Abstract
OBJECT
The surgical management of brainstem arteriovenous malformations (AVMs) might benefit from the definition of anatomical subtypes and refinements of resection techniques. Many brainstem AVMs sit extrinsically on pia mater rather than intrinsically in the parenchyma, allowing treatment by occluding feeding arteries circumferentially, interrupting draining veins after arteriovenous shunting is eliminated, and leaving the obliterated nidus behind. The authors report here the largest series of brainstem AVMs to define 6 subtypes, assess this “occlusion in situ” technique, and analyze the microsurgical results.
METHODS
Brainstem AVMs were categorized as 1 of 6 types: anterior midbrain, posterior midbrain, anterior pontine, lateral pontine, anterior medullary, and lateral medullary AVMs. Data from a prospectively maintained AVM registry were reviewed to evaluate multidisciplinary treatment results.
RESULTS
During a 15-year period, the authors treated 29 patients with brainstem AVMs located in the midbrain (1 anterior and 6 posterior), pons (6 anterior and 7 lateral), and medulla (1 anterior and 8 lateral). The nidus was pial in 26 cases and parenchymal in 3 cases. Twenty-three patients (79%) presented with hemorrhage. Brainstem AVMs were either resected (18 patients, 62%) or occluded in situ (11 patients, 38%). All lateral pontine AVMs were resected, and the occlusion in situ rate was highest with anterior pontine AVMs (83%). Angiography confirmed complete obliteration in 26 patients (89.6%). The surgical mortality rate was 6.9%, and the rate of permanent neurological deterioration was 13.8%. At follow-up (mean 1.3 years), good outcomes (modified Rankin Scale [mRS] score ≤ 2) were observed in 18 patients (66.7%) and poor outcomes (mRS score of 3–5) were observed in 9 patients (33.3%). The mRS scores in 21 patients (77.8%) were unchanged or improved. The best outcomes were observed with lateral pontine (100%) and lateral medullary (75%) AVMs, and the rate of worsening/death was greatest with posterior midbrain and anterior pontine AVMs (50% each).
CONCLUSIONS
Brainstem AVMs can be differentiated by their location in the brainstem (midbrain, pons, or medulla) and the surface on which they are based (anterior, posterior, or lateral). Anatomical subtypes can help the neurosurgeon determine how to advise patients, with lateral subtypes being a favorable surgical indication along with extrinsic pial location and hemorrhagic presentation. Most AVMs are dissected with the intention to resect them, and occlusion in situ is reserved for those AVMs that do not separate cleanly from the brainstem, that penetrate into the parenchyma, or are more anterior in location, where it is difficult to visualize and preserve perforating arteries (anterior pontine and lateral medullary AVMs). Although surgical morbidity is considerable, surgery results in a better obliteration rate than nonoperative management and is indicated in highly selected patients with high rerupture risks.
Keywords: brainstem, arteriovenous malformations, subtypes, surgery, occlusion in situ, vascular disorders
Brainstem arteriovenous malformations (AVMs) are intimidating lesions, because they sit in one of the inviolable areas of the brain among cranial nerves, their nuclei, and critical fiber tracts. Surgical exposures are not ideal, particularly when the skull base makes the brainstem so inaccessible. Many neurosurgeons consider these lesions inoperable; in addition, they are rare, accounting for only 2%–6% of all intracranial AVMs.1,2,4,17,19,23 Consequently, microsurgical experience with brainstem AVMs has been limited.1,3,4,6,12,16,19,21,23 There is growing evidence that AVMs of the brainstem have a more aggressive clinical course than their supratentorial counterparts. Observations of infratentorial AVMs presenting more frequently with hemorrhage than supratentorial AVMs (76%–100% of cases studied) have been consistently reported.4,7,8,10,19 Published studies in which only brainstem AVMs were considered have also reported significantly higher annual hemorrhage rates, as high as 15%–17.5%,11,15 which make them difficult to observe. Hemorrhage from brainstem AVMs is associated with a poor prognosis, with death in as many as one-third of treated patients and two-thirds of untreated patients,4,5,14 likely because of the lesions’ proximity to highly eloquent structures. Some authors have postulated that the higher observed rates of hemorrhagic presentation are partly a result of the lower propensity of these lesions to cause seizures,9 whereas some have noted a higher association with intranidal aneurysms in deeply seated AVMs.10
Brainstem AVMs today may be like brainstem cavernous malformations (CMs) were 20 years ago, when they too were considered inoperable lesions. The management of brainstem CMs evolved dramatically in the last 20 years into a surgically curable lesion with acceptable operative morbidity rates and established exposures, brainstem entry zones, and resection techniques. A similar evolution in surgical management may also be possible for brainstem AVMs with better definitions of the anatomical subtypes of brainstem AVMs and refinements of resection techniques. Many brainstem AVMs sit extrinsically on the pial surface rather than intrinsically in the parenchyma, making them different from cerebral and cerebellar AVMs. This unique feature allows some brainstem AVMs to be treated by occluding the feeding arteries circumferentially, interrupting draining veins when arteriovenous shunting has been eliminated, and leaving the obliterated nidus on the brainstem, thereby minimizing the extent of parenchymal dissection and its associated morbidity. This “occlusion in situ” technique is an alternative to conventional resection. Here, we report our surgical experience with brainstem AVMs, in the largest microsurgical series in the literature, to define 6 AVM subtypes, assess the occlusion in situ technique, and analyze the microsurgical results. We hypothesized that lateral brainstem AVMs are more favorable for surgery than medial/midline AVMs and that “occlusion in situ” offers a conservative alternative to resection with comparable occlusion and morbidity rates for difficult AVMs with some intrinsic component.
Methods
Data Collection
This study was approved by the institutional review board of the University of California, San Francisco, and conducted in compliance with Health Insurance Portability and Accountability Act regulations. The prospective registry of the University of California, San Francisco Brain Arteriovenous Malformation Study Project was searched to identify patients with brainstem AVMs who were evaluated and managed between 1997 and 2012. Patients were included if their AVM was located in the midbrain, pons, or medulla and excluded if their AVM was located only in the cerebellum. Patients were included if they were treated surgically. Six patients were treated radiosurgically, by embolization only, or were observed, and they were excluded. The database, medical records, pre- and postoperative radiographs, intraoperative photographs, and clinical follow-up evaluations were retrospectively reviewed.
The Spetzler-Martin AVM grading system incorporates AVM size (< 3 cm, 3–6 cm, or > 6 cm), venous drainage pattern (deep vs superficial), and eloquence of the AVM location (eloquent vs noneloquent).20 The Lawton-Young AVM grading system incorporates patient age (< 20 years, 20–40 years, or > 40 years), hemorrhagic presentation (ruptured vs unruptured), and compactness of the nidus (compact vs diffuse).13 The supplemented Spetzler-Martin grade is the sum of the Spetzler-Martin and Lawton-Young grades.
