Abstract
Neural crest cells (NCCs) are vertebrate-specific transient, multipotent, migratory stem cells that play a crucial role in many aspects of embryonic development. These cells emerge from the dorsal neural tube and subsequently migrate to different regions of the body, contributing to the formation of diverse cell lineages and structures, including much of the peripheral nervous system, craniofacial skeleton, smooth muscle, skin pigmentation, and multiple ocular and periocular structures. Indeed, abnormalities in neural crest development cause craniofacial defects and ocular anomalies, such as Axenfeld-Rieger Syndrome and primary congenital glaucoma. Thus, understanding the molecular regulation of neural crest development is important to enhance our knowledge of the basis for congenital eye diseases, reflecting the contributions of these progenitors to multiple cell lineages. Particularly, understanding the underpinnings of NC formation will help to discern the complexities of eye development, as these NCCs are involved in every aspect of this process. In this review, we summarize the role of ocular NCCs in eye development, particularly focusing on congenital eye diseases associated with anterior segment defects and the interplay between three prominent molecules, Pitx2, Cyp1b1, and RA, which act in concert to specify a population of neural crest-derived mesenchymal progenitors for migration and differentiation, to give rise to distinct anterior segment tissues. We also describe recent findings implicating this stem cell population in ocular coloboma formation, and introduce recent evidence suggesting the involvement of NCCs in optic fissure closure and vascular angiogenesis.
Introduction
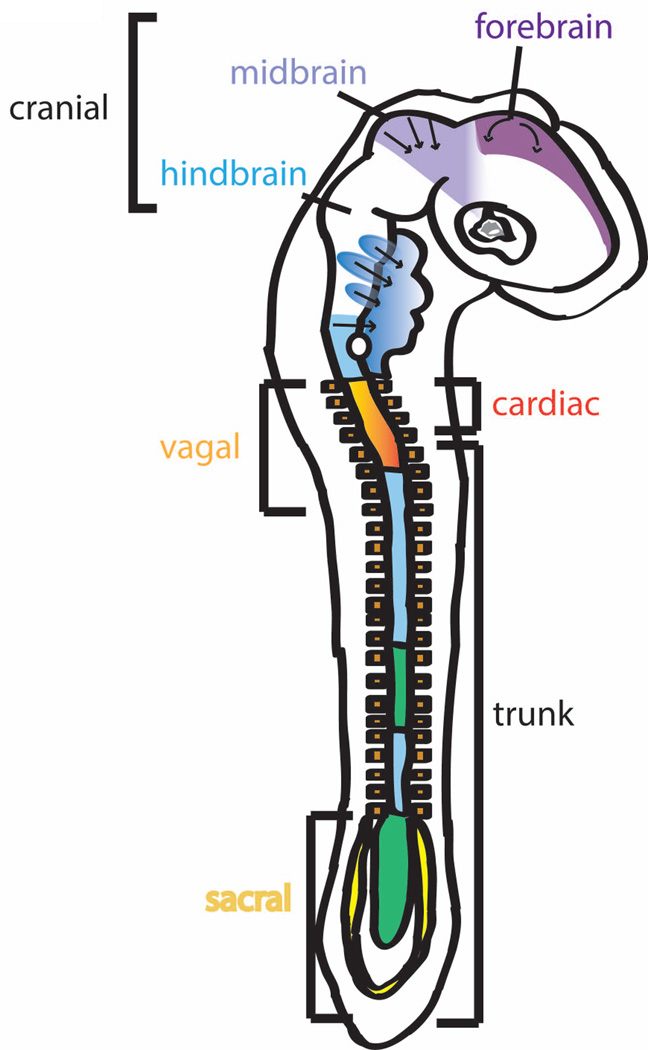
Neural crest cells (NCCs) are a population of multipotent embryonic stem cells that give rise to a wide range of cell and tissue types throughout the body. During gastrulation, NCCs originate at the neural plate border and migrate from folds of the neural ectoderm as the neuroepithelium closes to form the neural tube (Beebe and Coats, 2000; Creuzet et al., 2005; Whikehart, 2010). These cells subsequently migrate, pervading different regions of the embryo and yielding a broad range of tissues (from myofiboblasts, melanocytes, endocrine cells, neurons, and glial cells to cartilage and bone) (Beebe and Coats, 2000; Creuzet et al., 2005; Whikehart, 2010). Both migratory routes and derivatives of neural crest vary with rostrocaudal position along the neural tube. The vagal crest, derived from the caudal hindbrain, contributes to the heart, and together with the sacral neural crest, also form the enteric nervous system that innervates the gut (Creuzet et al., 2005). At the cephalic level, NCCs form the mesectoderm, which subsequently gives rise to craniofacial connective, dermal and skeletal tissues, neurons, and the cranial ganglia (Creuzet et al., 2005; Gage et al., 2005; Kish et al., 2011)(Fig. 1). With respect to ocular development, the NCCs migrating to the eye are primarily derived from the prosencephalon (developing forebrain and brainstem) and mesencephalon (developing midbrain) (Whikehart, 2010). These cells give rise to portions of the corneal endothelium and stroma, iris stroma, ciliary body stroma and muscles, sclera, and trabecular meshwork of the eye(Beebe and Coats, 2000; Cvekl and Tamm, 2004; Gage et al., 2005; Hay, 1980; Kish et al., 2011; Whikehart, 2010).
