Abstract
Background
The renal dopaminergic system plays an important role in the pathogenesis of hypertension. Dopamine D1-like receptors (D1R and D5R) decrease reactive oxygen species (ROS) production via inhibition of pro-oxidant enzymes such as NADPH oxidase. Paraoxonase 2 (PON2) is also involved in the inhibition of NADPH oxidase activity. Therefore, we tested the hypothesis that D1R and D5R inhibit ROS production by increasing the expression of PON2, including those in membrane microdomains.
Methods and results
PON2 colocalized with D1R and D5R in mouse renal proximal tubules (RPTs), human RPT (hRPT) cells, and HEK293 cells heterologously expressing human D1R (HEK-hD1R) or D5R (HEK-hD5R). Fenoldopam, an agonist for both D1R and D5R, increased PON2 co-immunoprecipitation with D1R and D5R in HEK-hD1R and HEK-hD5R cells, respectively. Silencing PON2 increased ROS production and NADPH oxidase activity, and impaired the inhibitory effect of fenoldopam. Fenoldopam increased PON2 protein in both lipid rafts (LRs) and non-LRs in HEK-hD1R cells, but only in non-LRs in HEK-hD5R and hRPT cells. Long-term (hrs) fenoldopam stimulation increased PON2 protein in a time-dependent manner in HEK-hD5R, but not in HEK-hD1R cells. Because the effects of fenoldopam on non-LR and total PON2 expressions were similar in HEK-hD5R and hRPT cells, additional studies were performed to determine the relationship between D5R and PON2. Renal PON2 protein was decreased in D5−/− mice. In hRPT cells, silencing D5R decreased PON2 expression and increased ROS production.
Conclusions
We conclude that D1-like receptors inhibit ROS production by altering PON2 distribution in membrane microdomains in the short-term, and by increasing PON2 expression in the long-term.
Keywords: dopamine receptors, paraoxonase 2, reactive oxygen species, NADPH oxidase, lipid rafts
Introduction
Dopamine, produced in non-neural tissues, is an important regulator of epithelial sodium transport, vascular smooth muscle contractility, and production of ROS [1– 3]. Dopamine exerts its actions via dopamine receptors that belong to the G protein-coupled receptor superfamily. Dopamine receptors are classified into D1-like (D1R and D5R) and D2-like (D2R, D3R, and D4R) receptors, based on their structure and pharmacology. D1-like receptors couple to Gαs and stimulate adenylyl cyclase activity, whereas D2-like receptors couple to Gαi/Gαo and inhibit adenylyl cyclase activity [1–4]. Abnormalities of dopamine production and receptor function are involved in the pathogenesis of essential hypertension [3,5–8]. Because the kidney plays an important role in the regulation of blood pressure, many studies have focused on the renal dopaminergic system. Dopamine receptors contribute to the regulation of blood pressure by subtype-specific mechanisms [3,5–7]. Among the dopamine receptors, D1R, D2R, and D5R have been reported to play important roles in maintaining normal redox (reduction/oxidation) balance [3,5,9,10].
ROS are byproducts of oxygen metabolism that include a number of oxygen intermediates, such as superoxide anion, hydrogen peroxide, hydroxyl radical, and hypochlorous acid. ROS are involved in multiple cellular functions such as intra-cellular signaling and cell defense [11–13]. A balance between the production and breakdown of ROS is important in the maintenance of healthy cells. Excessive ROS production can cause cell damage and cell death, which contribute to the pathogenesis of various diseases such as cancer, diabetes, atherosclerosis, and hypertension [14–16]. The important role of oxidative stress in the pathogenesis of hypertension is recognized [15,17–20]. Of the pro-oxidant enzymes, such as NADPH oxidase (NOX), cyclooxygenases, and lipoxygenases, NOX enzymes are the main sources of ROS generation in the cardiovascular and renal systems. Thus, many studies have concentrated on the role of NOX enzymes in the pathogenesis of hypertension [13–26].
Paraoxonases (PONs), classified as lactonases, have antioxidant properties [27–29]. The PON family is composed of three members: PON1, PON2, and PON3. PON1 and PON3 are associated with circulating high-density lipoproteins [30]. By contrast, PON2 is not associated with circulating high-density lipoproteins, but is cell-associated, and protects against cellular oxidative stress [31]. PON1 and PON2 can be inactivated by oxidative stress; but unlike PON1, marked oxidative stress can actually increase PON2 expression [32]. PON2 reduces vascular oxidative stress, which contributes to the prevention of atherosclerosis [33]. We have also reported that PON2 inhibits NOX isoform expression and activity and ROS production, in mouse kidney and hRPT cells [34].
LRs are membrane microdomains composed of sphingolipids, cholesterol, glycolipids, and specific proteins that serve as platforms to recruit signaling components, form new mixtures of signaling molecules and enzymes, and increase the efficiency of intracellular signal transduction. We and others have reported that membrane LRs are involved in redox signaling in several cells, including endothelial and kidney cells [35– 38]. Renal ROS production and NOX activity are increased when hRPT cells are treated with LR disruptors, such as methyl-β-cyclodextrin (βCD), which suggest that LRs play an important role in redox signal transduction [36]. G protein-coupled receptors, including dopamine receptors, are associated with LRs [35,39– 46]. Dopamine receptors, including D1R, D2R, and D5R, have been reported to decrease the production of ROS by dispersing NOX subunits in LRs and non-LRs in hRPT cells [34,36,46]. The D2R interacts with PON2 in LRs and non-LRs in hRPT cells [34], but it is not known whether D1R and D5R can regulate PON2 in these membrane microdomains. Therefore, in the current study, we tested the hypothesis that D1R and D5R inhibit ROS production by increasing the expression of PON2 in those in membrane microdomains.
Materials and methods
Materials
The reagents were obtained from the following sources: polyclonal rabbit anti-PON2 (Abcam Inc., Cambridge, MA); monoclonal anti-NOX2 (a gift from Dr. M. T. Quinn); monoclonal anti-β-actin (SIGMA Aldrich, St. Louis, MO); polyclonal rabbit anti-D1R and anti-D5R antibodies generated in Dr. Pedro A. Jose’s laboratory, the specificities of which have been reported [47–49]; goat anti-rabbit or goat anti-mouse horseradish peroxidase-conjugated secondary antibodies for immunoblotting studies (Jackson Immuno Research Laboratories Inc., West Grove, PA); ECL detection reagents (Millipore Corporation, Billerica, MA); polyclonal rabbit anti-PON2 antibody, labeled directly by the Mix-n-Stain™ CF488A Antibody labeling kit (Biotium Inc., Hayward, CA); secondary antibodies for immunofluorescence studies (Molecular Probes Inc., Eugene, OR); fenoldopam, SCH 23390, methyl- β-cyclodextrin (βCD), protease inhibitor cocktail, lucigenin, NADPH, and other chemicals (SIGMA Aldrich); protein G beads (Roche, Indianapolis, IN); cell culture reagents (Invitrogen, Rockville, MD); and BCA protein assay reagent (PIERCE, Rockford, IL); 26-well 4–20% gradient gels (Bio-Rad, Hercules, CA).
Methods
Cell culture
HEK-293 cells stably transfected with full-length hD1R tagged with Myc-His (HEK-hD1R) [50], or full-length hD5R tagged with V5/His (HEK-hD5R) [51,52] were cultured in αMEM, containing 10% fetal bovine serum. The transfectants were selected by blasticidin (5 µg/ml). As described previously, hRPT cells (passage 10–30) were cultured in Dulbecco’s modified Eagle’s medium/F12 with 10% fetal bovine serum, insulin, transferrin (5 µg/ml), selenium (5 µg/ml), triiodothyronine (4 pg/ml), hydrocortisone (36 ng/ml), and epidermal growth factor (10 ng/ml) [36,53,54]. All cells were cultured in humidified 95% air and 5% CO2 at 37 °C
Subcellular fractionation
The cells were treated with vehicle, fenoldopam (1 µM, 15 min), or methyl-β-cyclodextrin (βCD, 2%/1 h/37°C). LR and non-LR fractions were prepared by sucrose gradient centrifugation, using a detergent-free protocol [50,55]. In brief, the cells were washed with cold serum-free-medium, and pelleted. Each pellet was mixed with 1.5 ml of 500 mM sodium carbonate (pH 11) and homogenized by a Dounce homogenizer (10 strokes), a Polytron tissue grinder (three 10-second bursts), and a sonicator equipped with a conical tip (three 30-second bursts). The homogenates were then mixed with 1.5 ml of 80% sucrose solution. The same amount of proteins (concentration measured using a BCA kit) were loaded into the bottom of each centrifuge tube, overlaid with 4.5 ml of 35% sucrose and 4.5 ml of 5% sucrose, and centrifuged at 180,000 X g in a Beckman SW 41 rotor for 16 h at 4°C. All sucrose solutions were prepared in MES-buffered saline containing protease inhibitors. After centrifugation, 12 fractions of 1 ml each were collected and mixed with Laemmli buffer before boiling for immunoblotting.
D5R gene-deficient (D5−/−) mice
D5−/− mice and their wild-type littermates (D5+/+) mice have increased blood pressure, due in part to oxidative stress, caused by increased expression of NOX subunits and phospholipase D2 (PLD2), and decreased expression of hemeoxygenase 1 (HO-1) [51,52,56]. Six to eight-month old male mice were genotyped using a PCR-based protocol; genomic DNA was isolated from tail biopsies and renal tissue using standard methods. The mice were sacrificed by pentobarbital (100 mg/kg), and the kidneys were harvested and stored at −70°C for subsequent study
RNA interference
The cells were seeded in 6-well plates, cultured to 60 – 70% confluence, and transfected for 48 h with predesigned Flexi-Tube siRNA (Qiagen), targeting human PON2 mRNA (10 nM) or human D5R- siRNA (5 nM) using HiPerFect (Qiagen), following the manufacturer’s instructions. AllStars siRNA (Qiagen) was used as scrambled (non-silencing) siRNA.