Six Types of Brainstem AVMs
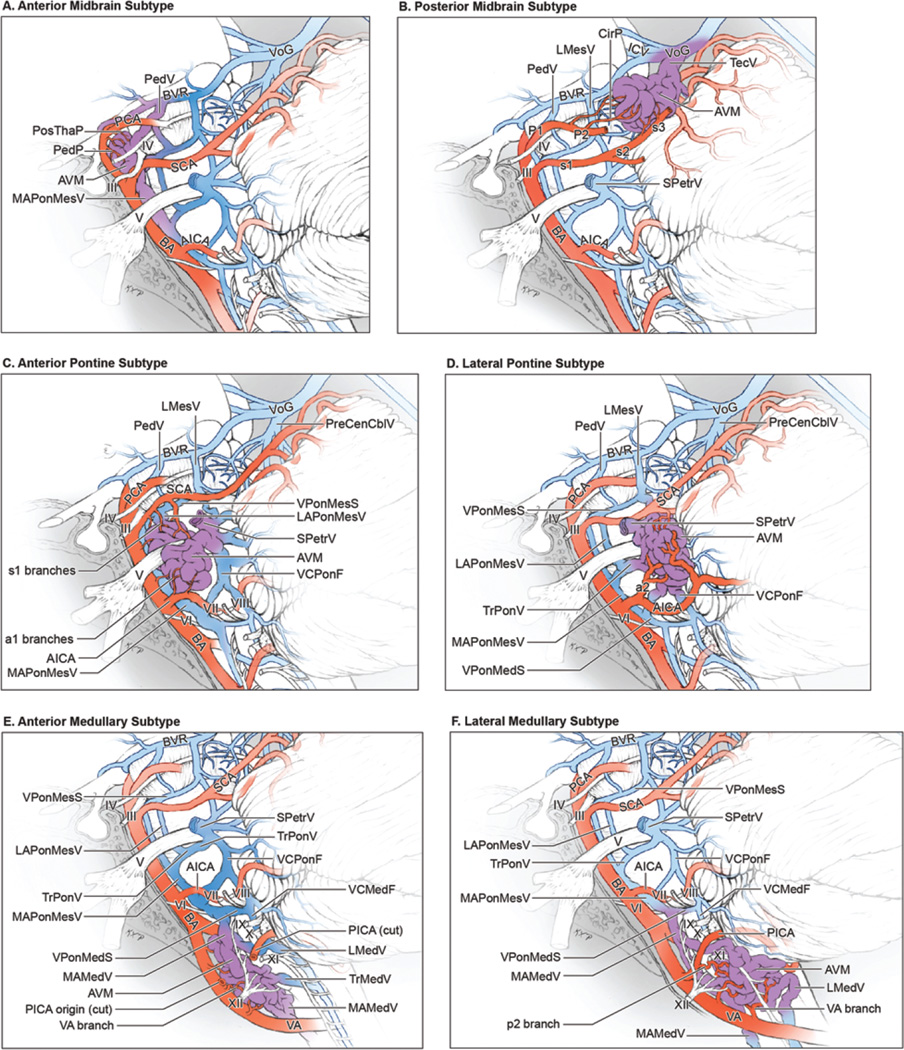
Brainstem AVMs were categorized as 1 of 6 types according to the following descriptions. The anterior midbrain AVM is located on the anterior midbrain surface on, in, or between the cerebral peduncles (Fig. 1A). This subtype is associated with the oculomotor nerve (cranial nerve [CN] III) and lies behind the P1 segment of the posterior cerebral artery (PCA) and posterior thalamoperforating arteries in the interpeduncular fossa. Perforating arteries from both P1 PCAs supply the nidus, and it is drained by the median anterior mesencephalic vein, the peduncular vein, and the posterior communicating vein, which then collect in the basal vein of Rosenthal.
FIG. 1.
The 6 brainstem AVM subtypes. A: The anterior midbrain AVM, lateral view. This AVM is located on the anterior midbrain surface, is supplied by P1 PCA branches (posterior thalamoperforating artery [PosThaP] and peduncular perforator [PedP]) and is drained by anterior veins (median anterior mesencephalic vein [MAPonMesV], peduncular vein [PedV], and posterior communicating vein [PComV]). B: The posterior midbrain AVM, lateral view. This AVM is located on the posterior midbrain surface, is supplied bilaterally by the PCA (long circumflex perforators from P1 and P2 segments) and SCA (s3 branches), and is drained by veins ascending to the vein of Galen (tectal vein [TecV], vein of the cerebellomesencephalic fissure [VCMesF], and superior vermian vein [SVerV]). C: The anterior pontine AVM, lateral view. This AVM is located on the rectangular surface between the basilar sulcus medially, the trigeminal root laterally, and the pontomesencephalic and pontomedullary sulci. It is supplied unilaterally by the s1 SCA and a1 AICA branches, as well as direct branches from the basilar trunk, and drains to the MAPonMesV medially or the superior petrosal vein [SPetrV] laterally. D: The lateral pontine AVM, lateral view. This AVM is located on the triangular surface of the pons and brachium pontis between the CN V root medially and the V-shaped cerebellopontine fissure laterally. It is supplied unilaterally by the a2 and a3 AICA branches and is drained by the SPetrV. E: The anterior medullary AVM, lateral view. This AVM is located below the pontomedullary sulcus, between the anterolateral sulci, and anterior to hypoglossal nerve rootlets. It is supplied by branches from the distal VA and is drained in the midline by the median anterior medullary vein (MAMedV) and MAPonMesV. F: The lateral medullary AVM, lateral view. This AVM is located below the pontomedullary sulcus, lateral to the anterolateral sulcus, and posterior to hypoglossal nerve rootlets. It is supplied by branches from the VA and PICA and drains medially (MAMedV) and/or laterally (lateral medullary vein [LMedV]). PreCenCblV = precentral cerebellar vein; ICV = internal cerebral vein; VPonMesS = vein of the pontomesencephalic sulcus; LAPonMesV = lateral anterior pontomesencephalic vein; VCPonF = vein of the cerebellopontine fissure; TrPonV = transverse pontine vein; VCMedF = vein of the cerebellomedullary fissure. Reproduced with permission from Lawton: Seven AVMs: Tenets and Techniques for Resection, Thieme, 2014.
The posterior midbrain AVM sits on the tectum/quadrigeminal plate, below the pineal gland and above the superior cerebellar peduncles, often intimate with trochlear nerves (Fig. 1B). These AVMs are fed by long circumflex perforators from the P1 and P2 PCA segments, as well as branches from the s3 segment of the superior cerebellar artery (SCA), typically bilaterally. Venous drainage into the tectal vein and vein of the cerebellomesencephalic fissure collects in the vein of Galen.
The anterior pontine AVM sits on the rectangular surface between the basilar sulcus medially and trigeminal (CN V) root laterally and between the pontomesencephalic sulcus superiorly and pontomedullary sulcus inferiorly (Fig. 1C). Despite its anterior location, this AVM is unilateral. It is located between the s1 SCA superiorly and the a1 segment of the anterior inferior cerebellar artery (AICA) inferiorly, and it is supplied by both arteries. Enlarged perforating branches from the basilar trunk also feed this AVM directly. Venous drainage collects medially in the median anterior mesencephalic vein or, more commonly, in the superior petrosal vein and the superior petrosal sinus (SPS) laterally.
The lateral pontine AVM sits on the triangular surface between the CN V root medially and the cerebellopontine fissure laterally (Fig. 1D). The extent of pial invasion varies among these AVMs, with some lying on the pial surface and others penetrating deeply into the middle cerebellar peduncle. These AVMs are supplied by the AICA (a2 and a3 segments) and, unlike anterior pontine AVMs, rarely receive input from the SCA. Venous drainage collects in the superior petrosal vein and superior petrosal sinus.
The anterior medullary AVM is located on the anterior surface below the pontomedullary sulcus, between the anterolateral sulci and anterior to hypoglossal nerve (CN XII) rootlets (Fig. 1E). This AVM sits posteroinferior to the vertebrobasilar junction (VBJ) in the midline and is supplied bilaterally by vertebral artery (VA) branches at the level of the anterior spinal artery origins (distal V4 segment). The posterior inferior cerebellar artery (PICA) originates lateral to this area and does not contribute. Venous drainage is through the median anterior medullary vein.
