Abstract
Chronic disabilities in multiple sclerosis are believed to be due to neuron damage and degeneration, which follow remyelination failure. Due to the presence of numerous oligodendrocyte precursors inside demyelination plaques, one reason for demyelination failure could be the inability of oligodendrocyte precursor cells to turn into myelinating oligodendrocytes. In this study, we show that thyroid hormone enhances and accelerates remyelination in an experimental model of chronic demyelination, i.e., experimental allergic encephalomyelitis in congenic female Dark Agouti rats immunized with complete guinea pig spinal cord. Thyroid hormone, when administered during the acute phase of the disease, increases expression of platelet-derived growth factor α receptor, restores normal levels of myelin basic protein mRNA and protein, and allows an early and morphologically competent reassembly of myelin sheaths. Moreover, thyroid hormone exerts a neuroprotective effect with respect to axonal pathology.
Keywords: neuroprotection, multiple sclerosis, oligodendrocyte precursor cells, axonal pathology, rat
Multiple sclerosis (MS) is a disorder of the central nervous system (CNS) that manifests as acute focal inflammatory demyelination with limited remyelination, usually culminating in chronic multifocal sclerotic plaques (1). Early axonal injury and loss followed by neuron distress (2) and death (3) occur in MS (4-6), accounting for brain and spinal cord atrophy. Irreversible axonal damage is an essential cause of nonremitting sensory, motor, and cognitive disabilities in MS (7). Although remyelination occurs in most experimental models of demyelination, this beneficial process is undoubtedly inadequate in MS. The reasons for this inadequacy are unknown, also because the oligodendrocyte precursor cells (OPCs), the cell population that is considered to be the most important source of remyelinating oligodendrocytes in the adult CNS (8-10), are present in early (fresh) demyelinating lesions in MS (11, 12).
There are many possible speculative explanations for remyelination failure in MS (9, 10), including quantitatively inadequate recruitment and/or differentiation of OPCs (13); axons not receptive to remyelination (14); and inappropriate support of growth factors by astrocytes and/or other inflammatory cells (15), such as the extracellular microenvironment with regard to matrix proteins and adhesion molecules (16).
Because the number of oligodendrocytes is greater than before demyelination in early MS, meaning that new oligodendrocytes are generated (17), another possibility is that OPCs are unable to turn into myelinating oligodendrocytes in chronic MS. Thus, extensive studies are under way to identify factors involved in OPC differentiation during remyelination. It is generally accepted that the process of remyelination represents a recapitulation of myelination during development, and so the key factors affecting the developmental maturation of OPCs into myelinating oligodendrocytes also should favor remyelination in the adult CNS. It is well established that thyroid hormone (TH) is required for the normal timing of OPC differentiation and maturation (18). Cell-cycle stopping mechanism, terminal differentiation, and myelin production require TH (19). Studies in genetically modified animals (20, 21), such as the analysis of myelination in hypo- and hyperthyroid animals (22), have provided abundant evidence that TH plays an important part in regulating oligodendrocyte lineage and maturation also in vivo and that the TH receptor α1 seems to be responsible for this process (23).
Accordingly, we previously reported that the administration of TH (T4) during the acute phase of experimental allergic encephalomyelitis (EAE) in Lewis rats, the most commonly used experimental model for MS, is able to channel OPCs and/or progenitors into oligodendroglial lineage (24). However, these results were obtained during acute EAE in a rat strain that develops only sparse demyelination. Here, we report that this treatment is able to contrast molecular and anatomical features of the chronic stage of the disease in congenic Dark Agouti (DA) female rats immunized with spinal cord guinea pig homogenate. Compared with Lewis rats, DA rats develop a severe, protracted, and relapsing encephalitis, which has two hallmarks of MS, demyelination and chronic relapsing disease course (25, 26). Pulsed administration of T4 during the acute phase improves and accelerates remyelination by increasing OPC and oligodendrocyte markers, including myelin basic protein (MBP) expression, thus leading to a faster morphological reorganization of myelin sheaths in the white matter during the chronic phase of the disease. Moreover, administration of T4 is also effective in axonal pathology, as indicated by morphometric analysis. We also included comparative experiments in Lewis rats.
Materials and Methods
Animals and Treatment. Female pathogen-free Lewis (Charles River Laboratories) and DA (Elevage Janvier, Le Genest Saint Isle, France) rats were used. At the beginning of the experiments, 30 DA and 30 Lewis rats were included in the study. A group of rats from each strain was sensitized with a medium containing 0.15 g/ml guinea pig spinal cord tissue in 50% vol/vol complete Freund's adjuvant (Sigma) to which 5 mg/ml heat-inactivated Mycobacterium (Difco, H37Ra) was added (100 μl for each hind paw). Uninjected and complete Freund's adjuvant-injected rats were used as controls. Clinical disease was scored according to the following scale: 1, loss of tail tone; 2, weakness in one or both hind legs or middle ataxia; 3, ataxia or paralysis; 4, severe hind-leg paralysis; 5, severe hind-leg paralysis accompanied by urinary incontinence. Rats were assigned randomly to the different experimental groups, and treatment was administered blindly. When rats displayed severe signs of neurological defect, some of the rats were treated with T4 (0.2 mg s.c.) at 11, 13, and 15 days postimmunization (dpi). This treatment has been proven to recruit OPCs and stem cells in EAE (24). Groups of DA rats also were treated with the same three-injection scheme administered at relapse (days 21, 23, and 25) or both in the acute and the relapse phases. All animal protocols described here were carried out according to the European Community Council Directive of 24 November 1986 (86/609/EEC) and approved by our intramural committee and the Ministero della Salute, in compliance with the guidelines published in Guide for the Care and Use of Laboratory Animals (27).