Fig. 1. Migration pathways of neural crest cells in the developing neural tube.
The migratory routes and derivatives of neural crest cells vary with rostrocaudal position along the neural tube. The vagal crest, derived from the caudal hindbrain, contributes to the heart, and together with the sacral neural crest, also form the enteric nervous system that innervates the gut. At the cephalic level, NCCs form the mesectoderm, which subsequently gives rise to craniofacial connective, dermal and skeletal tissues, neurons, and the cranial ganglia. With respect to ocular development, the NCCs migrating to the eye are primarily derived from the prosencephalon (developing forebrain and brainstem) and mesencephalon (developing midbrain).
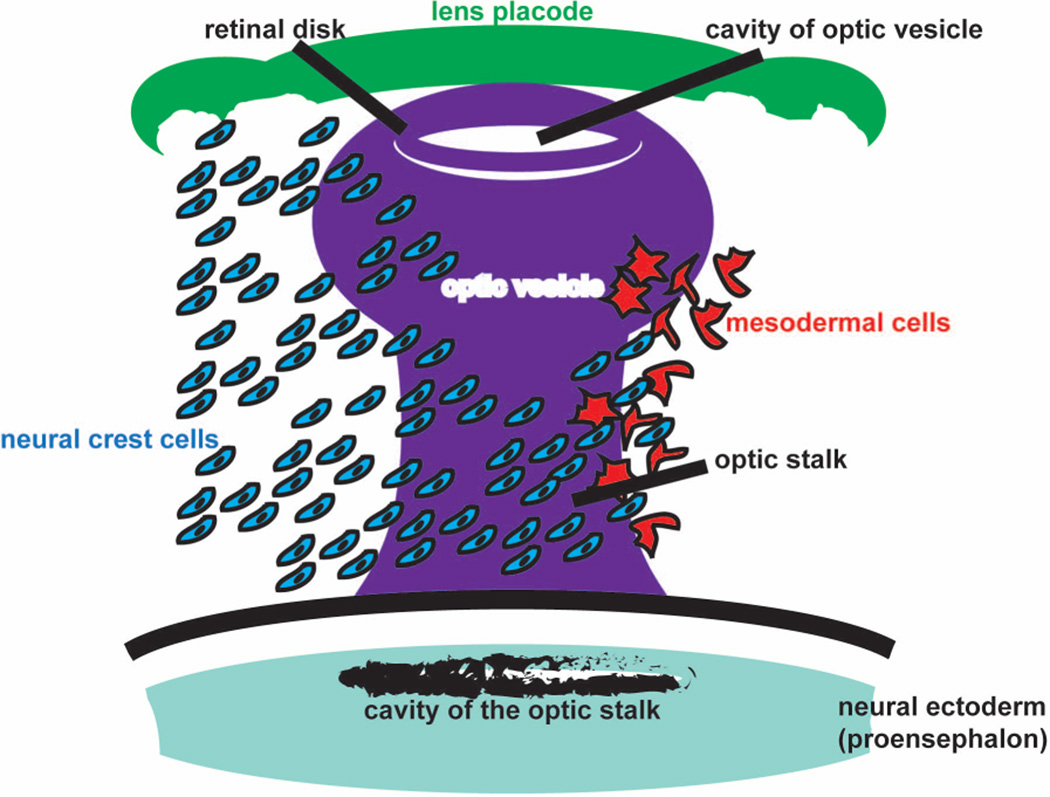
While the NCCs are migrating from the edge of the neural tube, the neuroectodermal-derived optic sulci appear as shallow pits along the neural plate and form the optic vesicles that protrude laterally from the prosencephalon until apposed to the surface ectodermal-derived optic placode (Creuzet et al., 2005) (Fig. 2). Concomitant with surface ectoderm thickening for the differentiation of the lens, morphogenetic movements involving the invagination of the optic vesicles leads to formation of a bi-layered optic cup (Beebe and Coats, 2000; Creuzet et al., 2005; Harada et al., 2007; Kish et al., 2011; Whikehart, 2010).
Fig. 2. Cell migration pattern to the optic cup during eye development.
Neural crest cells are indicated in blue and mesodermal cell are indicated in red.
