Abstract
Introduction: The aim of this study was to determine the histologic effect of low level laser therapy (LLLT) with or without the use of mineral trioxide aggregate (MTA) on exposed pulp tissues of dogs.
Methods: Twenty-five premolar teeth in three healthy mature dogs were randomly divided into five groups. In group 1, the pulp tissue was capped with MTA. In groups 2 and 3, before capping with MTA, the exposure site was irradiated by low power lasers at 630 nm (20 mW, 150 seconds, 7.5 J/cm2) and 810 nm (peak power 80 W, average power 50 mW, 1500 Hz, 50 seconds, 6.25 J/cm2) wavelengths, respectively. In groups 4 and 5, the exposure site was irradiated similar to that described in groups 2 and 3, but the pulp tissue was covered by gold foil instead of MTA. After 2 months, the animals were sacrificed and the samples were prepared for histologic evaluation.
Results: There were differences in pulpal response and dentinal barrier formation among the study groups. The morphology of pulpal tissue and the integrity of dentinal barrier and formation of odontoblastic layer were more favorable in the first three groups. The occurrence of extensive and intense inflammation and necrosis was more frequent in groups 4 and 5.
Conclusion: Under the conditions used in this study, the presence of MTA as a pulp capping material was more important than laser therapy in the success of direct pulp capping (DPC) treatment. MTA proved to be an effective material either alone or in combination with laser irradiation in vital pulp therapy.
Keywords: low level laser therapy, vital pulp therapy, dentinal bridge, gold foil, mta, pulp capping, pulp morphology
Introduction
Dental pulp is a unique soft tissue containing blood vessels, nerve fibers, collagen fibers, fibroblasts, odontoblasts, and immune cells. Like other connective tissues, dental pulp has the capacity of healing against external stimuli.1 Direct pulp capping (DPC) is one of the vital pulp therapy modalities, attempting to maintain the integrity of pulpal tissue. In this technique, a biocompatible material is placed on the pulp exposed by caries or trauma in order to protect the pulpal tissue from bacterial microleakage.2 This can promote dentinal bridge formation and lead to maintenance of pulp vitality. It is believed that an important marker of pulpal wound repair is dentinal bridge formation.1 The presence of reparative dentin is a barrier against the penetration of external stimuli, therefore, the amount and homogeneity, and the presence or absence of dentinal tubules within reparative dentin are important factors in the success of DPC treatment.
Historically, DPC was introduced by Philip Pfaff in 1765 by using a gold foil.3 From that time, various materials have been used for DPC including glass ionomers, adhesive resins, zinc oxide eugenol (ZOE) and calcium hydroxide [Ca(OH)2]. The use of these materials is associated with some disadvantages, such as microleakage, and some physical or chemical problems that may lead to pulp injuries before complete dentinal bridge formation , hence these materials are not commonly used today for DPC.4 In 1995, the mineral trioxide aggregate (MTA) was introduced by Torabinejad as a retrofill material in endodontic treatments. The excellent properties of MTA including high biocompatibility, good marginal seal, alkaline pH, slow release of Ca2+, and stimulation of cytokine release made this material as a suitable substance for DPC. It has been demonstrated that the use of MTA can lead to an increased rate of cell proliferation and the induction of homogenous calcified bridge underneath the exposed pulp tissue.1
Low level laser therapy (LLLT) has been extensively used in medicine and dentistry due to its anti-inflammatory, analgesic and biostimulative effects and its great benefits in accelerating the wound healing process.5-10 These excellent properties suggest that LLLT could play a great role in the success of DPC treatment by promoting hard tissue formation and accelerating the inflammatory process in damaged pulp tissues. Although some recent studies investigated the clinical, radiographic and histopathologic outcomes of LLLT in pulpotomy of primary teeth,11-13 but there are few studies regarding the effect of LLLT on DPC,14 and there has not been any comparison done between the effectiveness of low power red and infrared lasers in DPC. Furthermore, the quality of reparative dentin formed following laser treatment of exposed pulpal tissue has not been evaluated in previous investigations. Therefore, this study was conducted to determine the histologic effect of irradiation from low power red and infrared lasers with or without using MTA on exposed pulp tissues of dogs.
Methods
The study consisted of 25 maxillary and mandibular premolar teeth in three healthy mature dogs (Iranian mix, 15-30 kg weight) with mean age of 2 years 2 months old. The study protocol was approved by the ethics committee of Mashhad University of Medical Sciences (the approval code: 89309). Before performing the experiment, the periodontal condition of premolar teeth was assessed with a periodontal probe and the health of the pulp and periapical tissues was evaluated by periapical radiographs. The teeth that had caries and periodontal or periapical problems were excluded from the sample.