BrdUrd Administration. For proliferation studies, three to five rats per group were analyzed. A single bolus of BrdUrd (50 mg/kg) was administered i.p. Rats were killed 24 h after injection, and sections were processed for immunodetection of BrdUrd by using the monoclonal anti-BrdUrd antibody (Boehringer Mannheim).
Immunocytochemistry. For immunocytochemistry studies, five rats per group were analyzed. Brains and spinal cord were fixed (4% paraformaldehyde), and indirect immunofluorescence procedures were used to visualize the following antigens in the lumbar tract of the spinal cord: anti-oligodendrocyte antibody (MAB 1580, clone RIP, Chemicon), anti-NG2 chondroitin sulfate proteoglycan (Chemicon), anti-MBP (DAKO), anti-2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase, Chemicon), anti-galactocerebroside (GalC, Chemicon), and β-tubulin (Chemicon). For double experiments with BrdUrd, the immunocytochemical procedure was applied after BrdUrd visualization. Confocal laser scan microscopy (CLSM, Olympus FV500, Ar/HeNe lasers and appropriate filters for green and red fluorescence) was used for spinal cord sampling. Morphometry was performed on confocal images by using the AIS image analyzer (Analytic Imaging Station, Toronto).
Western Blotting. Tissue homogenates from the lumbar tract of the spinal cord were prepared by using a lysis buffer (pH 7.5) containing 10 mM Hepes, 1 mM DTT, and a protease inhibitor mixture (Sigma). Aliquots of protein were separated in 15% SDS-polyacrylamide gels and electroblotted to nitrocellulose membranes. Filters were incubated with blocking solution (Pierce) for 2 h at room temperature, and the primary antibody rabbit polyclonal anti-myelin basic protein, dilution 1:2,000 (DAKO), then was incubated overnight at 4°C. After washing for 1 h with 20 mM Tris·HCl and 0.5 M NaCl (TBS) with 0.05% Tween 20 (TTBS), filters were incubated with the secondary antibody anti-rabbit conjugated to horseradish peroxidase (Santa Cruz Biotechnology), dilution 1:2,000, for 30 min at room temperature and washed again for another hour. The proteins were detected by using an enhanced chemiluminescence kit (Pierce). Densitometric analysis was performed by using the AIS imaging system.
Semiquantitative RT-PCR. mRNA was prepared from the lumbar tract of the spinal cord and from the optic nerve by using a Roche kit (Roche Molecular Biochemicals). The concentration of the mRNAs obtained was determined spectrophotometrically at A260. First-strand cDNAs were obtained by following the specifications of the Moloney murine leukemia virus (MLV) reverse transcriptase (GIBCO/BRL), incubating the mRNA in the presence of 1× first-strand buffer, 0.5 mM each dNTP (Roche Molecular Biochemicals), 50 M p(dN)6 random primers (Roche Molecular Biochemicals), and 200 units of the enzyme MLV reverse transcriptase (GIBCO/BRL) at 37°C for 50 min, followed by a termination step at 70°C for 15 min. Before setting the conditions of amplification for each primer, linear regression curves assaying different amounts of cDNAs (corresponding to different mRNA concentrations) and number of cycles of amplification (data not shown) were performed. Both of these parameters (cDNA amount and number of cycles) were chosen in the linear range. The oligonucleotides used as specific primers were as follows: platelet-derived growth factor α receptor (PDGFαR) sense, 5′-AGATAGCTTCATGAGCCGAC-3′, and antisense, 5′-GGAACAGGGTCAATGTCTGG-3′; MBP sense, 5′-CAGCAAGTACCATGGACCAT-3′, and antisense, 5′-ATGTTCTTGAAGAAGTGGAC-3′ (28); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sense, 5′-TCCATGACAACTTTGGCATCGTGG-3′, and antisense, 5′-GTTGCTGTTGAAGTCACAGGAGAC-3′. Amplifications were performed in a mix reaction containing 1× PCR buffer (pH 8.3), 0.2 mM each dNTP, 2 mM MgCl, 0.05 unit/liter TaqDNA polymerase (Sigma), and the corresponding oligonucleotides (sense-antisense). The analysis of GAPDH mRNA was used as a control for cDNA quantities used as templates for PCR assays. PCR products were electrophoresed on agarose gels, stained with ethidium bromide (Sigma), and visualized under UV light. Specificity of amplifications was confirmed by the appearance of a single band of the expected size. Densitometric analysis was performed by using the AIS imaging system.
Statistical Analysis. In the descriptive analysis, data were expressed as mean ± SEM. Statistical analysis was carried out by using one-way ANOVA and Dunnett's test to compare the different experimental groups; Student's t test (prism software package, GraphPad, San Diego) also was used when appropriate. The probability level was set at 5% (two-tailed).