Immunoblotting
The cell lysates and mouse kidney homogenates were subjected to immunoblotting, as reported previously [57]. Equal amounts of protein were separated using SDS-PAGE and transferred onto nitrocellulose membranes. For immunoblotting of the sucrose gradient fractions, the twelve-fraction samples collected from each tube were loaded in precast 26-well 4 – 20% gradient gels and transferred onto membranes. The membranes were probed with anti-PON2, anti-D1R, anti-D5R, anti-NOX2, and anti-β-actin antibodies, then exposed to HRP-conjugated secondary antibodies. The immunoreactive bands were quantified by the NIH ImageJ program.
Co-immunoprecipitation
HEK-hD1R and HEK-hD5R cells were treated with fenoldopam (1 µM, 15 min), then lysed using buffer containing MBS [MES (25 mM, pH 6.7), NaCl (150 mM), EDTA (5 mM), PMSF (1 mM), DTT (5 mM), protease inhibitors] and noctyl-β-D-glucopyranoside buffer. The cell lysates (protein amount 300 µ g) were mixed with anti-D1R or anti-D5R antibodies (1 µg), by rocking at 4°C overnight. Then, protein G beads (50 µl) were added to each sample, and mixed by rocking for 2 h at room temperature. The immunocomplexes were washed and pelleted, and then eluted with 40 µl of Laemmli buffer, and boiled for 5 min. The samples were immunoblotted with anti-PON2 antibody.
RNA extraction and quantitative RT-PCR
Total RNA was extracted from HEK-hD1R and HEK-hD5R cells using the RNeasy Mini Kit (Qiagen). Total RNA samples were converted into first strand cDNA using RT First Strand Kit (Qiagen). Real-time PCR was performed using predesigned QuantiTect primers (Qiagen) for PON2 or GAPDH and SYBR Green PCR Master Mix (Qiagen). All measurements were performed as described in the manufacturer’s manuals, and in triplicate, to ensure reproducibility.
Immunofluorescence and confocal microscopy
In the kidney tissue immunofluorescence experiments, the sections (3 µm) of formalin-fixed, paraffin-embedded mouse kidney slices were deparaffinized in xylene, and rehydrated in step-down concentrations of ethanol, then subjected to antigen retrieval using citric acid buffer (10 mM, pH 6.0), and blocked with 5% goat serum. In the cell immunofluorescence experiments, after treatment with the indicated drugs, the coverslips inoculated with cells were fixed with 3% formaldehyde. The rabbit anti-PON2 antibody was directly labeled using the Mix-n-Stain™ CF488A antibody labeling kit. D1R and D5R were visualized using rabbit anti-D1R and anti-D5R antibody, respectively, followed by Alexa Fluor® 568-goat anti-rabbit IgG antibody. For the negative control, the primary antibodies were replaced by normal serum at the appropriate dilution. The fluorescence images were obtained using laser confocal scanning microscopy (Zeiss LSM 510) at excitation and emission wavelengths of 579/603 nm and 499/519 nm, respectively.
Measurement of NOX activity
Membrane proteins from HEK-hD1R, HEK-hD5R, and hRPT cells were prepared as reported previously [36]. Briefly, the cells were treated with drugs, then pelleted after centrifugation (1000 × g, 2 min) at 4°C, washed with PBS, and resuspended in lysis buffer (pH 7.4) containing Tris-HCl (25 mM), EDTA (1 mM), and EGTA (1 mM), along with protease inhibitors. Then, the cell suspensions were sonicated and centrifuged at 1000 ×g, for 10 min. The supernatants were collected, the pellets were resuspended in lysis buffer, and subjected to sonication and recentrifugation. The supernatants were combined and centrifuged at 425,000 × g, for 60 min. Then, the pellets were resuspended in the assay buffer containing KCl (10 mM), NaCl (150 mM), MgCl2 (2 mM), EDTA (1 mM), triethanolamine (50 mM), sodium phosphate (10 mM), potassium phosphate (2 mM), and protease inhibitors. NOX activity was measured (in triplicate) in the presence of lucigenin (5 µ M) and NADPH (100 µM). The specificity of the NOX activity was confirmed by treatment of the cells with a NOX inhibitor, diphenyleneiodonium chloride (DPI).
Detection of reactive oxygen species
Intracellular ROS were assayed (in duplicate) by quantifying the oxidation of 2’, 7’-dichlorofluoresce in diacetate (DCFDA). Briefly, cells were seeded in 96-well plates and grown to 90% confluence. The cells were incubated with freshly prepared DCFDA (10 µ M/30 min) at 37°C. Fluorescence was measured using a microplate reader at an excitation wavelength of 485 nm and an emission wavelength of 530 nm. ROS production was expressed in arbitrary units corrected by protein concentration (arbitrary units/per mg protein).
Statistical analysis
Data are expressed as mean ± SEM. A significant difference between two groups was determined by the Student’s t-test, whereas that among three or more groups was determined by one-way factorial ANOVA, followed by the Newman–Keuls post hoc test, as indicated. A value of P < 0.05 was considered significant.
Results
D1 R and D5R colocalize and physically interact with PON2
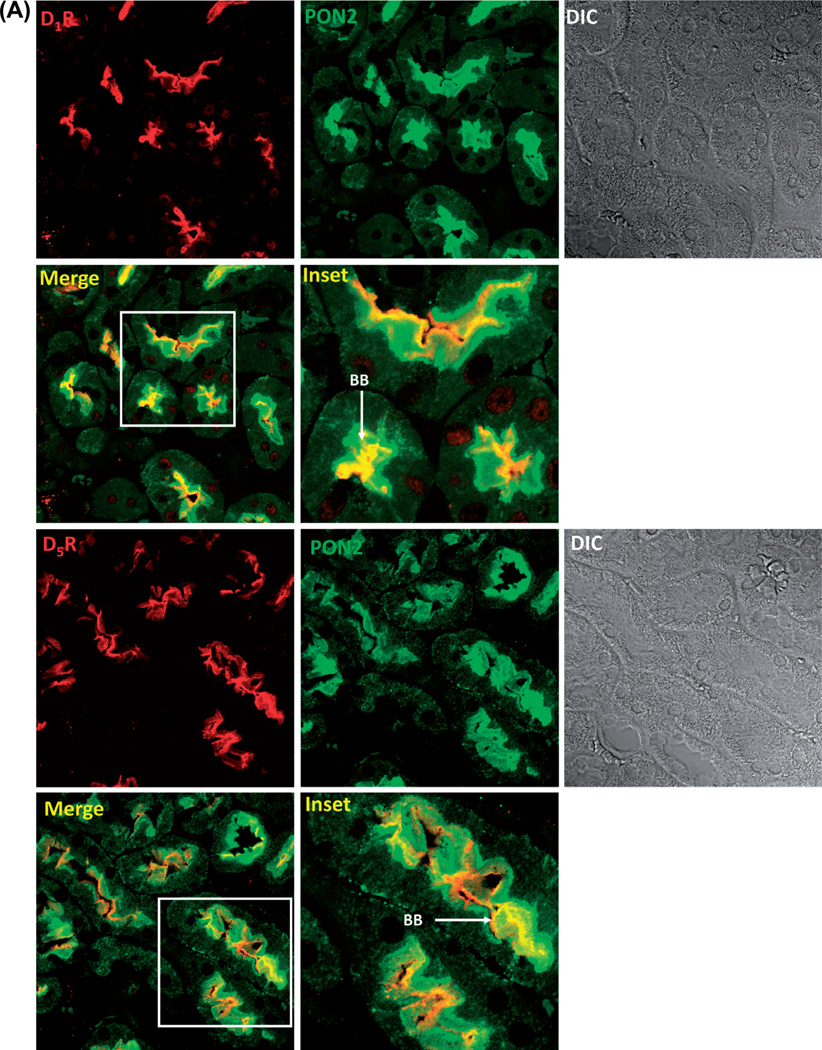
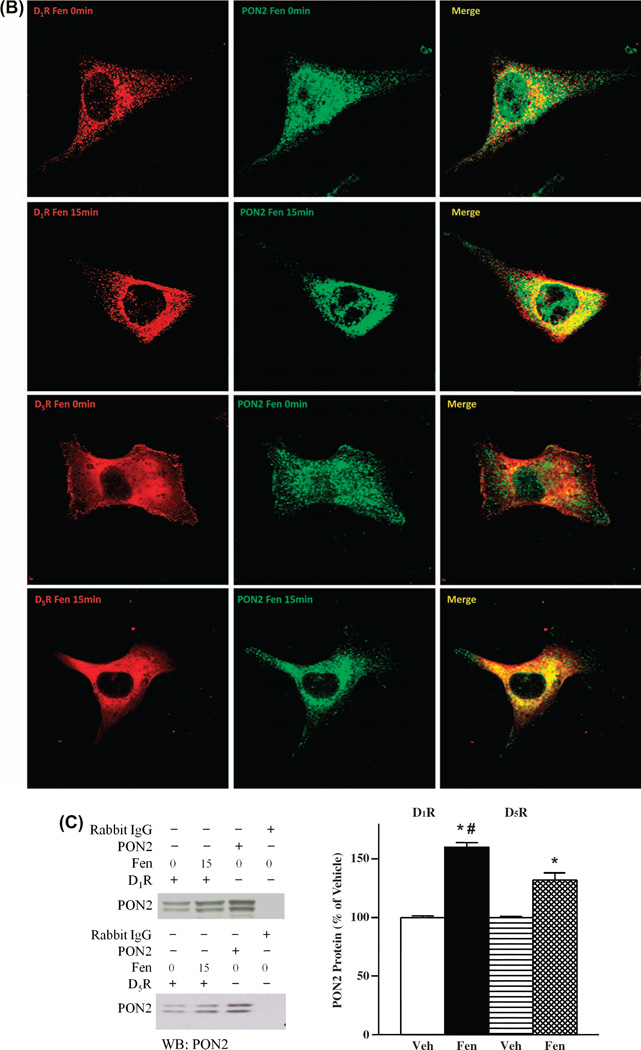
We first determined whether there was colocalization between D1R and PON2 and D5R and PON2 in the mouse kidney, using laser confocal microscopy. We found that D1R or D5R colocalized with PON2 in the brush borders of RPTs in the mouse kidney (Figure 1A). We next determined the cellular distribution of D1R and PON2 in hRPT cells. Using confocal immunofluorescence microscopy, we found that in the basal state, D1R was expressed at the plasma membrane and in the cytoplasm, while PON2 was found mainly in the cytoplasm; there was some colocalization of D1R and PON2 in the cytoplasm. Treatment with the D1-like dopamine receptor agonist fenoldopam (Fen, 1 µM, 15 min) promoted the internalization of some D1Rs and increased their colocalization with PON2 in the cytoplasm. In the basal state, D5R, as with D1R, was also localized in both the plasma membrane and the cytoplasm; there was also some colocalization between D5R and PON2 in the cytoplasm. Fenoldopam treatment (Fen, 1 µM, 15 min) also promoted the internalization of some D5Rs and increased their colocalization with PON2 in the perinuclear area (Figure 1B).