Acepromazine, diazepam, and ketamine were used for general anesthesia. The teeth were locally anesthetized by injection of 2% lidocaine combined with 1:100000 epinephrine (Darou Pakhsh, Iran) and then were isolated with a rubber dam (Supa, Iran) (Figure 1). A 0.2% chlorhexidine mouthrinse (Hexodine, World Health Laboratories Co., Tehran, Iran) was used for the disinfection of teeth and rubber dam. The pulpal tissue of each tooth was exposed by a round bur (Dia Dent Co., Seoul, Korea,) with a terminal diameter of 1.5 mm. The dentinal chips were removed by normal saline irrigation. Pulp bleeding was controlled by a slight pressure from some cotton pieces wetted by hypochlorite sodium (Razi Serum, Iran). The teeth in the study groups underwent DPC by one of the following methods:
Figure 1 .
The teeth were isolated with a rubber dam.
Group 1: The teeth were capped by MTA (Proroot, Dentsply, Tusla, USA).
Group 2: The exposed pulp areas in this group were irradiated by a low level red laser (LLRL) (Mustang 2000+, Moscow, Russia), emitting a wavelength of 630 nm. The laser was operated in continuous wave mode at a power of 20 mW and was held manually at an approximate distance of 2 mm from the exposure site. The beam was delivered through a nozzle, which was held manually at an approximate distance of 2 mm from the target area. The duration of irradiation was 150 seconds and the spot size was approximately 0.2 cm2 . Considering the 50% energy loss through the nozzle, the effective energy delivered to the tissue was estimated to be 1.5 J and the energy density was calculated to be 7.5 J/cm2 . Following laser therapy, the teeth were capped by MTA.
Group 3: The teeth in this group were irradiated with a low level infrared laser (LLIL; wavelength 810 nm; Mustang 2000+, Moscow, Russia). The laser was applied with a peak power of 80 W, average power of 50 mW and frequency of 1500 Hz, pulse length of 1 μs, and spot size of 0.2 cm2 for 50 seconds per tooth. The beam was delivered through a nozzle which was held manually at an approximate distance of 2 mm from the target area. Considering the 50% energy loss through the nozzle, the effective energy delivered to the tissue was estimated to be 1.25 J and the energy density was calculated to be 6.25 J/cm2 . The teeth were then capped by MTA.
Group 4: The low power red laser was irradiated to the exposed pulp tissue similar to that described in group 2. However, instead of MTA, a gold foil was used for capping the exposure site.
Group 5: A low power infrared laser was employed to irradiate the exposed pulp tissue similar to that described in group 3, but a gold foil was used, instead of MTA, for capping the exposure area.
In all groups, the cavities were filled with glass ionomer cement (GC Industrial Co., Tokyo, Japan). After 8 weeks, the dogs were sacrificed and a vital perfusion was accomplished. The teeth and surrounding tissues were block sectioned and immersed in 10% formalin. Then the tissues were decalcified with 17% EDTA over a period of 6 months and embedded in paraffin. After serial sectioning of the samples in 4-6 µm thickness, the specimens were stained with hematoxylin and eosin (H & E). The specimens were then coded and evaluated by a blind investigator under an optical microscope at 40× and 100× magnifications. The histopathological assessment performed in this study consisted of 3 parts and each part was classified as described below.15
Part 1. Inflammatory Reaction, Including Type, Intensity and Extension
(a) Type
Grade 1: acute and chronic inflammation; Grade 2: chronic inflammation; Grade 3: without inflammation.
(b) Intensity
Grade 1: severe inflammation (more than 60 inflammatory cells) or abscess formation; Grade 2: mild (0-30 inflammatory cells) to moderate (30-60 inflammatory cells) inflammation; Grade 3: without inflammation.
(c) Extension
Grade 1: entire coronal pulp; Grade 2: under the exposure area; Grade 3: without inflammation.
Part 2. Hard Tissue Formation Including Continuity, Morphology, and Thickness
(a) Continuity
Grade 1: absence of hard tissue formation; Grade 2: moderate contact of capping material with the dental pulp; Grade 3: complete bridge formation.
(b) Morphology
Grade 1: without dentinal tubules; Grade 2: irregular tubular dentin; Grade 3: regular tubular dentin.
(c) Thickness
Grade 1: less than 100 µm; Grade 2: 100-250 µm; Grade 3: more than 250 µm.
Part 3. Histopathological Changes in Soft Tissue Including Necrosis, Odontoblastic Layer Formation and Calcification
(a) Necrosis
Grade 1: complete necrosis; Grade 2: partial necrosis; Grade 3: without necrosis.
(b) Odontoblastic Layer Formation
Grade 1: absence of odontoblastic layer; Grade 2: presence of odontoblastic layer; Grade 3: parallel pattern of cells (palisade form).
(c) Calcification
Grade 1: diffuse calcification; Grade 2: pulp stone; Grade 3: without calcification.