Results
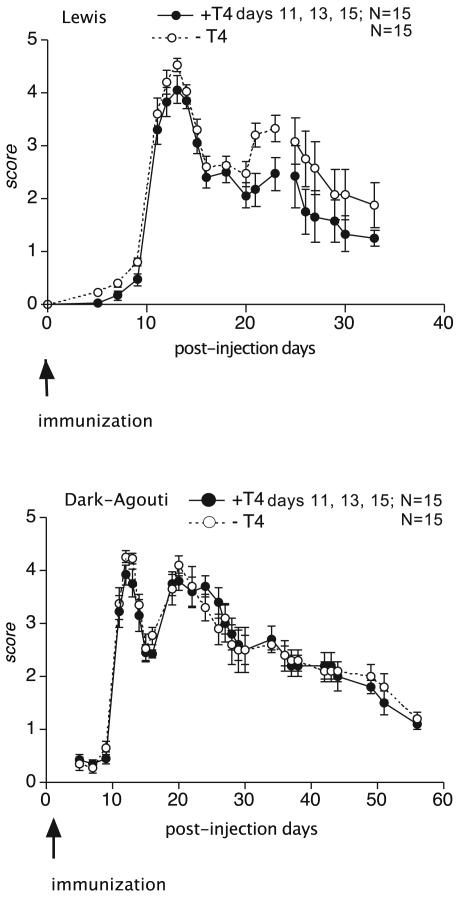
The schedule of the experiments, i.e., T4 administration, molecular biology, and immunohistochemistry, was designed according to the clinical progression of the disease in the two strains. In both Lewis and DA female rats, severity of EAE gradually increased, reaching its peak between 8 and 14 dpi and then partially recovering. In our experience (2, 29), which was confirmed in this experiment, in the Lewis strain the disease relapses with lower severity in 60% of rats (Fig. 1). In DA rats, disease relapses in 100% of rats, the relapse having a neurological score comparable to that observed in the acute phase. Moreover, relapse is resolved more slowly than in Lewis rats.
Fig. 1.
Clinical score of EAE in Lewis (Upper) and DA (Lower) rats. T4 treatment is effective in reducing the severity of the relapse in Lewis, but not DA, rats. Statistical analysis was performed with ANOVA and Dunnett's test. *, P < 0.05.
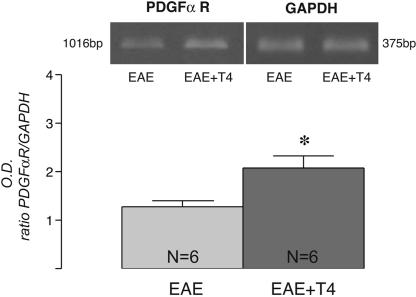
TH Facilitates Myelin Sheath Reassembly in EAE. We carried out remyelination analysis, studying coronal sections from the lumbar spinal cord serially collected from 500 μm of rostrocaudal extension. We investigated myelin-sheath organization in the lumbar tract of the spinal cord (ventral funiculus) by using histological and immunohistochemical staining, followed by CLSM of oligodendrocyte-associated markers, as visualized by the RIP antibody, which produces relatively complete staining of oligodendrocytes and their processes in the adult CNS (30); GalC and CNPase, which are expressed by immature and mature oligodendrocytes; and MBP, which is expressed by mature myelinating oligodendrocytes (10). By using these markers, we observed extensive loss and desegregation of myelin-associated staining already in the acute phase of the disease in both Lewis and DA rats. At this stage in disease progression, we administered T4 a total of three times, once a day on alternate days, in both strains. We then analyzed the effect of this treatment on markers for OPCs immediately after T4 administration (18 dpi in both DA and Lewis rats), and we found an increase in PDGFαR mRNA expression (Fig. 2), suggesting that a larger number of OPCs are formed in treated rats.
Fig. 2.
T4 significantly increases PDGFαR mRNA expression in the spinal cord of EAE-DA rats, as measured by semiquantitative RT-PCR 18 dpi. Data are from six rats per group. Statistical analysis was performed with Student's t test. *, P < 0.05.
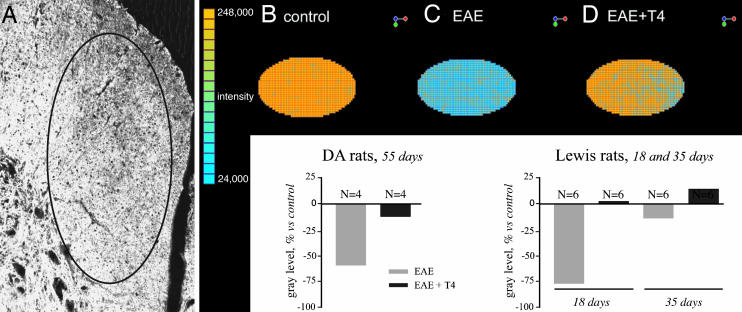
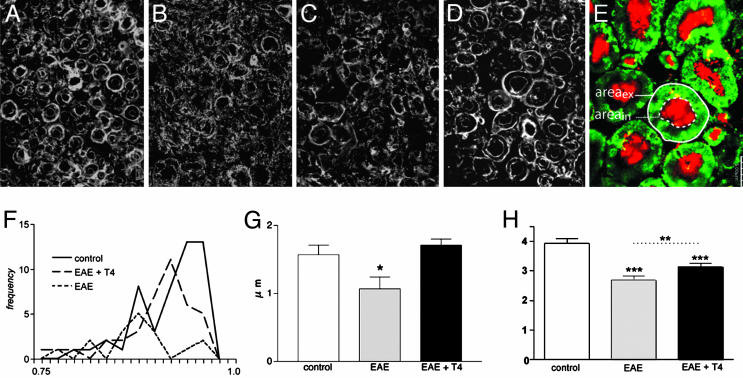
Differentiation of OPCs into myelinating oligodendrocytes and the efficacy of remyelination were investigated by molecular and morphological experiments performed at the end of the relapse phase, i.e., 55 and 35 dpi in DA and Lewis rats, respectively. We first analyzed the general structure of white matter in the ventral funiculus of the spinal cord by semiquantitative RIP-immunostaining analysis using CLSM. One single z-plane was bilaterally collected from 10 random nonconsecutive sections (five rats per group) by using the same loading parameters for all images to be compared (Fig. 3A). The gray level over the entire area then was measured by using Olympus flowview 500 software (intensity value on a planar region). Representative color-coded areas from control (Fig. 3B), EAE (55 dpi, Fig. 3C), and EAE + T4 (55 dpi, Fig. 3D) DA rats also are illustrated in Fig. 3, as well as graphs reporting results obtained in both DA and Lewis rats. Morphological and quantitative analysis reveals that T4 treatment almost completely restores the severe decline in RIP immunostaining in EAE. In DA rats, 55 dpi, there is a 50% reduction of immunostaining, which is resolved by T4 treatment. In Lewis rats, remyelination seems to be completed in EAE rats regardless of the T4 treatment 35 dpi, so we studied