Abstract
There is increasing interest among developmental psychopathologists in broad transdiagnostic factors that give rise to a wide array of clinical presentations (multifinality), but little is known about how these processes lead to particular psychopathological manifestations over the course of development. We examined whether individual differences in the error related negativity (ΔERN), a neural indicator of error monitoring, predicts whether early persistent irritability—a prototypical transdiagnostic construct— is associated with later internalizing versus externalizing outcomes. When children were 3 years old, mothers were interviewed about children’s persistent irritability and completed questionnaires about their children’s psychopathology. Three years later, EEG was recorded while children performed a go/no-go task to measure the ΔERN. When children were approximately 9 years old, mothers again completed questionnaires about their children’s psychopathology. Results indicated that among children who were persistently irritable at age 3, an enhanced or more negative ΔERN at age 6 predicted the development of internalizing symptoms at age 9, whereas a blunted or smaller ΔERN at age 6 predicted the development of externalizing symptoms. Our results suggest that variation in error monitoring predicts, and may even shape, the expression of persistent irritability and differentiates developmental trajectories from preschool persistent irritability to internalizing versus externalizing outcomes in middle-late childhood.
Keywords: irritability, transdiagnostic, multifinality, error monitoring, event-related potentials
Comorbidity, transdiagnostic factors, and multifinality
Psychopathology presents with dizzying arrays and combinations of symptoms, spurring numerous attempts over time to bring order by imposing different classification systems. For much of the history of modern psychiatric classification, imposing diagnostic hierarchies created order. “Organic” conditions were at the top of the hierarchy, followed by psychotic disorders, then mood disorders, and then all remaining disorders (e.g. Foulds, 1976). With the introduction of explicit diagnostic criteria in the third edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-III; American Psychiatric Association, 1980), these hierarchies were implemented as exclusion criteria, in which one diagnosis could not be made if it was “due to” another disorder (e.g. an individual could only receive an anxiety disorder diagnosis once “organic”, psychotic, and mood disorders had been ruled out). It was within this same context that Robins and Guze (1970) proposed their highly influential criteria for validating psychiatric diagnoses, which argued that for a psychiatric disorder to be considered a valid construct, it must be clearly delimited from other disorders and it must remain stable over time rather than being associated with the subsequent development of other disorders.
The DSM-III-Revised (DSM-III-R; American Psychiatric Association, 1987) relaxed diagnostic hierarchies due to evidence that they eliminated meaningful information (e.g., Boyd et al., 1984; Leckman, Weissman, Merikangas, Pauls, & Prusoff, 1983). This created a massive unanticipated problem – comorbidity. In the first nationally representative epidemiological study of psychiatric disorders in the United States, the DSM-III-R-based National Comorbidity Survey found that more than half of adults with one DSM diagnosis met criteria for at least one additional diagnosis (Kessler et al., 1994). Moreover, comorbidity appears to be even higher in children and adolescents (Angold, Costello & Erkanli, 1999; Rohde, Lewinsohn, & Seeley, 1991).
Comorbidity can reflect a number of processes, which can be divided into four general types (Klein & Riso, 1993; Lilienfeld, 2003): chance (i.e., some individuals have the misfortune to experience multiple independent disorders); spurious (e.g., comorbidity resulting from shared diagnostic criteria across distinct disorders); artifactual (e.g., comorbidity derived by mistakenly splitting one disorder into multiple diagnoses; these diagnoses therefore have the same or highly overlapping etiologies); and causal (i.e., one disorder influences the development of another disorder). We can also distinguish between concurrent and sequential comorbidity – that is, two or more disorders co-occurring at overlapping versus different times.
Comorbidity raises challenges for the Robins and Guze approach to validating psychiatric disorders, as clear delimitation of disorders is difficult when co-occurrence with other disorders is the norm. Moreover, sequential comorbidity suggests that individuals can develop different disorders at different points in time, and that this may not necessarily indicate that the initial diagnosis was incorrect or reflects an invalid diagnostic construct.
The concept of sequential comorbidity is also valuable in that it introduces a temporal perspective to discussions of the co-occurrence of disorders, suggesting that comorbidity is dynamic and can unfold over time. Sequential comorbidity is, however, agnostic regarding the processes by which this occurs. Moreover, a temporal perspective is part of, but not the same as, a developmental perspective. In developmental psychopathology, the concepts of diagnostic stability and sequential comorbidity are altered in subtle but very important ways by considering two related concepts: homotypic and heterotypic continuity. Similar to Robins and Guze’s concept of diagnostic stability, homotypic continuity describes a pattern in which a disorder has a similar manifestation across multiple time points. However, heterotypic continuity represents a very different perspective on both diagnostic stability and sequential comorbidity, as it suggests that the presentation of disorders can change or become more complex over time without necessarily invalidating the initial diagnosis or presuming that the two presentations reflect different disorders. Instead, there may be a form of continuity in which one clinical presentation morphs into, or expands to include, another presentation over the course of development.
Unfortunately, the concept of heterotypic continuity is often invoked in a promiscuous fashion - we rarely know what the underlying process is, so when investigators refer to heterotypic continuity, they are usually making unwarranted assumptions about the developmental continuity of underlying processes or misusing the term when what they really mean is the much broader concept of sequential comorbidity. That is, when one disorder follows another in time, it could truly reflect heterotypic continuity (i.e., factors or processes that are expressed differently over the course of development). However, it might also reflect the occurrence of two independent syndromes at different points in time or a causal relationship between two distinct syndromes (e.g., depression arising in adolescence as a consequence of peer neglect and rejection stemming from childhood social anxiety; Cummings, Caporino, & Kendall, 2014).
A more recent perspective on comorbidity stems from the growing emphasis on understanding transdiagnostic factors that cut across, or are shared by, multiple diagnostic categories (Nolen-Hoeksema & Watkins, 2011). Examples of potential transdiagnostic factors (which themselves overlap) are neuroticism (or negative emotionality), maladaptive emotion regulation, and rumination. Transdiagnostic factors have the potential to account for comorbidity because they may reflect shared etiological, pathophysiological, or psychopathological processes. Transdiagnostic factors may also be clinical features that contribute to multiple diagnoses. For example, irritability appears in the criteria sets for multiple DSM disorders, and may contribute to comorbidity through shared (transdiagnostic) processes, as well as overlapping diagnostic criteria. However, there has been little consideration of how transdiagnostic processes evolve over time and therefore contribute to heterotypic continuity.
To summarize, by inadvertently bringing the pervasiveness of comorbidity to light, DSM-III-R revealed a landscape in which multiple syndromes co-occur, suggesting that some clinical phenomena and underlying processes are transdiagnostic. Moreover, clinical presentations change shape and morph into other forms over time, as suggested by the concepts of sequential comorbidity and heterotypic continuity. Taken together, these concepts are consistent with the view that psychopathology is characterized by both multifinality (i.e, a single risk factor or clinical problem may develop into multiple clinical phenotypes) and equifinality (i.e., different risk factors or clinical problems may eventually converge in the same phenotypic presentation).
Empirically-derived models of classification and the Research Domains Criteria
With the abandonment of a priori diagnostic hierarchies, and the resulting disappearance of a semblance of nosological clarity and parsimony, dissatisfaction with the DSM has mounted, and several alternative approaches to conceptualizing psychopathology have emerged. First, it has been proposed that the traditional top-down approach rooted in the clinical observation and theory that formed the foundation of our current diagnostic system should be replaced by a bottom-up, empirically-derived classification system based on latent variable modeling (primarily factor analysis). This approach also tends to be associated with a rejection of the traditional categorical approach to classification in favor of a dimensional perspective, although one does not necessarily entail the other.
Bottom-up empirical classification systems explain comorbidity between categorical disorders (or covariation between symptom dimensions) by modeling higher order latent constructs that account for the associations between the lower order constructs. This approach has produced a consensus model that consists of at least two higher-order dimensions, Internalizing or the propensity to express distress inwards (including anxiety and depression) and Externalizing or the propensity to express distress outwards (including aggression, delinquency and hyperactivity-impulsivity), with some evidence for a third higher order dimension of Psychosis (e.g., Kotov et al., 2011; Wright et al., 2013). While there is less agreement on lower levels of classification, there is evidence for further dividing Internalizing into Distress vs. Fear. However, as the higher order factors of Internalizing and Externalizing are themselves moderately correlated, an alternative model positing a general psychopathology factor has also been supported in both adults (Caspi et al., 2014; Lahey et al., 2012) and children (Lahey et al., 2015; Olino, Dougherty, Bufferd, Carlson & Klein, 2014). While the nature of this general factor is unknown, several investigators have proposed that it involves individual differences in neuroticism (or negative emotionality), which refers to the propensity to experience and react to stress and frustration with negative affects such as anger/irritability, sadness, and fear, and/or to have difficulty regulating these negative emotional reactions (Hink, et al., 2013; Rhee, Lahey & Waldman, 2015).