Results
The outcome of histological assessment is explained separately regarding the inflammatory reaction, hard tissue formation, and histopathological changes in soft tissue in the following:
Inflammatory Reaction
No inflammatory reaction was observed in group 3 (LLIL+MTA), but chronic inflammation (grade 2) with mild to moderate intensity (grade 2) was observed in 20% of the specimens in groups 1 and 2, 60% of the specimens in group 4 and 40% of the specimens in group 5 under the exposure site (Figure 2). Furthermore, acute and chronic inflammation (grade 1) with mild to moderate intensity (grade 2) was observed in 20% of the specimens in group 5 under the exposed tissue. The remaining specimens in the study groups had no sign of inflammation (Table 1).
Figure 2 .
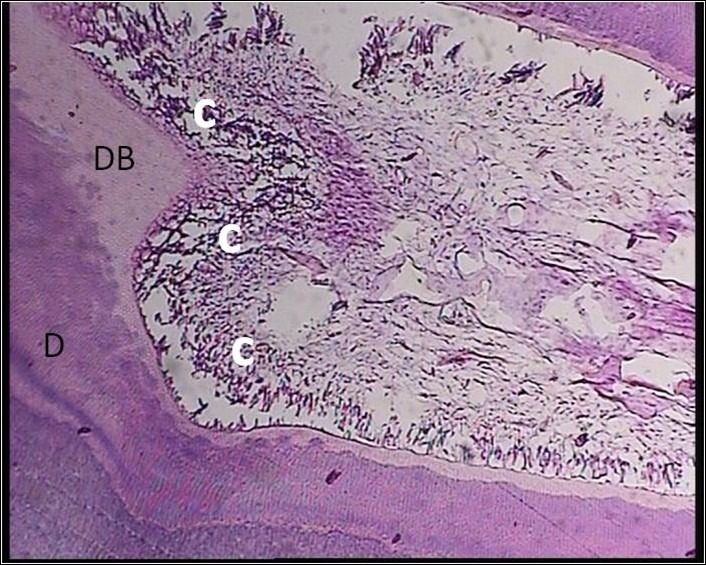
A Sample Treated With MTA+ LLRL (Group 2). Chronic inflammatory cells without any center of necrosis were seen. Abbreviations: MTA, mineral trioxide aggregate; LLRL, low level red laser; D, dentin; DB, dentinal bridge; C, chronic inflammation.
Table 1 . The Frequency (%) of Inflammatory Reactions, Including Type, Intensity and Extension, in the Study Groups .
| Inflammation | Grade | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | ||
| Type | I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (20) |
| II | 1 (20) | 1 (20) | 0 (0) | 3 (60) | 2 (40) | |
| III | 4 (80) | 4 (80) | 5 (100) | 2 (40) | 2 (40) | |
| Intensity | I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| II | 1 (20) | 1 (20) | 0 (0) | 3 (60) | 3 (60) | |
| III | 4 (80) | 4 (80) | 5 (100) | 2 (40) | 2 (40) | |
| Extension | I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| II | 1 (20) | 1 (6.2) | 0 (0) | 3 (60) | 3 (60) | |
| III | 4 (80) | 4 (93.8) | 5 (100) | 2 (40) | 2 (40) |
Hard Tissue Formation
A dentinal bridge was formed in 100% of the specimens in groups 1 to 3 and 80% of the specimens in groups 4 and 5 (Figure 3). In groups 1 to 3, the continuity of the dentinal bridge was mainly classified as grade 3 (complete bridge formation), whereas in groups 4 and 5, moderate contact of capping material with the dental pulp (grade 2) was more frequent. In groups 1 to 3, the dentinal bridge thickness was mainly in the range of 100-250 µm (grade 2) or more than 250 µm (grade 3) , whereas groups 4 and 5 frequently showed dentinal bridge with less than 250 µm thickness (grades 1 and 2) . Regarding dentin morphology, most of the specimens in the study groups exhibited irregular tubular structure (grade 2). Only 20% of the specimens in groups 1, 3, 4, and 5 showed regular tubular dentin (grade 3) and 20% of the specimens in groups 4 and 5 indicated atubular structures (grade 1) (Table 2).
Figure 3 .
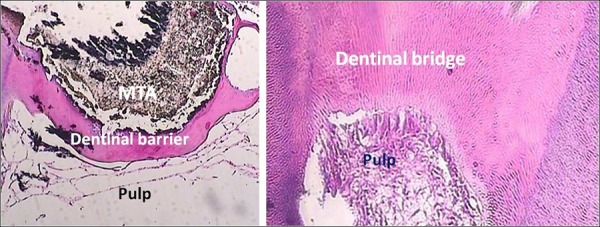
A Sample Treated With MTA (Group 1). A continuous dentinal bridge with regular tubules was formed at the pulp-MTA interface (×40 at the left side and ×100 at the right side). Odontoblast-like cells and healthy pulp were seen. Abbreviation: MTA, mineral trioxide aggregate.
