Abstract
IMPORTANCE
Most patients with relapsing-remitting (RR) multiple sclerosis (MS) who receive approved disease-modifying therapies experience breakthrough disease and accumulate neurologic disability. High-dose immunosuppressive therapy (HDIT) with autologous hematopoietic cell transplant (HCT) may, in contrast, induce sustained remissions in early MS.
OBJECTIVE
To evaluate the safety, efficacy, and durability of MS disease stabilization through 3 years after HDIT/HCT.
DESIGN, SETTING, AND PARTICIPANTS
Hematopoietic Cell Transplantation for Relapsing-Remitting Multiple Sclerosis (HALT-MS) is an ongoing, multicenter, single-arm, phase 2 clinical trial of HDIT/HCT for patients with RRMS who experienced relapses with loss of neurologic function while receiving disease-modifying therapies during the 18 months before enrolling. Participants are evaluated through 5 years after HCT. This report is a prespecified, 3-year interim analysis of the trial. Thirty-six patients with RRMS from referral centers were screened; 25 were enrolled.
INTERVENTIONS
Autologous peripheral blood stem cell grafts were CD34+ selected; the participants then received high-dose treatment with carmustine, etoposide, cytarabine, and melphalan as well as rabbit antithymocyte globulin before autologous HCT.
MAIN OUTCOMES AND MEASURES
The primary end point of HALT-MS is event-free survival defined as survival without death or disease activity from any one of the following outcomes: (1) confirmed loss of neurologic function, (2) clinical relapse, or (3) new lesions observed on magnetic resonance imaging. Toxic effects are reported using National Cancer Institute Common Terminology Criteria for Adverse Events.
RESULTS
Grafts were collected from 25 patients, and 24 of these individuals received HDIT/HCT. The median follow-up period was 186 weeks (interquartile range, 176–250) weeks). Overall event-free survival was 78.4% (90% CI, 60.1%–89.0%) at 3 years. Progression-free survival and clinical relapse-free survival were 90.9% (90% CI, 73.7%–97.1%) and 86.3% (90% CI, 68.1%–94.5%), respectively, at 3 years. Adverse events were consistent with expected toxic effects associated with HDIT/HCT, and no acute treatment-related neurologic adverse events were observed. Improvements were noted in neurologic disability, quality-of-life, and functional scores.
CONCLUSIONS AND RELEVANCE
At 3 years, HDIT/HCT without maintenance therapy was effective for inducing sustained remission of active RRMS and was associated with improvements in neurologic function. Treatment was associated with few serious early complications or unexpected adverse events.
Multiple sclerosis (MS) is an autoimmune disease characterized by the migration of immune cells into the central nervous system, production of proinflammatory cytokines, demyelination, and neuronal damage. A neurodegenerative process may contribute to loss of neurologic function during later secondary progressive MS.1 Active inflammation is most evident in early relapsing-remitting (RR) MS. Most patients with RRMS who receive approved disease-modifying therapies experience breakthrough disease activity.2,3 For example, in the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study4 comparing natalizumab with placebo for RRMS, only 37% of the patients who received natalizumab were without radiologic or clinical activity after 2 years of treatment. Furthermore, in Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis, Study Two (CARE-MS II),5 a trial of first-line treatment for refractory RRMS, 32% of the patients who received alemtuzumab and 14% of those who received interferon beta-1a showed no evidence of disease on magnetic resonance imaging (MRI) and clinical examination at 2 years of treatment. Discontinuation of disease-modifying therapies typically leads to reactivation of disease activity within months and is therefore not recommended.3 Consequently, patients are exposed to agents with potentially serious adverse effects (AEs) for years.
Autologous hematopoietic cell transplant (HCT) has been studied in MS for more than 20 years,6,7 with the goal of removing disease-causing immune cells and inducing a reset of the immune system. Early clinical trials8–10 of high-dose immunosuppressive therapy (HDIT)/HCT were conducted in patients with advanced disabilities and progressive forms of MS. Many patients continued to lose neurologic function, consistent with the contribution of noninflammatory factors and progressive neurodegeneration. After monitoring for 15 years, patients with active central nervous system inflammation before HDIT/HCT had a significantly better outcome compared with those without active inflammation before HDIT/HCT.11 Thus, HDIT/HCT may be more successful if instituted in the earlier inflammatory stages of MS.
We hypothesized that control of inflammation in earlier RRMS may provide prolonged remission with the potential to reverse neurologic dysfunction. The Hematopoietic Cell Transplantation for Relapsing-Remitting Multiple Sclerosis (HALT-MS) study is investigating the efficacy of early intervention with HDIT/HCT for patients with RRMS and breakthrough disease. A comprehensive assessment of MS disease activity, including progression, clinical relapse, or new lesions documented on MRI, was used as a composite primary end point in contrast to most previous HCT studies6 in which progression was the standard measure. To fully evaluate the safety of HDIT/HCT in RRMS, the protocol for HALT-MS includes collection and analysis of AEs through completion of the study.
Methods
Patients
The clinical study (protocol ITN033AI; BB-IND 12164 with type II DMF BB-IND 11821) was approved by the institutional review boards at participating sites, and the participants provided written informed consent. The participants self-identified race and ethnicity at screening. No financial compensation was provided.
Eligible patients (eFigure 1 in the Supplement) were aged 18 to 60 years and had a diagnosis of MS according to the McDonald criteria12 with: (1) RRMS, (2) Kurtzke Expanded Disability Status Scale (EDSS)13 scores of 3.0 to 5.5 at baseline, (3) lesions demonstrated on brain MRI that were consistent with MS, (4) disease duration of less than 15 years, and (5) failure of disease-modifying therapies, defined as 2 or more clinical relapses during 18 months of therapy that were associated with an increase in the EDSS score (by 1.0 for pre-relapse EDSS score of 3.0–3.5 or by 0.5 for an EDSS score of 4.0–5.5 and sustained for ≥4 weeks). Patients “off therapy” during an 18-month period in which they had 2 relapses and an increase in the EDSS score were also eligible if they had previously met the criteria of treatment failure “on therapy” within 4 years of determining study eligibility. Patients with comorbidities precluding safe HDIT were excluded. Patient data were evaluated by an MS review panel composed of 2 neurologists and a transplant physician.