The lateral medullary AVM is located below the pontomedullary sulcus, lateral to the anterolateral (preolivary) sulcus and posterior to hypoglossal nerve rootlets, in the location of the p2 segment of the PICA (Fig. 1F). It tends to be small and respects the pia. This AVM is unilateral and fed by small branches directly from the VA and the PICA. It is drained by the lateral medullary vein and the median anterior medullary vein.
Outcome Evaluation
Neurological outcomes were assessed by using the modified Rankin Scale (mRS). Neurological assessments were performed by a nurse clinician, under the supervision of a neurologist, preoperatively, at the time of postoperative discharge, and up to 2 years postoperatively. Follow-up information was obtained during routine clinic visits or telephone interviews. A good outcome was defined as a final mRS score of 0–2, whereas a poor outcome was defined as a final mRS score greater than 2. Functional improvement was defined as a decrease in the mRS score between the preoperative examination and the final follow-up examination. Deterioration was likewise defined as an increase in the mRS score during this time. The angiographic outcome after surgery was defined as either complete or incomplete AVM resection.
Statistical Analysis
Statistical analysis included Fisher’s exact test and Pearson’s chi-square test, when an appropriate sample size was available.
Results
Patients and Brainstem AVM Characteristics
During a 15-year period (1997–2012), 29 patients with brainstem AVMs were treated by our multidisciplinary team, which included neurosurgeons, neurologists, interventional neuroradiologists, and radiation oncologists. There were 18 male and 11 female patients, and the mean age at treatment was 54 years (range 3 months to 81 years). A total of 23 AVMs had hemorrhaged at presentation. In the remaining 6 patients, the most common presentations included headache, dizziness, facial pain, hydrocephalus, and weakness.
The AVM nidus was located in the midbrain in 7 patients (1 anterior and 6 posterior), in the pons in 13 patients (6 anterior and 7 lateral), and in the medulla in 9 patients (1 anterior and 8 lateral). The AVM nidus was pial in 26 cases and parenchymal in 3 cases. The average nidus size was 1.5 cm (range 3 mm to 2.9 cm). The most common Spetzler-Martin grade was III (24 cases), and the most common Lawton-Young grade was also III (21 cases) (Table 1).
TABLE 1.
Summary of brainstem AVM grading*
| Characteristic | Midbrain | Pons | Medulla | Total |
|---|---|---|---|---|
| AVM size in cm | ||||
| <3 | 7 | 13 | 9 | 29 |
| 3–6 | 0 | 0 | 0 | 0 |
| >6 | 0 | 0 | 0 | 0 |
| Venous drainage | ||||
| Superficial | 0 | 3 | 2 | 5 |
| Deep | 7 | 10 | 7 | 24 |
| Eloquence | ||||
| No | 0 | 0 | 0 | 0 |
| Yes | 7 | 13 | 9 | 29 |
| Spetzler-Martin grade | ||||
| I | 0 | 0 | 0 | 0 |
| II | 0 | 3 | 2 | 5 |
| III | 7 | 10 | 7 | 24 |
| IV | 0 | 0 | 0 | 0 |
| V | 0 | 0 | 0 | 0 |
| Age at treatment (yrs) | ||||
| <20 | 1 | 1 | 0 | 2 |
| 20–40 | 3 | 1 | 0 | 4 |
| >40 | 3 | 11 | 9 | 23 |
| Hemorrhagic presentation | ||||
| Ruptured | 5 | 11 | 7 | 23 |
| Unruptured | 2 | 2 | 2 | 6 |
| Compactness | ||||
| Compact | 7 | 13 | 9 | 29 |
| Diffuse | 0 | 0 | 0 | 0 |
| Lawton-Young grade | ||||
| I | 1 | 1 | 0 | 2 |
| II | 1 | 1 | 0 | 2 |
| III | 5 | 9 | 7 | 21 |
| IV | 0 | 2 | 2 | 4 |
| V | 0 | 0 | 0 | 0 |
| Supplemented AVM grade | ||||
| 1–3 (low) | 0 | 0 | 0 | 0 |
| 4–6 (moderate) | 7 | 12 | 7 | 26 |
| 7–10 (high) | 0 | 1 | 2 | 3 |
Values are numbers of patients.
The pial or parenchymal location was a major anatomical factor affecting surgical indication. All of the 26 pial AVMs involving the pia or invading minimally into the brainstem parenchyma were selected for surgery. In contrast, only 3 parenchymal AVMs were selected for surgery, and the 6 patients who did not undergo surgery and were excluded from the study had parenchymal brainstem AVMs. MRI was critical in differentiating pial from parenchymal location, with pial AVMs appearing exophytic in the subarachnoid spaces around the brainstem and parenchymal AVMs being buried in the brainstem and surrounded on 4 or more sides by normal tissue. This determination could not be made angiographically. Hemorrhagic presentation and clinical condition were the other major clinical factors affecting surgical indication. Twenty-three patients (79%) presented with hemorrhage, from either AVM bleeding or rupture of an associated aneurysm. Hemorrhagic presentation indicated a more aggressive natural history, and immediate treatment without delay for neurological recovery or rehabilitation was pursued to prevent rehemorrhage. In addition, hemorrhaging was associated with significant presenting neurological deficits (mRS score of ≥ 2) in 19 (83%) of these 23 patients. Hematoma evacuation was an important consideration in 5 patients to relieve mass effect in a tight posterior fossa.
Overall, 19 patients were treated with microsurgery only, 7 were treated with endovascular embolization and microsurgery, 1 was treated with stereotactic radiosurgery and microsurgery, and 2 were treated with stereotactic radiosurgery, endovascular embolization, and microsurgery (Table 2).
TABLE 2.
Summary of surgical management of brainstem AVMs*
| Midbrain | Pons | Medulla | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | Ant | Pst | Ant | Lat | Ant | Lat | Total |
| Previous radiosurgery | |||||||
| No | 0 | 6 | 6 | 5 | 1 | 8 | 26 |
| Yes | 1 | 0 | 0 | 2 | 0 | 0 | 3 |
| Preop embolization | |||||||
| No | 1 | 2 | 5 | 4 | 1 | 7 | 20 |
| Yes | 0 | 4 | 1 | 3 | 0 | 1 | 9 |
| Surgical approach | |||||||
| Orbitozygomatic | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Supracerebellar-infratentorial | 0 | 4 | 0 | 0 | 0 | 0 | 4 |
| Posterior interhemispheric | 0 | 2 | 0 | 0 | 0 | 0 | 2 |
| Retrosigmoid | 0 | 0 | 6 | 7 | 0 | 0 | 13 |
| Far lateral | 0 | 0 | 0 | 0 | 1 | 8 | 9 |
| AVM management | |||||||
| Resection | 1 | 4 | 1 | 7 | 1 | 4 | 18 |
| Occlusion in situ | 0 | 2 | 5 | 0 | 0 | 4 | 11 |
| Staged AVM resection | |||||||
| Single | 1 | 5 | 6 | 7 | 1 | 8 | 28 |
| Multiple | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Angiographic result | |||||||
| Complete obliteration | 1 | 5 | 4 | 7 | 1 | 8 | 26 |
| Incomplete obliteration | 0 | 1 | 2 | 0 | 0 | 0 | 3 |
| Postop radiosurgery | |||||||
| No | 1 | 5 | 5 | 7 | 1 | 8 | 27 |
| Yes | 0 | 1 | 1 | 0 | 0 | 0 | 2 |
| Preop mRS score | |||||||
| 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 |
| 1 | 0 | 1 | 1 | 2 | 0 | 4 | 8 |
| 2 | 0 | 2 | 2 | 1 | 0 | 1 | 6 |
| 3 | 0 | 0 | 2 | 1 | 0 | 2 | 5 |
| 4 | 1 | 0 | 0 | 1 | 0 | 0 | 2 |
| 5 | 0 | 2 | 0 | 2 | 1 | 1 | 6 |
| Postop mRS score | |||||||
| 0 | 0 | 0 | 1 | 1 | 0 | 2 | 4 |
| 1 | 1 | 3 | 1 | 1 | 0 | 3 | 9 |
| 2 | 0 | 1 | 1 | 1 | 0 | 2 | 5 |
| 3 | 0 | 1 | 2 | 2 | 0 | 0 | 5 |
| 4 | 0 | 0 | 0 | 2 | 0 | 0 | 2 |
| 5 | 0 | 0 | 1 | 0 | 1 | 0 | 2 |
| 6 | 0 | 1 | 0 | 0 | 0 | 1 | 2 |
| mRS score change | |||||||
| Improved or unchanged | 1 | 3 | 3 | 7 | 1 | 6 | 21 |
| Worsened or dead | 0 | 3 | 3 | 0 | 0 | 2 | 8 |
Ant = anterior; Pst = posterior.
