Abstract
Regulatory T cells (Tregs) are essential for the establishment and maintenance of immune tolerance, suggesting a potential therapeutic role for Tregs in transplantation. However, Treg administration alone is insufficient in inducing long-term allograft survival in normal hosts, likely due to the high frequency of alloreactive T cells. We hypothesized that a targeted reduction of alloreactive T effector cells would allow a therapeutic window for Treg efficacy. Here we show that preconditioning recipient mice with donor-specific transfusion followed by cyclophosphamide treatment deleted 70–80% donor-reactive T cells, but failed to prolong islet allograft survival. However, infusion of either 5 ×106 Tregs with direct donor reactivity or 25 ×106 polyclonal Tregs led to indefinite survival of BALB/c islets in more than 70% of preconditioned C57BL/6 recipients. Notably, protection of C3H islets in autoimmune nonobese diabetic mice required islet autoantigen-specific Tregs together with polyclonal Tregs. Treg therapy led to significant reduction of CD8+ T cells and concomitant increase in endogenous Tregs among graft-infiltrating cells early after transplantation. Together, these results demonstrate that reduction of the donor-reactive T cells will be an important component of Treg-based therapies in transplantation.
Keywords: CD8+ T cell, diabetes, T cell deletion, Treg therapy
Introduction
The alloimmune response is exceptionally robust in part due to the high frequency of alloreactive T cells, which is estimated to be 0.1–10% of an individual’s T cell repertoire. In contrast, the frequency for nominal peptide antigens is 100–10 000-fold lower (1,2).
Regulatory T cells (Tregs) are a small subset (2–10%) of CD4+T cells that express CD25 and the transcription factor, Foxp3. Deficiency in Tregs leads to fatal autoimmune diseases, demonstrating that Tregs are essential for ensuring immune self-tolerance and suggesting therapeutic potential of Treg administration. In many experimental models of autoimmunity, Tregs are efficacious in preventing and even reversing disease (3–6). Ample data from various transplantation models demonstrate that Tregs are an essential component of allograft tolerance (7–14). However, Treg infusion alone into lymphoreplete hosts only marginally prolongs allograft survival (15). Significant prolongation of allograft survival has been generally achieved only in the setting of lymphopenic hosts, and usually requires co-transfer of one to five Tregs per effector T cells (14), not a feasible ratio in normal circumstances. Thus, reduction of the alloreactive T effector pool is likely to be an important factor in Treg therapy in transplantation (16).
In this study, we tested therapeutic efficacy of combining Treg infusion and donor-reactive T cell deletion in protecting allogeneic grafts in lymphoreplete hosts using a fully MHC-mismatched murine islet transplantation model. We report that long-term survival of islet allografts can be achieved using Treg therapy when 70–80% of donor-reactive T cells have been deleted from nonautoimmune and autoimmune recipients. We further demonstrated that Treg therapy reduced CD8+ T cell infiltrates early after transplant and created an immune-privileged microenvironment locally in the grafts to confer long-term protection. These results provide important conceptual framework for designs of future clinical trials using Tregs in transplantation.
Materials and Methods
Mice
C57BL/6 (B6; H-2b), C57BL/6.RAG1−/− (B6.RAG1KO; H-2b), BALB/c (H-2d), C3H/HeJ (C3H; H-2k), CB6F1 (BALB/c ×B6 F1; H-2b/d) and B6C3F1 (C3H ×B6 F1; H-2b/k) were purchased from the National Cancer Institute (Frederick, MD), and nonobese diabetic (NOD) (H-2g7) mice were from the Jackson Laboratory (Bar Harbor, ME). The following TCR-transgenic (TCR-tg) mouse strains were used: 4C (17), TEa (18), OT-II (19) and BDC2.5 (20). Mice were bred and maintained in specific pathogen-free facilities at the University of California at San Francisco. All experiments were conducted according to Institutional Animal Care and Use Committee-approved protocols.
Precondition of recipient mice
Recipient mice were intravenously (i.v.) injected with 2 ×107 splenocytes through the retroorbital venous plexus as donor-specific transfusion (DST) 7 days before transplantation. Hundred or 200 mg/kg of cyclophosphamide (CY) was given by intraperitoneal injection 2 days after DST treatment.
Islet isolation and transplantation
Islets were isolated from donor pancreata (21) and 450–500 hand-picked islets were transplanted into the renal subcapsular space of recipient mice as previously described (22). B6 recipient mice were rendered diabetic using a single i.v. injection of streptozotocin (5–6 mg) 3 days before transplantation. Spontaneously diabetic NOD mice between 2 and 6 weeks of being diagnosed as diabetic (two blood glucose readings of >250 mg/dL) were selected as recipients. Diabetic mice were maintained with subcutaneous LinBit insulin implants (LinShin Canada, Inc., Ontario, Canada) until transplantation. Graft function was monitored using blood glucose measurements. Mice with successful islet engraftment within the first 7 days after transplant were entered into the experiments. Graft rejection was defined as a rise in blood glucose above 250 mg/dL for two consecutive readings.
In vitro expansion of Tregs
Treg isolation and expansion were carried out as described previously (3). Cultures were routinely checked for expression of CD4, CD25 and Foxp3, prior to use in experiments.
In vivo mixed lymphocyte reaction
Splenocytes and lymph node cells were collected from naïve and DST +CY preconditioned B6.Thy1.1 mice on day 7 after DST treatment and labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) before i.v. injecting into CB6F1 and B6C3F1 recipients through the retro-orbital venous plexus. Splenocytes and lymph node cells were collected 72 h after and stained with antibodies against Thy1.1, CD4, CD8 and Foxp3 before flowcytometric analysis of CFSE dilution of Thy1.1+ cells. Frequencies of BALB/c-reactive CD8+, CD4+Foxp3− T conventional (CD4+ Tconv) and CD4+Foxp3+ Treg precursors were calculated as described previously (2).
Adoptive transfer of TCR-tg T cells
Lymph node cells were isolated from the following three TCR-tg mice: 4C—direct alloreactive; TEa—indirect alloreactive; and OT-II—nonalloreactive control. The cells were labeled with CFSE and mixed together before i.v. injection in B6 recipients as previously described (23) 1 day before DST +CY treatment. Seven days later, total numbers of TCR-tg T cells in spleens and lymph nodes were determined using flow cytometry.
Skin transplantation
Ear skin (1–1.5 cm2) was transplanted unilaterally onto the dorsal thorax of mice with long-term protected BALB/c islet grafts for more than 100 days after DST +CY 200 mg/kg and Treg therapy and their age-matched naïve B6 mice as described previously (24). Graft rejection was defined as ~90% necrosis of graft tissue.
Immunofluorescent confocal microscopy
The islet graft-harboring kidneys were harvested and frozen in O.C.T. (Optimal Cutting Temperature) compound. Six-micron cryosections were fixed in acetone or 70% ethanol and incubated with primary antibodies, rabbit anti-mouse Foxp3 antisera (provided by Dr. Roli Khattri), biotinylated anti-mouse Ly5.1 (BD Bioscience, San Jose, CA), guinea pig anti-insulin (Dako, Carpinteria, CA) followed by goat anti-rabbit Alexa 555 (Invitrogen, Carlsbad, CA), streptavidin DyLight594 (Jackson Immunogenics, West Grove, PA), anti-guinea pig-Alexa 564 (Invitrogen) or anti-CD4 Alexa 488 (Invitrogen), anti-CD8 Alexa 647 (UCSF hybridoma core). Images were acquired on a Leica SP5 AOBS (Wetzlar, Germany) and analyzed using ImageJ software (NIH, Bethesda, MD).