samples from 18 dpi on, when myelin disaggregation was quite evident and RIP immunoreactivity in EAE dropped to <75% with respect to control rats. T4-treated rats at 18 dpi displayed immunostaining that was indistinguishable from control rats. We then subjected to deeper analysis the morphological features of myelin sheets, as stained by RIP, CNPase, and GalC antisera. Sampled high-power images obtained with RIP antiserum in DA rats (Fig. 4) showed the substantial destruction of myelin sheets in ventral funiculus in EAE and an inability to remyelinate 35 and 55 dpi (Fig. 4 A, control; B, EAE 35 dpi; and C, EAE 55 dpi). Images collected by CLSM according to the criteria already described were analyzed to measure the thickness of the myelin sheath, form factor of the myelinated fibers (assuming value 1 as a circle), and axon diameter (see Fig. 4E for sampling and legend for calculations). The results shown in Fig. 4 referring to RIP-immunostaining in DA rats indicate that myelin sheet thickness, as measured in the few morphologically intact myelin profiles, is decreased in EAE (Fig. 4G), and the form factor value has shifted to a lower value (Fig. 4F), indicating an irregular shape in pathological rats. Moreover, mean axonal diameter is lower in EAE rats (Fig. 4H). T4 treatment improves morphology (Fig. 4D) and all these indices, restoring myelin thickness to control values. Analysis of CNPase- and GalC-immunostained section confirms these results.
Fig. 3.
Effect of T4 treatment on oligodendrocytes in the spinal cord of DA-EAE rats. T4 treatment accelerates recovery of RIP immunostaining in both DA and Lewis EAE rats. (A) Confocal microscopy of RIP immunostaining in DA control rat, showing the sample area for semiquantitative analysis. (B-D) Color-coded confocal images of RIP immunoreactivity in the different experimental groups in DA strain.
Fig. 4.
Effect of T4 treatment on myelin sheath. (A-D) Confocal images of RIP immunoreactivity in control (A), EAE 35 dpi (B), EAE 55 dpi (C), and EAE 55 dpi + T4 (D) rats. (E) Confocal image of MBP (green) and β-tubulin (red) double-stained section, illustrating the sampling strategy. Areas of the entire fiber (areaex) and of the axon (areain) are measured. Form factor is calculated on the base of areaex values, assuming 1 as circle (F), axonal diameter as square root of areain/π (H), with myelin thickness according to the formula square root of areaex/π- square root of areain/π (G). Data were obtained from three to five rats per group. EAE caused a severe shift from 1 of the form factor value (F), myelin sheath is thinner (G), and axonal diameter is reduced (H). T4 treatment significantly improved all these indices. Statistical analysis was performed with Student's t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (Bars, 5 μm.)
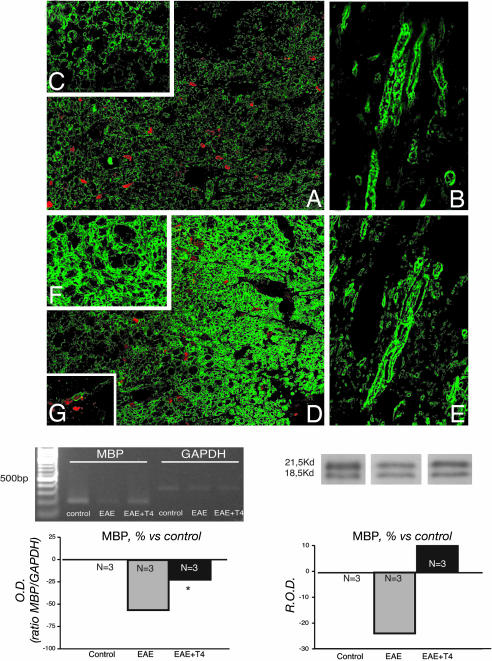
TH Increases MBP Expression in EAE. We then analyzed MBP, which accounts for 30% of the protein content in myelin sheath, by measuring semiquantitative mRNA and protein expression, as well as morphological features. Results are shown in Fig. 5. All of the methods indicate a decrease in MBP expression in DA-EAE rats 55 dpi. CLSM illustrates the severe decline in immunostaining in EAE rats and the clear morphological disaggregation in both coronal (Fig. 5 A, low power, and C, high power) and longitudinal (Fig. 5B) sections. Fig. 5 D-F shows corresponding areas in EAE + T4 rats, 55 dpi, illustrating the substantial recovery of morphological features of myelin sheath. This finding also is confirmed by molecular analysis, which indicates a 50% and 25% decline of MBP mRNA and protein expression, respectively, in EAE rats and a substantial recovery in T4-treated rats, in which expression values are not significantly different from controls. Similar results were obtained in the Lewis strain, confirming previously published results (22).
Fig. 5.