Figure 1.
Colocalization of and interaction between PON2 and D1R or D5R. (A). D1-like receptors, D1R and D5R, colocalize with PON2 in the mouse kidney. Formalin-fixed, paraffin-embedded mouse kidney sections were studied for the colocalization of D1R (red) and PON2 (green), as well as D5R (red) and PON2 (green), by confocal microscopy. The colocalization is shown as yellow in brush border membranes (BB) in the merge images. DIC = differential interference contrast. (B). D1-like receptors, D1R and D5R, colocalize with PON2 in hRPT cells. D1R (red) and D5R (red) are expressed at the plasma membrane and cytosol, while PON2 (green) is mainly distributed in the cytosol. Fenoldopam treatment (1 µM, 15 min) increased the colocalization of D1R and PON2, as well as D5R and PON2, primarily in the cytosol, shown as yellow in the merge images. Images are representative of three separate experiments. (C). D1R and PON2 and D5R and PON2 physically interact in HEK-hD1R and HEK-hD5R cells, respectively. The cells were treated with vehicle (Veh) or fenoldopam (Fen, 1 µM) for 15 min. The cell lysates were immunoprecipitated with anti-D1R, anti-D5R, and anti-PON2 (positive control) antibodies and normal rabbit IgG (negative control). The immunocomplexes were then immunoblotted with PON2 antibody. The blots are representative of one of four separate experiments. Values are mean ± SEM, *P < 0.05 vs Veh (control), #P < 0.05 vs others, the one-way factorial ANOVA, and the Newman–Keuls test.
We also performed the same immunofluorescence microscopy studies in HEK-hD1R and HEK-hD5R cells. We found fenoldopam (Fen, 1 µM, 15 min) also increased the colocalization in the cytosol of D1R with PON2 in HEK-hD1R cells, as well as D5R with PON2 in HEK-hD5R cells (data not shown), which is consistent with the results obtained using hRPT cells. There was also physical interaction between D1R and PON2, as well as D5R and PON2, because D1R and PON2, as well as D5R and PON2, co-immunoprecipitated in homogenates of HEK-hD1R, and HEK-hD5R, respectively. Fenoldopam (1 µM, 15 min) increased PON2 co-immunoprecipitation with D1R in HEK-hD1R cells (160.0 ±4.0% vs 100.0 ±1.4%, vehicle) and with D5R in HEK-hD5R cells (132.3 ±6.1% vs 100.0 ± 1.2%, vehicle) (P < 0.05, n = 4) (Figure 1C).
D1R and D5R decrease ROS production in HEK-hD1R and HEK-hD5R cells
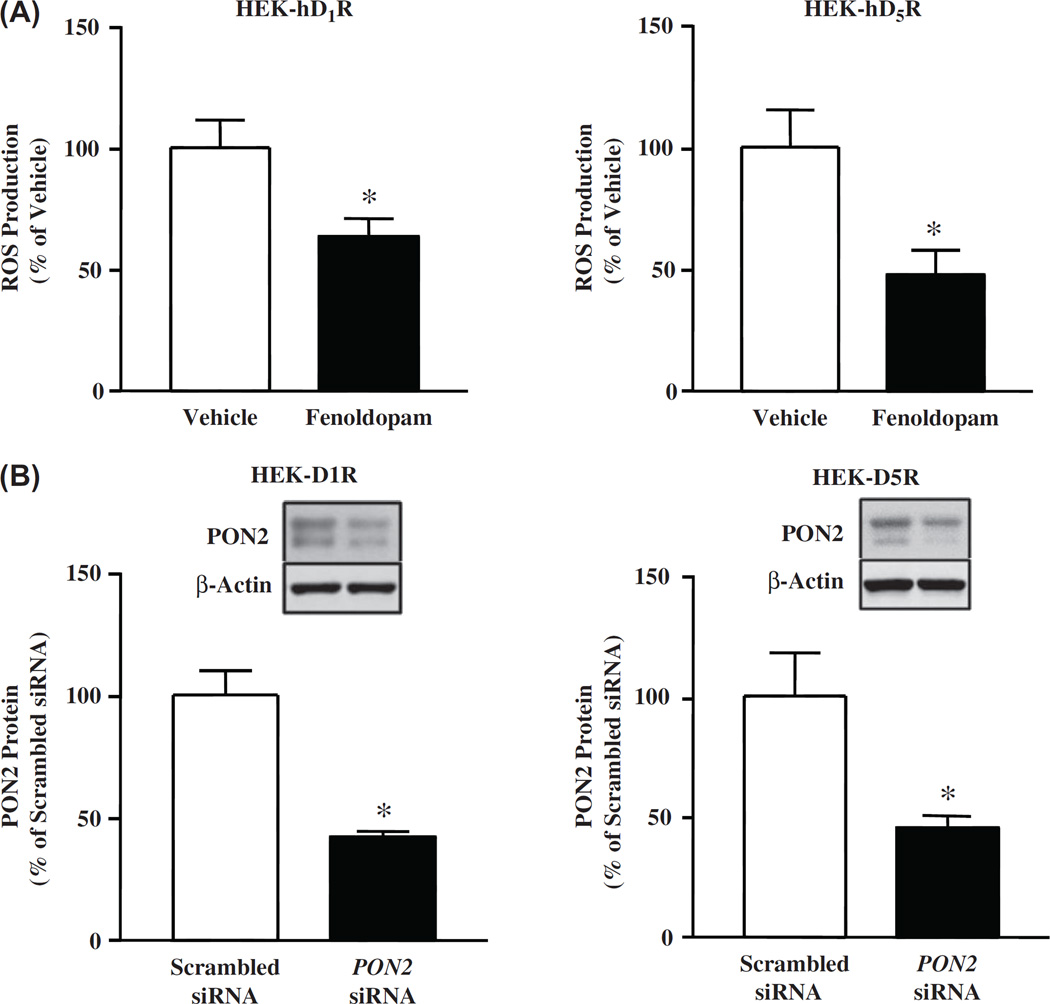
The next series of experiments were performed to confirm our previous reports [52,58] that fenoldopam decreased ROS production in HEK-hD1R and HEK-hD5R cells. In agreement with our previous reports, fenoldopam (1 µM, 15 min) decreased ROS production in HEK-hD1R (63.6 ± 6.7% vs 100.0 ± 11.4%, vehicle) and HEK-hD5R cells (47.9 ±9.8% vs 100 ±15.2%, vehicle) (Figure 2A).
Figure 2.