Procedures
Peripheral blood stem cells were mobilized with filgrastim (16 μg/kg/d for 4 days), collected by leukapheresis, CD34 selected (Isolex 300I; Baxter), and cryopreserved6,14 (minimum dose, 2.0 × 106 CD34+ cells/kg with purity of ≥70% viable CD34+ cells). To prevent MS relapse during mobilization, prednisone, 1 mg/kg/d, was given for 10 days beginning 1 day before filgrastim therapy was started. High-dose BEAM chemotherapy was composed of carmustine, 300 mg/m2, on day −6; etoposide, 200 mg/m2, and cytarabine, 200 mg/m2, daily from day −5 to −2; and melphalan, 140 mg/m2, on day −1.8 Rabbit antithymocyte globulin, 2.5 mg/kg/d, was administered on days −2 and −1. On day 0, CD34+ cells were thawed and infused. Filgrastim, 5 μg/kg/d, was administered from day 5 until the absolute neutrophil count was greater than 500/μL (to convert to ×109/L, multiply by 0.001). To prevent fever associated with engraftment syndrome, prednisone, 0.5 mg/kg/d, was administered from day 7 to 21 and then tapered over a 2-week period. Supportive care was administered as previously described.9
Study Evaluations
Clinical evaluations, including EDSS, the MS Functional Composite (MSFC),15 and the 29-item Multiple Sclerosis Impact Scale (MSIS-29),16 were performed at baseline before stem cell mobilization, at 6 and 12 months, and annually thereafter to 5 years after HCT. Patients were contacted by telephone every 3 months between annual visits; if they reported neurologic changes, they returned for evaluation, including MRI. The MSFC consisted of the (1) Timed 25-Foot Walk, (2) 9 Hole Peg Test, and (3) Paced Auditory Serial Addition Test. The MSFC is the mean of the z scores from each of 3 components. Each z score component is a relative measure that indicates how many SDs the current observation is from the mean of those in the task force database. Negative MSFC values indicate worse health.
Brain MRI17–19 was performed as a component of screening procedures at baseline before transplant and then at week 8, month 6, month 12, and annually through 5 years after HCT. Follow-up scans were performed on the same type of scanner used at baseline or, if a scanner had been replaced (retired), an assigned replacement. Scans were analyzed centrally (NeuroRx). The brain MRI at 2 months was considered the post-treatment reference scan for assessment of treatment failure. The screening scan was the reference for brain volume changes. For brain volume changes, if scanner hardware had been replaced or upgraded, volume changes measured across the hardware adjustments were unreliable and therefore were not reported. When this occurred, the reference visit was updated to the most recent visit that used the new hardware, and the missing data across the scanner were imputed using the mean percent volume change of other patients who had not undergone a hardware change at that visit.17
Toxic effects were reported according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.20 Adverse events of grade 2 or higher were recorded throughout the study; however, from the start of conditioning through day 60, only events that were grade 3 or higher were recorded.
For flow cytometry, blood was collected in 10-mL glass tubes containing sodium heparin (Vacutainer, Becton Dickinson) and shipped ambient overnight to the Immune Tolerance Network Flow Cytometry Core at Roswell Park Cancer Institute. Using a stain-lyse method, cells from blinded samples were stained with 5-color monoclonal antibody panels conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein complex (PERCP), phycoerythrin with cyano dye Cy7 (PECy7), or allophycocyanin (APC). The T cells were analyzed using antihuman CD3-PECy7, CD4-PERCP, CD4-PECy7, CD8-PERCP, CD31-FITC, CD45RA-FITC, CD45RA-APC, CD45RO-PE, and CD62L-APC (BD Biosciences). The B cells were analyzed using antihuman CD1c-FITC (An-cell), IgD-PE, CD27-PERCP, CD19-PECy7, and IgM-APC (BD Biosciences). Samples were acquired on a flow cytometer (Canto A; BD Biosciences) and then were gated and analyzed using FlowJo, Macintosh version 9.6.4 (Tree Star Inc).
Neutrophil engraftment was defined as the first of 2 consecutive days of neutrophil levels of 500/μL or more. Platelet engraftment was defined as the first day of a platelet count of 20 × 103/μL or more and sustained without transfusions during the previous 7 days. (Conversion of the platelet count to ×109/L is 1:1.)
Study Design and Primary End Point
HALT-MS is a prospective, open-label, single-arm, multicenter phase 2 clinical trial (clinicaltrials.gov Identifier: NCT00288626). The primary end point is the time to treatment failure during 5 years post HCT, defined as the first event of death from any cause or MS disease activity or disability defined by any of the following: (1) change in EDSS score of more than 0.5 points compared with EDSS at baseline when the test was performed 6 months or more post HCT and confirmed at least 3 months later; (2) relapse, defined as worsening or development of new neurologic sign and corresponding symptom lasting for more than 48 hours; and (3) 2 or more independent MS disease-related lesions (either gadolinium-enhancing or new T2-weighted lesions) on brain MRI performed 1 year or more after HCT.
Statistical Analysis
Analyses were conducted on interim data captured after the last participant completed the year 3 visit. SAS software, version 9.2 or higher (SAS Institute Inc), was used.
The probability of overall event-free survival (as well as progression-free, relapse-free, or MRI event-free survival) at 1 through 5 years was calculated. Analyses were conducted using Kaplan-Meier estimates with Wald-type 90% CIs based on Greenwood's formula for SE.21 The percentage of change in brain volume was calculated from screening and analyzed by end point status at year 3 with an exact Wilcoxon rank sum test using mean scores when the results were identical. The null hypothesis tests whether the median percentage of change in brain volume among participants who met the end point by year 3 is equal to the median of those who have not met the end point by year 3 at the baseline, month 6, and year 1 through 3 visits.
