling pressure and increased myocardial stretch in moderate and severe LV diastolic dysfunction.
Given the significant associations of NT-proBNP with LVH, LV systolic and diastolic dysfunction, we evaluated whether NT-proBNP could function as a screening test to select a subgroup of patients with CKD who should proceed to further evaluation with echocardiography. Despite the robust associations of NT-proBNP with LV systolic function and LVH and a weaker association with LV diastolic dysfunction, NT-proBNP did not effectively distinguish prevalent cardiac abnormalities. This suggests that NT-proBNP has limited utility in isolation for screening patients with CKD for cardiac structural and functional abnormalities. In contrast, when we added NT-proBNP to a model that included information available to a practicing clinician, NT-proBNP did succeed in improving the prediction of LVH and LV systolic dysfunction, as demonstrated by the category-less NRI. We chose this metric because there are no established risk categories for these echocardiographic outcomes, and we elected not to create arbitrary cut-points. These results indicate that NT-proBNP could be integrated with other clinical information to help decide which CKD patients should undergo echocardiography. The magnitude of improvement in reclassification of participants for LV systolic dysfunction (0.31) was similar to that obtained with the addition of flow-mediated dilation or the coronary artery calcium score to the Framingham Risk Score.27,28
This analysis is by far the largest exploration to date to determine the strength of association between NT-proBNP levels and cardiac structural and functional abnormalities among persons with CKD. Nonetheless, this study does have certain limitations. Most importantly, the analysis is cross-sectional and thus the direction of association cannot be determined. However, since NT-proBNP is being evaluated as a marker of prevalent cardiac disease, the cross-sectional design is appropriate for this clinical perspective. A second limitation is that coronary artery disease (CAD) and myocardial ischemia may be important intermediary mechanisms leading to LV structural and functional abnormalities, but CAD was not formally nor systematically assessed using either noninvasive or angiographic techniques in this study. Evaluation of LV diastolic function was accomplished using standard Doppler echocardiography. Newer techniques such as tissue Doppler or myocardial strain imaging may be better able to distinguish categories of diastolic dysfunction, yet these were not widely available when echocardiography was initially performed in this cohort. Exclusion of subjects with HF at entry was defined by self-report, rather than by an objective measure of HF.
Acknowledgments
Sources of Funding
This project was supported by M.S.’s R01 DK066488 award (principal investigator M.S.). In addition, we would like to acknowledge the CRIC GCRC and CTSA awards: University of Pennsylvania (UL1 RR-024134) Philadelphia, PA, USA; Johns Hopkins University (UL1 RR-025005) Baltimore, MD, USA; University of Maryland (M01RR-16500) College Park, MD, USA; Case Western Reserve University (UL1 RR-024989) Cleveland, OH, USA; University of Michigan (M01 RR-000042, UL1 RR-024986) Ann Arbor, MI, USA; University of Illinois at Chicago (UL1 RR-029879) Chicago, IL, USA; and Kaiser Permanente Division of Research in Oakland (2 U01 DK060902) Oakland, CA, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Drs. Mishra and Shlipak had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of Interest
None declared.
References
- 1.Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, Ogawa H, Okumura K, Mukoyama M, Nakao K. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. doi: 10.1161/01.cir.90.1.195. [DOI] [PubMed] [Google Scholar]
- 2.Anwaruddin S, Lloyd-Jones DM, Baggish A, Chen A, Krauser D, Tung R, Chae C, Januzzi JL., Jr Renal function, congestive heart failure, and amino-terminal pro-brain natriuretic peptide measurement: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Study. J Am Coll Cardiol. 2006;47:91–97. doi: 10.1016/j.jacc.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 3.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293:1609–1616. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 4.DeFilippi CR, Fink JC, Nass CM, Chen H, Christenson R. N-terminal pro-B-type natriuretic peptide for predicting coronary disease and left ventricular hypertrophy in asymptomatic CKD not requiring dialysis. Am J Kidney Dis. 2005;46:35–44. doi: 10.1053/j.ajkd.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Zoccali C, Mallamaci F, Benedetto FA, Tripepi G, Parlongo S, Cataliotti A, Cutrupi S, Giacone G, Bellanuova I, Cottini E, Malatino LS. Cardiac natriuretic peptides are related to left ventricular mass and function and predict mortality in dialysis patients. J Am Soc Nephrol. 2001;12:1508–1515. doi: 10.1681/ASN.V1271508. [DOI] [PubMed] [Google Scholar]