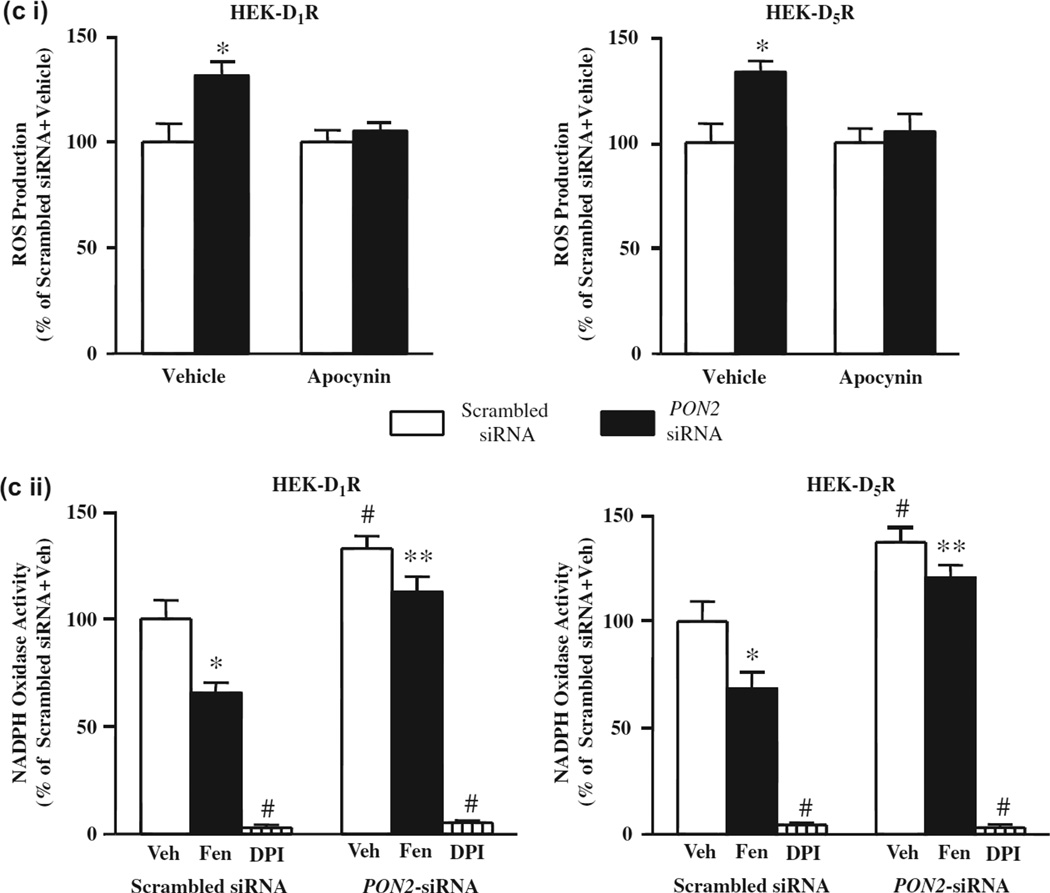
Role of PON2 in the inhibitory effect of D1R and D5R on oxidative stress. (A). Fenoldopam decreases ROS production in HEK-hD1R and HEK-hD5R cells. The cells were treated with vehicle, which served as control, or fenoldopam (1 µM, 15 min). ROS production was measured using DCFDA, normalized by protein concentration. The vehicle was assigned a value of 100%. Data are mean ± SEM, n = 4/group, *P < 0.05 vs vehicle, t-test. (B). Decrease in PON2 protein, normalized by β-actin, in HEK-hD1R and HEK-hD5R cells transfected with PON2-siRNA (10 nM), but not scrambled siRNA (10 nM), for 48 h; Scrambled non-silencing siRNA was assigned a value of 100%. The data, normalized by β-actin, are shown as mean ± SEM, n = 3/group, *P < 0.05 vs scrambled siRNA, t-test. The inserted blots are representative one of three immunoblots. (C). Decreasing PON2 expression increases ROS production, via an increase in NOX activity, in HEK-hD1R and HEK-hD5R cells, (i) PON2 silencing increases ROS production that is blocked by the NOX inhibitor apocynin. HEK-hD1R and HEK-hD5R cells transfected with PON2-siRNA (10 nM) or scrambled siRNA (10 nM) for 48 h were treated with apocynin (10 µM) or vehicle for 60 min. ROS production was measured using DCFDA. Scrambled siRNA was assigned a value of 100%. The data, normalized by protein concentration, are shown as mean ± SEM, n = 6/group. *P < 0.05 vs others, one-way factorial ANOVA, Newman–Keuls test, (ii) PON2 silencing increases NOX activity and impairs the inhibitory effect of fenoldopam. HEK-hD1R and HEK-hD5R cells, transfected with PON2-siRNA (10 nM) and scrambled non-silencing siRNA (10 nM, 48 h), were treated with vehicle (Veh), fenoldopam (Fen, 1 µM, 15 min), or DPI (5 µM, 60 min). NOX activity in the cell membrane was measured in the presence of 5 µM lucigenin and 100 µM NADPH. Veh + scrambled siRNA was assigned a value of 100%. The data, normalized by protein concentration, are shown as mean ± SEM, n = 6–8/group. *P < 0.05 vs. Veh + scrambled siRNA, #P < 0.05 vs others, **P < 0.05 vs Fen + scrambled siRNA, one-way factorial ANOVA, Newman–Keuls test. DPI = diphenyleneiodonium chloride.
Silencing PON2 increases ROS production via NOX in HEK-hD1R and HEK-hD5R cells
Our group has reported that PON2-siRNA-induced silencing of PON2 in the mouse kidney and hRPT cells in culture increased renal ROS production [34]. To evaluate the role of PON2 in the redox status of HEK-hD1R and HEK-hD5R cells, we decreased PON2 expression with PON2-siRNA (10 nM, 48 h). Relative to scrambled non-silencing siRNA, PON2-siRNA decreased PON2 expression to a similar extent in HEK-hD1R (42.4 ± 2.2% vs 100.0 ± 10.7%, scrambled siRNA) and HEK-hD5R (45.7 ±5.0% vs 100.0 ±18.1%, scrambled siRNA) cells (Figure 2B). PON2-siRNA increased basal ROS production to a similar extent in HEK-hD1R (131.3 ±6.0% vs 100.0 ±8.7%, scrambled siRNA) and HEK-hD5R (133.5 ±5.2% vs 100 ±9.0%, scrambled siRNA) cells (Figure 2Ci). The increase in ROS production caused by the decrease in PON2 expression was probably related to increased NOX activity, because the NOX inhibitor apocynin (10 µM) completely reversed the increased ROS production in both cells transfected with PON2-siRNA (Figure 2Ci).
PON2 partially mediates the inhibitory effect of D1R and D5R on NOX activity in HEK-hD1R and HEK-hD5R cells
We have reported that short-term fenoldopam stimulation (≤60 min) decreased NOX activity in HEK-hD1R and HEK-hD5R cells [52,58]. To confirm the role of PON2 in the inhibitory effect of short-term fenoldopam (1 µM, 15 min) stimulation on NOX activity, NOX activity was measured in cells transfected with PON2-siRNA. PON2-siRNA increased basal NOX activity to a similar extent in HEK-hD1R and HEK-hD5R cells (HEK-hD1R: 132.8 ± 5.7% vs 100.0 ± 8.7%, HEK-hD5R: 137.3 ± 7.0% vs 100.0 ± 5.1%) (Figure 2Cii). The inhibitory effect of fenoldopam on NOX activity was reduced to a similar extent in PON2-siRNA-transfected HEK-hD1R and HEK-hD5R cells (HEK-hD1R: −34.4% ±5.4 vs - 15.2 ± 5.1%, PON2-siRNA; HEK-hD5R - 31.5 ± 4.9% vs - 12.1 ± 2.9%, PON2-siRNA). Thus, the inhibitory effect of short-term fenoldopam stimulation on NOX activity, which was similar in HEK-hD1R and HEK-hD5R cells, was, in part, via PON2. DPI, another NOX inhibitor, abolished NOX activity in both cells, indicating the specificity of the assay for NOX activity (Figure 2Cii) [59].
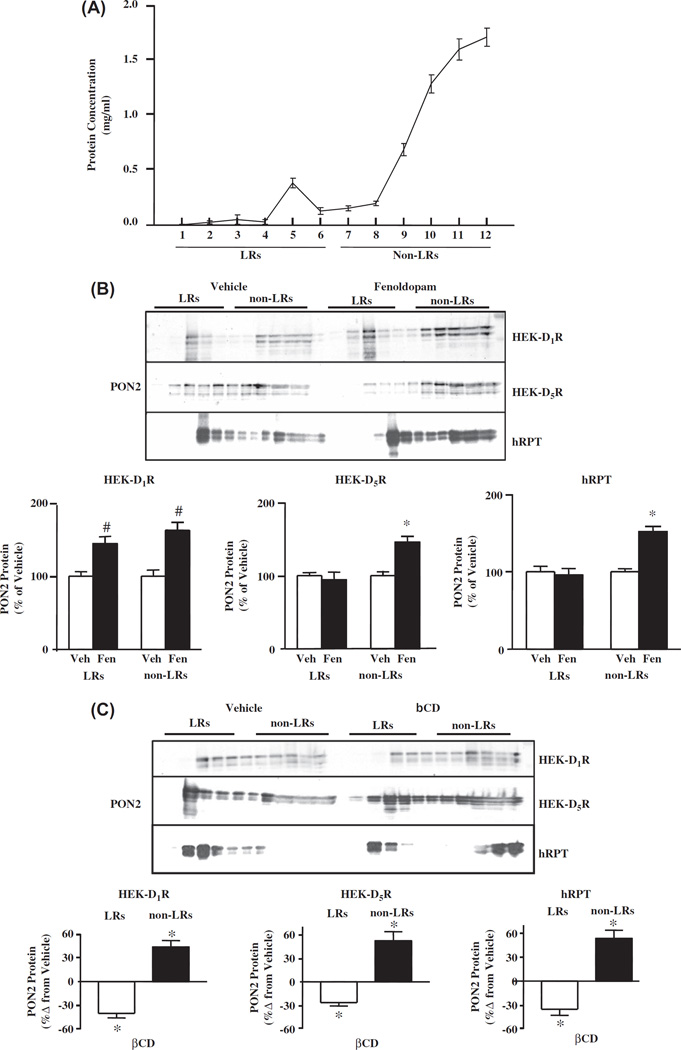
Sucrose gradient analysis of PON2 protein in HEK-hD1R, HEK-hD5R, and hRPT cells
To determine whether the inhibitory effect of PON2 on NOX activity is influenced by membrane microdomains, we analyzed the membrane distribution of PON2 in kidney cells. LRs are characterized by their relative insolubility in non-ionic detergents, such as sodium carbonate, and light buoyant density on sucrose gradient [55,60]. This method allows the separation of LRs from the bulk of cellular membranes and cytosolic proteins. LRs are best recovered in the low-density sucrose fractions (fractions 1–6); the high-density fractions (7–12) contain non-LRs [55,60]. In hRPT cells, less than 10% of the membrane proteins were found in LRs (Figure 3A), similar to that reported in HEK-hD1R and hRPT cells [36,50]. The distribution of PON2 in LRs and non-LRs of HEK-hD1R, HEK-hD1R, and hRPT cells are shown in Table I and Figure 3B. In vehicle-treated cells, the distribution of PON2 in LRs was 37.4 ±2.1%, 41.3 ± 4.9%, and 45.0 ± 2.4% in HEK-hD1R, HEK-hD5R, and hRPT cells, respectively. Fenoldopam treatment (1 µM, 15 min) of HEK-hD1R cells increased PON2 protein in both LRs (144.5 ± 9.3% vs 100.0 ± 6.3%, vehicle) and non-LRs (162.9 ±11.2% vs 100.0 ±8.7%, vehicle). By contrast, in HEK-hD5R cells, fenoldopam increased PON2 protein only in non-LRs (145.9 ±7.8% vs 100.0 ±5.2%, vehicle); expression in LR was not affected (94.4 ± 10.3% vs 100.0 ±4.0%, vehicle) (Figure 3B), indicating that fenoldopam induced the recruitment of PON2 to non-LRs in HEK-hD5R cells. The effect of fenoldopam on PON2 distribution in LRs and non-LRs in hRPT cells was similar to that found in HEK-hD5R cells; fenoldopam increased PON2 protein only in non-LRs (151.8 ±6.7% vs 100.0 ± 3.9%, vehicle) (Figure 3B).