All other measures were analyzed using a Wilcoxon signed rank test. The null hypothesis was that the median difference from baseline measurement in the values at the month 6, year 1, year 2, and year 3 visits was significantly different from zero, with baseline measurement defined as the screening assessment for the percentage of change in brain volume and the baseline visit for all other end points.To calculate MSFC, results from each of the 3 components were transformed into a z score and averaged to yield a composite for each patient at each time point. The z scores that compose the MSFC score were calculated using the reference population from the National Multiple Sclerosis Society Task Force database.22 For secondary end points, P values are presented for descriptive purposes.
Results
Patient Characteristics
From August 24, 2006, to August 28, 2009, 36 patients with RRMS were screened and 25 eligible individuals were enrolled (eFigure 1 in the Supplement). Among those eligible, 7 patients were not enrolled owing to insurance denial and 1 patient withdrew consent. The median age of the 25 participants was 38 years (interquartile range [IQR], 32–42 years); 17 were women (68%) (Table 1). The median disease duration was 4.9 years (IQR, 2.7–7.3 years); the baseline median EDSS score was 4.5 (IQR, 4.0–5.0). Baseline MSFC score is presented in Table 1, and the component raw scores of the baseline MSFC are presented in eTable 1 in the Supplement. Patients previously failed a median of 3 (IQR, 2–4) MS medications before transplant. Twenty-four participants proceeded to undergo autologous HCT; 1 individual was withdrawn from the study following an AE that occurred during mobilization.
Table 1.
Demographic and Baseline Characteristics
| Characteristic | Total (N = 25) |
|---|---|
| Age at mobilization, y | |
| Mean (SD) | 37.3 (7.7) |
| Median (range) | 38 (27 to 53) |
| Sex, No. (%) | |
| Male | 8 (32) |
| Female | 17 (68) |
| Race, No. (%) | |
| White | 24 (96) |
| Multiracial | 1 (4) |
| Years since diagnosis of MS | |
| No. | 25 |
| Mean (SD) | 5.7 (3.7) |
| Median (range) | 4.9 (0.6 to 12.0) |
| Lifetime MS medications reported at screening, No. (%)a | |
| 1 | 5 (20) |
| 2 | 5 (20) |
| 3 | 8 (32) |
| 4 | 6 (24) |
| 6 | 1 (4) |
| Baseline EDSS score | |
| No. | 25 |
| Mean (SD) | 4.4 (0.6) |
| Median (range) | 4.5 (3.0 to 5.5) |
| Baseline MSIS-29 score | |
| No. | 23 |
| Mean (SD) | 79.7 (25.8) |
| Median (range) | 79 (40 to 137) |
| Baseline MSFC scoreb | |
| No. | 24 |
| Mean (SD) | −0.10 (0.56) |
| Median (range) | −0.2 (−1.4 to 1.0) |
Abbreviations: EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; MSFC, MS Functional Composite; MSIS-29, 29-item Multiple Sclerosis Impact Scale.
Participants reported these previous medications for MS: 21 (84%) interferon beta-1a, 18 (72%) glatiramer acetate, 8 (32%) mitoxantrone, 6 (24%) natalizumab, 5 (20%) interferon beta-1b, 6 (24%) methylprednisolone or dexamethasone, and 1 (4%) each for cyclophosphamide, methotrexate, minocycline, and plasma exchange.
Details on the MSFC component scores appear in eTable 1 in the Supplement.
Peripheral Blood Stem Cell Collection and Engraftment
Participants underwent a median of 2 (IQR, 2–2) aphereses for graft collection. Mobilization with filgrastim failed in one individual but was successful after the administration of cyclophosphamide. Patients received a median of 4.58 × 106/kg (IQR, 3.49 × 106 to 5.95 × 106/kg) CD34-selected cells of 91.6% purity (IQR, 87.0%–95.8%) on day 0. The median time to neutrophil and platelet engraftment was 11 days (IQR, 10–11 days) and 18 days (IQR, 16.5–20 days), respectively.
Adverse Events
Grade 3 and 4 AEs are listed by time of occurrence in Table 2. Among 25 patients with evaluable data for mobilization AEs, one individual (4%) experienced a grade 4 pulmonary embolus associated with heparin-induced thrombocytopenia and was withdrawn from further study. Seven patients experienced 10 grade 3 AEs that were not hematopoietic or gastrointestinal, with most being line-associated thromboses and infections. One patient was nonadherent to prophylaxis therapy with prednisone during administration of filgrastim and experienced a grade 3 MS relapse. A brain MRI demonstrated new gadolinium-enhancing lesions with no change in the EDSS score.
Table 2.