A second, more top-down and theory-driven, approach to conceptualizing psychopathology has been proposed by the National Institute of Mental Health’s Research Domains Criteria (RDoC) initiative. While RDoC is not a classification system, it provides a framework for research in psychopathology that aims to produce a more valid classification system in the future. Like the empirically-based approach, RDoC emphasizes the use of dimensional constructs that range from normal to abnormal functioning. However, unlike the DSM and hierarchical models of psychopathology which focus almost entirely on psychological symptoms, the RDoC approach is rooted in the literature on biobehavioral systems and emphasizes neural circuits that are common across species, focusing on a handful of domains (e.g. positive valence, negative valence, cognition, arousal/regulation, and social processes) that can be examined across multiple within-person units of analysis (e.g. genes, molecules, cells, circuits, physiology, behavior, and self-report). RDoC explains comorbidity by positing that biobehavioral systems, or domains, cut across traditional diagnostic categories and create heterogeneity within those categories. Thus, the domains in the RDoC matrix are assumed to be transdiagnostic, at least with respect to the traditional diagnostic system.
Although neither of these approaches to conceptualizing psychopathology has systematically incorporated a developmental perspective, both are increasingly recognizing the importance of development (e.g., Casey, Oliveri, & Insel, 2014; Lahey et al., 2015). This is important, as a developmental psychopathology framework has the potential to complement both approaches through its emphasis on the dynamic interplay of different units of analysis across development, and its interest in whether risk factors operate uniformly for all people or in the same way for an individual across the lifespan (Cicchetti, 2008).
In sum, both empirically-derived classification and RDoC suggest that broad transdiagnostic factors give rise to a wide array of clinical presentations, but how these processes lead to particular psychopathological manifestations over the course of development is likely to depend on a host of other biological and environmental features. An important next step is to understand how transdiagnostic factors lead to multiple forms of psychopathology (or multifinality), and how variables at other levels influence the differential development and expression of these factors over time.
These issues are particularly relevant to irritability, which is a prototypical transdiagnostic construct as well as a common focus of clinical concern at all stages of human development. Historically, irritability has been a diagnostic orphan with no formal category of its own, yet it is a criterion for multiple diagnoses. DSM-5 tried to address this problem by introducing the categories of dysphoric mood dysregulation disorder (DMDD) for youth and intermittent explosive disorder for adults, both of which give irritability a central role. Irritability may help account for a great deal of comorbidity, both due to overlapping diagnostic criteria and to common underlying processes (e.g., negative emotional reactivity and dysregulation). In addition, as discussed in the next section, follow-up studies indicate that childhood irritability predicts multiple forms of later psychopathology, including both internalizing and externalizing disorders. Hence, it may explain much sequential comorbidity and heterotypic continuity and therefore play a significant role in multifinality. Hence, the current study explores what shapes the expression of irritability over the course of childhood and predicts whether it takes an internalizing versus externalizing path.
Irritability
Irritability is defined as low frustration tolerance characterized by anger and temper outbursts. As a symptom, irritability plays a prominent role in the current psychiatric nosology spanning both the internalizing and externalizing spectrums (e.g., major depressive disorder [MDD] in youth, DMDD, bipolar disorder, generalized anxiety disorder [GAD], oppositional defiant disorder [ODD], intermittent explosive disorder, and borderline and antisocial personality disorder). Additionally, it spans the normal-abnormal continuum in that it is a dimensional trait that is common, heritable (Roberson-Nay, et al., 2015), and relatively stable in typically developing youth that, at even relatively low levels, places young individuals at risk for later psychopathology and social maladjustment (Copeland, Brotman & Costello, 2015; Wakschlag et al., 2015).
To better understand the role of irritability in psychopathology, an emerging body of research has begun to examine persistent irritability’s relationship to later psychopathology. There is some evidence that persistent irritability in school-aged children and adolescents is specifically associated with depressive and anxiety disorders in adulthood (Brotman et al., 2006; Burke, 2012; Leibenluft, Cohen, Gorrindo, Brook, & Pine, 2006; Stringaris, Cohen, Pine, & Leibenluft, 2009), leading some to speculate that irritability is a mood manifestation that shares common risk factors with affective disorders (e.g. Stringaris & Taylor, 2015). However, other studies find that irritability is heterogeneous and its associations with psychopathology are characterized by less specificity. For example, among juvenile offenders, high levels of irritability predicted anxiety and depressive disorders and criminal violent reoffending even when controlling for conduct disorder (Aebi et al., in press). In a study examining longitudinal outcomes of the irritable dimension of ODD from ages 12 to 25 in large community sample, there were no differences in the strength of paths from prior irritability to later internalizing and externalizing problems across several assessments (Leadbeater & Homel, 2015). Similarly, in another large longitudinal study, childhood DMDD, a condition characterized by severe and persistent irritability and temper loss, was found to predict greater levels of risky and illegal behaviors, in addition to internalizing symptoms in adulthood (Copeland et al., 2014).
This lack of specificity is particularly apparent in studies examining very early manifestations of persistent irritability. Controlling for baseline symptoms, preschool persistent irritability has been shown to predict behavioral disorders (ODD, conduct disorder [CD], and attention deficit hyperactivity disorder [ADHD]) in addition to depressive and anxiety disorders across childhood (Dougherty et al., 2013; 2015; Waskchlag et al., 2015). When irritability symptoms are removed from the diagnostic criteria, longitudinal associations between irritability and both later internalizing and externalizing symptoms persist arguing against the notion that these associations are solely artifacts of overlapping diagnostic criteria (Dougherty et al., 2013).
The fact that one individual with persistent irritability can develop internalizing psychopathology while another can develop externalizing psychopathology (and some develop both) points to the possibility that persistent irritability is a transdiagnostic phenotype characterized by multifinality, where a similar initial presentation predicts a variety of dissimilar outcomes. Indeed, some researchers have postulated that irritability may be a nonspecific marker of risk that may help us identify etiological processes that are both common and unique across a wide range of psychopathology (Mulraney, Melvin, & Tonge, 2014; Wakschlag et al., 2015)
Currently, we know very little about the developmental mechanisms through which irritability transitions into subsequent internalizing and externalizing psychopathology across childhood, and what distinguishes longitudinal trajectories of irritability characterized by internalizing outcomes from those characterized by externalizing outcomes. Rather than waiting for serious problem behaviors to emerge, the identification of factors that moderate such pathways could allow us to predict the direction in which an individual’s psychopathology will manifest. This would have significant implications for the implementation and development of intervention and prevention strategies that specifically target internalizing and externalizing conditions.
One method to identify such mechanisms is to examine neural correlates of cognitive-affective processes, such as error monitoring, which have been implicated in multiple forms of psychopathology (Weinberg, Dieteric, & Riesel, 2015). Event-related potentials are an ideal tool to identify such neural correlates given their low cost and ability to be used in children and adolescents across development (Nelson & McCleery, 2008). Specifically, the error-related negativity (ERN), an event-related potential (ERP) that represents a neural index of error monitoring, may be a promising candidate to identify divergent developmental pathways that are associated with risk for different forms of later psychopathology. The ERN is a negative deflection of the ERP that is apparent at fronto-central sites approximately 50 ms after the commission of an error. The ERN has excellent internal consistency (Meyer, Bress & Proudfit, 2014; Riesel, Weinberg, Endrass, Meyer & Hajcak, 2013; Olvet & Hajcak, 2009; Segalowitz et al., 2010) and is stable over a two-year period (Meyer, Bress, et al., 2014; Weinberg & Hajack, 2011), suggesting that it is relatively trait-like. However, the ERN is also potentially malleable, as environmental influences that relate to error sensitivity like punishment (Riesel et al., 2012), uncertainty (Jackson, Nelson & Hajcak, 2015) and punitive parenting (Meyer, Proudfit et al., 2015) potentiate the ERN.
While there is a general consensus that the ERN indexes the activity of a performance monitoring system that serves the individual to adjust her/his behavior to meet environmental demands, several theories provide competing explanations of the ERN’s functional significance. For example, conflict monitoring theory suggests that the ERN is elicited by the detection and processing of cognitive conflict (Gehring & Fencsik; 2001), whereas reinforcement learning theory (Holroyd & Coles, 2002) suggests that the ERN may have an evaluative functioning which signals when an event is worse than expected. Recent conceptualizations also point to the ERN’s affective relevance and relationship to threat sensitivity (Proudfit, Inzlicht & Mennin, 2013). Indeed, the commission of an error prompts defense system activation response, including skin conductance (Hajcak, McDonald & Simons, 2003), pupil dilation (Critchley, Tang, Glaser, Butterworth & Dolan, 2005), and the startle reflex (Hajcak & Foti, 2008). However, these perspectives may not be mutually exclusive, as the negative affective experience of conflict motivates the immediate initiation of cognitive control (Inzlicht, Bartholow & Hirsch, 2015).