Values are numbers of patients.
Surgical Management
The anterior midbrain AVM was approached through an orbitozygomatic/transsylvian exposure (Table 2). Posterior midbrain AVMs were approached through a torcular craniotomy/supracerebellar-infratentorial approach, except for 2 cases in which a posterior interhemispheric approach was used to occlude the portion of the AVM involving the vein of Galen. Pontine AVMs, both the anterior and lateral subtypes, were accessed via an extended retrosigmoid craniotomy.18 Medullary AVMs were resected through a far-lateral craniotomy.
Brainstem AVMs were either resected (18 patients, 62%) or occluded in situ (11 patients, 38%). Occlusion in situ obliterated the AVM with circumferential pial dissection that did not transgress the brainstem tissue, leaving the occluded AVM in place rather than resecting it to avoid intraparenchymal dissection. Obliteration rates were highest with lateral pontine and anterior midbrain AVMs (100% each), and the occlusion in situ rate was highest with anterior pontine AVMs (83%) (Table 2). Indocyanine green videoangiography was used liberally throughout the dissection to identify the feeding arteries, monitor the extent of arteriovenous shunting, and confirm stasis in the draining vein at the end of the circumdissection. AVMs were treated in a single surgical stage except for one, which required 2 stages.
Postoperative angiography confirmed complete AVM obliteration in 26 patients (surgical obliteration rate 89.6%). Three patients had residual AVM, 2 of whom underwent postoperative radiosurgery in pursuit of complete obliteration.
Clinical Outcomes
Two patients died in the perioperative period (surgical mortality rate 6.9%). Both patients presented in coma with ruptured AVMs (posterior midbrain and lateral medulla) and large posterior fossa hematomas. Their hematomas were urgently evacuated, and their AVMs were resected completely; however, they failed to improve neurologically, and support was withdrawn. Six patients experienced a decline in their overall functional status. Three of these patients had anterior pontine AVMs: 1 patient suffered a lateral pontine infarct from a perforating artery occlusion that left him hemiplegic and dysarthric; 1 patient developed some mild ataxia postoperatively; and 1 patient experienced new ipsilateral hearing loss (mRS score of 0–1). A new, mild hemiparesis was observed in 1 patient with a lateral medullary AVM. The condition of 2 patients with posterior midbrain AVMs was worse after surgery, one because of increased dizziness and ataxia (mRS score of 0–1) and the other because of mild-to-moderate akinetic mutism. Excluding the 2 patients with an mRS score change from 0 to 1, the rate of permanent neurological deterioration was 13.8%.
At late follow-up of the surviving 27 patients (mean length of follow-up 1.3 years; range 2 weeks to 7.5 years), good outcomes (mRS score ≤ 2) were observed in 18 patients (66.7%), and poor outcomes (mRS score 3–5) were observed in 9 patients (33.3%). Overall, the condition of 21 patients (77.8%) was unchanged or improved according to their mRS score.
Relative outcomes were best in patients with AVMs located in the pons and the medulla (77% and 78% of the patients, respectively, had improved/unchanged mRS scores), but these differences were not significant (Table 3). According to brainstem subtypes, the best outcomes were observed with lateral pontine AVMs (100% improved/unchanged) and lateral medullary AVMs (75% improved/unchanged). Good relative outcomes were also observed in patients with anterior midbrain and anterior medullary AVMs, but there was only 1 patient in each of these subgroups. Compared with all other brainstem AVMs, lateral pontine AVMs had a significantly higher percentage of patients whose mRS score had improved/remained unchanged after resection (p = 0.04). The rate of worsening/death was greatest in individuals with posterior midbrain and anterior pontine AVMs (50% each).
TABLE 3.
Relative outcome analysis of patients with brainstem AVMs treated microsurgically
| Characteristic | No. of Patients (%) | ||
|---|---|---|---|
| Improved/Unchanged | Worse/Dead | Total | |
| Location | |||
| Midbrain | 4 (57) | 3 (43) | 7 |
| Pons | 10 (77) | 3 (23) | 13 |
| Medulla | 7 (78) | 2 (22) | 9 |
| Subtypes | |||
| Anterior midbrain | 1 (100) | 0 (0) | 1 |
| Posterior midbrain | 3 (50) | 3 (50) | 6 |
| Anterior pontine | 3 (50) | 3 (50) | 6 |
| Lateral pontine | 7 (100) | 0 (0) | 7 |
| Anterior medullary | 1 (100) | 0 (0) | 1 |
| Lateral medullary | 6 (75) | 2 (25) | 8 |
| Technique | |||
| Resection | 15 (83) | 3 (17) | 18 |
| Occlusion in situ | 6 (55) | 5 (45) | 11 |
| Presentation | |||
| Unruptured | 4 (66) | 2 (33) | 6 |
| Ruptured | 17 (74) | 6 (26) | 23 |
Overall, 18 brainstem AVMs were resected (62.1%), and 11 were occluded in situ (37.9%). All 3 incompletely obliterated AVMs were left in situ rather than resected, yielding complete occlusion rates of 72.7% (8 of 11 cases) with the occlusion in situ technique and 100% with AVM resection (all 18 cases). This difference did not reach significance (p = 0.07). Patients whose AVMs were resected fared better than those whose AVMs were occluded in situ, with rates of improvement/unchanged functional status of 83% and 55%, respectively, although this difference did not reach significance (p = 0.09). Patients presenting with ruptured brainstem AVMs fared slightly better than those with unruptured AVMs (rates of improved/unchanged status 74% and 66%, respectively), but this difference was not significant (Table 3).
Discussion
Brainstem AVM Subtypes
This series demonstrates the diversity of brainstem AVMs, each one differentiated by its location in the brainstem (midbrain, pons, or medulla) and the surface on which it is based (anterior, posterior, or lateral). Although each AVM has unique anatomy, technical challenges, and risks, these lesions are often analyzed collectively because they are so rare. As such, published surgical series of brainstem AVMs have been small; Yaşargil23 had experience with 14 patients, Drake el al.4 with 15 patients, Solomon and Stein19 with 12 patients, Spetzler and Martin20 with 10 patients, and Nozaki et al.15 with 20 patients. Limited experiences with brainstem AVMs prevent the categorization of recognizable AVM subtypes. Our series of 29 patients is the largest in the literature and has informed our characterization of 6 AVM subtypes. Some of these subtypes can be identified in other reports: anterior midbrain AVMs have been described as ventral; posterior midbrain AVMs as dorsal, tectal, and quadrigeminal; and lateral pontine AVMs as cerebellopontine angle and peduncular. Anterior pontine and lateral medullary AVMs have not been specifically described previously, but they were common in our experience. Posterior pontine and fourth ventricle AVMs were reported by Batjer and Samson1 (4 cases) and Solomon and Stein19 (2 cases), but none were encountered in our experience. Therefore, there may be other brainstem AVM subtypes beyond the 6 described here.