Adverse Events
| Grade 3 | Grade 4 | |||
|---|---|---|---|---|
|
|
|
|||
| Characteristic | Patients, No. (%)a | AEs, No. | Patients, No. (%)a | AEs, No. |
| AE Group and Time of Occurrence b,c | ||||
|
| ||||
| All AEs | ||||
|
| ||||
| Prior to start of mobilization | 1 (4.0) | 1 | 0 | 0 |
|
| ||||
| Mobilization until start of conditioning | 10 (40) | 19 | 4 (16) | 4 |
|
| ||||
| Start of conditioning to day 29 | 20 (80) | 51 | 24 (96) | 83 |
|
| ||||
| Days 30–99 | 7 (28) | 12 | 1 (4) | 1 |
|
| ||||
| Days 100–364 | 6 (24) | 11 | 2 (8) | 4 |
|
| ||||
| Year 1 to year 2 | 4 (16) | 15 | 1 (4) | 1 |
|
| ||||
| >Year 2 | 9 (36) | 21 | 1 (4) | 1 |
|
| ||||
| Nonhematopoietic/nongastrointestinal AEs | ||||
|
| ||||
| Prior to start of mobilization | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Mobilization until start of conditioning | 7 (28) | 10 | 1 (4) | 1 |
|
| ||||
| Start of conditioning to day 29 | 16 (64) | 32 | 2 (8) | 2 |
|
| ||||
| Days 30–99 | 6 (24) | 7 | 0 | 0 |
|
| ||||
| Days 100–364 | 5 (20) | 8 | 2 (8) | 4 |
|
| ||||
| Year 1 to year 2 | 4 (16) | 15 | 1 (4) | 1 |
|
| ||||
| >Year 2 | 7 (28) | 15 | 1 (4) | 1 |
|
| ||||
| Organ Class and AE Term b | ||||
|
| ||||
| Any AE | 24 (96) | 130 | 25 (100) | 94 |
|
| ||||
| Cytopenias | 16 (64) | 33 | 24 (96) | 85 |
|
| ||||
| Infections | 14 (56) | 23 | 0 | 0 |
|
| ||||
| Bacterial | 12 (48) | 17 | 0 | 0 |
|
| ||||
| Viral reactivation (including CMV and EBV)d | 3 (12) | 3 | 0 | 0 |
|
| ||||
| Pneumonia | 1 (4) | 2 | 0 | 0 |
|
| ||||
| Clostridium difficile colitis | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Gastrointestinal disorders | 9 (36) | 16 | 0 | 0 |
|
| ||||
| Metabolism | 8 (32) | 11 | 3 (12.0) | 3 |
|
| ||||
| Alanine aminotransferase increased | 5 (20) | 6 | 1 (4.0) | 1 |
|
| ||||
| Hyperglycemia | 3 (12) | 3 | 0 | 0 |
|
| ||||
| Aspartate aminotransferase increased | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Hyperuricemia | 0 | 0 | 1 (4.0) | 1 |
|
| ||||
| Hypokalemia | 0 | 0 | 1 (4.0) | 1 |
|
| ||||
| Hyponatremia | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Nervous system, including visual disorders | 6 (24) | 14 | 0 | 0 |
|
| ||||
| Headache | 3 (12) | 5 | 0 | 0 |
|
| ||||
| Multiple sclerosis exacerbatione | 2 (8) | 2 | 0 | 0 |
|
| ||||
| Sensory disturbance | 1 (4) | 2 | 0 | 0 |
|
| ||||
| Cognitive disorder | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Hypoesthesia | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Motor dysfunction | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Paresthesia | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Vision blurred | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Cardiovascular disorders | 5 (20) | 6 | 0 | 0 |
|
| ||||
| Deep vein thrombosis | 3 (12) | 3 | 0 | 0 |
|
| ||||
| Atrial thrombosis | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Atrioventricular block | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Cardiomyopathy | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Respiratory, thoracic, and mediastinal | 3 (12) | 11 | 3 (12) | 3 |
|
| ||||
| Pulmonary embolism | 1 (4) | 2 | 1 (4) | 1 |
|
| ||||
| Respiratory arrest/failure | 0 | 0 | 2 (8) | 2 |
|
| ||||
| Asthma | 1 (4) | 3 | 0 | 0 |
|
| ||||
| Dyspnea | 1 (4) | 3 | 0 | 0 |
|
| ||||
| Pneumonitis | 1 (4) | 2 | 0 | 0 |
|
| ||||
| Chest pain | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Immune system disorders | 4 (16) | 4 | 0 | 0 |
|
| ||||
| Contrast media allergy | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Engraftment syndrome, autologous | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Hypogammaglobulinemia | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Aseptic meningitis | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Musculoskeletal disorders | 4 (16) | 4 | 0 | 0 |
|
| ||||
| Back pain | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Neck pain | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Osteopenia | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Pain in extremity | 1 (4) | 1 | 0 | 0 |
|
| ||||
| General disorders | 3 (12) | 3 | 0 | 0 |
|
| ||||
| Epistaxis | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Pain | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Pyrexia | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Psychiatric disorders | 1 (4) | 2 | 2 (8) | 3 |
|
| ||||
| Suicide attempt | 0 | 0 | 2 (8) | 2 |
|
| ||||
| Depression | 1 (4) | 1 | 1 (4) | 1 |
|
| ||||
| Mania | 1 (4) | 1 | 0 | 0 |
|
| ||||
| TPN related | 2 (8) | 2 | 0 | 0 |
|
| ||||
| Congenital, familial, and genetic disorders | 1 (4) | 1 | 0 | 0 |
|
| ||||
| Arteriovenous malformation | 1 (4) | 1 | 0 | 0 |
Abbreviations: AE, adverse event; CMV, cytomegalovirus; EBV, Epstein-Barr virus; TPN, total parenteral nutrition.
Patients who experienced 1 or more AEs were counted only once. Percentages for the number of patients with AEs are based on the 25 patients enrolled.
Adverse events are presented for the 25 patients who underwent mobilization; all AEs from the time of informed consent to the database cut are included.
The study day was calculated from the date of the transplant.
Grade 3 viral reactivations included CMV in 1 patient and EBV in 2 patients.
Two patients experienced AEs related to a multiple sclerosis relapse. One individual experienced this complication during the screening period but before mobilization. Another patient had a relapse during mobilization (see the Results section).
Among 24 participants proceeding to HCT, the median follow-up at interim analysis was 186 weeks (IQR, 176–250 weeks). Among the 25 enrolled patients, 130 grade 3 and 94 grade 4 AEs occurred (Table 2); most were cytopenias or infections. Of the grade 4 AEs that occurred after transplant, only 8 were not hematopoietic or gastrointestinal; these included depression, suicide attempts (1 with associated respiratory failure), respiratory arrest, hyperuricemia, hypokalemia, and an increased alanine aminotransferase level. Two patients attempted suicide late after the transplant; the clinical course of both individuals was previously unremarkable.
Two patients who experienced grade 5 AEs died. One death was related to MS progression more than 2½ years after transplant; the patient's condition had been stable until meeting the study end point owing to loss of neurologic function (increased EDSS score) at 1.6 years. No other cause for loss of neurologic function was identified. The second individual had preexisting asthma; although she was evaluated and approved by pulmonary medicine physicians for transplant, she died more than 3½ years after HCT from worsening asthma. She had met the study end point earlier at 5 months after HCT owing to clinical relapse in the setting of aseptic meningitis after receiving trimethoprim-sulfamethoxazole.