Isolation of islet allograft-infiltrating leukocytes
Islet grafts were peeled off and digested with collagenase D and DNase I at 37°C for 30 min. The mixture was then treated with nonenzymatic cell dissociation buffer (Sigma–Aldrich, St. Louis, MO) for an additional 30 min and made into a single-cell suspension using gentle pipetting.
RNA isolation and quantitative real-time reverse transcription polymerase chain reaction
Islet infiltrating cells were sorted into TRIzol reagent (Invitrogen) and total RNA was isolated using the RNeasy Microkit (Qiagen, Hilden, Germany), followed by reverse transcription polymerase chain reaction (PCR) using SuperScript III First-Strand Synthesis System (Invitrogen) according to the manufacturer’s protocols. The cDNA template was then used for quantitative real-time PCR with Bio-Rad CFX96 system (Hercules, CA) and SYBR Green PCR kit (Qiagen). Level of gene expression was calculated as percentage relative to housekeeping genes beta actin or GAPDH.
Statistics
Data were analyzed using Prism5 (GraphPad Software, Inc., La Jolla, CA) and the results were expressed as mean ±SEM. Comparisons were made using the Student’s t-test, except log-rank (Mantel–Cox) test for Kaplan–Meier survival curves. A p-value <0.05 was considered statistically significant.
Results
Donor antigen-reactive Tregs alone are unable to prolong islet allograft survival
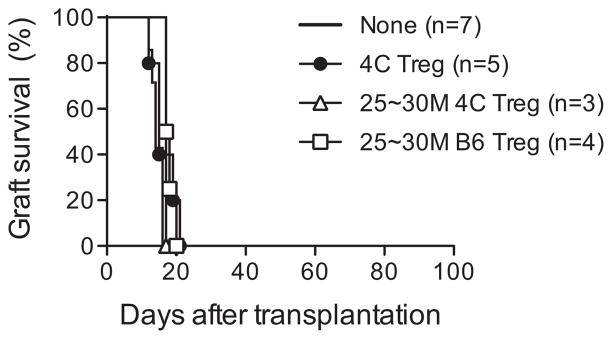
Previously we and others reported that Tregs with direct (15) or indirect (25) donor antigen reactivity and Tr1 cells (26) could modestly prolong graft survival using different transplantation setting in immunocompetent mice. To determine whether donor antigen-reactive Tregs alone can prolong the survival of fully MHC-mismatched grafts in immunocompetent hosts with high stringency, we used a murine islet transplantation model (BALB/c (H-2d) donors →C57BL/6 (H-2b) hosts). We used previously characterized 4C TCR-tg mice, which recognize I-Ad expressing donor cells, as a source of donor antigen-reactive Tregs (17). A group of streptozotocin-induced diabetic recipients received i.v. infusion of 5 ×106 Tregs isolated and expanded from 4C TCR-tg mice (designated 4C Tregs) 1 day before islet transplantation. This approach did not prolong islet allograft survival (Figure 1, filled circle). Increasing the number of infused Tregs to 25–30 ×106, either from 4C TCR-tg mice or from B6 mice (Figure 1, open circle and open triangle), similarly failed to prolong islet allograft survival, suggesting that Tregs were insufficient to protect allograft rejection as a stand-alone therapy.
Figure 1. Tregs alone were unable to prolong islet allograft survival.
B6 mice were rendered diabetic with streptozotocin before receiving 450–500 BALB/c islets under their left renal capsules. One day before transplantation, a group of mice received an intravenous infusion of 5 ×106 or 25–30 ×106 Tregs isolated and expanded from either 4C TCR-transgenic mice or B6 mice. Graft survival was assessed by monitoring blood glucose and calculated using the Kaplan–Meier method. Tregs, regulatory T cells.
Deletion of donor-reactive T cells using DST +CY preconditioning
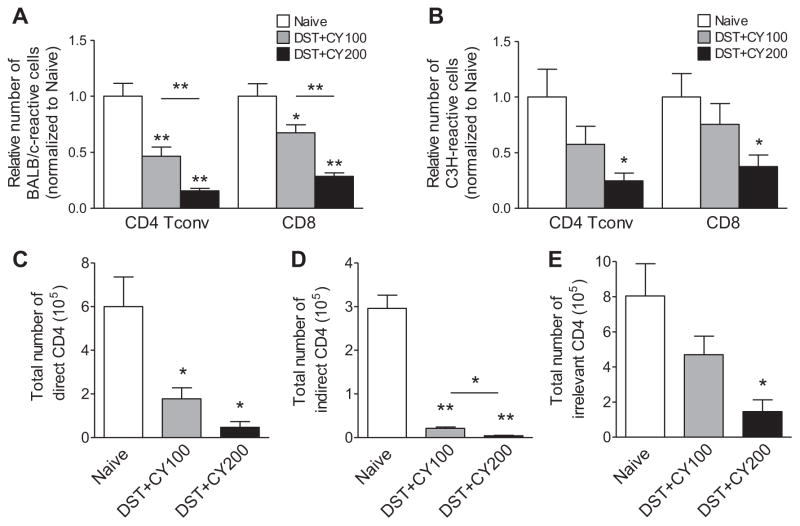
We hypothesized that the failure was due to high frequency of alloreactive T cells and reducing frequencies of donor-reactive T cells will provide a therapeutic window for Tregs to induce allograft tolerance. To preferentially delete donor-reactive T cells, we chose a previously described protocol to treat recipient mice with DST to induce proliferation of donor-reactive T cells, followed by CY to kill proliferating cells (designated as DST +CY hereafter) (27). To determine the efficacy of DST +CY preconditioning in reducing alloreactive T cells, we used an in vivo mixed lymphocyte reaction (MLR) assay (2) to calculate the number of donor-reactive T cells before and after DST +CY treatment. Preconditioning with DST followed by 100 mg/kg of CY (designated as DST +CY100) led to an average of 57% decrease in BALB/c-reactive CD4+ Tconv and 37% reduction in CD8+T cells (Table 1 and Figure 2A). Increasing the CY dose to 200 mg/kg (designated as DST +CY200) reduced the total numbers of BALB/c-reactive CD4+ Tconv by 82% and CD8+ T cells by 67% (Table 1 and Figure 2A). The total numbers of third party–reactive (C3H-reactive) T cells also decreased after DST +CY treatment (Figure 2B). Deletion of third-party cells was less efficient and it was mostly due to the nonspecific lymphotoxic effect of CY. These results demonstrated that DST +CY efficiently deleted donor-reactive T cells in a CY dose-dependent manner.
Table 1.