Subtyping brainstem AVMs may be useful when selecting patients for surgery, planning surgical strategy, and standardizing outcome assessments. Our experience demonstrated that lateral brainstem AVMs were more favorable for surgery than medial/midline AVMs. The complete resection rate was 100% for lateral pontine and lateral medullary AVMs, and the rates of improved/unchanged outcomes were 100% and 75%, respectively. The rate of worsening/death was greatest with posterior midbrain and anterior pontine AVMs (50% each). The careful determination of a brainstem AVM’s anatomical subtype can help the neurosurgeon advise patients, with lateral brainstem AVM subtype being a favorable indication for surgery, as are an extrinsic pial location and a hemorrhagic presentation.
AVM Resection Versus Occlusion In Situ
Complete AVM resection is the goal of surgery, but so too is the preservation of neurological function, which requires meticulous handling of normal brainstem perforating arteries and the parenchyma. The circumferential dissection needed to resect a brainstem AVM increases parenchymal manipulation, risk to perforators, and bleeding. Occlusion in situ is an alternative that circumferentially occludes feeding arteries in the pia, minimizes intraparenchymal dissection, closes the draining vein, and leaves the obliterated AVM behind.22 The extrinsic location of operable brainstem AVMs and their small size support this simpler strategy. This strategy was used most with anterior pontine (5 cases) and lateral medullary (4 cases) AVM subtypes, with which exposure and visualization are limited and the danger of vital perforator occlusion is greatest. Most AVMs are dissected with the intention to resect, and occlusion in situ is reserved for those AVMs that do not separate cleanly from the brainstem or that penetrate into the parenchyma. Therefore, occluded AVMs are more complex than resected AVMs, and although we found differences in good outcomes (83% vs 55% improved/unchanged status with resected and occluded AVMs, respectively) and complete obliteration rates (100% vs 73% in resected and occluded AVMs, respectively), these differences were not statistically significant and were affected by differences in AVM complexity and surgeon selection. We conclude that brainstem AVMs should be resected when pial location and AVM anatomy are judged intraoperatively to be conducive, but occlusion in situ may be more appropriate with penetrating AVMs in more anterior locations where it is more difficult to visualize and preserve normal perforators. Uninvolved perforators are small in caliber, immediately adjacent to the nidus, and often indistinguishable from AVM feeders, and the loss of even one perforator can devastate a patient.
This occlusion in situ technique has been previously described.22 In 1 of their case illustrations, Solomon and Stein cauterized feeding arteries on the pial surface, and when “the vein became smaller and turned blue, no further intramedullary dissection was necessary.”19 Some neurosurgeons have alternatively described a “pial resection technique” with circumdissection that occludes arterial input at the pial margins and resects the portion of the nidus that extends beyond the brainstem surface. After arterial input is eliminated and the draining vein darkens, it is important to divide the in situ AVM’s vein. The color change in the draining vein from red to blue indicates complete obliteration, and indocyanine green videoangiography is a valuable adjunct that confirms AVM occlusion.
Surgical Approaches
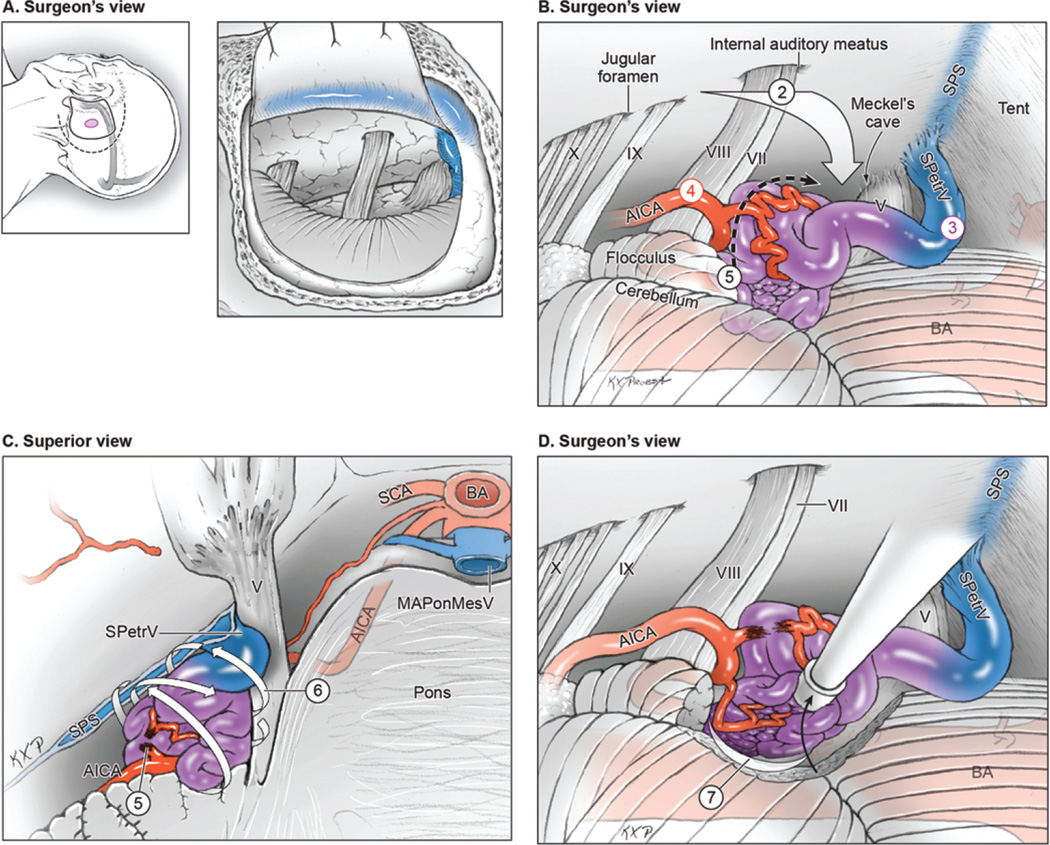
In the present series, lateral pontine and lateral medullary AVMs were the most common subtypes and had the best microsurgical results. Lateral pontine AVMs are resected through an extended retrosigmoid craniotomy (Fig. 2, Step 1), and initial subarachnoid dissection releases CSF from the cisterna magna, opens the cerebellopontine cistern, and frees the flocculus from the lower cranial nerves (Step 2). Lateral pontine AVMs sit lateral to CN V and do not need to be accessed through supra- and infratrigeminal triangles. Surgical access is the wide-open space of the cerebellopontine angle above CN VII/VIII. The superior petrosal vein drains the nidus but is not obstructive (Step 3), and the AICA supply is visualized in the infratrigeminal triangle proximally (Step 4). This AICA input may extend anteriorly to the AVM’s free surface but is controlled on the medial AVM margin, between the trigeminal and vestibulocochlear nerves (Step 5). This AVM is based medially on the belly of the pons and posteriorly on or in the middle cerebellar peduncle, which comprise its eloquent borders. However, its superior, inferior, and lateral borders abut the noneloquent cerebellum, which is unusual for a brainstem AVM. These noneloquent borders facilitate access to the AVM’s deep borders, meaning that the lateral half of the nidus can be mobilized with parenchymal circumdissection that closes feeders to these planes and rolls the nidus into the cerebellopontine angle (Step 6). The plane involving the middle cerebellar peduncle is dissected posteriorly, and the plane involving the lateral pons is dissected medially. Remaining feeders along these deep margins are visualized increasingly as the AVM mobilizes forward out of its resection bed (Step 7). This “back-door” technique makes the lateral pontine AVM the easiest brainstem AVM to resect. Occlusions in situ were not performed with these AVMs, and all were resected completely. The middle cerebellar peduncle tolerates parenchymal dissection without neurological consequences, and the best surgical outcomes were achieved for all the patients with these brainstem AVMs. Preserving the arachnoid over CN V and CN VII–VIII adjacent to these AVMs helps protect the nerves (Fig. 3).