Evidence from ERP source localization studies indicates that the ERN originates in the anterior cingulate cortex (ACC; Carter & van Veen, 2007; Holroyd & Coles, 2002), a part of the frontostriatal system implicated in numerous complex functions including error and performance monitoring, decision-making, and reward-punishment processing (Carter & van Veen, 2007). The ACC is comprised of two functional subdivisions: the dorsal, or cognitive, part which projects onto the motor cortex and prefrontal cortex (PFC) and the rostral, or affective part which projects onto the amygdala, nucleus accumbens, hypothalamus, and insula (Carlson et al., 2013). While some studies have found that the dACC is the principal generator of the ERN, consistent with the notion that the ERN may be linked to more cognitive processes (Ridderinkhof et al. 2004), other studies have found the ERN to be generated by the rostral or more affective region of the ACC (Taylor et al. 2007) lending support to the view that variation in the ERN may reflect individual differences in the integration of affective and cognitive processes during error detection (Weinberg, Reisel & Hajcak, 2012).
Research suggests that an enhanced (i.e., more negative) ERN is associated with internalizing disorders, particularly GAD and OCD but also other anxiety disorders and MDD (Olvet & Hajcak, 2009). An enhanced ERN has been consistently identified in both adults (Weinberg, Dietric & Riesel, 2015) and children (Ladouceur et al. 2006; Meyer et al., 2013) with anxiety disorders, as well as in adult depressive disorders (Chiu & Deldin, 2007; Holmes & Pizzagalli, 2008, 2010), though the data on the latter are mixed (e.g. Weinberg, Klein & Hajcak, 2012). There is also evidence that an enhanced ERN may indicate vulnerability for the later development of anxiety. For example, an increased ERN at age 6 predicted the first onset of anxiety disorders by age 9, even when controlling for baseline anxiety symptoms (Meyer, Hajcak, Torpey-Newman, Kujawa & Klein, 2015). Furthermore, in another longitudinal study, the ERN moderated the association between early temperamental behavioral inhibition (BI)—a temperament profile associated with risk for anxiety disorders—and the development of later anxiety symptoms, such that high levels of early temperamental BI was predictive of later anxiety symptoms only in the presence of an increased ERN (Lahat et al., 2014; McDermott et al., 2009).
At the same time, research has indicated that an attenuated (i.e., less negative) ERN is associated with externalizing psychopathology (Hall, Bernat & Patrick, 2007). For example, studies have shown that aggression, antisocial behaviors, substance use disorders (SUD), and impulsivity in adults are individually (Pailing, Segalowitz, Dywan, & Davies, 2002; Potts, George, Martin, & Barratt, 2006) and collectively (Hall et al., 2007) associated with a reduced ERN. A diminished ERN has also been identified in juvenile offenders (Vila-Ballo et al., 2014), pediatric ADHD patients (Van De Voorde, Roeyers & Wiersema, 2010), children who exhibit disruptive behaviors (Stieben et al., 2007), and adolescents who have a parent with a substance use disorder (Euser, Evans, Greaves-Lord, Hulzink, & Franken, 2013). Interestingly, in one study aiming to differentiate children with externalizing problems, researchers found the ERN was more blunted among individuals with pure externalizing problems compared to children who exhibited comorbid internalizing and externalizing problems and healthy controls (Stieben et al., 2007). Furthermore, a blunted ERN during early adolescence prospectively predicts the future initiation of substance use (Anokhin & Golosheykin, in press). Thus, a reduced ERN may be a specific marker of externalizing vulnerability.
As the ERN demonstrates opposing associations with internalizing and externalizing psychopathology and can be reliably elicited in young children, this raises the possibility that the ERN can demarcate distinct developmental pathways from early persistent irritability to later internalizing and externalizing outcomes. That is, the ERN may help identify which children with persistent irritability develop internalizing versus externalizing psychopathology later in life.
The current study sought to examine whether variation in the ERN moderates developmental pathways between preschool persistent irritability and the subsequent emergence of internalizing and externalizing symptoms 6 years later. We hypothesized that among persistently irritable 3-year olds, an enhanced ERN at age 6 would predict an increase in internalizing symptoms at age 9. We also hypothesized that among those same children, a blunted ERN at age 6 would predict an increase in externalizing symptoms at age 9. We used a structured interview to assess persistent irritability in a large community sample of 3-year old children. Three years later, children completed a simple Go/No-Go task while ERPs were recorded. When the children were 9 years old, mothers completed the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2000) to assess their child’s internalizing and externalizing symptoms. To ensure that we were examining the impact of preschool persistent irritability and the ERN on the subsequent development, rather than the continuation, of preexisting internalizing and externalizing symptoms at age 9, we controlled for age 3 CBCL internalizing and externalizing symptoms. Additionally, given that internalizing and externalizing symptomatology often co-occur (Anderson, Williams, McGee, & Silva, 1987; Angold Costello & Erkanai, 1999; Lahey et al., 2015), we also controlled for age 9 externalizing symptoms when predicting internalizing symptoms and vice versa to identify unique predictors of internalizing and externalizing outcomes, respectively.
Method
Participants
Families (N = 559) with 3-year-old children (M =3.55 years, SD = 0.43) were recruited through commercial mailing lists. Children with no significant medical condition or developmental disabilities who were living with at least one English-speaking biological parent were eligible to participate. 541 parents completed a diagnostic clinical interview and questionnaires regarding their 3-year-old child. Of those 541 families, 409 families participated in the age 6 (M =6.11 years, SD = 0.43) follow-up visit assessment and completed electroencephalogram (EEG) tasks. 83 children were excluded due to poor EEG quality and data from one participant was lost due to technical error. Of the remaining 325 families, 304 mothers completed questionnaires about the child’s current internalizing and externalizing symptomatology when s/he was 9 years old (M =9.14 years, SD = 0.32). This report’s final sample (N = 304; 131 females) was 94.7% Caucasian, 3.0% Black or African American, and 2.3% Asian. Ethnically, 8.9% of the final sample was of Hispanic or Latino origin. In 69.3% of families, at least one parent had a college degree. The Institutional Review Board approved all study procedures. Informed consent was obtained from parents, and families were financially compensated for their time.
Procedure
When the children were approximately 3 years old, mothers reported on their child’s current psychopathology. Families were invited back to the lab as close as possible to the child’s 6th birthday. After verbal assents from children were obtained, children began the EEG portion of the visit, including a 20-minute go/no-go task. At age 9, mothers completed questionnaires to assess for current child internalizing and externalizing symptoms.
Measures
Preschool Persistent Irritability
Preschool persistent irritability at age 3 was assessed with the Preschool Age Psychiatric Assessment (PAPA; Egger et al., 2004). A 3-month primary period was used to enhance recall, but symptom onset dates were obtained for all criteria. PAPA items were rated for intensity, frequency, and duration. The intensity rating indicates whether a symptom was absent or present and the extent to which it was intrusive, interfering, and generalizable across activities. A rating of 2 or higher indicates that the symptom was present at a threshold level of intensity. Six items from the PAPA were used to assess irritability (see Dougherty et al., 2013, 2015). Items corresponded to items from the Affective Reactivity Index, a parent-and child-reported chronic irritability scale for older youth (Stringaris et al., 2012). The PAPA items used were: irritable mood (depression section), prone to feelings of anger under minor provocation (depression section), prone to displays of anger under minor provocation (depression section), prone to feelings of frustration under minor provocation (depression section), discrete episodes of temper without violence (ODD section), discrete episodes of excessive temper with violence or attempts at damage directed against oneself, others, or property (ODD section).
Frequency items reflect the number of occurrences during the last 3 months. Following Brotman and colleagues’ (2006) and Copeland and colleagues’ (2013) procedures for assessing chronic irritability, each item was coded as present if a child was prone to the behavior/feeling at least 45 times in the past 3 months. In addition, to assess whether the child experienced irritable mood states for a long time, this criterion was coded present if the child was rated as having at least a 30-minute duration on irritable mood, prone to frustration, annoyance or anger, or difficulty to recover from temper tantrums. The total irritability scale consisted of the sum of symptoms coded as present according to the intensity, frequency, and duration criterion described above (see Dougherty et al. 2013 for further details). The Cronbach alpha coefficient of internal consistency (α) for the persistent irritability scale was .74.
Age 3 and Age 9 Child Internalizing and Externalizing Symptoms
At the age 3 and age 9 assessment, mothers completed the Child Behavior Checklist 1½-5 and 6–18 (CBCL; Achenbach & Rescorla, 2000, 2001), respectively. The CBCL assesses broadband internalizing (36 and 32 items at age 3 and 9, respectively) and externalizing (24 and 33 items at age 3 and 9, respectively) behaviors in children using a 3-point likert scale (0=never true to 2=very true or often true). Coefficient alphas for the internalizing scale were .84 and .86 at age 3 and age 9, respectively; for the externalizing scale, α = .91 at age 3 and α = .88 at age 9. At age 9, we also examined the CBCL internalizing (anxious/depression; 13 items, α = 79; withdrawn/depression: 8 items, α = 71; somatic complaints: 11 items, α = .69) and externalizing (rule breaking: 15 items, α = .58; aggression: 18 items, α = .86; attention problems: 10 items, α = .85) subscales.