Table 2 . The Frequency (%) of Hard Tissue Formation Including Continuity, Morphology and Thickness in the Study Groups.
| Dentinal Bridge | Grade | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | ||
| Continuity | I | 0 (0) | 0 (0) | 0 (0) | 1 (20) | 1 (20) |
| II | 1 (20) | 0 (0) | 1 (20) | 4 (80) | 3 (60) | |
| III | 4 (80) | 5 (100) | 4 (80) | 0 (0) | 1 (20) | |
| Morphology | I | 0 (0) | 0 (0) | 0 (0) | 1 (20) | 1 (20) |
| II | 4 (80) | 5 (100) | 4 (80) | 3 (60) | 3 (60) | |
| III | 1 (20) | 0 (0) | 1 (20) | 1 (20) | 1 (20) | |
| Thickness | I | 1 (20) | 0 (0) | 0 (0) | 2 (40) | 2 (40) |
| II | 2 (40) | 2 (40) | 2 (40) | 2 (40) | 2 (40) | |
| III | 2 (40) | 3 (60) | 3 (60) | 1 (20) | 1 (20) |
Histopathologic Changes in Soft Tissue
None of the specimens in groups 1 to 3 showed pulp necrosis, whereas partial pulp necrosis was observed in 20% of the specimen in groups 4 and 5 (Figure 4). An odontoblastic layer was formed adjacent to the dentinal bridge in 100% of the specimens in groups 1 to 3. Twenty percent of the specimens in groups 4 and 5 presented no sign of odontoblastic layer formation. Regarding calcification, none of the specimens in the study groups showed diffuse calcification. There was no pulp stone in groups 1 and 2, but 20% of the specimens in groups 3 and 4 exhibited pulp stone (Figure 5; Table 3).
Figure 4 .
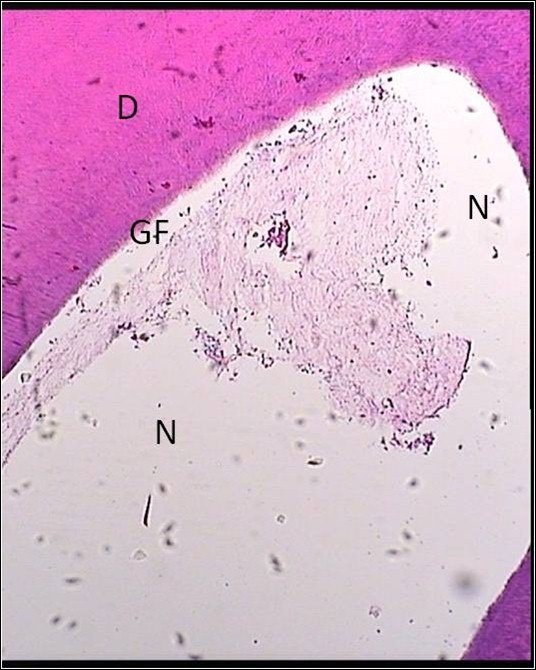
A Sample Treated With Gold Foil + LLRL (Group 4). Zone of necrosis was observed under the material. Also the dentinal bridge was not formed. Abbreviations: LLRL, low level red laser; D, dentin, GF, gold foil; N necrosis.
Figure 5 .
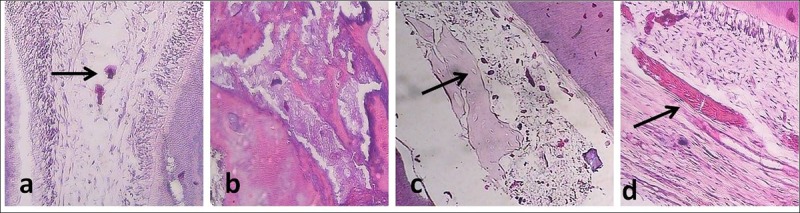
A Sample Treated With MTA+ LLIL (Group 3). Calcified basophilic nodules were seen in the pulp. (a) A sample treated with MTA+ LLRL (group 2). The calcified mass was seen in the pulp (×100). (b) A sample treated with MTA+ LLIL (group 3). Calcified basophilic nodules and osteodentin tissue with lacuna containing cells were seen in the pulp. (c) A sample treated with MTA (group 1). Calcification along the collagen fibers was seen. (d) A sample treated with MTA (group 1). Calcification along the collagen fibers was seen. Abbreviations: MTA, mineral trioxide aggregate; LLRL, low level red laser; LLIL, low level infrared laser.