Effect of T4 administration on MBP expression during EAE in DA rats. Low (A and D) and high (C and F, coronal; B and E, longitudinal) confocal images of MBP immunoreactivity in the ventral funiculus of the lumbar spinal cord of untreated EAE-DA rats (A-C) and EAE-DA rats treated with T4 (D-F), at dpi 55. Images show a clear decline in staining intensity and myelin sheet disaggregation in EAE rats and substantial recovery in EAE rats treated with T4. Red spots indicate the extensive infiltration of BrdUrd-positive cells. Graphs show the quantification of MBP mRNA and protein expression, indicating both the decline in EAE rats compared with control rats and recovery due to T4 treatment. Data were obtained from six rats per group. R.O.D., relative OD.
We also investigated the possible involvement of newly generated cells in these molecular effects. To perform this investigation, we injected BrdUrd during the acute phase of the disease, when an increase in the number of proliferating cells, both stem and OPCNG2-positive cells, was found by us (24, 31) and others (32, 33). Also for DA rats, we confirmed the extensive presence of BrdUrduptaking cells >4 weeks after BrdUrd administration in the white matter of the spinal cord of EAE rats (Fig. 5 A and D). We found NG2-positive cells that were also positive for BrdUrd uptake, and thus possibly newly generated OPCs, in EAE rats treated with T4 (Fig. 5G).
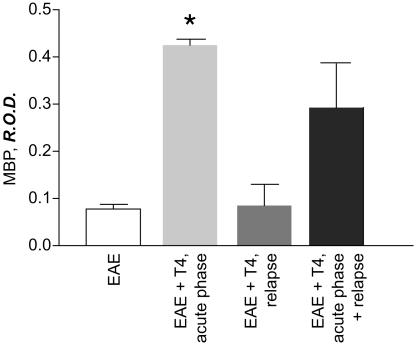
To obtain further indications concerning the possible cellular substrate for T4 action in remyelination in EAE and particularly in restoring MBP content (see Discussion), we also tested T4-administration schedules different from the reference schema used in this study (acute phase of EAE), i.e., in the relapse phase (21, 23, and 25 dpi in DA rats). We found that MBP content in the optic nerve increased when T4 was administered during the acute phase of EAE, but not during relapse (Fig. 6). Administration in both phases also increased MBP to a level not significantly different from that found after T4 treatment in the acute phase.
Fig. 6.
Effect of different schedules of T4 administration on MBP protein content in the optic nerve of EAE-DA rats. The bar legends indicate the phase of the disease at which T4 was administered. Samples all were analyzed at dpi 55. Data were obtained from six rats per group. Statistical analysis was performed with ANOVA and Dunnett's test. *, P < 0.05. R.O.D., relative OD.
Finally, we observed a slight but significant effect of TH treatment in improving the clinical score of the relapse phase in Lewis, but not in DA, rats (Fig. 1).
Discussion
Remyelination failure is a major and frustrating problem in MS, and extensive research is directed to stimulate remyelination also postulating invasive transplantation strategies involving stem and precursor cells (10, 34). In this paper, we suggest a noninvasive approach to enhance remyelination by stimulating endogenous OPCs. Positive effects on remyelination and also neuroprotection are obtained by T4 administration in DA rats affected by EAE. This is a model of chronic relapsing-remitting EAE, which is characterized by extensive demyelination (35) and axonal, dendritic, and synaptic damage (36). When administered during the acute phase of the disease, T4 favors lineage toward oligodendrocytes, as indicated by increased expression of the OPC marker PDGFαR, increases expression of MBP, and favors myelin sheath reorganization, which returns to a thickness comparable to that in control rats. This result occurs only if T4 is administered in a time window, e.g., acute EAE, in which a large number of stem cells and OPCs are mobilized and extensive proliferative activity takes place (24, 31-33). We also found that axonal diameter of fibers in ventral funiculus of the spinal cord is increased in T4-treated compared with untreated EAE rats, suggesting a neuroprotective effect of T4. We postulate that T4 is able to induce maturation of OPCs into myelinating oligodendrocytes by acting on cell-cycle regulation, as it does during development (18). In fact, this effect is not observed if T4 is administered when OPCs are no longer cycling. The effectiveness of remyelination is supported by the morphometric parameters of newly formed myelin sheets, which are similar to control rats, while in untreated rats, such as in MS, newly formed myelin sheets are thinner and shorter with regard to internode distance (37).
Cellular Targets for TH During Remyelination. OPCs and oligodendrocytes are the most obvious cellular targets to explain the effect of TH on remyelination in EAE, because oligodendrocyte development is under TH control (23). TH action at the cellular level is mediated by ligand-regulated transcription factors that belong to the family of nuclear receptors. There are two TH receptor genes (α and β), each generating two isoforms (α1 and α2 and β1 and β2) by alternative splicing (38, 39), and expression of TH receptors in the different tissues and cell types is temporally and regionally regulated. OPCs are disseminated within the white and gray matter of the adult CNS, but they also can be generated from stem cells present in different areas of the CNS (8). Consequently, a potentially unlimited number of myelinating cells could be recruited in the adult CNS.
OPC differentiation into myelinating oligodendrocytes is regulated by two functional components: the so-called timing component, which depends on PDGF and involves the cyclin-dependent protein kinase inhibitor p27/Kip1 (40-42), inducing cells to divide for a defined number of cycles (estimated as eight cell divisions in vitro) over a defined time interval, and the effector component, which is regulated by TH, involves p53 family proteins (43), and stops cell division and initiates differentiation (19). We found that TH is effective in improving remyelination in EAE rats only if administered when intense proliferation takes place and new OPCs are generated, as indicated by increased expression of PDGFαR. Thus, we may speculate that the disease itself, probably through cytokines and growth factors (32, 44, 45), released either from widespread inflammatory and glial cells (46) or from damaged axons after demyelination (47), induces adult OPCs to reenter the cell cycle after demyelination. This proliferative activity is believed to set the intrinsic timer in OPCs to become sensitive to TH, possibly through the expression of suitable amounts of nuclear receptors (23, 48, 49). A pulsed stimulus by TH at this time favors oligodendrocyte maturation. This hypothesis is supported by the absence of any effect produced by hyperthyroidism on OPCs and oligodendrocyte marker expression in nonpathological rats (24, 50).