Error Monitoring
The Go/No-Go task is a computerized task that requires participants to respond to upward-pointing triangles by pressing a mouse button, and to withhold a response to all other triangles. As previously reported (Torpey et al., 2011), the task was administered using Presentation software (Neurobehavioral Systems). The stimuli were green equilateral triangles in four orientations. On 60% of trials, the triangles were vertically aligned and pointed up; on 20% of the trials; the triangles were vertically aligned and pointed down; on 10% of the trials, the triangles were tilted slightly to the right; and on 10% of trials triangles we tilted slightly to the left.
EEG Data Acquisition and Processing
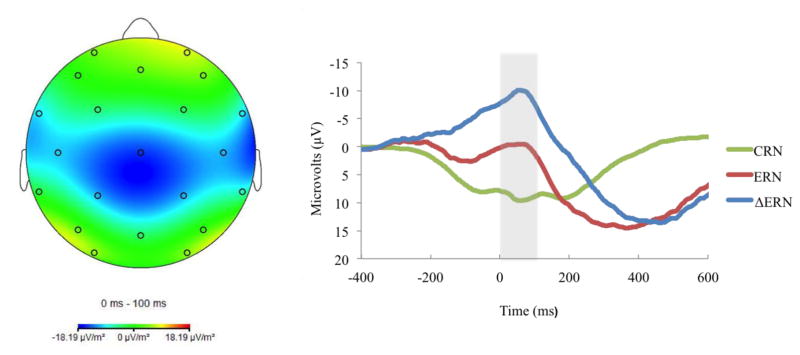
Continuous EEG was recorded using a 32-channel Biosemi system based on the 10/20 system. Data were processed offline with Brain Vision Analyzer (Brain Products, Gilching, Germany). EEG data were referenced to the nose, and high and low pass were filtered at .1 and 30 Hz, respectively. Segments were extracted from the continuous EEG, beginning 500 ms prior to responses. ERP data were corrected for eye movements and blinks using the Gratton, Coles, and Donchin (1983) method. Artifacts were automatically rejected if a voltage step of more than 50 μV between data points occurred, or if a voltage difference of less than .5 μV within a 100-ms interval occurred. After this, data were visually inspected for remaining artifacts. ERP averages were created for incorrect and correct trials. Only errors of commission were classified as incorrect. The baseline of the average activity from −500 to −300 ms prior to the response was subtracted from each data point. The ERN was examined as the difference between the mean amplitude on error relative to correct trials (ΔERN; see Figure 1)1 in the window from 0 to 100 ms after the response, at electrode Cz2 where the difference between error and correct trials was maximal. ERP and behavioral results have been previously reported in the full sample (Meyer et al., 2013; Torpey et al., 2011; Torpey et al., 2013).
Figure 1.
Topographic map of activity (error minus correct) on the left; and response-locked ERP waveforms for correct and error trials, as well as the difference waves at electrode Cz on the right.
Data Analysis
To examine whether error monitoring moderates longitudinal associations between preschool persistent irritability and later internalizing outcomes in middle/late childhood, multiple regression analysis was performed, in which the dependent variable was child internalizing symptoms at age 9 and independent variables included demographics (child gender and age at ERN assessment), age 3 symptoms (broadband internalizing, externalizing and persistent irritability), and age 9 externalizing symptoms, number of errors committed and the interaction between ΔERN and age 3 irritability. As previously mentioned, we included age 3 internalizing and externalizing symptoms to ensure that we were examining the subsequent development of, rather than the continuation of preexisting, internalizing and externalizing symptoms at age 9. We included age 9 externalizing symptoms in order to identify unique predictors of internalizing symptoms, given their frequent-occurrence with internalizing symptoms. We included age at the ERN assessment and number of errors committed as covariates in the model given their significant associations with the ΔERN and ERN and the significant association of number of errors with internalizing symptoms at age 9 (see Table 1). To determine whether error monitoring moderates associations between preschool persistent irritability and later externalizing outcomes, we repeated the multiple regression with age 9 externalizing symptoms as the dependent variable, and replaced age 9 externalizing symptoms with age 9 internalizing symptom as an independent variable. In addition, as follow-up analyses, we repeated the multiple regressions, replacing age 9 internalizing and externalizing symptoms as the dependent variable with each of their respective subscales (internalizing: anxious/depressed, withdrawn/depressed and somatic complaints; and externalizing: attention problems, aggressive behavior and rule breaking). We continued to adjust for age 3 internalizing and externalizing in these models as some of the subscales in the CBCL 1½-5 do not correspond to the subscales in the CBCL 6–18.
Table 1.
Descriptive Statistics and Bivariate Associations between Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gender (Female) | __ | −.11 | −.08 | −.10 | −.06 | −.04 | −.08 | −.07 | −.03 | −.07 | −.06 |
| 2. Age at ERN | __ | .09 | .02 | .07 | −.14* | −.11* | .05 | .00 | .08 | −.05 | |
| 3. Age 3 Irritability | __ | .28** | .49** | −.00 | .00 | .00 | .31** | .38** | −.04 | ||
| 4. Age 3 Internalizing | __ | .56** | −.02 | −.02 | .01 | .44** | .36** | .03 | |||
| 5. Age 3 Externalizing | __ | −.11* | .07 | −.08 | .38** | .53** | 00 | ||||
| 6. Age 6 ΔERN | __ | .79** | −.33** | −.03 | .04 | .17** | |||||
| 7. Age 6 ERN | __ | .31** | .02 | .03 | .13* | ||||||
| 8. Age 6 CRN | __ | .08 | −.02 | −.06 | |||||||
| 9. Age 9 Internalizing Symptoms | .58** | .15* | |||||||||
| 10. Age 9 Externalizing Symptoms | .09 | ||||||||||
| 11. Number of Error Responses | __ | __ | |||||||||
|
| |||||||||||
| M (SD) | 6.11(.43) | .77(1.32) | 8.85(6.29) | 12.92(7.63) | −9.10(9.10) | −.06(9.05) | 9.03(5.83) | 4.20(4.97) | 4.79(5.54) | 16.12(7.67) | |
| Range | 5.15–7.51 | 0–6 | 0–33 | 0–38 | −41.27–25.08 | −30.48–34.88 | −6.15–30.26 | 0–34 | 0–28 | 6–45 | |
p ≤ .05,
p < .01
Results
Table 1 presents the descriptive statistics and the bivariate correlations of the major study variables. As expected, internalizing and externalizing symptoms at both ages 3 and age 9 showed moderate concurrent associations. From age 3 to age 9, both internalizing and externalizing symptoms showed moderate and between construct associations. Consistent with the literature (Dougherty et al., 2013, 2015; Leadbeater & Homel, 2015), age 3 persistent irritability was moderately positively correlated with both concurrent and later internalizing and externalizing symptoms. Both the ΔERN and the ERN were correlated with age and total number of errors, such that a greater differentiation between error and correct trials and a larger negativity to errors was associated with older age and fewer errors. Errors were also associated with greater age 9 internalizing symptoms. A smaller (or more blunted) ΔERN was associated with greater age 3 externalizing symptoms.
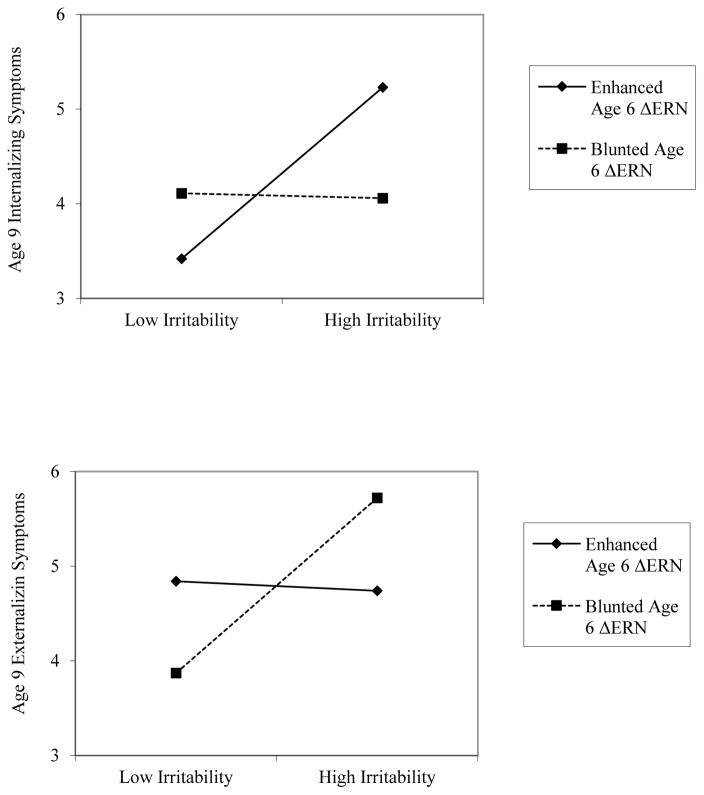
First, a multiple regression analysis was computed with demographic variables (gender and age at ΔERN assessment), age 3 internalizing and externalizing symptoms, age 6 Go/No-Go behavioral performance, and age 9 externalizing symptoms to examine whether the ΔERN at age 6 moderates the longitudinal association between age 3 persistent irritability and age 9 internalizing symptoms. Entry standardized regression coefficients, partial rs, ΔR2 and total model R2, are presented in Table 2. Greater age 3 internalizing symptoms, age 9 externalizing symptoms, and Go/No-Go errors significantly predicted greater age 9 internalizing symptoms. There were no significant main effects of the ΔERN or age 3 persistent irritability on age 9 internalizing symptoms. However, the interaction between early persistent irritability and the ΔERN was significant. To probe this interaction, simple slope terms were tested (Aiken, West & Reno, 1991) (see Figure 1). For children with a more enhanced or negative ΔERN, greater early persistent irritability predicted increased internalizing symptoms at age 9, (b=.69, t=2.90, p=.004). However, among children with a smaller, or more blunted ΔERN, greater early persistent irritability did not predict age 9 internalizing symptoms (b=−.02, t=−.10, p=.91). To determine the values of irritability at which varying levels of ΔERN differ significantly on age 9 internalizing symptoms, we conducted a regions of significance test (RoS; Hayes & Matthes, 2009). The RoS revealed that when irritability was .74 SD above the mean, an enhanced ΔERN was associated with an increase in internalizing symptoms.