Reduction of donor-reactive T cells using DST +CY in B6 mice
| Precursor frequency (%)
|
Total cellularity (106) | |||
|---|---|---|---|---|
| CD4+ Tconv | CD8+ | Treg | ||
| BALB/c reactive (donor reactive) | ||||
| Naïve (n =6) | 9.3 ±1.2 | 4.0 ±0.4 | 17.6 ±2.8 | 147.6 ±9.3 |
| DST +CY100 (n =5) | 5.7 ±1.0 (53.5%)a | 2.8 ±0.3 (32.5%)a | 12.7 ±2.5 (44.2%)a | 94.8 ±15.2 |
| DST +CY200 (n =5) | 4.2 ±0.6 (74.4%)a | 2.4 ±0.3 (61.6%)a | 10.7 ±2.1 (78.4%)a | 45.5 ±1.9 |
| C3H/HeJ reactive (third-party reactive) | ||||
| Naïve (n =4) | 9.2 ±2.3 | 4.8 ±1.0 | 16.6 ±4.2 | 147.6 ±9.3 |
| DST +CY100 (n =4) | 7.5 ±2.1 (42.5%)a | 4.1 ±1.0 (24.5%)a | 17.0 ±5.1 (57.4%)a | 94.8 ±15.2 |
| DST +CY200 (n =4) | 6.0 ±1.7 (75.3%)a | 3.6 ±1.0 (62.4%)a | 11.6 ±2.8 (85.1%)a | 45.5 ±1.9 |
CFSE, carboxyfluorescein diacetate succinimidyl ester; CY, cyclophosphamide; DST, donor-specific transfusion; Tconv, T conventional; Tregs, regulatory T cells.
CD90.1+/+ congenic C57BL/6 mice were treated with DST (20 ×106 BALB/c splenocytes) followed by CY (100 or 200 mg/kg) 2 days later. Seven days after DST treatment, cells from spleens and peripheral lymph nodes were collected and labeled with CFSE. 20 ×106 CFSE-labeled cells were intravenously injected into CB6F1 or B6C3F1 mice to measure the reactivity against BALB/c (donor) and C3H/HeJ (third party), respectively. Donor-reactive precursor frequencies for conventional CD4+ Tconv, CD8+ and Tregs were calculated as described in the Materials and Methods section. Data shown are mean ±SEM and are a summary of four to six independent experiments. The number of CB6F1 or B6C3F1 mice per each experimental group is indicated.
Deletional efficacy (shown as percentage) in each subset of T cells compared to naïve.
Figure 2. DST +CY treatment significantly reduced donor-reactive T cells.
Total numbers of donor-reactive (A) and third party–reactive (B) CD4+ Tconv and CD8+ T cells in B6 mice treated with BALB/c DST and CY (100 and 200 mg/kg) were calculated from the total cellularity of spleens and lymph nodes and the frequencies of donor-reactive or third party–reactive cells (shown in Table 1). Untreated naïve B6 mice were used as controls and all numbers were normalized to the mean of the naïve B6 mice for the ease of comparison. To assess the efficacy in reducing T cells of direct versus indirect alloreactivity, B6 mice were intravenously injected with 1 ×106 of each tracer CD4+ T cells from 4C (C, direct), TEa (D, indirect) and OT-II (E, irrelevant) TCR-transgenic mice. All the mice were subjected to BALB/c DST and CY treatment. Seven days after DST treatment, total numbers of the three tracer cell populations in spleens and lymph nodes were calculated using flow cytometry and cell counting. Data are shown as mean ±SEM. Data are representative of at least four independent experiments using one mouse per group. *p <0.05, **p <0.01. A p-value <0.05 was considered statistically significant. CY, cyclophosphamide; DST, donor-specific transfusion; Tconv, T conventional.
We further analyzed the deletional efficacy of DST +CY on direct and indirect donor-reactive TCR-tg CD4+ T cells as previously described (23). We used T cells from 4C TCR-tg mice that recognize I-Ad as a source of CD4 T cells with direct BALB/c reactivity. We also used TEa TCR-tg mice that recognize a peptide derived from the MHC class II molecule I-Eα (aa 52–68) presented by I-Ab as a source of indirectly BALB/c-reactive CD4+ T cells. In order to monitor deletion of nonspecific CD4+T cells, OT-II TCR-tg mice that recognize OVA323–339 bound to I-Ab were used. Both direct and indirect CD4+ TCR-tg T cells were significantly reduced after DST +CY, and the reduction was more profound in DST +CY200 (Figure 2C and D), in agreement with the reduction of endogenous T cells. The number of irrelevant CD4+ OT-II TCR-tg T cells was also reduced (Figure 2E), reflecting lymphopenia-driven nonspecific deletion. Taken together, our results demonstrated that DST +CY deleted donor-reactive CD4+ T cells of direct and indirect reactivity with comparable efficacy.
Efficacy of Treg infusion in DST +CY preconditioned recipients
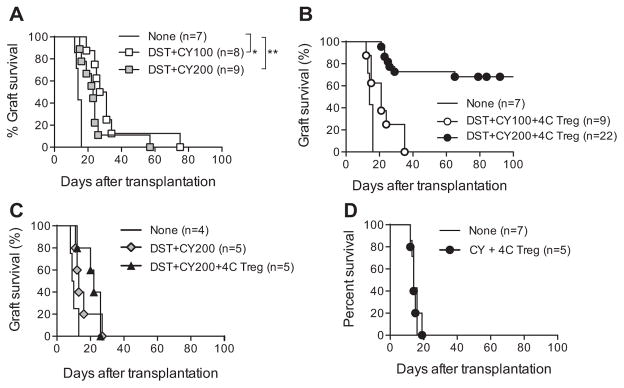
We transplanted BALB/c islets to B6 mice preconditioned with BALB/c DST and CY to determine whether DST +CY treatment was sufficient to protect islet allografts from rejection. Despite significant reduction of BALB/c-reactive T cells, DST with either dose of CY was only able to prolong graft survival by 1–2 weeks (Figure 3A, mean survival time [MST] of DST +CY100 =33, MST of DST +CY200 =25, MST of none =14; p =0.02 (DST +CY100), 0.03 (DST + CY200) compared to none). Additional infusion of 4C Tregs 1 day before transplantation did not improve graft survival when combined with DST +CY100 (Figure 3B, open circle; MST =22, p =0.04 compared to none, p =0.14 compared to DST +CY100). In contrast, when combined with DST + CY200 preconditioning, 4C Tregs induced indefinite graft survival in majority (72.7%, n =22) of recipients (Figure 3B, closed circle; MST >114, p <0.00001 compared to either none or DST +CY200). Removal of the islet-bearing kidney uniformly resulted in hyperglycemia, demonstrating that euglycemia in these mice was graft dependent (data not shown). In addition, these mice were fully capable of rejecting third-party C3H islets without significant delay (Figure 3C, MST of DST +CY200 =16, MST of DST + CY200 +4C Treg =21, p =0.2). Moreover, 4C Tregs combined with CY-driven general lymphopenia did not prolong graft survival (Figure 3D, MST of CY +4C Treg =15), suggesting that the overall protective effect was antigen-specific.
Figure 3. Donor-antigen-reactive Tregs induced long-term islet allograft survival in DST +CY conditioned recipients.