Figure 3.
Localization of PON2 in membrane LRs and non-LRs (A). Protein concentrations of sucrose gradient fractions of hRPT cells. LRs are in fractions 1 to 6, and non-LRs are in fractions 7 to 12. (B). Effect of fenoldopam on the expression of PON2 protein in LRs and non-LRs of HEK-hD1R, HEK-hD5R and hRPT cells. HEK-hD1R, HEK-hD5R and hRPT cells were treated with vehicle (Veh, which served as control) or fenoldopam (Fen, 1 µM, 15 min), and then subjected to sucrose gradient centrifugation, as described in “Methods”. The blots are representative of one of five to six separate experiments. Veh was assigned a value of 100%. The data are mean ± SEM, n = 5–6/group, #P < 0.05 vs Veh, HEK-D1R, *P < 0.05 vs others, one-way factorial ANOVA, Newman–Keuls test. (C). Effect of methyl-β-cyclodextrin (βCD) on PON2 protein in LRs and non-LRs of HEK-hD1R, HEK-hD5R and hRPT cells. HEK-hD1R, HEK-hD5R and hRPT cells were treated with vehicle (which served as control) or βCD (2%) for 1 h, then subjected to sucrose gradient centrifugation, as described in “Methods”. The blots are representative of three to four separate experiments. The percent change from vehicle was assigned a value of 0%. The data are shown as mean ± SEM, n = 3–4/group, *P < 0.05 vs vehicle, one-way factorial ANOVA, Newman–Keuls test.
Table I.
Distribution of the PON2 protein in LRs and non-LRs.
| Cell type | LRs (%) | Non-LRs (%) |
|---|---|---|
| HEK-hD1R | 37.4 ± 2.1* | 62.6 ± 2.1 |
| HEK-hD5R | 41.3 ± 4.9* | 58.7 ± 4.9 |
| hRPT | 45.0 ± 2.4* | 55.0 ± 2.4 |
Proteins from sucrose gradient fractions were probed with PON2 antibody and quantified, as described in ‘Methods’. The results are expressed as percentage of lipidrafts (LRs) + non-LRs, set as 100%. The values are mean ± SEM from 5–6 experiments, t-test,
P < 0.05, LRs vs non-LRs.
Effect of methyl-β-cyclodextrin (βCD) on PON2 distribution in membrane microdomains
To confirm the expression of PON2 in LRs, βCD was used to deplete the plasma membranes of cholesterol [36,44]. βCD treatment shifted PON2 protein expression from LRs to non-LRs in HEK-hD1R, HEK-hD5R, and hRPT cells (Figure 3C).
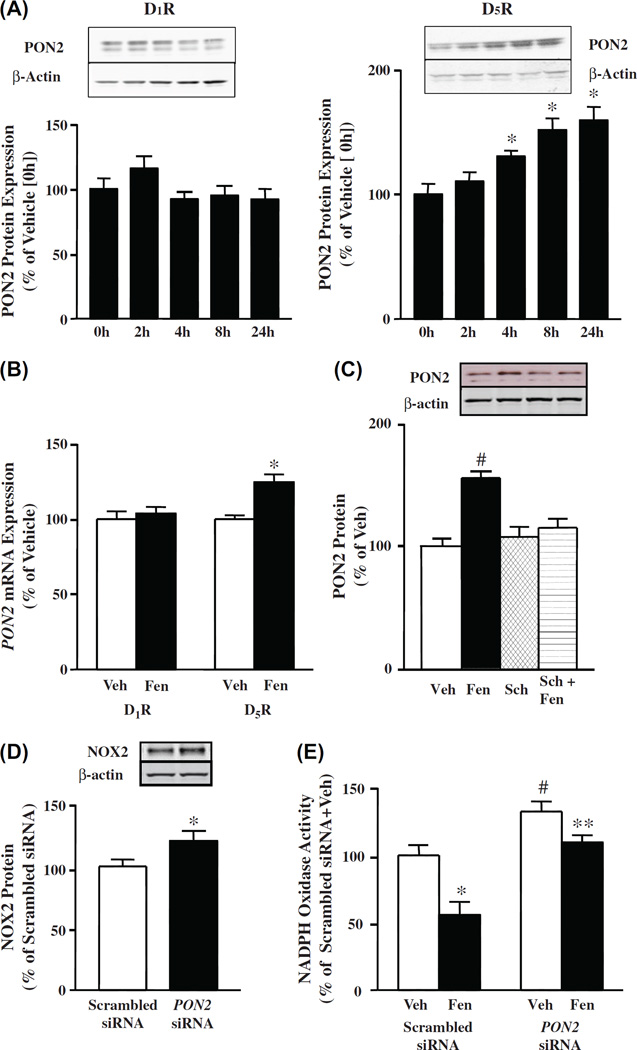
Effect of long-term stimulation of D1R and D5R on PON2 protein in HEK-hD1R and HEK-hD5R cells
To determine the effect of long-term fenoldopam treatment on PON2 protein expression, HEK-hD1R and HEK-hD5R cells were treated with vehicle or fenoldopam (1 µM) for 2–24 h. Fenoldopam increased PON2 protein expression in a time-dependent manner (130.6 ±4.4%/4 h, 151.8 ±9.3%/8 h, and 159.7 ± 10.6%/24 h vsl00.0± 8.5%, vehicle) in HEK-hD5R cells (Figure 4A, right graph), but had no effect in HEK-hD1R cells (Figure 4A, left graph). Fenoldopam also increased PON2 mRNA (124.7 ±4.9% vs 100.0 ±2.6%) at 24 h in HEK-hD5R cells (Figure 4B), similar to the effect observed with D2R stimulation [34], but not in HEK-hD1R cells (Figure 4B). The fenoldopam-induced increase in PON2 protein in HEK-hD5R cells was completely prevented by the D,-like receptor antagonist SCH23390 (Sch), (in the absence of D1R, Sch is a specific D5R antagonist), which by itself had no effect on PON2 protein expression (Figure 4C). Therefore, the D5R, but not the D1R, can regulate PON2 expression in the long-term. Similar studies were also performed in hRPT cells (see below).
Figure 4.
Effect of long-term stimulation of D1R and D5R on PON2 protein in HEK-hD1R and HEK-hD5R cells. (A). Time course of the effect of fenoldopam on PON2 protein in HEK-hD1R and HEK-hD5R cells. The cells were treated with fenoldopam (1 µM) at varying durations (0, 2, 4, 8, 24 h) as described in “Methods”. Zero h (0 h) time was assigned a value of 100%. The data, normalized by β-actin, are shown as mean ± SEM, n = 4–6/group; *P < 0.05 vs control (0 h), one-way factorial ANOVA, Newman–Keuls test. (B). Effect of fenoldopam on PON2 mRNA expression in HEK-hD1R and HEK-hD5R cells. The cells were treated with fenoldopam (Fen, 1 µM) for 24 h. mRNA was prepared from the cell pellets, as described in “Methods”. Vehicle (Veh, control) was assigned a value of 100%. The data, normalized by GAPDH, are shown as mean ± SEM, n = 6/group, *P < 0.05 vs Veh, one-way factorial ANOVA, Newman–Keuls test. (C). Effect of fenoldopam and D1-like receptor antagonist Sch 23390 on PON2 protein in HEK-hD5R cells. The cells were treated with vehicle (Veh, which served as control, 24 h), fenoldopam (Fen, 1 µM, 24 h), Sch23390 (Sch, 1 µM, 24 h), or pretreated with Sch (1 µM, 1 h) and then incubated with Fen (1 µM, 24 h) (Sch + Fen). Veh was assigned a value of 100%. The data, normalized by β-actin, are shown as mean ± SEM, n = 4/group, #P < 0.05 vs others, one-way factorial ANOVA, Newman–Keuls test. (D). Effect of silencing PON2 on NOX2 protein in HEK-hD5R cells. Cells were transfected with PON2-siRNA (10 nM) or scrambled siRNA (10 nM) for 48 h. Scrambled siRNA was assigned a value of 100%. The data, normalized by β-actin, are shown as mean ± SEM, n = 3/group, compared with scrambled siRNA assigned a value of 100%, *P < 0.05 vs scrambled siRNA, t-test. (E). Effect of silencing of PON2 on NOX activity in HEK-hD5R cells. Cells were transfected with PON2-siRNA (10 nM) or scrambled non-silencing siRNA (10 nM) for 48 h. The cells were then treated with fenoldopam (Fen, 1 µM) or vehicle (Veh) for 24 h. Membrane NOX activity was measured in the presence of 5 µM lucigenin and 100 µM NADPH. Veh + scrambled siRNA was assigned a value of 100%. Data, normalized by protein concentration, are shown as mean ± SEM, n = 6/group, *P < 0.05 vs Veh + scrambled siRNA, #P < 0.05 vs others, **P < 0.05 vs Fen + scrambled siRNA, one-way factorial ANOVA, Newman–Keuls test.