Table 2.
Multiple Regressions with Interactions between Age 3 Persistent Irritability and Age 6 ΔERN Predicting Children’s Internalizing and Externalizing Symptoms at Age 9
| Age 9 Internalizing | ||
|---|---|---|
|
| ||
| Predictor | Entry β | Partial r |
| ΔR2 = .01, F(1, 294) = 6.66** | ||
|
| ||
| Gender | .03 | .04 |
| Age at ERN | −.05 | −.06 |
| Age 3 Persistent Irritability | .10 | .11 |
| Age 3 Internalizing Symptoms | .28*** | .29**. |
| Age 3 Externalizing Symptoms | −.07 | −.07 |
| Age 6 ΔERN | −.06 | −.05 |
| Age 6 Number of Errors | .11* | .14* |
| Age 9 Externalizing Symptoms | .48** | .47** |
| Age 3 Irritability X Age 6 ΔERN | −.15** | |
|
| ||
| Total Model R2 = .43, F(9, 294) = 25.04*** | ||
|
| ||
| Age 9 Externalizing | ||
|
| ||
| ΔR2 = .01, F(1, 294) = 6.32* | ||
|
| ||
| Gender | −.03 | −.03 |
| Age at ERN | .05 | .07 |
| Age 3 Persistent Irritability | .08 | .10 |
| Age 3 Internalizing Symptoms | −.07 | −.07 |
| Age 3 Externalizing Symptoms | .35*** | .34*** |
| Age 6 ΔERN | .01 | −.01 |
| Age 6 Number of Errors | .03 | .04 |
| Age 9 Internalizing Symptoms | .44*** | .47** |
| Age 3 Irritability X Age 6 ΔERN | .15* | |
|
| ||
| Total Model R2 = .69, F(9, 294) = 29.55*** | ||
p ≤ .05,
p < .01
Next, we re-ran the model to examine change in age 9 externalizing symptoms, while controlling for age 9 internalizing symptoms (see Table 2). Greater age 3 externalizing and age 9 internalizing symptoms significantly predicted greater age 9 externalizing symptoms. There were no significant main effects of the ΔERN or age 3 persistent irritability on age 9 externalizing symptoms. However, the interaction between age 3 persistent irritability and ΔERN significantly predicted age 9 externalizing symptoms (see Figure 2). For children with a smaller or less negative ΔERN, greater early persistent irritability predicted increased externalizing symptoms (b=.71, t= 2.04, p=.04). However, there was no significant association between early persistent irritability and age 9 externalizing symptoms in children with a greater or more enhanced ΔERN (b=.01, t=.04 p=.83). The RoS revealed that when irritability was 1.96 SD above the mean, a blunted compared to an enhanced ΔERN was associated with an increase in externalizing symptoms.
Figure 2.
Significant interactions between early persistent irritability and the age 6 ΔERN at 1 SD above and below the mean in predicting age 9 internalizing (top) and externalizing (bottom) symptoms. ERN = error related negativity
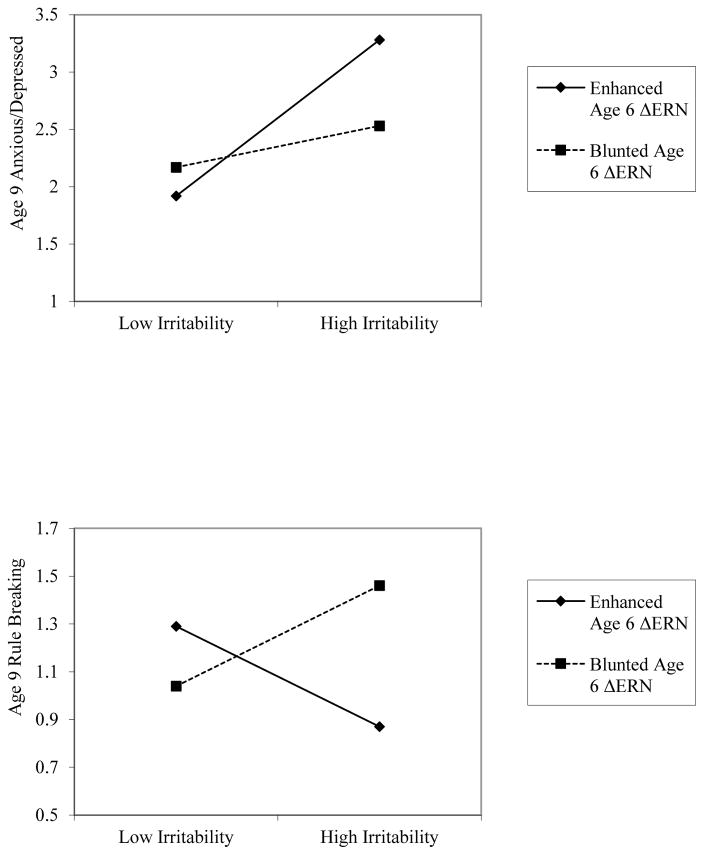
To further examine the specificity of the interaction between early persistent irritability and the ΔERN on age 9 internalizing symptoms, we reran the model examining the development of age 9 internalizing symptoms 3 more times, replacing age 9 internalizing symptoms with the CBCL anxious/depression, withdrawn/depression and somatic complaints subscales as the dependent variable. There was a significant interaction between age 3 persistent irritability and ΔERN predicting age 9 anxious/depression (partial r = −.13, p < .05). For children with a larger or more negative ΔERN, greater early persistent irritability predicted increased anxious/depression (b=.54, t=3.61, p < .001; see Figure 3). There was no significant association between early persistent irritability and age 9 anxious/depression in children with a smaller or less negative ΔERN (b=.14, t=.95 p=.34). There were no significant interactions between age 3 persistent irritability and ΔERN predicting withdrawn/depression (p = .18) or somatic complaints (p = .07). The RoS revealed that when irritability was .91 SD above the mean, an enhanced compared to a blunted ΔERN was associated with an increase in anxious/depression.
Figure 3.
Significant interactions between early persistent irritability and the age 6 ΔERN at 1 SD above and below the mean in predicting age 9 anxious/depression (top) and rule breaking (bottom) symptoms. ERN = error related negativity
Finally, to examine the specificity of the interaction on age 9 externalizing symptoms, we also reran the model examining the development of age 9 externalizing symptoms 3 more times, replacing age 9 externalizing symptoms with the CBCL attention problems, aggressive behavior and rule breaking subscales as the dependent variable. There was a significant interaction between age 3 persistent irritability and ΔERN predicting age 9 rule breaking (partial r = .17, p < .001). For children with a smaller or less negative ΔERN, greater early persistent irritability predicted increased rule breaking (b=.17, t= 2.22, p < .05; see Figure 3). There was also a significant negative association between early persistent irritability and age 9 rule breaking in children with a larger or more negative ΔERN (b=−.15, t=−1.94 p = .05), such that greater early persistent irritability predicted less rule breaking. There were no significant interactions between age 3 persistent irritability and ΔERN predicting attention problems (p = .93) or aggressive behavior (p = .07). The RoS revealed that when irritability was 0.40 SD above the mean, a blunted compared to an enhanced ΔERN was associated with an increase in rule breaking behavior.
Discussion
The current study used a multilevel approach to explore how irritability, a transdiagnostic construct that likely contributes to comorbidity, leads to a broad range of multifinal outcomes. We examined if individual differences in the ΔERN, a neural indicator of error monitoring, can predict whether early persistent irritability—a prototypical transdiagnostic construct— is associated with later internalizing versus externalizing outcomes. We found that among children with higher levels of irritability at age 3, an enhanced or more negative ΔERN at age 6 predicted the development of more internalizing symptoms at age 9, whereas a blunted ΔERN at age 6 predicted the development of more externalizing symptoms at age 9. Importantly, these longitudinal associations were evident after adjusting for preexisting internalizing and externalizing symptoms at age 3.