However, TH also could act on neural stem cells, favoring the generation of more oligodendrocytes (24). Indeed, the TRα gene is essential for stem cell differentiation, as indicated by gene expression analysis of embryonic stem cells in which a dominant negative knock-in point mutation was introduced into this gene (51). It also has been reported that brief in vitro exposure to TH induces more oligodendrocytes to form from neural stem cells derived from embryonic and adult brain cultured as neurospheres (45).
Molecular Targets for TH During Remyelination. Positive effects on remyelination also could be due to direct TH control of gene expression for several genes involved in myelin formation in oligodendrocytes. A specific hormone-receptor interaction with the MBP promoter region indicates that this is a TH-responsive gene (52). However, TH regulates MBP expression only for a limited period during OPC differentiation, and then the gene becomes refractory to TH in the mature brain. No effects on MBP expression actually were found in this and previous studies in nonpathological rats (24, 50). Finally, TH also affects survival of developing oligodendrocytes, also overcoming proapoptotic effects mediated by the inflammatory cytokines tumor necrosis factor α and interleukin 1 (53).
We cannot exclude other molecular targets for the positive action of TH on remyelination in EAE rats. A brief exposure to TH can increase the endogenous synthesis of growth factors, including nerve growth factor in the CNS (54) and also in the case of the severe deficit observed during EAE (24), and nerve growth factor could in turn produce positive effects on EAE, as proven by the nerve growth factor administration in the marmoset (55). This effect has been attributed to an immunomodulatory role played by nerve growth factor. However, recent in vitro and in vivo studies have shown that neurotrophins influence proliferation, differentiation, survival, and regeneration of mature oligodendrocytes and OPCs in favor of a myelin repair (56). Thus, a wide-ranging neuroprotective role could be obtained through brief exposure to TH. Indeed, the increase in trophic factors for different classes of neurons could support nude axons, allowing them to express the appropriate molecular signals required for OPCs to engage demyelinated axons for proper myelination and remyelination (57). Electrical activity seems in fact to promote myelination (58), and axon-derived molecules are temporally and spatially required to coordinate oligodendrocyte differentiation (59). Moreover, TH has been proven to increase axonal transport (60, 61), thus favoring intracellular trafficking of molecules, including survival molecules.
Finally, although a slight but significant improvement was observed in the clinical course of EAE in the relapse phase in Lewis rats, no effect was observed in the DA strain. However, despite the fact that effectiveness of therapeutic intervention in EAE is usually related to the neurological score for motor disabilities, the cause of the neurological deficit in EAE is still poorly understood. It is clear that CNS inflammation and demyelination do not fully account for the neurological deficit (62).
In conclusion, remyelination in experimental and spontaneous diseases requires a complex interplay among different cellular partners, e.g., OPCs, oligodendrocytes, axons, inflammatory cells, and astrocytes, and related secretory and membrane proteins such as extracellular matrix, and the role of these partners in this representation changes according to the phase of the disease. Thus, multiple therapies should be the final goal for an effective treatment for MS. We suggest that TH could have a role in potentiating reluctant myelination by inducing OPCs to differentiate into myelinating oligodendrocytes during a specific phase of the disease. It is therefore proposed as a possible partner in restoring the proper signaling in the microenvironment required for successful remyelination also through an indirect neuroprotective role. In the hypothesis that remyelination fails in MS because of insufficient differentiation of recruited OPCs, one can speculate that the slight signals of altered thyroid function homeostasis observed in MS patients (63, 64) could take part in the decline in remyelination attempts.
Acknowledgments
We thank Nadia De Sordi for technical assistance. This work was supported by the Fondazione Carisbo (Bologna), L'Associazione Italiana Sclerosi Multipla/Fondazione Italiana Sclerosi Multipla Grant 2002/R/14, Istituto Superiore di Sanità project “Cellule Staminali” CS12, and University of Bologna.
Author contributions: L.G. and L.C. designed research; M.F., A.G., S.P., and G.D. performed research; L.A. contributed new reagents/analytic tools; L.G. and L.C. analyzed data; and L.A., R.L.-M., and L.C. wrote the paper.
Abbreviations: CLSM, confocal laser scan microscopy; DA, Dark Agouti; EAE, experimental allergic encephalomyelitis; MBP, myelin basic protein; MS, multiple sclerosis; OPC, oligodendrocyte precursor cell; PDGF, platelet-derived growth factor; PDGFαR, PDGF α receptor; TH, thyroid hormone; dpi, days postimmunization.