Silencing PON2 increases NOX2 protein in HEK-hD5R cells
We have reported that D1-like receptors [36,46,52] and D2R [34] inhibit NOX activity and NOX protein isoform expression, including NOX2 and NOX4. We have also reported that NOX2 and NOX4 proteins were increased in D5−/− mice, relative to the wild-type littermates [52]. In addition, we have reported that NOX2 protein was decreased in HEK-hD5R cells with long-term (24 h) fenoldopam treatment [52]. Long-term (24 h) treatment of hRPT cells with the D2R/D3R agonist quinpirole also decreased NOX2 and NOX4 protein expressions [34]. We now show that PON2 can also regulate NOX2 protein expression in HEK-hD5R cells, because PON2-siRNA, which decreased PON2 protein expression (45.7 ± 5.0% vs 100.0 ± 18.1%, vehicle) (Figure 2B), increased NOX2 protein (120.6 ±8.4% vs 100.0 ±5.7%, vehicle) (Figure 4D). Therefore, PON2 constitutively inhibits NOX2 protein expression.
PON2 partially mediates the long-term inhibitory effect of D5R on NOX activity in HEK-hD5R cells
We next determined whether PON2 is involved in the long-term inhibitory effect of D5R on NOX activity by silencing the PON2 gene in HEK-hD5R cells. The NOX activity in scrambled siRNA-transfected cells treated with fenoldopam (1 µM/24 h) was 55.8 ±9.6% vs 100.0 ±7.2%, vehicle; in PON2-siRNA-transfected cells, NOX activity was increased in vehicle-treated cells (132.4 ±7.5%) and decreased (109.6 ±5.4%) with fenoldopam treatment (1 µM/24 h) (Figure 4E). The inhibitory effect of fenoldopam on NOX activity was lower in PON2-siRNA-transfected cells (− 17.2 ± 4.1%) than the effect in scrambled siRNA-transfected cells (−44.2 ± 9.6%). Thus, PON2-siRNA partially reversed the inhibitory effect of fenoldopam on NOX activity by 63.3%. However, PON2-siRNA did not completely abolish the inhibitory effect of long-term (24 h) fenoldopam treatment on NOX activity, which may be attributed to other D5R-mediated antioxidant mechanisms, such as stimulation of HO-1 [56] and inhibition of PLD2 [51,52].
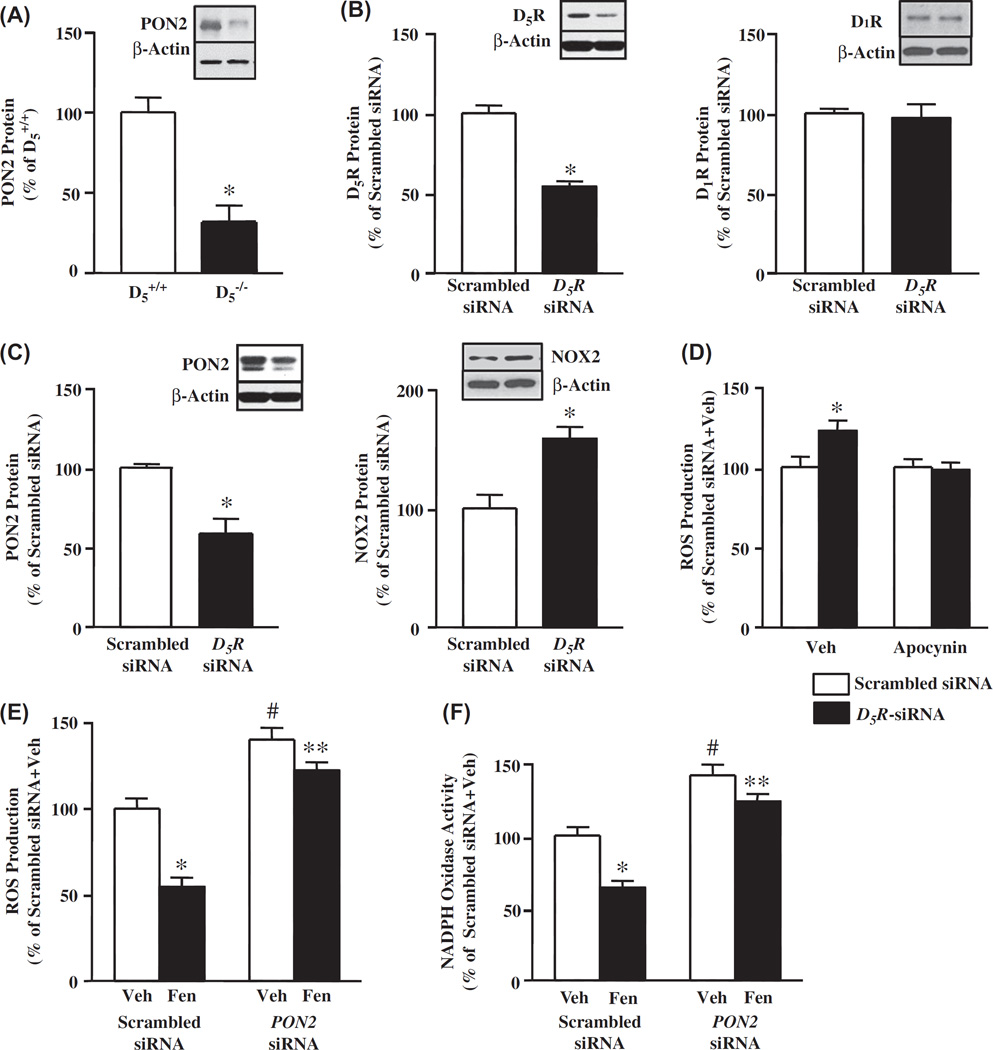
PON2 protein expression is decreased in D5−/− mice
To evaluate the relevance of the interaction between D5R and PON2 in vivo, we quantified renal PON2 protein in D5−/− mice and found that PON2 protein was decreased in D5−/− mice relative to D5+/+ mice (31.8 ± 10.2% vs 100.0 ± 9.1%, D5+/+) (Figure 5A).
Figure 5.
Effect of disruption of the D5R gene (Drd5) in mice, or silencing of Drd5 in hRPT cells, on PON2 and NOX2 protein and ROS production. (A). Renal PON2 protein is decreased in D5−/− mice relative to their wild-type (D5+/+) littermates. Renal cortical homogenates were immunoblotted with PON2 antibody. Data from D5+/+ mice were given a value of 100%. Data, normalized by β-actin, are shown as mean ± SEM, n = 4–6/group; *P < 0.05 vs D5+/+ mice, t-test. The blot is representative of four to six experiments. (B). Effect of silencing Drd5 on D5R and D1R proteins in hRPT cells. Cells were transfected with human D5R-siRNA (5 nM) or scrambled siRNA (5 nM) for 48 h. Scrambled siRNA was assigned a value of 100%. Data, normalized by β-actin, are shown as mean ± SEM, n = 3–4/group, *P < 0.05 vs scrambled siRNA, t-test. The blots are representative of three to four separate experiments. (C). Effect of silencing Drd5 on PON2 and NOX2 protein expression in hRPT cells. Cells were transfected with human D5R-siRNA (5 nM) or scrambled siRNA (5 nM) for 48 h. Scrambled siRNA was assigned a value of 100%. Data, normalized by β-actin, are shown as mean ± SEM of 3–4/groups, *P < 0.05, t-test vs scrambled siRNA. The blot is representative of three to four separate experiments. (D). Effect of silencing of Drd5 and apocynin on ROS production in hRPT cells. The cells were transfected with D5R-siRNA (5 nM) or scrambled siRNA (5 nM) for 48 h. The cells were then treated with vehicle (Veh) or apocynin (10 µM) for 30 min. ROS production was measured using DCFDA. Vehicle + scrambled siRNA was assigned a value of 100%. Data, normalized by protein concentration, are shown as mean ± SEM, n = 6/groups, *P < 0.05 vs others, one-way factorial ANOVA, Newman–Keuls test. (E). Effect of silencing PON2 on ROS production in hRPT cells. The cells were transfected with PON2-siRNA (10 nM) or scrambled siRNA (10 nM) for 48 h. The cells were then treated with fenoldopam (Fen, 1 µM) or vehicle (Veh) for 24 h. ROS production was measured using DCFDA. Veh + scrambled siRNA was assigned a value of 100%. Data, normalized by protein concentration, are shown as mean ± SEM, n = 6–9/group; *P < 0.05 vs Veh + scrambled siRNA, #P < 0.05 vs others, **P < 0.05 vs fenoldopam + scrambled siRNA, one-way factorial ANOVA, Newman–Keuls test. (F). Effect of silencing PON2 on NOX activity in hRPT cells. Cells were transfected with PON2-siRNA (10 nM) or scrambled siRNA (10 nM) for 48 h, then treated with fenoldopam (Fen, 1 µM) or vehicle (Veh) for 24 h. Membrane NOX activity was measured in the presence of 5 µM lucigenin and 100 µM NADPH. Veh + scrambled siRNA was assigned a value of 100%. Data, normalized by protein concentration, are shown as mean ± SEM, n = 6/group, *P < 0.05 vs Veh + scrambled siRNA, #P < 0.05 vs others, **P < 0.05 vs Fen + scrambled siRNA, one-way factorial ANOVA, Newman–Keuls test.
PON2 partially mediates the inhibitory effect of D5R on ROS production in hRPT cells
Because fenoldopam increased PON2 protein in non-LRs in hRPT cell membranes, similar to the effect seen in HEK-hD5R cells, we studied the relevance of PON2 in the antioxidant effect of D5R but not D1R in hRPT cells that endogenously express both D1R and D5R. Silencing the DRD5 (D5R gene) using human D5R-siRNA decreased D5R protein (54.1 ±1.7% vs 100.0 ±4.8%, scrambled siRNA) (Figure 5B), that was associated with a decrease in PON2 protein (58.8 ± 9.3% vs 100.0 ± 2.5%, scrambled siRNA) (Figure 5C, left graph). Human D5R-siRNA treatment did not affect D1R protein expression in hRPT cells (Figure 5B).