Prior research indicates that the ΔERN has opposing associations with externalizing and internalizing psychopathology (Hall et al., 2007; Olvet & Hajcak, 2009; Stieben et al., 2007). However, our findings are the first to suggest that in the same group of children, variation in the ΔERN can predict internalizing versus externalizing outcomes over the course of childhood. Thus, in contrast to several commonly researched biomarkers, such as the P300 and diminished autonomic responding, which likely reflect a general vulnerability to psychopathology (Beauchaine & Thayer, 2015), individual differences in the ΔERN reflect a more specific vulnerability and can distinguish between internalizing and externalizing psychopathology.
Our data raise the possibility that the degree to which children with persistent irritability monitor their behavior, as indexed by the ΔERN at age 6, influences how their emotional distress is experienced and expressed. The ΔERN currently appears as a measure in three RDoC domains: Cognitive Systems (Cognitive Control); Negative Valence Systems (Sustained Threat), and Positive Valence Systems (Reward Learning). Unfortunately, RDoC does not address the relationships, overlap, or interaction between domains. Thus, it is not clear if the ΔERN fits into any one domain better than others to or whether it derives from multiple, interacting biobehavioral systems. For example, irritable children who engage in more intensive self-monitoring—as indexed by a potentiated or more negative ΔERN at age 6—may be more sensitive to threat and prone to fear and anxiety, and/or they have greater cognitive control resources to inhibit angry and acting-out behavior. Conversely, young children who have a propensity to experience high levels of negative emotional reactivity but who do not monitor their performance may be disinhibited in expressing their distress because of deficient cognitive control and/or a lack of concern about the consequences of their behavior, and therefore manifest it externally in the form of conduct problems.
In the current study, early persistent irritability was associated with both later internalizing and externalizing symptoms. As noted earlier, there is an emerging body of research suggesting that there is a general psychopathology factor that accounts for the shared association between Internalizing and Externalizing (Caspi et al., 2013; Lahey et al., 2012, 2015; Olino et al., 2014). This factor is believed to reflect the liability to develop a broad range of psychopathologies (Caspi et al., 2013; Lahey et al., 2012). Interestingly, studies have shown that childhood negative emotionality and emotional dysregulation constructs that overlap substantially with irritability are salient early developmental features of the general factor (Caspi et al., 2013; Olino et al., 2014; Rhee et al., 2015). Thus, it is plausible that early persistent irritability reflects a nonspecific liability to develop psychopathology (Mulraney et al., 2014), and it is the interplay with other biological and environmental factors, such as the ΔERN, that steers the expression of this propensity along a continuum from internalizing to externalizing problems.
There was no main effect of irritability on the ΔERN, suggesting that irritability observed in very young children is heterogeneous with respect to error monitoring. Thus, it remains unclear why some irritable children have a relatively more enhanced or blunted ΔERN. Recent evidence indicates that the ΔERN is heritable (Anokhin et al., 2008), but the environment also influences its development as harsh parenting (Meyer, Proudfit et al., 2015) and uncertainty (Jackson, Nelson & Hajcak, 2014) have been found to potentiate the ΔERN.
In addition, the ΔERN at age 6 did not predict increases in either externalizing or internalizing symptoms in the absence of persistent irritability, suggesting that it is the combination of irritability and variation in error monitoring that contribute to the development of later internalizing and externalizing symptoms. Interestingly, similar results have been previously found in the context of BI—a temperament profile associated with risk for anxiety disorders—such that an enhanced ERN predicted the development of anxiety symptoms only among children who had a history of high BI (Lahat et al., 2014; McDermott et al., 2009). Our findings that, in irritable children, an increased ΔERN predicted increases in internalizing while a diminished ΔERN predicted increases in externalizing symptoms suggests that variation in the ΔERN may not exist on a continuum that ranges from normal to abnormal functioning, but rather that it may be related to differences in behavioral styles associated with cognitive control, sustained threat, and/or reward learning that may not be pathological on their own, but interact with or possibly even shape the specific expression of a more general liability for psychopathology, like irritability.
The follow-up analyses examining the specificity of early irritability and the ΔERN with respect to internalizing and externalizing outcomes suggest that our findings apply most specifically to anxious/depression symptoms and rule breaking behaviors, respectively. We also found that persistently irritable children who demonstrated an increased ΔERN at age 6 went on to develop fewer rule breaking behaviors at age 9. An enhanced ΔERN may therefore also play a protective role against the development of at least some forms of externalizing behaviors. The fact that the ΔERN did not interact with early persistent irritability to predict symptoms of withdrawn/depression or somatic complaints is in line with an accumulating body of research that suggests that associations between ΔERN and internalizing disorders may be primarily driven by the presence of punishment sensitivity that is most characteristic of anxiety disorders, and that the motivational deficits observed in some forms of depression (e.g. anhedonic depression) may blunt the ΔERN (Weinberg, Klein & Hajcak, 2012, Weinberg, Kotov & Proudfit, 2015). However, the lack of longitudinal associations between early irritability, the ΔERN and withdrawn/depression may also be due to the restricted range of depressive symptoms in our sample, owing to the young age of the children. Finally, childhood irritability, anxiety, and externalizing disorders all predict depressive disorders in adolescence and adulthood (Cummings et al., 2014; Stringaris & Goodman, 2009; Stringaris et al., 2009). As an enhanced and blunted ΔERN have both been associated with depression in adults (Chiu & Deldin, 2007; Holmes & Pizzagalli, 2008, 2010; Weinberg, Klein & Hajcak, 2012), it is conceivable that both developmental pathways from early irritability to age 9 internalizing and externalizing symptoms may subsequently converge on depressive outcomes in adolescence or adulthood. However, it will be necessary for future longitudinal studies to examine this possibility.
Although a more blunted ΔERN has previously been linked to aggression (von Borries et al., 2010) and ADHD (Van De Voorde et al., 2010), we found no association between the combination of early irritability and a blunted ΔERN to predict their subsequent development. Heterogeneity in aggressive behaviors and attention problems may account for these discrepancies (Costa Dias et al., 2015; Stieben et al., 2007). For example, persistently irritable children are more likely to exhibit reactive aggression or anger in response to a perceived threat (Berkowitz, 1983; Dodge, 1980), which may have dissociable neural correlates from proactive or instrumental aggression characterized by a blunted ΔERN (Stieben et al., 2007).
This study had several strengths. First, we used dimensional measures of internalizing and externalizing symptoms and multiple level of analysis (e.g., neural measures such as the ΔERN) to understand how transdiagnostic factors, such as irritability, lead to multifinality. This approach can guide further exploration of the mechanisms by which other transdiagnostic factors lead to multiple outcomes. Second, we used an unselected community sample, which is important as irritability is common in the course of development. Third, our sample size was large enough and had enough statistical power to examine irritability by ΔERN interactions. Finally, our prospective longitudinal design allowed us to examine development over a six-year period.
Despite these strengths, this study also has limitations. First, as there is no validated measure for persistent irritability in preschoolers, we derived our own measure using items from a well-validated diagnostic interview (Egger et al., 2004) and guided by the content of a well-validated scale for irritability in older children (Stringaris et al., 2010). Although it was derived ad hoc, our measure has shown good concurrent and predictive validity in previous reports from our group (Dougherty et al., 2013, 2015) and others (Brotman et al., 2006; Copeland et al., 2013). Second, we did not examine current manifestations of persistent irritability; thus we are unable to determine whether and the degree to which variation in the ΔERN influenced the homotypic continuity of irritability. Lastly, we were unable to assess ΔERN at age 3, hence we cannot determine how early in development error monitoring begins to influence the link between irritability and internalizing and externalizing symptoms.
The present study is the first to examine the developmental mechanisms through which the transdiagnostic construct of irritability leads to subsequent internalizing and externalizing psychopathology across childhood. Our results suggest that variation in a neural index of error monitoring differentiates, and may even shape, developmental trajectories from preschool persistent irritability to internalizing and externalizing outcomes in middle-late childhood. They also point to the possible utility of using the ΔERN to anticipate which direction a persistently irritable child’s subsequent psychopathology is likely to take in order to inform prevention and intervention efforts. An enhanced ΔERN may indicate that a child with persistent irritability will go on to develop primarily internalizing manifestations of psychopathology, whereas a blunted ΔERN may indicate the subsequent development of externalizing psychopathology. These findings underscore the importance of considering the dynamic interplay of different units of analysis across development when examining transdiagnostic constructs and multifinality in youth psychopathology.
Acknowledgments
This work was supported by National Institute of Mental Health grant R01 MH069942 (Klein).
Footnotes
To rule out the possibility that reactivity to correct as opposed to error trials were driving effects, we examined ERN and CRN residuals, which reflect the difference between an individual’s observed response to the outcome of interest and what would be predicted from an individual’s response to the alternate outcome. These residuals are independent from the average response to the alternate outcome, but correlated with the average response to the outcome of interest. When we examined the ERN residual, results were virtually identical. When we examined the CRN residual, there were no significant interactions with preschool persistent irritability to predict age 9 internalizing and externalizing outcomes.