Table 3 . The Frequency (%) of Histopathological Changes in Soft Tissue Including Necrosis, Odontoblastic Layer Formation and Calcification in the Study Groups.
| Soft Tissue Changes | Grade | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | ||
| Necrosis | I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| II | 0 (0) | 0 (0) | 0 (0) | 1 (20) | 1 (20) | |
| III | 5 (100) | 5 (100) | 5 (100) | 4 (80) | 4 (80) | |
| Odontoblastic layer formation | I | 0 (0) | 0 (0) | 0 (0) | 1 (20) | 1 (20) |
| II | 3 (60) | 2 (40) | 3 (60) | 3 (60) | 4 (80) | |
| III | 2 (40) | 3 (60) | 2 (40) | 1 (20) | 0 (0) | |
| Calcification | I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| II | 0 (0) | 0 (0) | 1 (20) | 1 (20) | 0 (0) | |
| III | 5 (100) | 5 (100) | 4 (80) | 4 (80) | 5 (100) |
Discussion
The main purpose in vital pulp therapy is to activate the odontoblast-like cells to generate dentinal bridges and thus protect the underling soft tissue.1 The DPC technique is an effort to maintain the vitality of dental pulp following exposure. In this method, the volume of the remaining dental pulp is greater than that of the other vital pulp therapy modalities. This greater amount of dental pulp tissue could be helpful in dentinal bridge formation.16 The dental pulp response to DPC technique has been investigated in previous studies using various pulp-capping materials.17 This study investigated the healing effect of low power laser therapy with or without MTA on exposed pulp tissue. This animal study was designed on histologic results, rather than on long- term clinical findings. The follow-up period was 8 weeks similar to most of the previous investigations,2,18,19 although some authors evaluated the pulpal effects of capping materials up to 150 days.15
Fibroblasts are the most frequent cells in the pulp and have a key role in the wound healing process.1 Previous studies demonstrated that low power laser therapy could increase the production of fibroblast growth factor (FGF) and reduce the formation of inflammatory mediators, and thus result in the promotion of remodeling and acceleration of wound healing process.20-23 However, under the conditions used in this study, LLLT had no additional benefit over MTA for improving the healing process in teeth underwent DPC treatment. This may be related to the excellent properties of MTA as a pulp capping material including perfect seal, high biocompatibility, stimulation of dentinal bridge formation, and stability in liquids. Furthermore, MTA indicates decontaminative, biostimulative, and hemostatic effects1 and these properties, altogether, could lead to a successful DPC treatment.
In the present study, the occurrence of pulp inflammation was greater in groups 4 and 5 where laser therapy was used in association with gold foil instead of MTA. Sixty percent of the specimens in groups 4 and 5 showed inflammation, generally observed under the exposure site and not over the entire coronal pulp. There was no inflammation when LLIL was used in combination with MTA (group 3), whereas 20% of the specimens in LLRL + MTA (group 2) and MTA alone (group 1) groups experienced the occurrence of inflammation. The continuity and morphology of the dentinal bridge could be considered as a sign of successful pulp healing.1 Dentinal bridge formation was observed in 100% of the specimens in groups 1 to 3 and 80% of the specimens in groups 4 and 5. However, in groups 1 to 3, complete bridge formation was observed in most of the specimens, whereas in groups 4 and 5, moderate contact of the capping material with the dental pulp was more frequent. The thickness of the dentinal bridge was also greater in MTA-treated (1 to 3) than gold foil-treated (4 and 5) groups. Twenty percent of the specimens in groups 4 and 5 showed atubular structure, whereas atubular dentin was not observed in any of the specimens in groups 1 to 3. Regarding histopathological changes in soft tissues, no pulpal necrosis was observed in MTA-capped groups (1 to 3), whereas 20% of the specimens in gold foil-capped groups (4 and 5) demonstrated partial necrosis. The formation of odontoblastic layer occurred in all of the specimens in MTA-treated groups, and 80% of the specimens in groups 4 and 5. Altogether, the findings of this study indicate that laser therapy could not be considered as an effective modality for treatment of exposed pulp tissue. The most influencing factor in pulpal response was the use of MTA against gold foil, and additional laser therapy did not enhance the healing process in teeth that underwent DPC treatment.
The reason why gold foil was used in groups 4 and 5 of this study was to determine the net effect of laser therapy on stimulating dentinal bridge formation and reducing inflammation in exposed pulp tissue, as the gold foil is an inert material. The specimens in groups 4 and 5, however, showed greater frequency of inflammation, necrosis and absence of odontoblastic layer formation compared to that observed in groups 1 to 3 where MTA was used instead of gold foil. It should be noted that gold foil does not have hemostatic and decontaminative properties of MTA, and provides less seal and marginal adaptation compared to MTA. Therefore, the poor results observed in groups 4 and 5 of this study could be attributed to improper marginal seal of gold foil that possibly neutralizes or decreases any healing effect of laser therapy on exposed pulp tissue. The results of this study indicated that low level laser irradiation on exposure site could not compensate the limitation of gold foil as a pulp capping material.