ROS production was greater in D5R-siRNA-transfected hRPT cells (122.7 ±6.3%) than in scrambled siRNA-transfected cells (100.0 ±6.5%) (Figure 5D). Apocynin (10 µM) completely reversed the increase in ROS production in D5R-siRNA-transfected cells (Figure 5D), indicating that the antioxidant effect of D5R in hRPT cells was mediated by the inhibition of NOX activity, via PON2.
We also studied NOX2 protein expression in hRPT cells transfected with D5R-siKNA and scrambled siRNA, and found that the NOX2 protein was increased in D5R-siRNA-transfected cells (158.6 ±9.8% vs 100.0 ± 11.4%, scrambled siRNA) (Figure 5C, right graph), which could be taken to indicate that the lower expression of NOX2 mediated by PON2 participates in the antioxidant effect of D5R in hRPT cells.
ROS production was also greater in PON2-siRNA-transfected hRPT cells (139.9 ± 6.7%) than in scrambled siRNA-transfected cells (100.0 ±5.3%) (Figure 5E). PON2 protein expression was decreased (60%) in hRPT cells transfected with PON2- siRNA [34]. PON2- siRNA impaired the ability of fenoldopam (1 µM/2A h) to inhibit ROS production (−12.7 ±3.2 vs - 45.5 ± 5.7%, scrambled siRNA).
To evaluate the relevance of PON2 in the long-term inhibitory effect of D5R on NOX in hRPT cells, we measured NOX activity in hRPT cells in which the PON2 gene was silenced. Fenoldopam decreased the NOX activity in scrambled siRNA-transfected cells (64.1 ±4.7% vs 100.0 ±6.5%, vehicle) and in PON2-siRNA-transfected cells (123.7 ± 4.6% vs 141.7 ± 7.4%, vehicle) (Figure 5F). The inhibitory effect of fenoldopam on NOX activity was lower in PON2- siRNA-transfected cells than in scrambled siRNA-transfected cells (− 12.7 ± 3.3 vs - 35.9 ± 4.7%). Thus, PON2-siRNA partially weakened the inhibitory effect of fenoldopam on NOX activity by 64.6%, which is similar to the result obtained in HEK-hD5R cells.
Discussion
The current studies confirmed our previous reports that dopamine D1-like receptors decrease ROS production by inhibiting NOX activity and NOX2 protein expression in HEK-hD1R and HEK-hD5R cells [36,52,58]. The ability of D5R and D2R to decrease ROS production may also be related to their ability to increase the activity of antioxidant enzymes, HO-1 [56] and HO-2 [61], respectively. We have also reported that D2R decreases ROS production by increasing PON2 expression [34]. We now report that the ability of both D1R and D5R to decrease ROS production can also be mediated by PON2. D1R and D5R colocalize and co-immunoprecipitate with PON2 in renal epithelial cells. Silencing PON2 with PON2-siRNA increases ROS production and impairs the ability of D1R and D5R to inhibit NOX activity in HEK-hD1R and HEK-hD5R cells, respectively.
Our previous studies have demonstrated that membrane microdomains play a role in the regulation of oxidative stress by dopamine receptors, including D1R and D2R [34,36,46]. We have suggested that LRs maintain NOX in a less active state in hRPT, but not in rat RPT cells [36,46]. Stimulation of D1-like receptors dispersed NOX subunits, including NOX2 and NOX4, into LRs, and decreased NOX activity. However, any differential effect between the two D1-like receptors, D1R and D5R, was not studied. In hRPT cells, NOX2, p22phox, Rac1, and D1R have similar distribution patterns in LRs (fractions 4–6, especially fraction 5) and non-LRs (fractions 9–12) [36]. Although the D5R is distributed in cell membranes more widely than the aforementioned proteins, its expression in sucrose gradient fraction 5 is also substantial [36]. In the current studies, we found that the PON2 distribution in these sucrose gradient fractions followed the distribution of the D1R in HEK-hD1R cells and D5R in HEK-hD5R cells [36]. The ability of short-term stimulation with fenoldopam to increase the expression of PON2 in LRs in HEK-hD1R cells, which may be a mechanism by which the D1R inhibits NOX activity.
The disruption of LRs with βCD increased ROS production in hRPT cells was associated with redistribution of NOX2, NOX4, and p22phox to non-LRs [36]. In the current studies, we found that βCD increased PON2 expression in non-LRs in HEK-hD1R, HEK-hD5R, and hRPT cells. Although, this may be a mechanism by which βCD treatment can increase ROS production, supportive data are not available. However, short-term fenoldopam treatment did increase PON2 expression only in non-LRs in HEK-hD5R and hRPT cells, and yet decreased NOX activity and ROS production. These could be taken to indicate that mechanisms in addition to dispersal in membrane domains are necessary for specific protein action, but this remains to be determined. We do know that the short-term (30 min) inhibitory effect of D5R on NOX activity is not related to protein kinase A, but may be partially related to inhibition of PLD2 activity [51,52]. The mechanisms by which D5R decreases ROS production in the short-term remain to be determined.
We have reported the D2R increased PON2 mRNA and protein expression in hRPT cells [34]. In the current study, we show that the D5R, but not D1R, increased PON2 mRNA and protein expression in the long-term (hrs). We also found that PON2 constitutively inhibited NOX2 expression in HEK-hD5R cells. The ability of D5R to inhibit NOX2 expression in the long-term is in agreement with our previous report that the long-term antioxidant effect of D5R was related, in part, to inhibition to NOX2 expression [52]. However, the inhibitory effect of D5R on NOX activity was not completely abolished by PON2-siRNA, which could be attributed to the partial gene silencing effect of PON2-siRNA, or the contribution of other anti-oxidative mechanisms, such as HO-1 [56].
In summary, our study demonstrates, for the first time, the specific roles of D1R and D5R in the negative regulation of oxidative stress. Both D1R and D5R positively regulate the antioxidant activity of PON2 in the short-term by altering its expression in lipid and non-lipid membrane microdomains, while D5R, but not D1R, regulates the antioxidant activity of PON2 in the long-term by increasing its transcription. Indeed, renal PON2 expression is decreased in D5−/− mice. These results have human translational potential because both D1R and D5R colocalize in the plasma membrane of hRPT cells, and silencing of PON2 in hRPT cells also impairs the antioxidant effect of fenoldopam, the long-term effect of which may be dependent on D5R. Whether or not D1-like receptors participate in the redox status and hypertension associated with silencing of PON2 gene, remains to be determined[34].
Acknowledgments
These studies were supported in part by grants from the National Natural Science Foundation of China (31130029, 81100500), and grant from the US National Institutes of Health, 5P01HL074940.