B6 mice were treated with BALB/c DST followed by CY (100 or 200 mg/kg) 2 days later. On day 4 after DST treatment, the mice were rendered diabetic with streptozotocin. On day 7 after DST, 450–500 BALB/c islets were transplanted under the left renal capsule (A). A group of the mice received an i.v. infusion of 5 ×106 Tregs isolated and expanded from 4C TCR-transgenic mice 1 day before islet transplantation (B). Islets from third-party (C3H/HeJ) donors were transplanted into B6 mice treated with BALB/c DST and CY (200 mg/kg) in the presence or absence of 4C Tregs (C). CY (200 mg/kg)-treated diabetic B6 mice without DST received an i.v. infusion of 5 ×106 4C Tregs 1 day before transplantation with BALB/c islets (D). Untreated diabetic B6 mice were similarly transplanted and used as controls. Graft survival was assessed by monitoring blood glucose and calculated using the Kaplan–Meier method, with comparisons among groups using the log-rank test. *p <0.05, **p <0.01. A p-value <0.05 was considered statistically significant. CY, cyclophosphamide; DST, donor-specific transfusion; i.v., intravenous; Tregs, regulatory T cells.
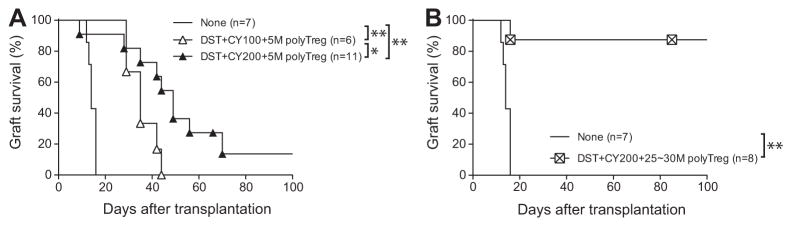
For clinical transplantation, manufacturing polyclonal Treg production is easier than donor-reactive Treg production. Therefore, we next determined whether polyclonal Tregs could also protect allogeneic grafts from rejection in preconditioned recipients. Administration of 5 ×106 polyclonal Tregs modestly prolonged graft survival, but all the grafts were progressively lost (Figure 4A), consistent with previous reports that donor-antigen-reactive Tregs are more effective than polyclonal Tregs (28,29). Using in vivo MLR, we determined that approximately 17.6% ±2.8% of B6 Tregs responded to BALB/c antigen (Table 1). Therefore, we hypothesized that increasing the number of polyclonal Tregs by five- to sixfold would deliver a similar number of donor-reactive Tregs as that used in the 4C Treg experiments. Indeed, infusion of 25–30 ×106 polyclonal Tregs was able to induce indefinite graft survival in a 87% of recipients (Figure 4B, MST >87, p =0.0006). Together, these data demonstrated that Treg infusion combined with donor-reactive T cell deletion (designated as Treg therapy) was sufficient to induce long-term graft acceptance and that the degree of deletion as well as the absolute number of donor-reactive Tregs was important for therapeutic efficacy.
Figure 4. Higher number of polyclonal Tregs was required to confer long-term protection of islet allografts.
(A) B6 mice were treated with BALB/c DST followed by CY (100 or 200 mg/kg) 2 days later, and rendered diabetic with streptozotocin (5–6 mg) 4 days later. On day 6 after DST, the conditioned mice received an i.v. infusion of 5 ×106 polyclonal B6 Tregs. On day 7 after DST, 450–500 BALB/c islets were transplanted under the left renal capsule. (B) B6 mice preconditioned with BALB/c DST and 200 mg/kg CY were rendered diabetic. They received an i.v. infusion of 25–30 ×106 polyclonal B6 Tregs on day 6 after DST and were transplanted with 450–500 BALB/c islets 1 day later. Untreated diabetic B6 mice were similarly transplanted and used as controls. Graft survival was assessed by monitoring blood glucose and calculated using the Kaplan–Meier method, with comparisons among groups using the log-rank test. *p <0.05, **p <0.01. A p-value <0.05 was considered statistically significant. CY, cyclophosphamide; DST, donor-specific transfusion; i.v., intravenous; Tregs, regulatory T cells.
Efficacy of Treg infusion in DST +CY preconditioned autoimmune diabetic mice
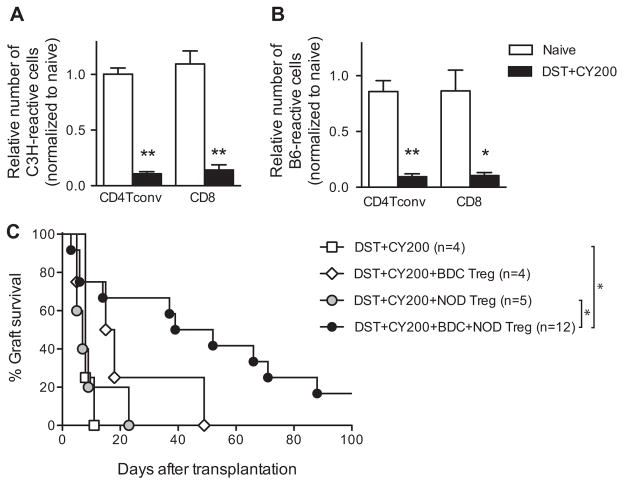
Considering the fact that most islet transplant recipients are likely type 1 diabetic patients, we decided to test the efficacy of our protocol in autoimmune NOD mice (H-2g7), a difficult setting to achieve long-term allograft survival due to recurrent autoimmunity and intrinsic defect in tolerance (30–32). We used C3H mice (H-2k) as islet donors and first measured total number of C3H-reactive CD4+ Tconv and CD8+ T cells in NOD mice before and after DST +CY treatment. Deletional efficiency of DST + CY200 in NOD was 90% both in the CD4+ Tconv and in the CD8+ T cell population (Table 2 and Figure 5A). As observed in B6 mice, the numbers of third party–reactive CD4+ Tconv and CD8+ T cell precursors were also decreased (Figure 5B).
Table 2.
Reduction of donor-reactive T cells using DST +CY in autoimmune NOD mice
| Donor-reactive precursor frequency (%)
|
Total cellularity (106) | |||
|---|---|---|---|---|
| CD4+ Tconv | CD8+ | Treg | ||
| C3H/HeJ reactive (donor reactive) | ||||
| Naïve (n =5) | 4.3 ±0.06 | 2.0 ±0.16 | 13.4 ±0.66 | 115.0 ±7.4 |
| DST +CY200 (n =3) | 1.2 ±0.01 (89.1%)a | 0.6 ±0.06 (87.2%)a | 6.6±0.19 (97.2%)a | 38.1 ±6.1 |
| C57BL/6 reactive (third-party reactive) | ||||
| Naïve (n =4) | 2.0 ±0.23 | 1.4 ±0.30 | 5.3 ±0.46 | 115.0 ±7.4 |
| DST +CY200 (n =4) | 0.7 ±0.19 (86.3%)a | 0.5 ±0.14 (87.2%)a | 2.9±0.48 (85.5%)a | 38.1 ±6.1 |
CFSE, carboxyfluorescein diacetate succinimidyl ester; CY, cyclophosphamide; DST, donor-specific transfusion; NOD, nonobese diabetic; Tconv, T conventional; Treg, regulatory T cell.