Given evidence that there may be topographical differences in the distribution of the ΔERN in anxious children, such that children with anxiety have a more frontally distributed ΔERN (Meyer, Proudfit, Torpey-Newman, Kujawa & Klein, 2015), we also examined the ΔERN at electrode Fz. All results were virtually identical.
Disclosures: G.A.C. has received funding from Otsuka-BMS, Schering, Pfizer and Merck.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA preschool forms and profiles: An integrated system of multi-informant assessment. Burlington: ASEBA; 2000. [Google Scholar]
- Achenbach TM, Rescorla L. ASEBA school-age forms & profiles. Burlington: ASEBA; 2001. [Google Scholar]
- Aebi M, Barra S, Bessler C, Steinhausen H, Walitza S, Plattner B. Oppositional defiant disorder dimensions and subtypes among detained male adolescent offenders. Journal of Child Psychology & Psychiatry. doi: 10.1111/jcpp.12473. in press. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG, Reno RR. Multiple regression: Testing and interpreting interactions. Sage; 1991. [Google Scholar]
- Anokhin AP, Golosheykin S. Neural correlates of error monitoring in adolescents prospectively predict initiation of tobacco use. Developmental Cognitive Neuroscience. 2015 doi: 10.1016/j.dcn.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S, Heath AC. Heritability of frontal brain function related to action monitoring. Psychophysiology. 2008;45(4):524–534. doi: 10.1111/j.1469-8986.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: Author; 1980. text rev. [Google Scholar]
- Anderson JC, Williams S, McGee R, Silva PA. DSM-III disorders in preadolescent children: Prevalence in a large sample from the general population. Archives of General Psychiatry. 1987;44(1):69–76. doi: 10.1001/archpsyc.1987.01800130081010. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A. Comorbidity. Journal of Child Psychology and Psychiatry. 1999;40(01):57–87. [PubMed] [Google Scholar]
- Beauchaine TP, Thayer J. Heart rate variability as a transdiagnostic biomarker of psychopathology. International Journal of Psychophysiology. 2015;98:338–350. doi: 10.1016/j.ijpsycho.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Boyd JH, Burke JD, Gruenberg E, Holzer CE, Rae DS, George LK, … Nestadt G. Exclusion criteria of DSM-III: A study of co-occurrence of hierarchy-free syndromes. Archives of General Psychiatry. 1984;41(10):983–989. doi: 10.1001/archpsyc.1984.01790210065008. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Schmajuk M, Rich BA, Dickstein DP, Guyer AE, Costello EJ, … Leibenluft E. Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biological Psychiatry. 2006;60(9):991–997. doi: 10.1016/j.biopsych.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Burke JD. An affective dimension within oppositional defiant disorder symptoms a mong boys: personality and psychopathology outcomes into early adulthood. Journal of Child Psychology and Psychiatry. 2012;53(11):1176–1183. doi: 10.1111/j.1469-7610.2012.02598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Cha J, Mujica-Parodi LR. Functional and structural amygdala–anterior cingulate connectivity correlates with attentional bias to masked fearful faces. Cortex. 2013;49(9):2595–2600. doi: 10.1016/j.cortex.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Carter CS, Van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(4):367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Oliveri ME, Insel T. A neurodevelopmental perspective on the Research Comain Criteria (RDoC) framework. Biological Psychiatry. 2014;76(5):350–353. doi: 10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, … Moffitt TE. The p factor one general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science. 2014;2(2):119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D. A multiple-levels-of-analysis perspective on research in development and psychopathology. In: Beauchaine TP, Hinshaw SP, editors. Child and Adolescent Psychopathology. Hoboken, NJ: Wiley; 2008. pp. 27–57. [Google Scholar]
- Chiu PH, Deldin PJ. Neural evidence for enhanced error detection in major depressive disorder. American Journal of Psychiatry. 2007;164(4):608–616. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Angold A, Costello EJ, Egger H. Prevalence, comorbidity, and correlates of DSM-5 proposed disruptive mood dysregulation disorder. American Journal of Psychiatry. 2013;170:173–179. doi: 10.1176/appi.ajp.2012.12010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Brotman MA, Costello EJ. Normative irritability in youth: Developmental findings from the Great Smoky Mountains Study. Journal of the American Academy of Child & Adolescent Psychiatry. 2015;54(8):635–642. doi: 10.1016/j.jaac.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Egger H, Angold A, Costello EJ. Adult diagnostic and functional outcomes of DSM-5 disruptive mood dysregulation disorder. American Journal of Psychiatry. 2014;171(6):668. doi: 10.1176/appi.ajp.2014.13091213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. Neuroimage. 2005;27(4):885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Cummings CM, Caporino NE, Kendall PC. Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychological Bulletin. 2014;140(3):816. doi: 10.1037/a0034733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias TGC, Iyer SP, Carpenter SD, Cary RP, Wilson VB, Mitchel SH, … Fair DA. Characterizing heterogeneity in children with and without ADHD based on reward system connectivity. Developmental Cognitive Neuroscience. 2015;11:155–174. doi: 10.1016/j.dcn.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty LR, Smith VC, Bufferd SJ, Stringaris A, Leibenluft E, Carlson GA, Klein DN. Preschool irritability: longitudinal associations with psychiatric disorders at age 6 and parental psychopathology. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52(12):1304–1313. doi: 10.1016/j.jaac.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty LR, Smith VC, Bufferd SJ, Kessel E, Carlson GA, Klein DN. Preschool irritability predicts child psychopathology, functional impairment, and service use at age nine. Journal of Child Psychology and Psychiatry. 2015 doi: 10.1111/jcpp.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A. Test-retest reliability of the preschool age psychiatric assessment (PAPA) Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- Euser AS, Evans BE, Greaves-Lord K, Huizink AC, Franken IH. Diminished error-related brain activity as a promising endophenotype for substanceuse disorders: evidence from high-risk offspring. Addiction Biology. 2013;18(6):970–984. doi: 10.1111/adb.12002. [DOI] [PubMed] [Google Scholar]
- Foulds GA. The hierarchical nature of personal illness. London: Academic Press; 1976. [Google Scholar]
- Gehring WJ, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. The Journal of Neuroscience. 2001;21(23):9430–9437. doi: 10.1523/JNEUROSCI.21-23-09430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Foti D. Errors are aversive defensive motivation and the error- related negativity. Psychological Science. 2008;19(2):103–108. doi: 10.1111/j.1467-9280.2008.02053.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: Error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology. 2003;40(6):895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- Hall JR, Bernat EM, Patrick CJ. Externalizing psychopathology and the error-related negativity. Psychological Science. 2007;18(4):326–333. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behavior Research Methods. 2009;41(3):924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Hink LK, Rhee SH, Corley RP, Cosgrove VE, Hewitt JK, Schulz-Heik RJ, … Waldman ID. Personality dimensions as common and broadband-specific features for internalizing and externalizing disorders. Journal of Abnormal Child Psychology. 2013;41(6):939–957. doi: 10.1007/s10802-013-9730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Archives of General Psychiatry. 2008;65(2):179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Effects of task-relevant incentives on the electrophysiological correlates of error processing in major depressive disorder. Cognitive, Affective, & Behavioral Neuroscience. 2010;10(1):119–128. doi: 10.3758/CABN.10.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):679. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Inzlicht M, Bartholow BD, Hirsch JB. Emotional foundations of cognitive control. Trends in Cognitive Sciences. 2015;19(3):126–132. doi: 10.1016/j.tics.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson F, Nelson BD, Proudfit GH. In an uncertain world, errors are more aversive: Evidence from the error-related negativity. Emotion. 2015;15(1):12. doi: 10.1037/emo0000020. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, … Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Klein DN, Riso LP. Psychiatric diagnoses: Problems of boundaries and co- occurrences. In: Costello CG, editor. Basic issues in psychopathology. New York: Guilford Press; 1993. pp. 19–66. [Google Scholar]
- Kotov R, Ruggero CJ, Krueger RF, Watson D, Yuan Q, Zimmerman M. New dimensions in the quantitative classification of mental illness. Archives of General Psychiatry. 2011;68(10):1003–1011. doi: 10.1001/archgenpsychiatry.2011.107. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry. 2006;47(10):1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Lahat A, Lamm C, Chronis-Tuscano A, Pine DS, Henderson HA, Fox NA. Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53(4):447–455. doi: 10.1016/j.jaac.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, Hakes JK, Zald DH, Hariri AR, Rathouz PJ. Is there a general factor of prevalent psychopathology during adulthood? Journal of Abnormal Psychology. 2012;121(4):971. doi: 10.1037/a0028355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Rathouz PJ, Keenan K, Stepp SD, Loeber R, Hipwell AE. Criterion validity of the general factor of psychopathology in a prospective study of girls. Journal of Child Psychology and Psychiatry. 2015;56(4):415–422. doi: 10.1111/jcpp.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Weissman MM, Merikangas KR, Pauls DL, Prusoff BA. Panic disorder and major depression: Increased risk of depression, alcoholism, panic, and phobic disorders in families of depressed probands with panic disorder. Archives of General Psychiatry. 1983;40(10):1055–1060. doi: 10.1001/archpsyc.1983.01790090017002. [DOI] [PubMed] [Google Scholar]