Footnotes
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med. 2003;228:134–142. doi: 10.1177/153537020322800202. [DOI] [PubMed] [Google Scholar]
- 2.Zeng C, Zhang M, Asico LD, Eisner GM, Jose PA. The dopaminergic system in hypertension. Clin Sci. 2007;112:583–597. doi: 10.1042/CS20070018. [DOI] [PubMed] [Google Scholar]
- 3.Armando I, Villar VA, Jose PA. Dopamine and renal function and blood pressure regulation. Compr Physiol. 2011;1:1075–1117. doi: 10.1002/cphy.c100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 5.Asghar M, Tayebati SK, Lokhandwala MF, Hussain T. Potential dopamine-1 receptor stimulation in hypertension management. Curr Hypertens Rep. 2011;13:294–302. doi: 10.1007/s11906-011-0211-1. [DOI] [PubMed] [Google Scholar]
- 6.Harris RC. Abnormalities in renal dopamine signaling and hypertension: the role of GRK4. Curr Opin Nephrol Hypertens. 2012;21:61–65. doi: 10.1097/MNH.0b013e32834de2cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soares-da-Silva P, Pestana M, Ferreira A, Damasceno A, Polonia J, Cerqueira-Gomes M. Renal dopaminergic mechanisms in renal parenchymal diseases, hypertension, and heart failure. Clin Exp Hypertens. 2000;22:251–268. doi: 10.1081/ceh-100100075. [DOI] [PubMed] [Google Scholar]
- 8.Carey RM. Theodore Cooper Lecture: Renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure. Hypertension. 2001;38:297–302. doi: 10.1161/hy0901.096422. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Cuevas S, Asico LD, Escano C, Yang Y, Pascua AM, et al. Deficient dopamine D2 receptor function causes renal inflammation independently of high blood pressure. PLoS One. 2012;7:e38745. doi: 10.1371/journal.pone.0038745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Armando I, Yu P, Escano C, Mueller SC, Asico L, et al. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Invest. 2008;118:2180–2189. doi: 10.1172/JCI33637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forman HJ, Torres M. Reactive oxygen species and cell signaling. Am J Respir Crit Care Med. 2002;166:S4–S8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 12.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datla SR, Griendling KK. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension. 2010;56:325–330. doi: 10.1161/HYPERTENSIONAHA.109.142422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briones AM, Touyz RM. Oxidative stress and hypertension: current concepts. Curr Hypertens Rep. 2010;12:135–142. doi: 10.1007/s11906-010-0100-z. [DOI] [PubMed] [Google Scholar]
- 16.Wilcox CS. Asymmetric dimethylarginine and reactive oxygen species: Unwelcome twin visitors to the cardiovascular and kidney disease tables. Hypertension. 2012;59:375–381. doi: 10.1161/HYPERTENSIONAHA.111.187310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuevas S, Villar VA, Jose PA, Armando I. Renal dopamine receptors, oxidative stress, and hypertension. Int J Mol Sci. 2013;14:17553–17572. doi: 10.3390/ijms140917553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Araujo M, Wilcox CS. Oxidative stress in hypertension: role of the kidney. Antioxid Redox Signal. 2014;20:74–101. doi: 10.1089/ars.2013.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sedeek M, Nasrallah R, Touyz RM, Hebert RL. NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J Am Soc Nephrol. 2013;24:1512–1518. doi: 10.1681/ASN.2012111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol. 2005;289:R913–R935. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 22.Schiffrin EL. Antioxidants in hypertension and cardiovascular disease. Mol Interv. 2010;10:354–362. doi: 10.1124/mi.10.6.4. [DOI] [PubMed] [Google Scholar]
- 23.Fontayne A, Dang PM, Gougerot-Pocidalo MA, El-Benna J. Phosphorylation of p47phox sites by PKC alpha, beta II, delta, and zeta: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- 24.Quinn MT, Gauss KA. Stucture and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidase. J Leukoc Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- 25.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, et al. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res. 2002;90:1205–1213. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- 26.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005;46:1239–1247. doi: 10.1194/jlr.M400511-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Aviram M, Vaya J. Paraoxonase 1 activities, regulation, and interactions with atherosclerotic lesion. Curr Opin Lipidol. 2013;24:339–344. doi: 10.1097/MOL.0b013e32835ffcfd. [DOI] [PubMed] [Google Scholar]
- 29.Martinelli N, Consoli L, Girelli D, Orison E, Corrocher R, Olivieri O. Paraoxonases: ancient substrate hunters and their evolving role in ischemic heart disease. Adv Clin Chem. 2013;59:65–100. doi: 10.1016/b978-0-12-405211-6.00003-6. [DOI] [PubMed] [Google Scholar]
- 30.Reddy ST, Wadleigh DJ, Grijalva V, Ng C, Hama S, Gango-padhyay A, et al. Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidized lipids. Arterioscler Thromb Vasc Biol. 2001;21:542–547. doi: 10.1161/01.atv.21.4.542. [DOI] [PubMed] [Google Scholar]
- 31.Précourt LP, Amre D, Denis MC, Lavoie JC, Delvin E, Seidman E, Levy E. The three-gene paraoxonase family: physiologic roles, actions and regulation. Atherosclerosis. 2011;214:20–36. doi: 10.1016/j.atherosclerosis.2010.08.076. [DOI] [PubMed] [Google Scholar]
- 32.Shiner M, Fuhrman B, Aviram M. A biphasic U-shape effect of cellular oxidative stress on the macrophage anti-oxidant paraoxonase 2 (PON2) enzymatic activity. Biochem Biophys Res Commun. 2006;349:1094–1099. doi: 10.1016/j.bbrc.2006.08.150. [DOI] [PubMed] [Google Scholar]
- 33.Horke S, Witte I, Wilgenbus P, Krüger M, Strand D, Forstermann U. Paraoxonase-2 reduces oxidative stress in vascular cells and decreases endoplasmic reticulum stress-induced caspase activation. Circulation. 2007;115:2055–2064. doi: 10.1161/CIRCULATIONAHA.106.681700. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, Zhang Y, Cuevas S, Villar VA, Escano C, Asico LD, et al. Paraoxonase 2 decreases renal reactive oxygen species production, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of NADPH oxidase. Free Radic Biol Med. 2012;53:437–446. doi: 10.1016/j.freeradbiomed.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li PL, Zhang Y, Yi F. Lipid raft redox signaling platforms in endothelial dysfunction. Antioxid Redox Signal. 2007;9:1457–1470. doi: 10.1089/ars.2007.1667. [DOI] [PubMed] [Google Scholar]
- 36.Han W, Li H, Villar VA, Pascua AM, Dajani MI, Wang X, et al. Lipid rafts keep NADPH oxidase in the inactive state in human renal proximal tubule cells. Hypertension. 2008;51:481–487. doi: 10.1161/HYPERTENSIONAHA.107.103275. [DOI] [PubMed] [Google Scholar]
- 37.Li PL, Zhang Y. Cross talk between ceramide and redox signaling: implications for endothelial dysfunction and renal disease. Handb Exp Pharmacol. 2013;216:171–197. doi: 10.1007/978-3-7091-1511-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burger D, Montezano AC, Nishigaki N, He Y, Carter A, Touyz RM. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/Rho kinase pathways targeted to lipid rafts. Arterioscler Thromb Vasc Biol. 2011;31:1898–1907. doi: 10.1161/ATVBAHA.110.222703. [DOI] [PubMed] [Google Scholar]
- 39.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 40.Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 41.Sowa G, Pypaert M, Sessa WC. Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc Natl Acad Sci USA. 2001;98:14072–14077. doi: 10.1073/pnas.241409998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foster LJ, De Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci USA. 2003;100:5813–5818. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lotocki G, Alonso OF, Dietrich WD, Keane RW. Tumor necrosis factor receptor 1 and its signaling intermediates are recruited to lipid rafts in the traumatized brain. J Neurosci. 2004;24:11010–11016. doi: 10.1523/JNEUROSCI.3823-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- 45.Ostrom RS, Post SR, Insel PA. Stoichiometry and compart-mentation in G protein-coupled receptor signaling: implications for therapeutic interventions involving Gs . J Pharmacol Exp Ther. 2000;294:407–412. [PubMed] [Google Scholar]
- 46.Li H, Han W, Villar VA, Keever LB, Lu Q, Hopfer U, et al. D1-like receptors regulate NADPH oxidase activity and subunit expression in lipid raft microdomains of renal proximal tubule cells. Hypertension. 2009;53:1054–1061. doi: 10.1161/HYPERTENSIONAHA.108.120642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng S, Yu P, Zeng C, Wang Z, Yang Z, Andrews PM, et al. Gα12-and Gα13-protein subunit linkage of D5 dopamine receptors in the nephron. Hypertension. 2003;41:604–610. doi: 10.1161/01.HYP.0000057422.75590.D7. [DOI] [PubMed] [Google Scholar]
- 48.Yu P, Asico LD, Luo Y, Andrews P, Eisner GM, Hopfer U, et al. D1 dopamine receptor hyperphosphorylation in renal proximal tubules in hypertension. Kidney Int. 2006;70:1072–1079. doi: 10.1038/sj.ki.5001708. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Luo Y, Escano CS, Yang Z, Asico L, Li H, et al. Upregulation of renal sodium transporters in D5 dopamine receptor-deficient mice. Hypertension. 2010;55:1431–1437. doi: 10.1161/HYPERTENSIONAHA.109.148643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu P, Yang Z, Jones JE, Wang Z, Owens SA, Mueller SC, et al. D1 dopamine receptor signaling involves caveolin-2 in HEK-293 cells. Kidney Int. 2004;66:2167–2180. doi: 10.1111/j.1523-1755.2004.66007.x. [DOI] [PubMed] [Google Scholar]
- 51.Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Bai RK, et al. D5 dopamine receptor regulation of phospholipase D. Am J Physiol Heart Circ Physiol. 2005;288:H55–H61. doi: 10.1152/ajpheart.00627.2004. [DOI] [PubMed] [Google Scholar]
- 52.Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Escano CS, et al. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R96–R104. doi: 10.1152/ajpregu.00434.2005. [DOI] [PubMed] [Google Scholar]
- 53.Felder RA, Sanada H, Xu J, Yu P, Wang Z, Watanabe H, et al. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci USA. 2002;99:3872–3877. doi: 10.1073/pnas.062694599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watanabe H, Xu J, Bengra C, Jose PA, Felder RA. Desensitization of human renal D1 dopamine receptors by G protein-coupled receptor kinase 4. Kidney Int. 2002;62:790–798. doi: 10.1046/j.1523-1755.2002.00525.x. [DOI] [PubMed] [Google Scholar]
- 55.Song KS, Li Shengwen, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains Detergent-free purification of caveolae microdomains. J Biol Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- 56.Lu Q, Yang Y, Villar VA, Asico L, Jones JE, Yu P, et al. D5 dopamine receptor decreases NADPH oxidase, reactive oxygen species and blood pressure via heme oxygenase-1. Hyper-tens Res. 2013;36:684–690. doi: 10.1038/hr.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu P, Asico LD, Eisner GM, Hopfer U, Felder RA, Jose PA. Renal protein phosphatase 2A activity and spontaneous hypertension in rats. Hypertension. 2000;36:1053–1058. doi: 10.1161/01.hyp.36.6.1053. [DOI] [PubMed] [Google Scholar]
- 58.Yu P, Han W, Villar VA, Li H, Arnaldo FB, Concepcion GP, et al. Dopamine D1 receptor-mediated inhibition of NADPH oxidase activity in human kidney cells occurs via protein kinase A-protein kinase C cross talk. Free Radic Biol Med. 2011;50:832–840. doi: 10.1016/j.freeradbiomed.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dodd-O JM, Pearse DB. Effect of the NADPH oxidase inhibitor apocynin on ischemia-reperfusion lung injury. Am J Physiol Heart Circ Physiol. 2000;279:H303–H312. doi: 10.1152/ajpheart.2000.279.1.H303. [DOI] [PubMed] [Google Scholar]
- 60.Yu P, Villar VA, Jose PA. Methods for the study of dopamine receptors within lipid rafts of kidney cells. Methods Mol Biol. 2013;964:15–24. doi: 10.1007/978-1-62703-251-3_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Armando I, Wang X, Villar VA, Jones JE, Asico LD, Escano C, Jose PA. Reactive oxygen species-dependent hypertension in dopamine D2 receptor-deficient mice. Hypertension. 2007;49:672–678. doi: 10.1161/01.HYP.0000254486.00883.3d. [DOI] [PubMed] [Google Scholar]