Most of the previous studies on the use of laser therapy in DPC employed high power lasers such as CO2 and diode,24-29 which have different mechanisms of action from low power lasers and so the results of those studies could not be compared with the present investigation. Regarding the effect of LLLT on DPC, Utsunomiya14 reported that the use of a gallium-aluminum-arsenide diode laser (300 mW) on exposed surface of the pulp resulted in earlier expression of lectins and collagens in laser irradiation group than in the control group, and thus they concluded that laser therapy lead to the acceleration of wound healing process in the pulp. In contrast, Ferriello et al20 evaluated the effect of a low level diode laser (wavelength 680 nm; fluence 4 J/cm2 , 60 s) and different pulp-capping substances on proliferation of L-929 fibroblasts. They reported that LLLT did not improve the performance of dental pulp-capping materials and showed no effect on proliferation of L929 cells. Instead of DPC, some studies evaluated the effect of LLLT on pulpotomy of primary teeth. Golpayegani et al30 compared the effectiveness of LLLT with a semiconductor diode laser (wavelength 632 nm, 151 s, 4.0 J/cm2 ) with that of conventional formocresol in healing of the remaining pulp following pulpotomy of primary teeth. The clinical and radiographic success rate with LLLT (100% and 89%, respectively) was comparable to that of formocresol technique (100% and 100%, respectively), suggesting that LLLT can be used successfully as a complementary step to pulpotomy procedure. Marques et al13 evaluated the effect of LLLT (wavelength 660 nm, 10 mW, 2.5 J/cm2 , 10 s) on pulpal response of primary teeth after pulpotomy. They found that the lowest degree of pulpal inflammation was present in LLLT + Ca(OH)2 group and thus recommended laser therapy prior to the use of Ca(OH)2 in order to provide satisfactory results regarding pulp tissue healing. On the other hand, De Coster et al11 in a systematic review concluded that the results of the LLLT plus either Ca(OH)2 or ZOE application must be cautiously interpreted. The benefits achieved by these interactions are still unclear due to the lack of supportive studies. Pulpal reactions depend on the status of the tissue, the systemic condition of the patient, and some characteristics of capping materials, such as mechanism of action, pH, antibacterial activity, and toxicity level. Improper restorations, lack of sealing which enables bacterial leakage and pulp inflammation, and even the irritating effect of the pulp capping materials can influence the results.
The energy density employed in this study was 7.5 J/cm2 when using 630 nm wavelength and 6.25 J/cm2 when using 810 nm laser. These energy densities were within the therapeutic window (between 0.01 and 10 J/cm2 ) where biostimulatory effects of low power lasers are presented, whereas the inhibitory effects on physiologic activity can occur at higher dosages (greater than 12 J/cm2 ).31,32 The results of a meta-analysis study indicate that the use of 632 nm wavelength and energy density in the range of 0.5 to 4.0 J/cm2 provided the most effective outcomes in cellular proliferation and thus caused a significant improvement in tissue repair.33 Generally, contact mode is recommended for almost all laser applications with one exception: treating an open wound requires a 2 to 4 mm distance between the laser and the target tissue, providing a condition called non-contact mode.31 In this study, the non-contact mode was employed for laser irradiation on pulp wounds.
The limitation of this study was the single application of LLLT on teeth under DPC treatment. However, the exposed teeth should be restored immediately after pulp capping and it was difficult, if not impossible, to continue LLLT on restored teeth of dogs on the following days. Matic et al34 and Ezzati et al35 reported that intermittent laser irradiation had excellent effects on healing of skin ulcers in humans. In another study, Neiburger36 showed acceleration in healing of gingival ulcers following intermittent laser irradiation. Further studies are warranted to investigate the effect of intermittent application of LLLT following DPC using a larger sample size and long-term follow ups in the clinical situation.
Conclusion
Under the conditions used in this study, the type of pulp capping material was a more important factor than low power laser therapy in the success of DPC treatment. The use of MTA either alone or in combination with low power red and infra red lasers provided successful results in teeth that underwent DPC treatment.
Ethical Considerations
This study have been approved by ethical committee of Mashhad University of Medical Science, Mashhad, Iran.
Conflict of Interests
The authors deny any conflicts of interest related to this study.
Acknowledgments
The authors would like to thank the vice chancellor for research of Mashhad University of Medical Sciences for the financial support of this project (grant No. 88367). The results presented in this paper have been taken from a postgraduate student thesis (No. 441).
Please cite this article as follows: Bidar M, Talati A, Moushekhian S, Gharechahi M, Ahrari F, Bojarpour M. The effect of low level laser therapy on direct pulp capping in dogs. J Lasers Med Sci. 2016;7(3):177-183. doi:10.15171/jlms.2016.31.