CD90.1+/+ congenic NOD mice were intravenously injected with 20 ×106 C3H/HeJ splenocytes followed by 200 mg/kg CY treatment 2 days later. Seven days after DST treatment, 20 ×106 CFSE-labeled NOD. CD90.1+/+cells were prepared as described in Table 1 and injected into NOD ×C3H F1 or NOD ×B6 F1 mice. Donor-reactive precursor frequencies for conventional CD4+, CD8+and Tregs were calculated as described in the Materials and Methods section. Data shown are mean ±SEM and are a summary of three independent experiments. The number of NOD ×C3H F1 or NOD ×B6 F1 mice per each experimental group is indicated.
Deletional efficacy (shown as percentage) in each subset of T cells compared to naïve.
Figure 5. Prolongation of islet allograft survival in spontaneously diabetic DST +CY-treated NOD mice required combination therapy using islet autoantigen-specific and polyclonal Tregs.
Total numbers of donor-reactive (A) and third party–reactive (B) CD4+ Tconv and CD8+T cells of NOD mice treated with C3H/HeJ DST and CY (200 mg/kg) were calculated from the total cellularity of spleens and lymph nodes and the frequencies of donor-reactive or third party–reactive cells (shown in Table 1). Untreated naïve NOD mice were used as controls and all numbers were normalized to the mean of the naïve NOD mice for ease of comparison. (C) Spontaneously diabetic female NOD mice received C3H/HeJ DST followed by 200 mg/kg CY 2 days later. On day 6 after DST, the mice were divided into four groups that received an i.v. infusion of 5 ×106 BDC2.5 Tregs, 5 ×106 polyclonal NOD Tregs, both, or no Tregs. On day 7 after DST, 450–500 C3H/HeJ islets were transplanted under the left renal capsule. Graft survival was assessed by monitoring blood glucose and calculated using the Kaplan–Meier method, with comparisons among groups using the log-rank test. *p <0.05, **p <0.01. A p-value <0.05 was considered statistically significant. CY, cyclophosphamide; DST, donor-specific transfusion; i.v., intravenous; NOD, nonobese diabetic; Tconv, T conventional; Tregs, regulatory T cells.
We next tested if Treg infusion in DST +CY conditioned NOD mice can induce long-term acceptance of C3H islet allografts. All the mice that were given DST +CY treatment alone rejected C3H grafts within 11 days (open square in Figure 5C, MST =9). Additional infusion of 5 ×106 polyclonal Tregs failed to protect (gray circle in Figure 5C, MST =10, p =0.8 compared to DST +CY200), consistent with other studies demonstrating the relative difficulty of prolonging islet allograft survival in the autoimmune setting. The addition of 5 ×106 islet autoantigen-specific BDC2.5 Tregs increased graft survival up to 49 days (open diamond in Figure 5C, MST =22, p =0.3 compared to DST +CY200). The combination of the polyclonal and BDC2.5 Tregs prolonged graft survival further (black circle in Figure 5C, MST =58, p =0.006 compared to DST +CY200). Taken together, prolongation of allogeneic islet graft survival in DST +CY preconditioned autoimmune NOD recipients required both islet autoantigen-reactive Tregs and polyclonal Tregs, which contain 13.4% ±0.66% alloantigen-reactive Tregs (Table 2).
Treg therapy does not induce systemic tolerance
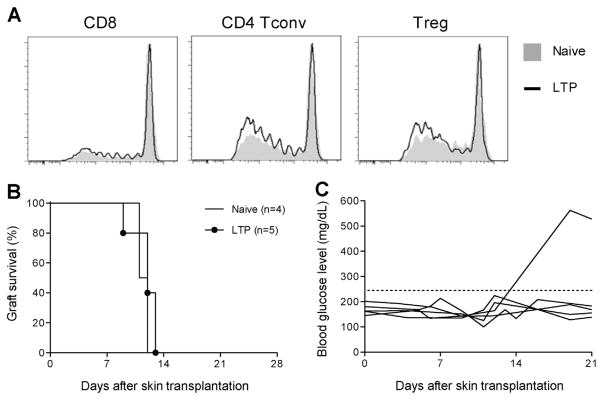
To determine whether our Treg therapy can diminish donor-specific alloimmune response as demonstrated by Miller et al (33), we analyzed mice with long-term protected grafts. We first determined the numbers of donor-reactive CD4+Tconv, CD8+T cells and Tregs in mice harboring long-term protected islet allografts (designated LTP mice) and found the numbers to be similar to those found in naïve mice (Figure 6A), suggesting no systemic deletional tolerance or persistent systemic increase of donor-reactive Tregs. Adoptive transfer of splenocytes from LTP mice into B6.Rag1−/− mice bearing established BALB/c islets led to graft rejection without delay when compared to grafts in mice that received naïve B6 splenocytes (data not shown). Last BALB/c skin grafts were all rejected without delay in LTP mice compared to unmanipulated B6 mice (n =5 out of 5, Figure 6B). It is important to note that while LTP mice rejected BALB/c skin grafts, most of their original islet grafts remained functional (n =4 out of 5, Figure 6C). These data collectively suggested that LTP mice had donor-reactive T cells capable of mediating graft rejection in periphery, and the protective effect was local to the islet grafts.
Figure 6. Treg therapy did not induce systemic tolerance.
(A) Overlaid histograms showing donor reactivity of CD8+T cells, CD4+Tconv cells and Tregs from naïve B6 mice (filled gray) and mice with long-term protected BALB/c islet grafts for more than 100 days after DST +200 mg/kg CY and Treg therapy (LTP, black line). Data are representative of four naïve B6 mice and four LTP mice. (B) Survival of BALB/c skin grafts in B6 mice with stably protected BALB/c islet grafts for more than 100 days or control age-matched naïve B6 mice. (C) Blood glucose measurement indicating the survival of the original BALB/c islet grafts in mice challenged with BALB/c skin grafts as shown in (B). The horizontal gray line indicates the threshold for rejection of islet grafts. CY, cyclophosphamide; DST, donor-specific transfusion; LTP, long-term protected; Tconv, T conventional; Tregs, regulatory T cells.
Impact of therapeutic Tregs on immunological milieu of the allografts
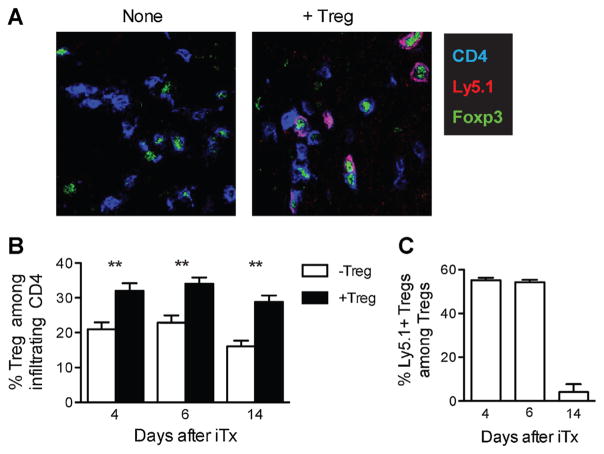
To determine the alterations of the local microenvironment within allografts by Treg therapy, we quantified total Tregs and transferred Tregs in islet grafts using multicolor immunofluorescence. Total Tregs were identified as CD4+Foxp3+ and the transferred 4C Tregs were identified by the additional expression of the CD45.1 congenic marker (Figure 7A). We found that the percentage of total Tregs within CD4+ T cell population was higher in Treg-treated group at every time point examined (Figure 7B). Transferred Tregs were nearly half of all Tregs on days 4 and 6 after transplantation, in accordance with their previously described trafficking pattern (34). However, they became barely detectable on day 14 after transplantation (Figure 7C). The loss was not specific to the graft sites because very few transferred Tregs were detected systemically in draining and nondraining lymph nodes and spleens. Together, our results show that transferred Tregs infiltrated the allografts early after transplant but were short-lived. They promoted recruitment of endogenous Tregs to the grafts.