FIG. 2.
Surgical approach for lateral pontine AVMs. A: Step 1, exposing the AVM with an extended retrosigmoid approach that includes limited mastoidectomy and skeletonization of the sigmoid sinus (surgeon’s view, with scalp incision [dashed line], craniotomy [solid line], and AVM [purple circle] shown on the left and a dural flap mobilizing the skeletonized sigmoid sinus anteriorly shown on the right). B: Step 2, approaching the AVM through the cerebellopontine cistern; Step 3, identifying ascending drainage laterally (SPetrV); Step 4, locating AICA feeders in the infratrigeminal triangle; and Step 5, interrupting the medial front between the trigeminal and vestibulocochlear nerves (surgeon’s view). C: Step 6, circumdissecting the lateral, superior, and inferior margins in the cerebellum (back-door technique, superior view of the pons and posterior fossa). D: Step 7, mobilizing the AVM anteriorly to dissect the posterior plane along the brachium pontis and the medial plane along the pons (surgeon’s view). BA = basilar artery. Reproduced with permission from Lawton: Seven AVMs: Tenets and Techniques for Resection, Thieme, 2014.
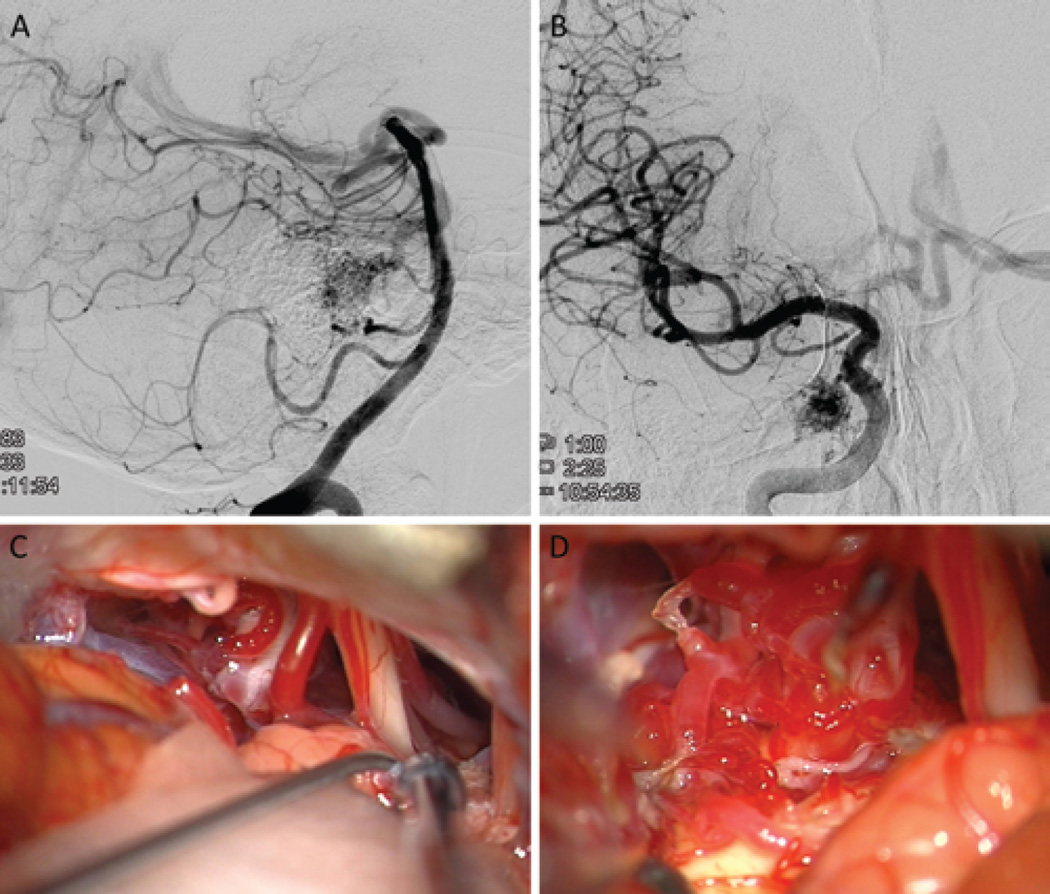
FIG. 3.
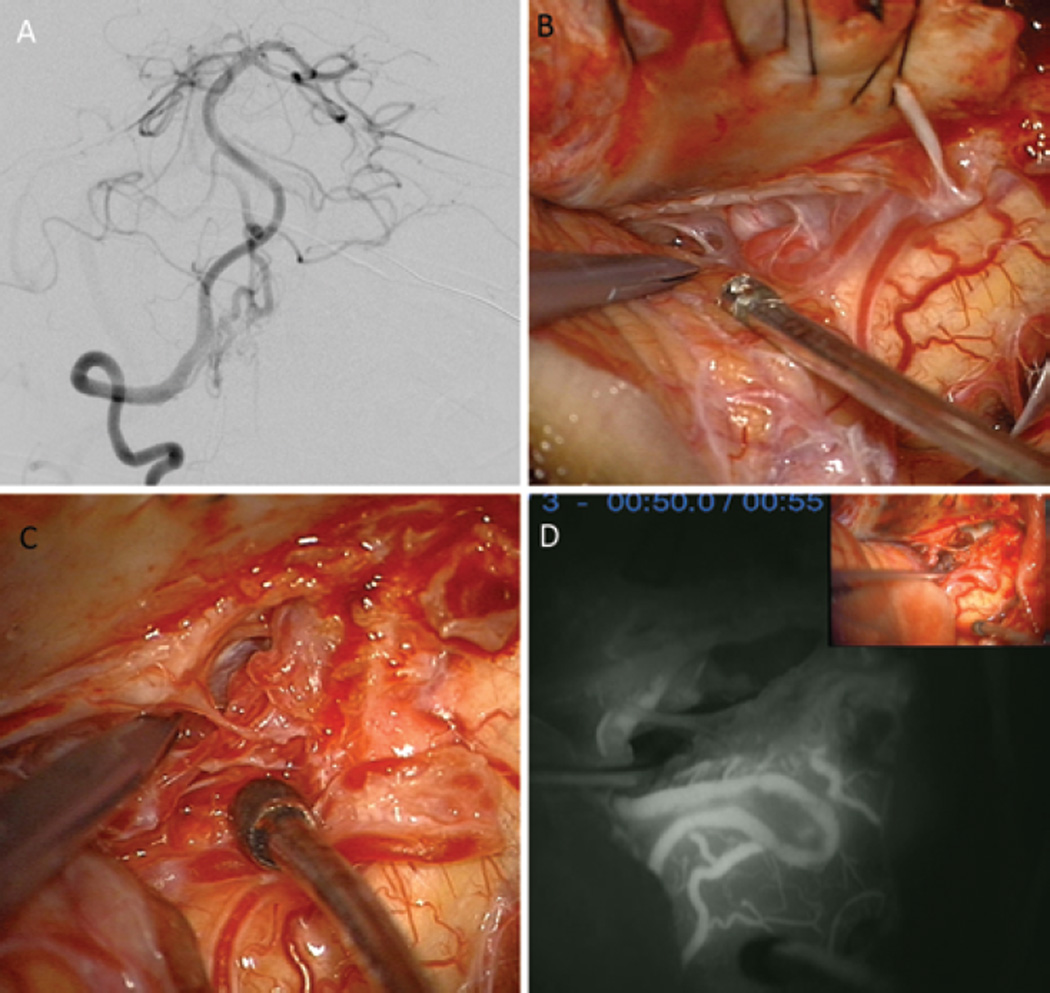
A 75-year-old man presented with a remote hemorrhage from a right lateral pontine AVM (supplemented Spetzler-Martin Grade 6 [S1V1E1/A3B0C0; < 3 cm in diameter, deep venous drainage, and in eloquent location/age > 40 years, bled, and compact nidus]), supplied by the AICA (right VA angiogram, lateral view; A) and dural arteries from the meningohypophyseal trunk (right ICA angiogram, anteroposterior view; B). The AVM was approached through a right extended retrosigmoid craniotomy, which exposed CN V, CN VII–VIII (at tips of Rhoton 6 and sucker, respectively), and CN IX (right of sucker tip) (C). The AVM infiltrated the trigeminal nerve root but was mostly lateral to it (D).