References
- 1.Ravindra SV, Mamatha GP, Sunita JD, Balappanavar AY, Sardana V. Morphometric analysis of pulp size in maxillary permanent central incisors correlated with age: An indirect digital study. J Forensic Dent Sci. 2015;7(3):208–214. doi: 10.4103/0975-1475.172438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nowicka A, Wilk G, Lipski M, Kołecki J, Buczkowska-Radlińska J. Tomographic evaluation of reparative dentin formation after direct pulp capping with Ca (OH) 2, MTA, biodentine, and dentin bonding system in human teeth. J Endod. 2015;41(8):1234–1240. doi: 10.1016/j.joen.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Small B, Johnson W. Gold foil and its use in modern dentistry. Dent Today. 2006;25(3):92–96. [PubMed] [Google Scholar]
- 4.Tabarsi B, Parirokh M, Eghbal M, Haghdoost AA, Torabzadeh H, Asgary S. A comparative study of dental pulp response to several pulpotomy agents. Int Endod J. 2010;43(7):565–571. doi: 10.1111/j.1365-2591.2010.01711.x. [DOI] [PubMed] [Google Scholar]
- 5.Ahrari F, Madani AS, Ghafouri ZS, Tuner J. The efficacy of low-level laser therapy for the treatment of myogenous temporomandibular joint disorder. Lasers Med Sci. 2014;29(2):551–557. doi: 10.1007/s10103-012-1253-6. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira AN, Silveira L Jr, Genovese WJ. et al. Effect of GaAIAs laser on reactional dentinogenesis induction in human teeth. Photomed Laser Ther. 2006;24(3):358–365. doi: 10.1089/pho.2006.24.358. [DOI] [PubMed] [Google Scholar]
- 7.Eshghpour M, Ahrari F, Najjarkar NT, Khajavi MA. Comparison of the effect of low level laser therapy with alvogyl on the management of alveolar osteitis. Med Oral Patol Oral Cir Bucal. 2015;20(3):e386–e392. doi: 10.4317/medoral.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moosavi H, Maleknejad F, Sharifi M, Ahrari F. A randomized clinical trial of the effect of low-level laser therapy before composite placement on postoperative sensitivity in class V restorations. Lasers Med Sci. 2015;30(4):1245–1249. doi: 10.1007/s10103-014-1565-9. [DOI] [PubMed] [Google Scholar]
- 9.Heravi F, Ahrari F, Mahdavi M, Basafa S. Comparative evaluation of the effect of Er:YAG laser and low level laser irradiation combined with CPP-ACPF cream on treatment of enamel caries. J Clin Exp Dent. 2014;6(2):e121–e126. doi: 10.4317/jced.51309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heravi F, Moradi A, Ahrari F. The effect of low level laser therapy on the rate of tooth movement and pain perception during canine retraction. Oral Health Dent Manag. 2014;13(2):183–188. [PubMed] [Google Scholar]
- 11.De Coster P, Rajasekharan S, Martens L. Laser‐assisted pulpotomy in primary teeth: a systematic review. Int J Paediatr Dent. 2013;23(6):389–399. doi: 10.1111/ipd.12014. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes AP, Lourenço Neto N, Teixeira Marques NC. et al. Clinical and radiographic outcomes of the use of Low‐Level Laser Therapy in vital pulp of primary teeth. Int J Paediatr Dent. 2015;25(2):144–150. doi: 10.1111/ipd.12115. [DOI] [PubMed] [Google Scholar]
- 13.Marques NC Neto NL, de Oliveira Rodini C. et al. Low-level laser therapy as an alternative for pulpotomy in human primary teeth. Lasers Med Sci. 2015;30(7):1815–1822. doi: 10.1007/s10103-014-1656-7. [DOI] [PubMed] [Google Scholar]
- 14.Utsunomiya T. A histopathological study of the effects of low-power laser irradiation on wound healing of exposed dental pulp tissues in dogs, with special reference to lectins and collagens. J Endod. 1998;24(3):187–193. doi: 10.1016/s0099-2399(98)80181-7. [DOI] [PubMed] [Google Scholar]
- 15.Asgary S, Eghbal MJ, Parirokh M, Ghanavati F, Rahimi H. A comparative study of histologic response to different pulp capping materials and a novel endodontic cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(4):609–614. doi: 10.1016/j.tripleo.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Witherspoon DE. Vital pulp therapy with new materials: new directions and treatment perspectives—permanent teeth. J Endod. 2008;34(7):S25–S28. doi: 10.1016/j.joen.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 17.Briso ALF, Rahal V, Mestrener SR, Dezan Junior E. Biological response of pulps submitted to different capping materials. Braz Oral Res. 2006;20(3):219–225. doi: 10.1590/s1806-83242006000300007. [DOI] [PubMed] [Google Scholar]