Figure 7. Treg therapy increased frequency of Tregs in the grafts early after transplantation.
B6 mice preconditioned with BALB/c DST +200 mg/kg CY were transplanted with BALB/c islets either with or without receiving 5 ×106 4C Tregs as described in the legend of Figure 3. Islet grafts were collected on days 4, 6 and 14 after transplantation and analyzed using confocal immunofluorescence microscopy. (A) Representative images of grafts from Ly5.1+ 4C Treg-treated (right) and untreated (left) mice showing CD4 (blue), Foxp3 (green) and Ly5.1 (red) staining on day 6 after transplantation. Original magnification was 640×. (B and C) Entire graft areas were captured by 640× magnification and all the fields were stitched together after image acquisition with the aid of ImageJ software to reconstruct the entire graft section. Numbers of CD4+, CD4+Foxp3+ and CD4+Foxp3+Ly5.1+ cells in each graft section were then determined by manual counting of the reconstructed micrographs. At least three sections more than 60 μm from each other were counted for each graft and more than three mice per experimental condition were analyzed. The average percentages of total Tregs (CD4+Foxp3+) among CD4+ cells (B) and percentages of CD4+Foxp3+Ly5.1+ transferred Tregs among total Tregs (C) were calculated. The data are presented as mean ±SEM of individual graft. **p <0.01. A p-value <0.05 was considered statistically significant. CY, cyclophosphamide; DST, donor-specific transfusion; iTx, islet transplantation; Tregs, regulatory T cells.
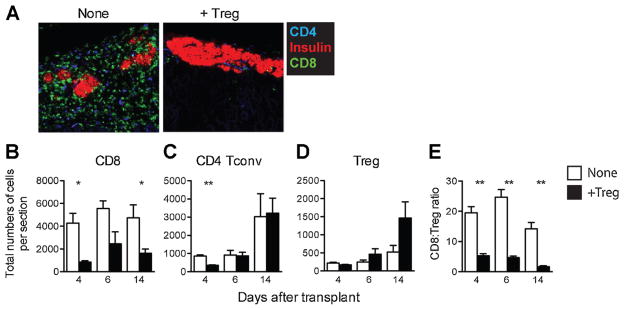
We also determined the impact of Treg therapy on the infiltration of conventional CD4+ and CD8+ T cells. In mice without Treg therapy, residual islets were surrounded by CD8+ T cell-dominant infiltrates on day 14 after transplantation (Figure 8A, left). Strikingly, the group with Treg therapy showed well-preserved islet morphology with minimal infiltrates, consisting of more CD4+ than CD8+ T cells (Figure 8A, right). The suppression of CD8+ T cell accumulation in the graft was maintained throughout the early posttransplant period (Figure 8B). In contrast, CD4+ Tconv cells arrived at the graft sites slower than CD8+ T cells and their presence in the grafts was not affected by Treg therapy (Figure 8C). The infiltration of Tregs followed the same delayed tempo as CD4+ Tconv cells, but their accumulation in the grafts was markedly increased by Treg therapy (Figure 8D). Thus, the ratio of CD8+ T cells to Treg was dramatically lower in Treg-treated group (Figure 8E). These data suggested that Tregs protected islet allografts through alteration of the balance between CD8+ T cells and Tregs by inhibiting CD8+ T cell accumulation while promoting Treg recruitment to the grafts.
Figure 8. Treg therapy reduced graft-infiltrating CD8+T cells.
Graft sections prepared as described in figure legend 7 were also analyzed for graft-infiltrating CD8+ T cells, CD4+ Tconv cells and Tregs using confocal immunofluorescence microscopy. (A) Representative images of 4C Treg-treated (left) and untreated (right) grafts showing CD4 (blue), CD8 (green) and insulin (red) staining on day 14 after transplantation. Original magnification was 640×. (B–E) Total numbers for CD8+ T cells (B), CD4+ Tconv cells (C), Tregs (D) and the ratio of CD8+ T cells to Tregs (E) per graft section were determined as described in figure legend 7 B and C. The data are presented as mean ±SEM of individual graft. *p <0.05, **p <0.01. A p-value <0.05 was considered statistically significant. Tconv, T conventional; Tregs, regulatory T cells.
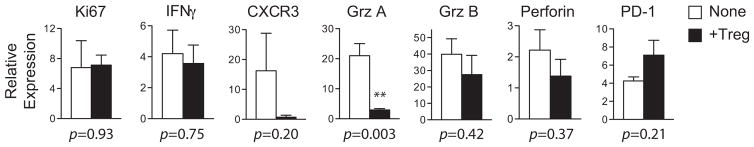
We next explored the basis of Treg-mediated suppression of CD8+ T cell accumulation. We sorted CD8+ T cells from transplanted islet grafts on day 6 after transplantation, the peak of CD8+ T cell accumulation, and analyzed their expression of a panel of genes implicated in CD8+ T cell activation and exhaustion (35). The expression of Ki67, a proliferation marker, and IFNγ did not change by Treg therapy (Figure 9). The expression of CXCR3, a chemokine receptor previously implicated in CD8+ T cell activation and recruitment to allografts (36), was markedly decreased. Similarly, mRNAs of CD8+ T cell effector molecules, granzyme A, granzyme B and perforin were also down-regulated by Treg therapy. The expression of PD-1, a CD8+ T cell exhaustion marker and negative regulator of T cell activation, was higher in mice that received Tregs. These results suggested that Treg therapy modulates the size of CD8+ T cell infiltrates by suppressing CD8+ T cell activation and promoting their exhaustion.
Figure 9. Graft-infiltrating CD8+ T cells exhibit exhausted phenotype in Treg-treated mice.
B6 mice preconditioned with BALB/c DST +200 mg/kg CY were transplanted with BALB/c islets either with or without receiving 5 ×106 4C Tregs as described in the legend of Figure 3. Graft-infiltrating CD8+ T cells were FACS sorted from dissociated islet grafts collected on day 6 after transplantation. Total RNA from CD8+ T cells was analyzed using quantitative real-time polymerase chain reaction for the mRNA levels for beta actin and genes indicated on the graph. Data presented is a summary of two independent experiments and each experiment includes 2–3 mice per group. **p <0.01. A p-value <0.05 was considered statistically significant. CY, cyclophosphamide; DST, donor-specific transfusion; Tregs, regulatory T cells.