Evaluation of Disease
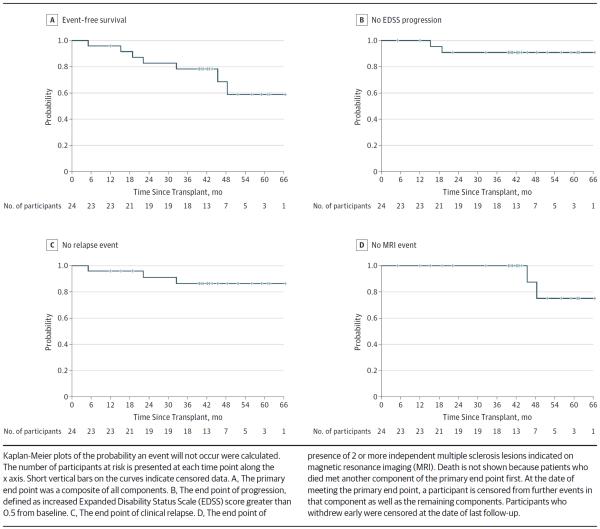
Treatment in 5 (21%) of the 24 patients who underwent transplant failed according to the criteria of the primary end point by 3 years (eTable 2 in the Supplement and Figure 1) either by an increase in the EDSS score of more than 0.5 or by clinical relapse. Beyond 3 years after HCT, an additional 2 patients met the study end point according to MRI criteria. The estimated event-free survival probability was 95.8% (90% CI, 80.2%–99.2%) at 1 year, 82.8% (90% CI, 65.0%–92.0%) at 2 years, 78.4% (90% CI, 60.1%–89.0%) at 3 years, 68.6% (90% CI, 44.9%–83.7%) at 4 years, and 58.8% (90% CI, 33.7%–77.2%) at 5 years (eTable 2 in the Supplement and Figure 1A). At 3 years, probabilities for the components of event-free survival were (1) EDSS progression-free survival, 90.9% (90% CI, 73.7%–97.1%); (2) clinical relapse-free survival, 86.3% (90% CI, 68.1%–94.5%); (3) MRI event-free survival, 100.0% (90% CI, 100%–100%); and (4) overall event-free survival probability, 78.4% (90% CI, 60.1%–89.0%) (eFigure 1 and eTable 2 in the Supplement and Figure 1B–D). Final survival probabilities using all 4- and 5-year data will be assessed in a future article once all participants have completed 5 years of follow-up.
Figure 1.
Neurologic Outcomes: Composite Primary End Point and Components
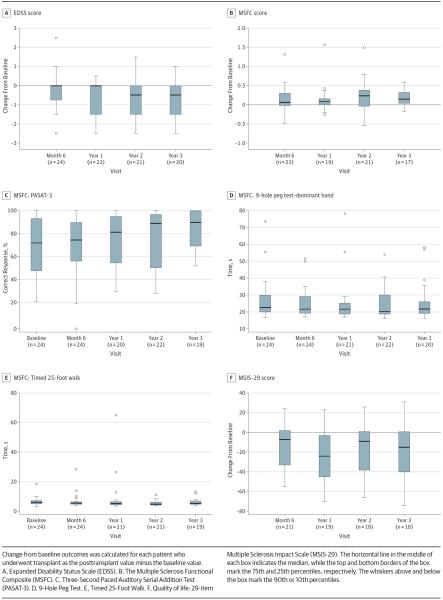
The EDSS score improved after HCT, with a median change from baseline of −0.50 (IQR, −1.5 to 0.0) at 3 years (P = .007) (Figure 2A). Improvement in the EDSS score was also demonstrated at year 1 (P =.003) and year 2 (P =.004). The MSFC score improved from baseline (Figure 2B) by a median of 0.15 (IQR, 0.04 to 0.32) at 3 years after HDIT (P = .01); components of the MSFC are illustrated in Figure 2C–E. The MSIS-29 quality-of-life score also improved, with a median change from baseline of −15.0 (IQR, −40.0 to 0.6) at 3 years after HCT (P = .02) (Figure 2F).
Figure 2.
Clinical Outcomes
MRI Assessments
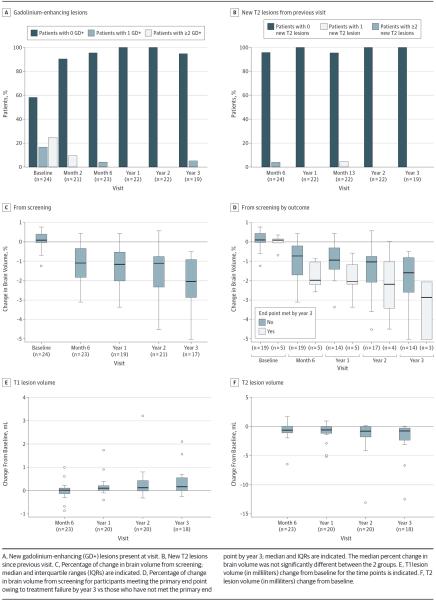
The observed MRI gadolinium-enhancing lesions and new T2 lesions are illustrated in Figure 3A and B, respectively. A reduction of gadolinium-enhancing lesions was observed immediately after transplant (Figure 3A, baseline vs months 2 and 6 after HCT). Two patients developed new brain gadolinium-enhancing and/or T2 lesions between 3½ and 4 years after transplant (Figure 3A and B), meeting the study end point (eTable 2 in the Supplement and Figure 1D). Median percent change in brain volume from screening was significantly different at month 6 and years 1 to 3 (P < .001 for each visit). The greatest change in brain volume occurred in the first months after transplant. Brain volume was decreased at 6 months compared with baseline but subsequently appeared to stabilize until year 3 (Figure 3C). Participants for whom treatment failed may have had a greater rate of atrophy (Figure 3D), although this finding was not statistically significant. The T1 lesion volume (Figure 3E) increased from baseline over time. The median change from baseline in the T1 lesion volume was significantly different from zero at year 1 (P = .019), year 2 (P = .018), and year 3 (P = .006). The T2 lesion volume decreased from baseline through 3 years (Figure 3F). The median change was significantly different at month 6 (P = .025), year 1 (P < .001), year 2 (P < .001), and year 3 (P < .001).