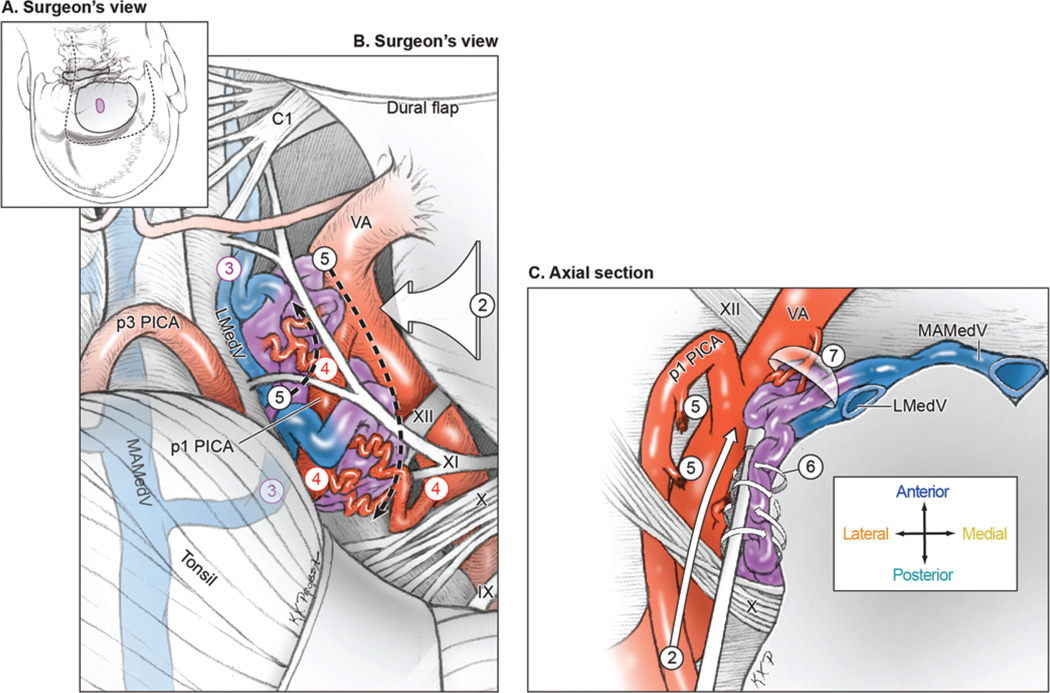
Lateral medullary AVMs are exposed through a farlateral craniotomy (Fig. 4, Step 1) by using a “hockey-stick” or “lazy S” scalp incision. Subarachnoid dissection releases CSF from the cisterna magna, identifies the VA where it pierces the dura, incises the overlying dentate ligament, opens the cerebellomedullary fissure, and elevates the tonsil (Step 2). The vagoaccessory triangle is opened to clarify the course of CNs IX/X/XI, and hypoglossal nerve roots are identified inside this triangle to define the supra- and infrahypoglossal triangles. The lateral medullary AVM sits on the lateral surface intermingled with lower cranial nerves. Lateral drainage through the lateral medullary vein is identified to monitor AVM occlusion, but medial drainage through the median anterior medullary vein may not be visible until later (Step 3). Feeding arteries originate from the posterolateral aspect of VA between its point of dural entry and vertebrobasilar junction (V4 segment), and from proximal PICA branches (P1 and P2 segments, Step 4). Feeding arteries are occluded at the lateral AVM margin, skeletonizing the VA so that it can continue to nourish medullary perforators and contribute to basilar circulation (Step 5). All of the lateral medullary AVMs in this series were pial and were circumdissected without parenchymal transgression (Step 6). Mobilization of the AVM medially toward the medulla and away from the VA helps to visualize any remaining inputs from the VA more distally as well as anterior venous drainage to the median anterior medullary vein (Step 7). Half of these AVMs separated cleanly from the medulla and lower cranial nerves and were resected, and half were occluded in situ, using indocyanine green videoangiography to demonstrate the cessation of arteriovenous shunting before coagulating the dark draining vein (Fig. 5).
FIG. 4.
Surgical approach for lateral medullary AVMs. A: Step 1, exposing the AVM with a far-lateral craniotomy (surgeon’s view, with the patient in the park-bench position). Shown are the scalp incision (dashed line), craniotomy (solid line), and AVM (purple circle). B: Step 2, opening the cisterna magna and dissecting the vagoaccessory, suprahypoglossal, and infrahypoglossal triangles; Step 3, identifying the lateral draining vein (LMedV); Step 4, locating feeding arteries from the VA and PICA; and Step 5, skeletonizing the VA and PICA to interrupt the lateral front while preserving distal flow (surgeon’s view). C: Step 6, circumdissecting in the cerebellomedullary cistern and along the pial margin; and Step 7, mobilizing the AVM medially away from the VA to interrupt deep arterial connections and visualize anterior drainage to the MAMedV (axial cross-sectional view). Reproduced with permission from Lawton: Seven AVMs: Tenets and Techniques for Resection, Thieme, 2014.
FIG. 5.
A 55-year-old woman presented with an intraventricular hemorrhage from a right lateral medullary AVM (supplemented Spetzler-Martin Grade 6 [S1V1E1/A3B0C0; < 3 cm in diameter, deep venous drainage, and in eloquent location/age > 40 years, bled, and compact nidus]), located on the pial surface of the medulla with dilated draining veins anteriorly. A: The AVM was supplied by PICA branches and the anterior spinal artery (AntSpA) and drained by the MAMedV (right VA angiogram, anteroposterior view). B: A right far-lateral craniotomy exposed the cerebellomedullary fissure and lateral medulla. C: The AVM was on the pial surface of the lateral medulla and extended inferiorly to the cervical spinal cord. D: As PICA branches were interrupted, the PICA separated from the AVM and MAMedV, seen on the anterior medullary surface, darkened. Indocyanine green videoangiography confirmed the absence of arteriovenous shunting. The AVM was occluded in situ, and angiography confirmed complete occlusion.
Conclusions
Brainstem AVMs can be differentiated by their location in the brainstem (midbrain, pons, or medulla) and the surface on which they are based (anterior, posterior, or lateral). Anatomical subtypes can help the neurosurgeon determine how to advise patients, with lateral subtypes being a favorable indication for surgery along with extrinsic pial location and hemorrhagic presentation. Most AVMs are dissected with the intention to resect, and occlusion in situ is reserved for those AVMs that do not separate cleanly from the brainstem, that penetrate into the parenchyma, or are in more anterior locations where it is difficult to visualize and preserve normal perforating arteries (anterior pontine and lateral medullary AVMs). Despite being the largest surgical series of brainstem AVMs, the small number of patients limited statistical comparisons of the outcomes. Although surgical morbidity is considerable, surgery resulted in improved/unchanged outcomes in 78% of the patients, has a better obliteration rate than nonoperative management, and is indicated in highly selected patients with high rerupture risks.