References
- 1.Compston, A. & Coles, A. (2002) Lancet 359, 1221-1231. [DOI] [PubMed] [Google Scholar]
- 2.Giardino, L., Giuliani, A., Fernandez, M. & Calzà, L. (2004) Neuropathol. Appl. Neurobiol., 30, 522-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed, Z., Doward, A. I., Pryce, G., Taylor, D. L., Pocock, J. M., Leonard, J. P., Baker, D. & Cuzner, M. L. (2002) Am. J. Pathol. 161, 1577-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferguson, B., Matyszak, M. K., Eiri, M. M. & Perry, V. H. (1997) Brain 120, 393-399. [DOI] [PubMed] [Google Scholar]
- 5.De Stefano, N., Matthews, P. M., Fu, L., Narayanan, S., Stanley, J., Francis, G. S., Antel, J. P. & Arnold, D. L. (1998) Brain 121, 1469-1477. [DOI] [PubMed] [Google Scholar]
- 6.Trapp, B. D., Peterson, J., Ransohoff, R. M., Rudick, R., Mork, S. & Bo, L. (1998) N. Engl. J. Med. 338, 278-285. [DOI] [PubMed] [Google Scholar]
- 7.Bjartmar, C. & Trapp, B. D. (2001) Curr. Opin. Neurol. 14, 271-278. [DOI] [PubMed] [Google Scholar]
- 8.Levine, J. M., Reynolds, R. & Fawcett, J. W. (2001) Trends Neurosci. 24, 39-47. [DOI] [PubMed] [Google Scholar]
- 9.Franklin, R. J. M. (2002) Nat. Neurosci. 3, 705-714. [Google Scholar]
- 10.Stangel, M. & Hartung, H.-P. (2002) Prog. Neurobiol. 68, 361-376. [DOI] [PubMed] [Google Scholar]
- 11.Wolswijk, G. (1998) J. Neurosci. 18, 601-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scolding, N. J., Rayner, P. J. & Compston, D. A. (1999) Neuroscience 89, 1-4. [DOI] [PubMed] [Google Scholar]
- 13.Keirstead, H. S., Levine, J. M. & Blakemore, W. F. (1998) Glia 22, 161-170. [PubMed] [Google Scholar]
- 14.Chang, A., Tourtellotte, W. W., Rudick, R. & Trapp, B. D. (2002) N. Engl. J. Med. 346, 165-173. [DOI] [PubMed] [Google Scholar]
- 15.Ridet, J. L., Malhotra, S. K., Privat, A. & Gage, F. H. (1997) Trends Neurosci. 20, 570-577. [DOI] [PubMed] [Google Scholar]
- 16.Sobel, R. A. (1998) J. Neuropathol. Exp. Neurol. 57, 205-217. [DOI] [PubMed] [Google Scholar]
- 17.Carrol, W. M. & Jennings, A. R. (1994) Brain 117, 563-578. [DOI] [PubMed] [Google Scholar]
- 18.Rogister, B., Ben-Hur, T. & Dubois-Dalcq, M. (1999) Mol. Cell. Neurosci. 14, 287-300. [DOI] [PubMed] [Google Scholar]
- 19.Durand, B. & Raff, M. (2000) BioEssays 22, 64-71. [DOI] [PubMed] [Google Scholar]
- 20.Baas, D., Legrand, C., Samarut, J. & Flamant, F. (2002) Proc. Natl. Acad. Sci. USA 99, 2907-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Shea, P. J. & Williams, G. R. (2002) J. Endocrinol. 175, 553-570. [DOI] [PubMed] [Google Scholar]
- 22.Jagannathan, N. R., Tandon, N., Raghunathan, P. & Kochupillai, N. (1998) Dev. Brain Res. 109, 179-186. [DOI] [PubMed] [Google Scholar]
- 23.Billon, N., Jolicoeur, C., Tokumoto, Y., Vennstrom, B. & Raff, M. (2002) EMBO J. 21, 6452-6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calzà, L., Fernandez, M., Giuliani, A., Aloe, L. & Giardino, L. (2002) Proc. Natl. Acad. Sci. USA 99, 3258-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorentzen, J. C., Issazadeh, S., Storch, M., Mustafa, M. I., Lassman, H., Linington, C., Klareskog, L. & Olsson, T. (1995) J. Neuroimmunol. 63, 193-205. [DOI] [PubMed] [Google Scholar]
- 26.Tanuma, N., Shin, T. & Matsumoto, Y. (2000) J. Neuroimmunol. 108, 171-180. [DOI] [PubMed] [Google Scholar]
- 27.National Research Council (1996) Guide for the Care and Use of Laboratory Animals (Natl. Acad. Press, Washington, DC).