Figure 3.
Magnetic Resonance Imaging Studies at Baseline and After Transplant
Immune Reconstitution
Depletion and reconstitution of immune cell populations were analyzed through 3 years after HCT (eFigure 2 in the Supplement). After HDIT/HCT, CD8 memory T-cell counts returned to baseline by 2 months, CD8-naive T-cell counts by 1 year, CD4 T-cell counts by longer than 3 years, and B-cell counts by 6 months (eFigure 2 in the Supplement).
Discussion
Since HDIT/HCT is associated with significant risks, this treatment would need to be highly effective to be considered as a reasonable alternative to non-HCT therapies for MS. In the HALT-MS study of individuals with RRMS, event-free survival was estimated to be 82.8% at 2 years and 78.4% at 3 years after HCT. In contrast, although comparisons across clinical trials can be problematic, in a retrospective analysis of the AFFIRM study that included patients with EDSS scores of 0.0 to 5.0 and used a composite end point,4 only 37% and 7% of patients who received natalizumab treatment or placebo, respectively, remained free from both clinical and MRI-documented disease activity at 2 years. Furthermore, in the HALT-MS study, there was improvement from baseline in the MSFC score and neurologic function (EDSS score) in contrast to observations for interferon therapy.5 Continued breakthrough disease activity with currently approved non-HCT treatments is an indicator of poor prognosis and may justify the risks associated with use of an intensive approach that may improve long-term outcome.
To better assess the feasibility of HDIT/HCT for treatment of MS, we characterized toxic effects by documentation of AEs for all participants through completion of the study at 5 years. Most AEs observed thus far in the HALT-MS study have been hematologic or gastrointestinal, which are expected and reversible after high-dose immunochemotherapy, and no early treatment-related mortality or organ failure has occurred. Only 11% of the National Cancer Institute Common Terminology Criteria for Adverse Events grade 4 or 5 AEs involved other organ systems, only 3 of these occurred in the first month after HCT when most regimen-related toxic effects develop, and the AEs consisted of transient changes in laboratory values that did not affect transplant outcome. Of the other grade 4 or 5 events, one occurred before transplant and the others developed late after HCT. Two suicide attempts occurred late after transplant. A higher rate of suicide attempts in patients with MS compared with the general population has been reported.23,24 Two deaths occurred, with one at more than 2½ years and the other more than 3½ years after HCT. Progression of disease with loss of neurologic function and death has been reported10 in European Group for Blood and Marrow Transplantation registry studies between 15 and 42 months after transplant. Two patients developed new immune-mediated events during HALT-MS, with one consisting of heparin-induced thrombocytopenia during mobilization and the other being trimethoprim-sulfamethoxazole-induced aseptic meningitis after HDIT/HCT (eTable 3 in the Supplement). Patients with autoimmune diseases may be predisposed to developing new immune-mediated disorders.25 The infrequent visit schedule may have led to an underestimation of AEs and relapses, both of which are participant generated; this concern is mitigated by telephone contact every 3 months and a detailed review of events at each annual study visit.
In general, there was no improvement in neurologic function in the early clinical trials6 of autologous HCT for patients with secondary progressive MS. In a more recent study,26 patients with RRMS and a median baseline EDSS score of 3.5 demonstrated improvement by greater than 1 point after 2 or more years. Although participants in the HALT-MS study had a median EDSS score of 4.5 at entry, improvement in neurologic function, as measured by MSFC, EDSS, and quality of life, was observed in the first 3 years.
Magnetic resonance imaging was included as a component of the primary end point for the present study since it is a sensitive indicator of MS activity. As measured by gadolinium-enhancing lesions and new T2 lesions, disease activity was controlled through 3 years after HCT. Subsequently, however, treatment failed in 2 patients beyond 3 years according to MRI criteria. Furthermore, in this study we observed a decrease in brain volume early after transplant that appeared to stabilize until year 3, similar to previous observations,18 except for patients whose treatment failed. Follow-up of all participants through 5 years for HALT-MS will be needed to confirm these observations and fully evaluate their implications.
In the early months after transplant, MS remissions are likely achieved as a result of lymphoablation. However, we speculated that long-term remission may result from persistent immunomodulatory effects of HDIT/HCT. We have confirmed the previous observation that CD4+ memory T cells have not recovered to baseline levels in peripheral blood even at 2 years after transplant.27 Other lymphocyte subsets recovered by 6 months to 1 year after HCT. Furthermore, in the HALT-MS study, we used high-throughput deep sequencing of T-cell receptor β chains to directly assess millions of individual T-cell receptors per patient sample to evaluate immune reconstitution at 1 year.28 For CD4+ T cells, dominant T-cell receptor clones present before treatment were not detectable following reconstitution, and patients usually developed a new repertoire consistent with an immune reset. In contrast, the CD8 repertoire reconstituted largely from clonal expansion of cells that were present before treatment.
Conclusions
In the present study, HDIT/HCT induced remission of MS disease activity for up to 3 years in most participants. It may therefore represent a potential therapeutic option for patients with MS in whom conventional immunotherapy fails, as well as for other severe immune-mediated diseases of the central nervous system. Most early toxic effects were hematologic and gastrointestinal and were expected and reversible. Longer follow-up is needed to determine the durability of the response. Careful comparison of the results of this investigation and other ongoing studies26,29–31 will be needed to identify the best approaches for high-dose immunosuppressive therapies for MS and plan the next clinical studies.32–34 We have presented the 3-year interim analysis of the HALT-MS trial; the prespecified full duration of observation is 5 years, after which the final report on the study will be prepared.