Abbreviations
- AICA
anterior inferior cerebellar artery
- AVM
arteriovenous malformation
- CM
cavernous malformation
- CN
cranial nerve
- mRS
modified Rankin Scale
- PCA
posterior cerebral artery
- PICA
posterior inferior cerebellar artery
- SCA
superior cerebellar artery
- SPS
superior petrosal sinus
- VA
vertebral artery
- VBJ
vertebrobasilar junction
Footnotes
Disclosure The authors report no conflict of interest concerning the materials or methods used in this study or the findings specific in this paper.
Author Contributions Conception and design: Lawton, Han. Acquisition of data: Han, Englot. Analysis and interpretation of data: all authors. Drafting the article: all authors. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Lawton. Statistical analysis: Han. Administrative/technical/material support: Kim. Study supervision: Lawton, Kim.
References
- 1.Batjer H, Samson D. Arteriovenous malformations of the posterior fossa. Clinical presentation, diagnostic evaluation, and surgical treatment. J Neurosurg. 1986;64:849–856. doi: 10.3171/jns.1986.64.6.0849. [DOI] [PubMed] [Google Scholar]
- 2.Batjer H, Samson D. Arteriovenous malformations of the posterior fossa: clinical presentation, diagnostic evaluation and surgical treatment. Neurosurg Rev. 1986;9:287–296. doi: 10.1007/BF01743635. [DOI] [PubMed] [Google Scholar]
- 3.Chyatte D. Vascular malformations of the brain stem. J Neurosurg. 1989;70:847–852. doi: 10.3171/jns.1989.70.6.0847. [DOI] [PubMed] [Google Scholar]
- 4.Drake CG, Friedman AH, Peerless SJ. Posterior fossa arteriovenous malformations. J Neurosurg. 1986;64:1–10. doi: 10.3171/jns.1986.64.1.0001. [DOI] [PubMed] [Google Scholar]
- 5.Fults D, Kelly DL., Jr Natural history of arteriovenous malformations of the brain: a clinical study. Neurosurgery. 1984;15:658–662. doi: 10.1227/00006123-198411000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Hosoda K, Fujita S, Kawaguchi T, Yamada H. A transcondylar approach to the arteriovenous malformation at the ventral cervicomedullary junction: report of three cases. Neurosurgery. 1994;34:748–753. doi: 10.1227/00006123-199404000-00029. [DOI] [PubMed] [Google Scholar]
- 7.Kader A, Young WL, Pile-Spellman J, Mast H, Sciacca RR, Mohr JP, et al. The influence of hemodynamic and anatomic factors on hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1994;34:801–808. doi: 10.1227/00006123-199405000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kano H, Kondziolka D, Flickinger JC, Yang HC, Flannery TJ, Niranjan A, et al. Stereotactic radiosurgery for arteriovenous malformations, Part 5: management of brainstem arteriovenous malformations. Clinical article. J Neurosurg. 2012;116:44–53. doi: 10.3171/2011.9.JNS11176. [DOI] [PubMed] [Google Scholar]
- 9.Khaw AV, Mohr JP, Sciacca RR, Schumacher HC, Hartmann A, Pile-Spellman J, et al. Association of infratentorial brain arteriovenous malformations with hemorrhage at initial presentation. Stroke. 2004;35:660–663. doi: 10.1161/01.STR.0000117093.59726.F9. [DOI] [PubMed] [Google Scholar]
- 10.Kiran NA, Kale SS, Kasliwal MK, Vaishya S, Gupta A, Singh Sharma M, et al. Gamma knife radiosurgery for arteriovenous malformations of basal ganglia, thalamus and brainstem—a retrospective study comparing the results with that for AVMs at other intracranial locations. Acta Neurochir (Wien) 2009;151:1575–1582. doi: 10.1007/s00701-009-0335-0. [DOI] [PubMed] [Google Scholar]
- 11.Koga T, Shin M, Terahara A, Saito N. Outcomes of radiosurgery for brainstem arteriovenous malformations. Neurosurgery. 2011;69:45–52. doi: 10.1227/NEU.0b013e31821421d1. [DOI] [PubMed] [Google Scholar]
- 12.Lawton MT, Hamilton MG, Spetzler RF. Multimodality treatment of deep arteriovenous malformations: thalamus, basal ganglia, and brain stem. Neurosurgery. 1995;37:29–36. doi: 10.1227/00006123-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Lawton MT, Kim H, McCulloch CE, Mikhak B, Young WL. A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery. 2010;66:702–713. doi: 10.1227/01.NEU.0000367555.16733.E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumura H, Makita Y, Someda K, Kondo A. Arteriovenous malformations in the posterior fossa. J Neurosurg. 1977;47:50–56. doi: 10.3171/jns.1977.47.1.0050. [DOI] [PubMed] [Google Scholar]
- 15.Nozaki K, Hashimoto N, Kikuta K, Takagi Y, Kikuchi H. Surgical applications to arteriovenous malformations involving the brainstem. Neurosurgery. 2006;58(4 Suppl 2):ONS-270–ONS-279. doi: 10.1227/01.NEU.0000210001.75597.81. [DOI] [PubMed] [Google Scholar]
- 16.Patil AA. Surgical excision of arteriovenous malformation of the cerebellum and brain stem: a case presentation. Acta Neurochir (Wien) 1980;54:117–125. doi: 10.1007/BF01401950. [DOI] [PubMed] [Google Scholar]
- 17.Perret G, Nishioka H. Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Section VI. Arteriovenous malformations. An analysis of 545 cases of cranio-cerebral arteriovenous malformations and fistulae reported to the cooperative study. J Neurosurg. 1966;25:467–490. doi: 10.3171/jns.1966.25.4.0467. [DOI] [PubMed] [Google Scholar]
- 18.Quiñones-Hinojosa A, Chang EF, Lawton MT. The extended retrosigmoid approach: an alternative to radical cranial base approaches for posterior fossa lesions. Neurosurgery. 2006;58(4 Suppl 2):ONS-208–ONS-214. doi: 10.1227/01.NEU.0000192714.15356.08. [DOI] [PubMed] [Google Scholar]
- 19.Solomon RA, Stein BM. Management of arteriovenous malformations of the brain stem. J Neurosurg. 1986;64:857–864. doi: 10.3171/jns.1986.64.6.0857. [DOI] [PubMed] [Google Scholar]
- 20.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476–483. doi: 10.3171/jns.1986.65.4.0476. [DOI] [PubMed] [Google Scholar]
- 21.Sugiura K, Baba M. Total removal of an arteriovenous malformation embedded in the brain stem. Surg Neurol. 1990;34:327–330. doi: 10.1016/0090-3019(90)90009-e. [DOI] [PubMed] [Google Scholar]
- 22.Velat GJ, Chang SW, Abla AA, Albuquerque FC, McDougall CG, Spetzler RF. Microsurgical management of glomus spinal arteriovenous malformations: pial resection technique. Clinical article. J Neurosurg Spine. 2012;16:523–531. doi: 10.3171/2012.3.SPINE11982. [DOI] [PubMed] [Google Scholar]
- 23.Yaşargil MG, editor. Microneurosurgery. Vol IIIB. AVM of the Brain, Clinical Considerations, General and Specific Operative Techniques, Surgical Results, Nonoperated Cases, Cavernous and Venous Angiomas, Neuroanesthesia. Stuttgart: Thieme; 1988. Infratentorial (central) AVMs; pp. 358–366. [Google Scholar]