- 18.Mestrener SR, Holland R, Dezan E. Influence of age on the behavior of dental pulp of dog teeth after capping with an adhesive system or calcium hydroxide. Dent Traumatol. 2003;19(5):255–261. doi: 10.1034/j.1600-9657.2003.00167.x. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Liu T, Li X, Li H, Pi G. Histologic evaluation of direct pulp capping with a self-etching adhesive and calcium hydroxide in beagles. Oral Sur Oral Med Oral Pathol Oral Radiol Endod. 2006;102(4):e78–e84. doi: 10.1016/j.tripleo.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 20.Ferriello V, Faria MR, Cavalcanti BN. The effects of low-level diode laser treatment and dental pulp-capping materials on the proliferation of L-929 fibroblasts. J Oral Sci. 2010;52(1):33–38. doi: 10.2334/josnusd.52.33. [DOI] [PubMed] [Google Scholar]
- 21.Yasukawa A, HRUI H, Koyama Y, Nagai M, Takakuda K. The effect of low reactive-level laser therapy (LLLT) with helium-neon laser on operative wound healing in a rat model. J Vet Med Sci. 2007;69(8):799–806. doi: 10.1292/jvms.69.799. [DOI] [PubMed] [Google Scholar]
- 22.Maiya GA, Kumar P, Rao L. Effect of low intensity helium-neon (He-Ne) laser irradiation on diabetic wound healing dynamics. Photomed Laser Ther. 2005;23(2):187–90. doi: 10.1089/pho.2005.23.187. [DOI] [PubMed] [Google Scholar]
- 23.Madani AS, Ahrari F, Nasiri F, Abtahi M, Tuner J. Low-level laser therapy for management of TMJ osteoarthritis. Cranio. 2014;32(1):38–44. doi: 10.1179/0886963413z.0000000004. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki M, Ogisu T, Kato C, Shinkai K, Katoh Y. Effect of CO2 laser irradiation on wound healing of exposed rat pulp. Odontology. 2011;99(1):34–44. doi: 10.1007/s10266-010-0140-5. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki M, Katsumi A, Watanabe R, Shirono M, Katoh Y. Effects of an experimentally developed adhesive resin system and CO2 laser irradiation on direct pulp capping. Oper Dent. 2005;30(6):702–718. [PubMed] [Google Scholar]
- 26.Moritz A, Schoop U, Goharkhay K, Sperr W. Advantages of a pulsed CO2 laser in direct pulp capping: a long-term. Lasers Surg Med. 1998;22:288–293. doi: 10.1002/(sici)1096-9101(1998)22:5<288::aid-lsm5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 27.Nammour S, Tielemans M, Heysselaer D, Pilipili Ch, De Moor R, Nyssen-Behets C. Nammour S, Tielemans M, Heysselaer D, Pilipili Ch, De Moor R, Nyssen-Behets CComparative study on dogs between CO2 laser and conventional technique in direct pulp capping . Rev Belge Med Dent. 2008;64(2):81–86. [PubMed] [Google Scholar]
- 28.Olivi G, Genovese M, Maturo P, Docimo R. Pulp capping: advantages of using laser technology. Eur J Paediatr Dent. 2007;8(2):89–95. [PubMed] [Google Scholar]
- 29.Yazdanfar I, Gutknecht N, Franzen R. Effects of diode laser on direct pulp capping treatment. Lasers Med Sci. 2015;30(4):1237–1243. doi: 10.1007/s10103-014-1574-8. [DOI] [PubMed] [Google Scholar]
- 30.Golpayegani MV, Ansari G, Tadayon N, Shams S, Mir M. Low-level laser therapy for pulpotomy treatment of primary molars. J Dent Tehran Univ Med Sci. 2009;6(4):168–174. [Google Scholar]
- 31.AlGhamdi KM, Kumar A, Moussa NA. Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci. 2012;27(1):237–249. doi: 10.1007/s10103-011-0885-2. [DOI] [PubMed] [Google Scholar]
- 32.Jahanbin A, Ramazanzadeh B, Ahrari F, Forouzanfar A, Beidokhti M. Effectiveness of Er:YAG laser-aided fiberotomy and low-level laser therapy in alleviating relapse of rotated incisors. Am J Orthod Dentofacial Orthop. 2014;146(5):565–572. doi: 10.1016/j.ajodo.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Nadin G, Goel BR, Yeung CA, Glenny AM. Pulp treatment for extensive decay in primary teeth. Cochrane Database Syst Rev. 2003;(1):CD003220. doi: 10.1002/14651858.cd003220. [DOI] [PubMed] [Google Scholar]
- 34.Matić M, Lazetić B, Poljacki M, Duran V, Ivkov-Simić M. Low level laser irradiation and its effect on repair processes in the skin. Med Pregl. 2002;56(3-4):137–141. doi: 10.2298/mpns0304137m. [DOI] [PubMed] [Google Scholar]
- 35.Ezzati A, Bayat M, Khoshvaghti A. Low-level laser therapy with a pulsed infrared laser accelerates second-degree burn healing in rat: a clinical and microbiologic study. Photomed Laser Surg. 2010;28(5):603–611. doi: 10.1089/pho.2009.2544. [DOI] [PubMed] [Google Scholar]
- 36.Neiburger E. Rapid healing of gingival incisions by the helium-neon diode laser. J Mass Dent Soc. 1998;48(1):8–13. [PubMed] [Google Scholar]