Discussion
In this study, we show that reducing 70–80% of donor-reactive T cells by DST +CY preconditioning leads to a successful Treg therapy to achieve long-term graft protection in a fully MHC-mismatched murine islet transplantation model. With alloreductive preconditioning, Tregs are able to induce indefinite graft survival in a majority of recipients without additional immunosuppression. Donor alloantigen-reactive Tregs are five to six times more potent than polyclonal Tregs, likely related to the inherent precursor frequency of alloreactive Treg. Mechanistically, Treg therapy suppresses the accumulation of CD8+ T cell infiltrates but promotes the presence of endogenous Tregs in the grafts early after transplantation, thus altering the balance between these two cell populations. Moreover, the graft-infiltrating CD8+ T cells in Treg-treated mice have a less activated and more exhausted phenotype when compared to their counterparts in untreated mice.
The DST +CY-mediated deletion was not restricted to donor-reactive T cells and was associated with significant lymphopenia. Despite the controversial role of lymphopenia on tolerance induction (37–39), we are encouraged to see long-term graft survival after DST +CY200 treatment combined with Treg infusion. This result is consistent with the notion that Treg therapy may overcome the resistance to tolerance in lymphopenic hosts (40,41). It further suggests that nonspecific lymphodepleting agents such as Thymoglobulin or Campath-1 may be effectively combined with Treg therapy. We found that 70–80% deletion of donor-reactive T cells was required to create a therapeutic window for Tregs to effectively control allograft rejection. This provides us a benchmark for designing clinical Treg therapy in combination with lymphodepletion.
Our finding of better efficacy for alloantigen-reactive Tregs in conferring long-term graft protection is in agreement with published studies in various models (15,25,28,29,42,43). Five to six times more polyclonal Tregs are needed to achieve the same efficacy, consistent with the frequency of donor-reactive Tregs in the polyclonal pool estimated using in vivo MLR (Table 2). Although polyclonal Tregs can confer long-term graft protection, the requirement for high numbers may lead to generalized immunosuppression. While polyclonal Tregs are more straightforward to manufacture for clinical application, the production of very large numbers of polyclonal Tregs while retaining their regulatory function remains a significant technical challenge (44,45). Recently, various protocols for selectively expanding human alloantigen-reactive Tregs have been demonstrated (43,44,46,47). Therefore, we consider using donor alloantigen-reactive Tregs as a better strategy in future clinical trials.
Surprisingly, we found no evidence of systemic tolerance to donor alloantigens in mice with long-term protected graft after Treg therapy. Histological analysis of graft-infiltrating cells revealed that the early action in preventing graft rejection was primarily within the grafts, consistent with previous reports (34,48). Our findings pointed to a primary impact on CD8+ T cells and their effector functions. CD8+ T cells dominated the infiltration prior to graft rejection. Treg therapy significantly reduced the number of intragraft CD8+ T cells. How Treg therapy suppresses CD8+ T cell accumulation remains to be determined. It is possible that Treg therapy accelerated Treg arrival in the grafts, and Tregs compete with CD8+ T cells for growth and survival factors such as IL-2 (49,50). In this regard, local administration of Tregs with the islet grafts may improve the efficacy of Treg therapy as shown previously (34). Interestingly, while the transferred Tregs were short-lived in this model, the grafts were protected long after the transferred Tregs were gone. We postulated that Treg therapy likely created an immune privileged local environment within the grafts through infectious tolerance (51–53).
In summary, we have explored the requirements for inducing long-term allograft survival using Treg therapy in a stringent murine islet transplantation model. Our study provides a rationale for using a combined regimen of lymphodepletion and donor-alloantigen-reactive Tregs (44,45). With the advent of good manufacturing practice–compliant production of human alloantigen-reactive Tregs, this strategy can be readily applicable to human studies. Currently, we are actively planning clinical trials to evaluate safety and efficacy of alloantigen-reactive Treg therapy in liver and kidney transplantation including the multi-national collaborative project the ONE Study (54). These efforts are the first steps toward realizing the goal of harnessing the tolerogenic potential of Tregs in solid organ transplantation.
Acknowledgments
This study was supported by Korea Foundation for International Cooperation of Science and Technology (KICOS) Grant (2007-00107), NIH (P30 DK063720) and donation from Joyce and Fred Nicholas Family. The authors thank Ninnia Lescano and Mariela Pauli for their technical assistance and members of UCSF Transplantation Research Laboratory for helpful discussions.
Abbreviations
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- CY
cyclophosphamide
- DST
donor-specific transfusion
- GMP
good manufacturing practice
- i.v
intravenous(ly)
- LN
lymph node
- LTP
long-term protected
- MLR
mixed lymphocyte reaction
- MST
mean survival time
- NOD
nonobese diabetic
- PCR
polymerase chain reaction
- STZ
streptozotocin
- Tconv
T conventional
- tg
transgene, transgenic
- Treg
regulatory T cell
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Matesic D, Lehmann PV, Heeger PS. High-resolution characterization of cytokine-producing alloreactivity in naive and allograft-primed mice. Transplantation. 1998;65:906–914. doi: 10.1097/00007890-199804150-00008. [DOI] [PubMed] [Google Scholar]
- 2.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: New answers to an old question. J Immunol. 2001;166:973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 3.Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Koldzic DN, Izikson L, et al. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+ CD4+ regulatory T cells. Int Immunol. 2004;16:249–256. doi: 10.1093/intimm/dxh029. [DOI] [PubMed] [Google Scholar]
- 6.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+ CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 7.Waldmann H, Adams E, Fairchild P, Cobbold S. Regulation and privilege in transplantation tolerance. J Clin Immunol. 2008;28:716–725. doi: 10.1007/s10875-008-9249-5. [DOI] [PubMed] [Google Scholar]
- 8.Long E, Wood KJ. Regulatory T cells in transplantation: Transferring mouse studies to the clinic. Transplantation. 2009;88:1050–1056. doi: 10.1097/TP.0b013e3181bb7913. [DOI] [PubMed] [Google Scholar]
- 9.Kang SM, Tang Q, Bluestone JA. CD4+ CD25+ regulatory T cells in transplantation: Progress, challenges and prospects. Am J Transplant. 2007;7:1457–1463. doi: 10.1111/j.1600-6143.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 10.Walsh PT, Taylor DK, Turka LA. Tregs and transplantation tolerance. J Clin Invest. 2004;114:1398–1403. doi: 10.1172/JCI23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeung MY, Sayegh MH. Regulatory T cells in transplantation: What we know and what we do not know. Transplant Proc. 2009;41(6 Suppl):S21–S26. doi: 10.1016/j.transproceed.2009.06.093. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Fueyo A, Sandner S, Habicht A, et al. Specificity of CD4+ CD25+ regulatory T cell function in alloimmunity. J Immunol. 2006;176:329–334. doi: 10.4049/jimmunol.176.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sagoo P, Lombardi G, Lechler RI. Regulatory T cells as therapeutic cells. Curr Opin Organ Transplant. 2008;13:645–653. doi: 10.1097/MOT.0b013e328317a476. [DOI] [PubMed] [Google Scholar]