- 28.Schaich, M., Budzinski, R. M. & Stoffel, W. (1986) Biol. Chem. Hoppe-Seyler 367, 825-834. [DOI] [PubMed] [Google Scholar]
- 29.Pozza, M., Bettelli, C., Aloe, L., Giardino, L. & Calzà, L. (2000) Brain Res. 855, 39-46. [DOI] [PubMed] [Google Scholar]
- 30.Friedman, B., Hockfield, S., Black, J. A., Woodruff, K. A. & Waxman, S. G. (1989) Glia 2, 380-390. [DOI] [PubMed] [Google Scholar]
- 31.Calzà, L., Giardino, L., Pozza, M., Bettelli, C., Micera, A. & Aloe, L. (1998) Proc. Natl. Acad. Sci. USA 95, 3209-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Decker, L., Picard-Riera, N., Lachapelle, F. & Baron-Van Evercooren, A. (2002) J. Neurosci. Res. 69, 763-771. [DOI] [PubMed] [Google Scholar]
- 33.Picard-Riera, N., Decker, L., Delarasse, C., Goude, K., Nait-Oumesmar, B., Liblau, R., Pham-Dinh, D. & Baron-Van Evercooren, A. (2002) Proc. Natl. Acad. Sci. USA 99, 13211-13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pluchino, S., Furlan, R. & Martino, G. (2004) Curr. Opin. Neurol. 17, 247-255. [DOI] [PubMed] [Google Scholar]
- 35.Weissert, R., Wallstrom, E., Storch, M. K., Stefferl, A., Lorentzen, J., Lassmann, H., Linington, C. & Olsson, T. (1998) J. Clin. Invest. 102, 1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu, B., Luo, L., Moore, G. R. W., Paty, D. W. & Cynader, M. S. (2003) Am. J. Pathol. 162, 1639-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blakemore, W. F. (1974) Nature 249, 577-578. [DOI] [PubMed] [Google Scholar]
- 38.Yen, P. M. (2001) Physiol. Rev. 81, 1097-1142. [DOI] [PubMed] [Google Scholar]
- 39.Forrest, D., Reh, T. A. & Rusch, A. (2002) Curr. Opin. Neurobiol. 12, 49-56. [DOI] [PubMed] [Google Scholar]
- 40.Casaccia-Bonnefil, P., Tikoo, R., Kiyokawa, H., Friedrich, V., Jr., Chao, M. V. & Koff, A. (1997) Genes Dev. 11, 2335-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durand, B., Gao, F. B. & Raff, M. (1997) EMBO J. 16, 306-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durand, B., Fero, M. L., Roberts, J. M. & Raff, M. (1998) Curr. Biol. 8, 431-440. [DOI] [PubMed] [Google Scholar]
- 43.Tokumoto, Y. M., Tang, S. G. & Raff, M. C. (2001) EMBO J. 20, 5261-5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barres, B. A. & Raff, M. C. (1994) Neuron 12, 935-942. [DOI] [PubMed] [Google Scholar]
- 45.Johe, K. K., Hazel, T. G., Muller, T., Dugich-Djordjevic, M. M. & McKay, R. D. G. (1996) Genes Dev. 10, 3129-3140. [DOI] [PubMed] [Google Scholar]
- 46.Levine, J. M. (1994) J. Neurosci. 14, 4716-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Bello, I. C., Dawson, M. R. L., Levine, J. M. & Reynolds, R. (1999) J. Neurocytol. 28, 365-381. [DOI] [PubMed] [Google Scholar]
- 48.Carre, J. L., Demerens, C., Rodriguez-Pena, A., Floch, H. H., Vincendon, G. & Sarlieve, L. L. (1998) J. Neurosci. Res. 54, 584-594. [DOI] [PubMed] [Google Scholar]
- 49.Gao, F. B., Apperly, J. & Raff, M. (1998) Dev. Biol. 197, 54-66. [DOI] [PubMed] [Google Scholar]
- 50.Fernandez, M., Pirondi, S., Manservigi, M., Giardino, L. & Calzà, L. (2004) Eur. J. Neurosci., 20, 2059-2070. [DOI] [PubMed] [Google Scholar]
- 51.Liu, Y.-Y., Tachiki, K. H. & Brent, G. A. (2002) Endocrinology 143, 2664-2672. [DOI] [PubMed] [Google Scholar]
- 52.Strait, K. A., Carlson, D. J., Schwartz, H. L. & Oppenheimer, J. H. (1997) Endocrinology 138, 635-641. [DOI] [PubMed] [Google Scholar]
- 53.Jones, S. A., Jolson, D. M., Cuta, K. K., Mariash, C. N. & Anderson, G. W. (2003) Mol. Cell. Endocrinol. 199, 49-60. [DOI] [PubMed] [Google Scholar]
- 54.Walker, P., Weichsel., M. E., Jr., Fisher, D. A., Guo, S. M. & Fisher, D. A. (1979) Science 204, 427-429. [DOI] [PubMed] [Google Scholar]
- 55.Villoslada, P., Hauser, S. L., Bartke, I., Unger, J., Heald, N., Rosemberg, D., Cheung, S. W., Mobley, W. C., Fisher, S. & Genain, C. P. (2000) J. Exp. Med. 191, 1799-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Althaus, H. H. (2004) Prog. Brain Res. 146, 415-432. [DOI] [PubMed] [Google Scholar]
- 57.Baumann, N. & Pham-Dinh, D. (2001) Physiol. Rev. 81, 871-927. [DOI] [PubMed] [Google Scholar]
- 58.Demerens, C., Stankof, B., Logak, M., Anglade, P., Allinquant, B., Couraud, F., Zalc, B. & Lubetzki, C. (1996) Proc. Natl. Acad. Sci. USA 93, 9887-9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu, Q.-D., Ang, B.-T., Karsak, M., Hu, W.-P., Cui, X.-Y., Duka, T., Takeda, Y., Chia, W., Sankar, N., Ng, Y.-K., et al. (2003) Cell 115, 163-175. [DOI] [PubMed] [Google Scholar]
- 60.Sidenius, P., Nagel, P., Larsen, J. R., Boye, N. & Laurberg, P. (1987) J. Neurochem. 49, 1790-1795. [DOI] [PubMed] [Google Scholar]
- 61.Tang, H. Z. & Hammerschlag, R. (1996) Neurochem. Res. 21, 489-494. [DOI] [PubMed] [Google Scholar]
- 62.'t Hart, B. A. & Amor, S. (2003) Curr. Opin. Neurol. 16, 375-383. [DOI] [PubMed] [Google Scholar]
- 63.Durelli, L., Oggero, A., Verdun, E., Isoardo, G. L., Barbero, P., Bergamasco, B., Brossa, P. C., Ghigo, E., Maccario, M. & Faggiano, F. (2001) J. Neurol. Sci. 193, 17-22. [DOI] [PubMed] [Google Scholar]
- 64.Zych-Twardowska, E. & Wajgt, A. (2001) Med. Sci. Monit. 7, 1005-1012. [PubMed] [Google Scholar]