Supplementary Material
Acknowledgments
Funding/Support: This work was sponsored by the Division of Allergy, Immunology and Transplantation, National Institute of Allergy and Infectious Diseases (DAIT-NIAID), NIH, and was conducted by the ITN (grant N01 AI015416), and the DAIT-NIAID funded statistical and clinical coordinating centers (contracts HHSN272200800029C and HHSN272200900057C). Baxter Healthcare Corporation supplied the Isolex 300i Magnetic Cell Selection System machines, Disposable Sets, and CD34 Reagent Kits used for the HALT-MS clinical trial to DAIT, NIAID, without charge.
Role of the Funder/Sponsor: The funding organization and sponsor, DAIT, NIAID, NIH, participated in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication.
Additional Contributions: We thank the patients and their families for their participation in this study and the research nurses, clinical coordinators, and data technicians for their many essential contributions to patient care and the conduct of the protocol. Noha Lim, PhD (Immune Tolerance Network), contributed statistical analysis for eFigure 2 in the Supplement. There was no financial compensation.
Dr Nash received a grant from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH)/Immune Tolerance Network (ITN) and nonfinancial support from Baxter. Dr Hutton received grants and personal fees from Biogen Idec, EMD Serono, Genzyme, Novartis, and Teva Neuroscience; personal fees from Bayer and Pfizer; and grants from Accorda, Avanir, Hoffman LaRoche, and Opexa Therapeutics. Dr Racke received grants from National Multiple Sclerosis Society and Teva and was a consultant for Biogen Idec, Novartis, Questcor, and Revalesio. Dr Popat received a grant from NIAID, NIH/ITN. Dr Devine has received grants from the National Heart, Lung, and Blood Institute and the National Cancer Institute. Dr Stüve has received grants from Teva Neuroscience, is an associate editor for Clinical & Experimental Immunology, serves on the editorial board for Multiple Sclerosis Journal, is the managing editor for Therapeutic Advances in Neurological Disorders, and serves on data monitoring committees for Pfizer and sanofi-aventis. Dr Arnold receives payments to NeuroRx from NIAID and NIH/ITN and personal fees from Acorda, Biogen Idec, Genzyme, Hoffman-La Roche, Innate Therapeutics, Medimmune, Mitsubishi, Novartis, Stem Cells Inc, and Teva. Dr Wundes receives grants and personal fees from Biogen Idec and personal fees from Acorda, Med-IQ (medical education organization), Multiple Sclerosis Association (nonprofit), and Teva. Dr Kraft is a member of Axon Council for Acorda. Dr Bowen receives grants from N01AI15416, NIH/NIAID; personal fees from Pfizer and Teva Neuroscience; personal fees and research contracts from Biogen, EMD Serono, and Novartis; and research contracts from Genentec, Genzyme, GlaxoSmithKline, Medimmune, and sanofi-aventis.
Footnotes
Author Contributions: Drs Nash and Spychala had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Nash, Griffith, Muraro, Openshaw, Sayre, Arnold, Kraft, Bowen.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Nash, Griffith, Spychala, McConville.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Spychala, McConville.
Obtained funding: Nash.
Administrative, technical, or material support: Nash, Racke, Popat, Sayre, Stüve, Arnold, Harris, Phippard, Georges, Kraft.
Study supervision: Nash, Racke, Griffith, Stüve, Arnold, Kraft.
Conflict of Interest Disclosures: No other disclosures were reported.
Publisher's Disclaimer: The opinions expressed are those of the authors and do not represent the position of the NIAID, NIH, or US government. Dr Stüve is an associate editor for JAMA Neurology but was not involved in the editorial review or decision to accept the manuscript for publication.
REFERENCES
- 1.Frischer JM, Bramow S, Dal-Bianco A, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132(pt 5):1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reingold SC, Steiner JP, Polman CH, et al. The challenge of follow-on biologics for treatment of multiple sclerosis. Neurology. 2009;73(7):552–559. doi: 10.1212/WNL.0b013e3181b2a6ce. [DOI] [PubMed] [Google Scholar]
- 3.O'Connor PW, Goodman A, Kappos L, et al. Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology. 2011;76(22):1858–1865. doi: 10.1212/WNL.0b013e31821e7c8a. [DOI] [PubMed] [Google Scholar]
- 4.Havrdova E, Galetta S, Hutchinson M, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol. 2009;8(3):254–260. doi: 10.1016/S1474-4422(09)70021-3. [DOI] [PubMed] [Google Scholar]
- 5.Coles AJ, Twyman CL, Arnold DL, et al. CARE-MS II Investigators. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829–1839. doi: 10.1016/S0140-6736(12)61768-1. [DOI] [PubMed] [Google Scholar]
- 6.Mancardi G, Saccardi R. Autologous haematopoietic stem-cell transplantation in multiple sclerosis. Lancet Neurol. 2008;7(7):626–636. doi: 10.1016/S1474-4422(08)70138-8. [DOI] [PubMed] [Google Scholar]
- 7.Nash RA. Hematopoietic cell transplantation for autoimmune diseases. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas' Hematopoietic Cell Transplantation. 4th ed Wiley-Blackwell; Oxford, England: 2009. pp. 1014–1029. [Google Scholar]
- 8.Fassas A, Anagnostopoulos A, Kazis A, et al. Peripheral blood stem cell transplantation in the treatment of progressive multiple sclerosis: first results of a pilot study. Bone Marrow Transplant. 1997;20(8):631–638. doi: 10.1038/sj.bmt.1700944. [DOI] [PubMed] [Google Scholar]
- 9.Nash RA, Bowen JD, McSweeney PA, et al. High-dose immunosuppressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Blood. 2003;102(7):2364–2372. doi: 10.1182/blood-2002-12-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saccardi R, Kozak T, Bocelli-Tyndall C, et al. Autoimmune Diseases Working Party of EBMT. Autologous stem cell transplantation for progressive multiple sclerosis: update of the European Group for Blood and Marrow Transplantation autoimmune diseases working party database. Mult Scler. 2006;12(6):814–823. doi: 10.1177/1352458506071301. [DOI] [PubMed] [Google Scholar]