- 14.Tang Q, Bluestone JA, Kang SM. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol. 2012;4:11–21. doi: 10.1093/jmcb/mjr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brennan TV, Tang Q, Liu FC, et al. Requirements for prolongation of allograft survival with regulatory T cell infusion in lymphosufficient hosts. J Surg Res. 2011;169:e69–e75. doi: 10.1016/j.jss.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells AD, Li XC, Li Y, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 17.Brennan TV, Hoang V, Garrod KR, et al. A new T-cell receptor transgenic model of the CD4+ direct pathway: Level of priming determines acute versus chronic rejection. Transplantation. 2008;85:247–255. doi: 10.1097/TP.0b013e31815e883e. [DOI] [PubMed] [Google Scholar]
- 18.Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- 19.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 20.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 21.Szot GL, Koudria P, Bluestone JA. Murine pancreatic islet isolation. J Vis Exp. 2007;7:255. doi: 10.3791/255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szot GL, Koudria P, Bluestone JA. Transplantation of pancreatic islets into the kidney capsule of diabetic mice. J Vis Exp. 2007;9:404. doi: 10.3791/404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brennan TV, Jaigirdar A, Hoang V, et al. Preferential priming of alloreactive T cells with indirect reactivity. Am J Transplant. 2009;9:709–718. doi: 10.1111/j.1600-6143.2009.02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrod KR, Cahalan MD. Murine skin transplantation. J Vis Exp. 2008;11:634. doi: 10.3791/634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsang JY, Tanriver Y, Jiang S, et al. Conferring indirect allospecificity on CD4+ CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008;118:3619–3628. doi: 10.1172/JCI33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gagliani N, Jofra T, Stabilini A, et al. Antigen-specific dependence of Tr1-cell therapy in preclinical models of islet transplant. Diabetes. 2010;59:433–439. doi: 10.2337/db09-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayumi H, Good RA. Long-lasting skin allograft tolerance in adult mice induced across fully allogeneic (multimajor H-2 plus multiminor histocompatibility) antigen barriers by a tolerance-inducing method using cyclophosphamide. J Exp Med. 1989;169:213–238. doi: 10.1084/jem.169.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golshayan D, Jiang S, Tsang J, Garin MI, Mottet C, Lechler RI. In vitro-expanded donor alloantigen-specific CD4+CD25+regulatory T cells promote experimental transplantation tolerance. Blood. 2007;109:827–835. doi: 10.1182/blood-2006-05-025460. [DOI] [PubMed] [Google Scholar]
- 29.Joffre O, Santolaria T, Calise D, et al. Prevention of acute and chronic allograft rejection with CD4+ CD25+ Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14:88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markees TG, Serreze DV, Phillips NE, et al. NOD mice have a generalized defect in their response to transplantation tolerance induction. Diabetes. 1999;48:967–974. doi: 10.2337/diabetes.48.5.967. [DOI] [PubMed] [Google Scholar]
- 31.Molano RD, Berney T, Li H, et al. Prolonged islet graft survival in NOD mice by blockade of the CD40–CD154 pathway of T-cell costimulation. Diabetes. 2001;50:270–276. doi: 10.2337/diabetes.50.2.270. [DOI] [PubMed] [Google Scholar]
- 32.Makhlouf L, Kishimoto K, Smith RN, et al. The role of autoimmunity in islet allograft destruction: Major histocompatibility complex class II matching is necessary for autoimmune destruction of allogeneic islet transplants after T-cell costimulatory blockade. Diabetes. 2002;51:3202–3210. doi: 10.2337/diabetes.51.11.3202. [DOI] [PubMed] [Google Scholar]
- 33.Luo X, Pothoven KL, McCarthy D, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci USA. 2008;105:14527–14532. doi: 10.1073/pnas.0805204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang N, Schroppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wherry EJ, Ha SJ, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Hancock WW, Lu B, Gao W, et al. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–1520. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Z, Bensinger SJ, Zhang J, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karim M, Feng G, Wood KJ, Bushell AR. CD25+CD4+regulatory T cells generated by exposure to a model protein antigen prevent allograft rejection: Antigen-specific reactivation in vivo is critical for bystander regulation. Blood. 2005;105:4871–4877. doi: 10.1182/blood-2004-10-3888. [DOI] [PubMed] [Google Scholar]
- 39.Dai Z, Li Q, Wang Y, et al. CD4+CD25+regulatory T cells suppress allograft rejection mediated by memory CD8+ T cells via a CD30-dependent mechanism. J Clin Invest. 2004;113:310–317. doi: 10.1172/JCI19727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Addio F, Yuan X, Habicht A, et al. A novel clinically relevant approach to tip the balance toward regulation in stringent transplant model. Transplantation. 2010;90:260–269. doi: 10.1097/tp.0b013e3181e64217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neujahr DC, Chen C, Huang X, et al. Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it. J Immunol. 2006;176:4632–4639. doi: 10.4049/jimmunol.176.8.4632. [DOI] [PubMed] [Google Scholar]
- 42.Trenado A, Charlotte F, Fisson S, et al. Recipient-type specific CD4+ CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003;112:1688–1696. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sagoo P, Ali N, Garg G, Nestle FO, Lechler RI, Lombardi G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med. 2011;3:83ra42. doi: 10.1126/scitranslmed.3002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang Q, Lee K. Regulatory T-cell therapy for transplantation: How many cells do we need? Curr Opin Organ transplant. 2012;17:349–354. doi: 10.1097/MOT.0b013e328355a992. [DOI] [PubMed] [Google Scholar]
- 45.Hippen KL, Merkel SC, Schirm DK, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3:83ra41. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peters JH, Hilbrands LB, Koenen HJ, Joosten I. Ex vivo generation of human alloantigen-specific regulatory T cells from CD4(pos)CD25(high) T cells for immunotherapy. PloS ONE. 2008;3:e2233. doi: 10.1371/journal.pone.0002233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veerapathran A, Pidala J, Beato F, Yu XZ, Anasetti C. Ex vivo expansion of human Tregs specific for alloantigens presented directly or indirectly. Blood. 2011;118:5671–5680. doi: 10.1182/blood-2011-02-337097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan Z, Spencer JA, Lu Y, et al. In vivo tracking of ‘color-coded’ effector, natural and induced regulatory T cells in the allograft response. Nat Med. 2010;16:718–722. doi: 10.1038/nm.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McNally A, Hill GR, Sparwasser T, Thomas R, Steptoe RJ. CD4+ CD25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasis. Proc Natl Acad Sci USA. 2011;108:7529–7534. doi: 10.1073/pnas.1103782108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kastenmuller W, Gasteiger G, Subramanian N, et al. Regulatory T cells selectively control CD8+ T cell effector pool size via IL-2 restriction. J Immunol. 2011;187:3186–3197. doi: 10.4049/jimmunol.1101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin S, Cobbold SP, Pope H, et al. “Infectious” transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 52.Cobbold SP, Adams E, Graca L, et al. Immune privilege induced by regulatory T cells in transplantation tolerance. Immunol Rev. 2006;213:239–255. doi: 10.1111/j.1600-065X.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 53.Fallarino F, Grohmann U, You S, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 54.Leslie M. Immunology, regulatory T cells get their chance to shine. Science. 2011;332:1020–1021. doi: 10.1126/science.332.6033.1020. [DOI] [PubMed] [Google Scholar]