- Leadbeater BJ, Homel J. Irritable and defiant sub-dimensions of ODD: Their stability and prediction of internalizing symptoms and conduct problems from adolescence to young adulthood. Journal of Abnormal Child Psychology. 2015;43(3):407–421. doi: 10.1007/s10802-014-9908-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Cohen P, Gorrindo T, Brook JS, Pine DS. Chronic versus episodic irritability in youth: a community-based, longitudinal study of clinical and diagnostic associations. Journal of Child & Adolescent Psychopharmacology. 2006;16(4):456–466. doi: 10.1089/cap.2006.16.456. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO. Comorbidity between and within childhood externalizing and internalizing disorders: Reflections and directions. Journal of Abnormal Child Psychology. 2003;31(3):285–291. doi: 10.1023/a:1023229529866. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65(5):445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Bress J, Proudfit GH. Psychometric properties of the error-related negativity in children and adolescents. Psychophysiology. 2014;51(7):602–610. doi: 10.1111/psyp.12208. [DOI] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey DC, Kujawa A, Kim J, Bufferd S, … Klein DN. Increased error-related brain activity in six-year-old children with clinical anxiety. Journal of Abnormal Child Psychology. 2013;41(8):1257–1266. doi: 10.1007/s10802-013-9762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Proudfit GH, Bufferd SJ, Kujawa AJ, Laptook RS, Torpey DC, Klein DN. Self-reported and observed punitive parenting prospectively predicts increased error-related negativity in six-year-old children. Journal of Abnormal Child Psychology. 2015;43:821–829. doi: 10.1007/s10802-014-9918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Proudfit GH, Torpey-Newman DC, Kujawa A, Klein DN. Increased error-related brain activity in children predicts the onset of anxiety disorders three years later. Journal of Abnormal Psychology. 2015;124:266–274. doi: 10.1037/abn0000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulraney M, Melvin G, Tonge B. Brief report: Can irritability act as a marker of psychopathology? Journal of Adolescence. 2014;37(4):419–423. doi: 10.1016/j.adolescence.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Nelson CA, McCleery JP. Use of event-related potentials in the study of typical and atypical development. Journal of American Acadamy of Child and Adolescent Psychiatry. 2008;47(11):1252–1261. doi: 10.1097/CHI.0b013e318185a6d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Watkins ER. A heuristic for developing transdiagnostic models of psychopathology explaining multifinality and divergent trajectories. Perspectives on Psychological Science. 2011;6(6):589–609. doi: 10.1177/1745691611419672. [DOI] [PubMed] [Google Scholar]
- Olino TM, Dougherty LR, Bufferd SJ, Carlson GA, Klein DN. Testing models of psychopathology in preschool-aged children using a structured interview-based assessment. Journal of Abnormal Child Psychology. 2014;42:1201–1211. doi: 10.1007/s10802-014-9865-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clinical Psychology Review. 2008;28(8):1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pailing PE, Segalowitz SJ, Dywan J, Davies PL. Error negativity and response control. Psychophysiology. 2002;39(2):198–206. doi: 10.1017/S0048577202010247. [DOI] [PubMed] [Google Scholar]
- Potts GF, George MRM, Martin LE, Barratt ES. Reduced punishment sensitivity in neural systems of behavior monitoring in impulsive individuals. Neuroscience Letters. 2006;397(1):130–134. doi: 10.1016/j.neulet.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Proudfit GH, Inzlicht M, Mennin DS. Anxiety and error monitoring: the importance of motivation and emotion. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Lahey BB, Waldman ID. Comorbidity among dimensions of childhood psychopathology: Converging evidence from behavior genetics. Child Development Perspectives. 2015;9(1):26–31. doi: 10.1111/cdep.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Kathmann N, Hajcak G. Punishment has a lasting impact on error-related brain activity. Psychophysiology. 2012;49(2):239–247. doi: 10.1111/j.1469-8986.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Meyer A, Hajcak G. The ERN is the ERN is the ERN? Convergent validity of error-related brain activity across different tasks. Biological Psychology. 2013;93(3):377–385. doi: 10.1016/j.biopsycho.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Robins E, Guze SB. Establishment of diagnostic validity in psychiatric illness: Its application to schizophrenia. American Journal of Psychiatry. 1970;126(7):983–7. doi: 10.1176/ajp.126.7.983. [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Seeley JR. Comorbidity of unipolar depression: II. Comorbidity with other mental disorders in adolescents and adults. Journal of Abnormal Psychology. 1991;100:214–222. [PubMed] [Google Scholar]
- Segalowitz SJ, Santesso DL, Murphy TI, Homan D, Chantziantoniou DK, Khan S. Retest reliability of medial frontal negativities during performance monitoring. Psychophysiology. 2010;47(2):260–270. doi: 10.1111/j.1469-8986.2009.00942.x. [DOI] [PubMed] [Google Scholar]
- Stieben J, Lewis MD, Granic I, Zelazo PD, Segalowitz S, Pepler D. Neurophysiological mechanisms of emotion regulation for subtypes of externalizing children. Development and Psychopathology. 2007;19(02):455–480. doi: 10.1017/S0954579407070228. [DOI] [PubMed] [Google Scholar]
- Stringaris A, Cohen P, Pine DS, Leibenluft E. Adult outcomes of youth irritability: a 20-year prospective community-based study. American Journal of Psychiatry. 2009;166(9):1048–1054. doi: 10.1176/appi.ajp.2009.08121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Goodman R. Longitudinal outcome of youth oppositionality: irritable, headstrong, and hurtful behaviors have distinctive predictions. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(4):404–412. doi: 10.1097/CHI.0b013e3181984f30. [DOI] [PubMed] [Google Scholar]
- Stringaris A, Goodman R, Ferdinando S, Razdan V, Muhrer E, Leibenluft E, Brotman MA. The Affective Reactivity Index: A concise irritability scale for clinical and research settings. Journal of Child Psychology and Psychiatry. 2012;53(11):1109–1117. doi: 10.1111/j.1469-7610.2012.02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Taylor E. Disruptive mood: Irritability in children and adolescents. Oxford University Press; 2015. [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13(2):160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Kim J, Kujawa A, Klein DN. Electrocortical and behavioral measures of response monitoring in young children during a Go/No-Go task. Developmental Psychobiology. 2012;54(2):139–150. doi: 10.1002/dev.20590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Kim J, Kujawa AJ, Dyson MW, Olino TM, Klein DN. Error-related brain activity in young children: associations with parental anxiety and child temperamental negative emotionality. Journal of Child Psychology and Psychiatry. 2013;54(8):854–862. doi: 10.1111/jcpp.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Voorde S, Roeyers H, Wiersema JR. Error monitoring in children with ADHD or reading disorder: An event-related potential study. Biological Psychology. 2010;84(2):176–185. doi: 10.1016/j.biopsycho.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Vilà-Balló A, Hdez-Lafuente P, Rostan C, Cunillera T, Rodriguez-Fornells A. Neurophysiological correlates of error monitoring and inhibitory processing in juvenile violent offenders. Biological Psychology. 2014;102:141–152. doi: 10.1016/j.biopsycho.2014.07.021. [DOI] [PubMed] [Google Scholar]
- Von Borries AKL, Brazil IA, Bulten BH, Buitelaar JK, Verkes RJ, De Bruijn ERA. Neural correlates of error-related learning deficits in individuals with psychopathy. Psychological Medicine. 2010;40(09):1559–1568. doi: 10.1017/S0033291709992017. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Estabrook R, Petitclerc A, Henry D, Burns JL, Perlman SB, … Briggs-Gowan ML. Clinical Implications of a Dimensional Approach: The Normal: Abnormal Spectrum of Early Irritability. Journal of the American Academy of Child & Adolescent Psychiatry. 2015;54(8):626–634. doi: 10.1016/j.jaac.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Dieterich R, Riesel A. Error-related brain activity in the age of RDoC: A review of the literature. International Journal of Psychophysiology. 2015 doi: 10.1016/j.ijpsycho.2015.02.029. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Longer term test–retest reliability of error-related brain activity. Psychophysiology. 2011;48(10):1420–1425. doi: 10.1111/j.1469-8986.2011.01206.x. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Klein DN, Hajcak G. Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. Journal of Abnormal Psychology. 2012;121(4):885. doi: 10.1037/a0028270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Kotov R, Proudfit G. Neural indicators of error processing in generalized anxiety disorder, obsessive-compulsive disorder, and major depressive disorder. Journal of Abnormal Psychology. 2015;124(1):172–185. doi: 10.1037/abn0000019. [DOI] [PubMed] [Google Scholar]
- Wright AG, Krueger RF, Hobbs MJ, Markon KE, Eaton NR, Slade T. The structure of psychopathology: toward an expanded quantitative empirical model. Journal of Abnormal Psychology. 2013;122(1):281–294. doi: 10.1037/a0030133. [DOI] [PMC free article] [PubMed] [Google Scholar]