- 11.Fassas A, Kimiskidis VK, Sakellari I, et al. Long-term results of stem cell transplantation for MS: a single-center experience. Neurology. 2011;76(12):1066–1070. doi: 10.1212/WNL.0b013e318211c537. [DOI] [PubMed] [Google Scholar]
- 12.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 13.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 14.Rowley SD, Loken M, Radich J, et al. Isolation of CD34+ cells from blood stem cell components using the Baxter Isolex system. Bone Marrow Transplant. 1998;21(12):1253–1262. doi: 10.1038/sj.bmt.1701257. [DOI] [PubMed] [Google Scholar]
- 15.Cutter GR, Baier ML, Rudick RA, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999;122(pt 5):871–882. doi: 10.1093/brain/122.5.871. [DOI] [PubMed] [Google Scholar]
- 16.Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain. 2001;124(pt 5):962–973. doi: 10.1093/brain/124.5.962. [DOI] [PubMed] [Google Scholar]
- 17.Caramanos Z, Narayanan S, Arnold DL. SIENA-based estimates of brain-volume change in a clinical-trial setting: use of an incremental-change-summation approach to evaluate different imputation methods used to estimate missing data. Mult Scler J. 2010;16(suppl 10):S109–S110. [Google Scholar]
- 18.Chen JT, Collins DL, Atkins HL, Freedman MS, Galal A, Arnold DL, Canadian MS. BMT Study Group. Brain atrophy after immunoablation and stem cell transplantation in multiple sclerosis. Neurology. 2006;66(12):1935–1937. doi: 10.1212/01.wnl.0000219816.44094.f8. [DOI] [PubMed] [Google Scholar]
- 19.Filippi M, Rocca MA, Arnold DL, et al. EFNS guidelines on the use of neuroimaging in the management of multiple sclerosis. Eur J Neurol. 2006;13(4):313–325. doi: 10.1111/j.1468-1331.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute . Common terminology criteria for adverse events. version 3.0. Cancer Therapy Evaluation Program; [Accessed November 18, 2014]. Mar 31, 2003. Published August 9, 2006. http://ctep.cancer.gov. [Google Scholar]
- 21.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. John Wiley & Sons; New York, NY: 1980. [Google Scholar]
- 22.Fischer JS, Jak AJ, Kniker JE, Rudick RA, Cutter G. [Accessed March 8, 2013];Multiple Sclerosis Functional Composite (MSFC) administration and scoring manual. National Multiple Sclerosis Society. http://main.nationalmssociety.org/docs/HOM/MSFC_Manual_and_Forms.pdf. Revised October 2001.
- 23.Sadovnick AD, Eisen K, Ebers GC, Paty DW. Cause of death in patients attending multiple sclerosis clinics. Neurology. 1991;41(8):1193–1196. doi: 10.1212/wnl.41.8.1193. [DOI] [PubMed] [Google Scholar]
- 24.Turner AP, Williams RM, Bowen JD, Kivlahan DR, Haselkorn JK. Suicidal ideation in multiple sclerosis. Arch Phys Med Rehabil. 2006;87(8):1073–1078. doi: 10.1016/j.apmr.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 25.Daikeler T, Labopin M, Di Gioia M, et al. EBMT Autoimmune Disease Working Party. Secondary autoimmune diseases occurring after HSCT for an autoimmune disease: a retrospective study of the EBMT Autoimmune Disease Working Party. Blood. 2011;118(6):1693–1698. doi: 10.1182/blood-2011-02-336156. [DOI] [PubMed] [Google Scholar]
- 26.Burt RK, Loh Y, Cohen B, et al. Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a phase I/II study. Lancet Neurol. 2009;8(3):244–253. doi: 10.1016/S1474-4422(09)70017-1. [DOI] [PubMed] [Google Scholar]
- 27.Muraro PA, Douek DC, Packer A, et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J Exp Med. 2005;201(5):805–816. doi: 10.1084/jem.20041679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muraro PA, Robins H, Malhotra S, et al. T cell repertoire following autologous stem cell transplantation for multiple sclerosis. J Clin Invest. 2014;124(3):1168–1172. doi: 10.1172/JCI71691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mancardi GL, Sormani MP, Di Gioia M, et al. Italian BMT Study Group. Autologous haematopoietic stem cell transplantation with an intermediate intensity conditioning regimen in multiple sclerosis: the Italian multi-centre experience. Mult Scler. 2012;18(6):835–842. doi: 10.1177/1352458511429320. [DOI] [PubMed] [Google Scholar]
- 30.Darlington PJ, Touil T, Doucet J-S, et al. Canadian MS/BMT Study Group. Diminished Th17 (not Th1) responses underlie multiple sclerosis disease abrogation after hematopoietic stem cell transplantation. Ann Neurol. 2013;73(3):341–354. doi: 10.1002/ana.23784. [DOI] [PubMed] [Google Scholar]
- 31.Berard JA, Bowman M, Atkins HL, Freedman MS, Walker LA. Cognitive fatigue in individuals with multiple sclerosis undergoing immunoablative therapy and hematopoietic stem cell transplantation. J Neurol Sci. 2014;336(1–2):132–137. doi: 10.1016/j.jns.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 32.Saccardi R, Freedman MS, Sormani MP, et al. European Blood and Marrow Transplantation Group; Center for International Blood and Marrow Research; HSCT in MS International Study Group. A prospective, randomized, controlled trial of autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: a position paper. Mult Scler. 2012;18(6):825–834. doi: 10.1177/1352458512438454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfender N, Saccardi R, Martin R. Autologous hematopoietic stem cell transplantation as a treatment option for aggressive multiple sclerosis. Curr Treat Options Neurol. 2013;15(3):270–280. doi: 10.1007/s11940-013-0234-9. [DOI] [PubMed] [Google Scholar]
- 34.Atkins HL, Freedman MS. Hematopoietic stem cell therapy for multiple sclerosis: top 10 lessons learned. Neurotherapeutics. 2013;10(1):68–76. doi: 10.1007/s13311-012-0162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.