- 6.Khan IA, Fink J, Nass C, Chen H, Christenson R, deFilippi CR. N-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide for identifying coronary artery disease and left ventricular hypertrophy in ambulatory chronic kidney disease patients. Am J Cardiol. 2006;97:1530–1534. doi: 10.1016/j.amjcard.2005.11.090. [DOI] [PubMed] [Google Scholar]
- 7.Vickery S, Price CP, John RI, Abbas NA, Webb MC, Kempson ME, Lamb EJ. B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis. 2005;46:610–620. doi: 10.1053/j.ajkd.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14:S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 9.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 11.de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–1062. doi: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- 12.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 13.Bergstrom A, Andersson B, Edner M, Nylander E, Persson H, Dahlstrom U. Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function. Results of the Swedish Doppler-echocardiographic study (SWEDIC) Eur J Heart Fail. 2004;6:453–461. doi: 10.1016/j.ejheart.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Pencina MJ, D’Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med. 2010;48:1703–1711. doi: 10.1515/CCLM.2010.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madsen LH, Ladefoged S, Corell P, Schou M, Hildebrandt PR, Atar D. N-terminal pro brain natriuretic peptide predicts mortality in patients with end-stage renal disease in hemodialysis. Kidney Int. 2007;71:548–554. doi: 10.1038/sj.ki.5002087. [DOI] [PubMed] [Google Scholar]
- 18.Satyan S, Light RP, Agarwal R. Relationships of N-terminal pro-B-natriuretic peptide and cardiac troponin T to left ventricular mass and function and mortality in asymptomatic hemodialysis patients. Am J Kidney Dis. 2007;50:1009–1019. doi: 10.1053/j.ajkd.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueller T, Gegenhuber A, Poelz W, Haltmayer M. Head-to-head comparison of the diagnostic utility of BNP and NT-proBNP in symptomatic and asymptomatic structural heart disease. Clin Chim Acta. 2004;341:41–48. doi: 10.1016/j.cccn.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Luchner A, Hengstenberg C, Lowel H, Riegger GA, Schunkert H, Holmer S. Effect of compensated renal dysfunction on approved heart failure markers: direct comparison of brain natriuretic peptide (BNP) and N-terminal pro-BNP. Hypertension. 2005;46:118–123. doi: 10.1161/01.HYP.0000170140.36633.8f. [DOI] [PubMed] [Google Scholar]
- 21.Ng LL, Geeranavar S, Jennings SC, Loke I, O’Brien RJ. Diagnosis of heart failure using urinary natriuretic peptides. Clin Sci (Lond) 2004;106:129–133. doi: 10.1042/CS20030234. [DOI] [PubMed] [Google Scholar]
- 22.Wahl HG, Graf S, Renz H, Fassbinder W. Elimination of the cardiac natriuretic peptides B-type natriuretic peptide (BNP) and N-terminal proBNP by hemodialysis. Clin Chem. 2004;50:1071–1074. doi: 10.1373/clinchem.2003.030692. [DOI] [PubMed] [Google Scholar]
- 23.van Kimmenade RR, Januzzi JL, Jr, Bakker JA, Houben AJ, Rennenberg R, Kroon AA, Crijns HJ, van Dieijen-Visser MP, de Leeuw PW, Pinto YM. Renal clearance of B-type natriuretic peptide and amino terminal pro-B-type natriuretic peptide a mechanistic study in hypertensive subjects. J Am Coll Cardiol. 2009;53:884–890. doi: 10.1016/j.jacc.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 24.Betti I, Castelli G, Barchielli A, Beligni C, Boscherini V, De Luca L, Messeri G, Gheorghiade M, Maisel A, Zuppiroli A. The role of N-terminal PRO-brain natriuretic peptide and echocardiography for screening asymptomatic left ventricular dysfunction in a population at high risk for heart failure. The PROBE-HF study. J Card Fail. 2009;15:377–384. doi: 10.1016/j.cardfail.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Niizuma S, Iwanaga Y, Yahata T, Tamaki Y, Goto Y, Nakahama H, Miyazaki S. Impact of left ventricular end-diastolic wall stress on plasma B-type natriuretic peptide in heart failure with chronic kidney disease and end-stage renal disease. Clin Chem. 2009;55:1347–1353. doi: 10.1373/clinchem.2008.121236. [DOI] [PubMed] [Google Scholar]
- 26.Otsuka T, Suzuki M, Yoshikawa H, Sugi K. Left ventricular diastolic dysfunction in the early stage of chronic kidney disease. J Cardiol. 2009;54:199–204. doi: 10.1016/j.jjcc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]