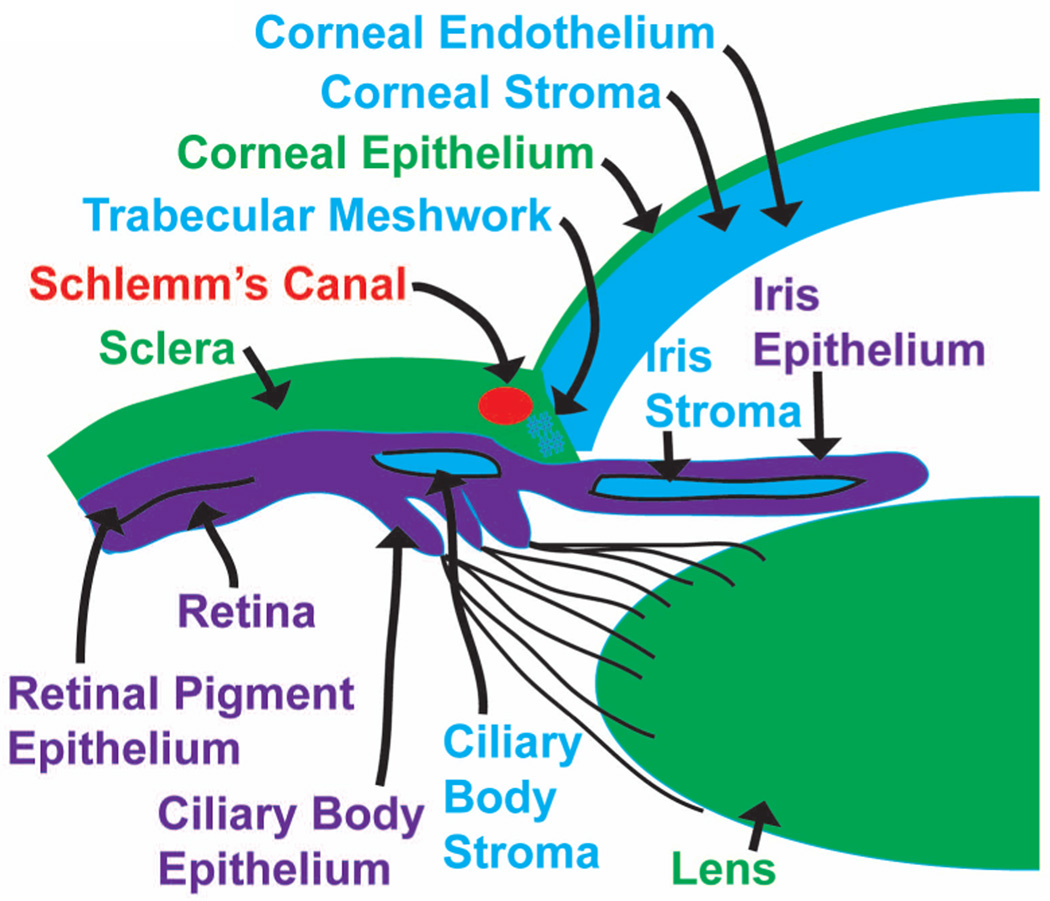
A loose array of neuroectoderm-derived cranial NCCs, termed the periocular mesenchyme (POM), migrate around the posterior of the optic cup. In humans, the NCC migrates in three waves, while in mice and chick there appears to be only two waves (Gage et al., 2005; Hay, 1980; Johnston et al., 1979). In humans, the first wave of NCCs migrates into the space between the anterior surface of the lens and the surface ectoderm destined to form the corneal epithelium to form the corneal endothelium (Gage et al., 2005; Whikehart, 2010) (Fig. 3). A second wave of cells migrates between the corneal epithelium and endothelium to become the keratinocytes of the corneal stroma. The corneal epithelium synthesizes components of the extracellular matrix for the formation of primary stroma when the lens detaches from the surface ectoderm, while a third wave of NCCs migrates to the angle between the posterior cornea (endothelium) and the anterior edge of the optic cup, eventually contributing to the ciliary body and iris stroma(Gage et al., 2005; Whikehart, 2010) (Fig. 3). The POM located in the tissues anterior to the chamber angle between the anterior edge of the eye cup and the endothelium initially remains undifferentiated at this stage, but subsequently develops into flat endothelial-like cells, comprising the trabecular meshwork and Schlemm’s canal, respectively (Gage et al., 2005; Whikehart, 2010) (Fig. 3).
Fig. 3. Overview of the embryonic derivatives in the developing eye.
Further development of the optic cup involves distinct regionalized differentiation into at least four different structures, including the retina, the retinal pigment epithelium (RPE), the iris epithelium, and the ciliary epithelium (Fig. 3). Notably, a second essential function of the POM during ocular development is to provide essential signals for the patterning of ocular ectoderm primordia, which not only includes the specification of the RPE from the optic cup, but also the induction of lacrimal glands from the surface ectoderm and the differentiation of the optic stalk from the neural ectoderm (Fuhrmann et al., 2000; Gage et al., 2005; Kao et al., 2013). Moreover, cells originating from the surface epithelium interact with the POM for proper eyelid development (Le Lievre and Le Douarin, 1975).
Over the last decade, studies have highlighted the contributions of the NCCs to ocular and periocular development, emphasizing the importance of these cells in vertebrate ocular evolution. Indeed, defects in neural crest formation lead to severe craniofacial defects and ocular anomalies, and a comprehensive understanding of the interactions involved in the molecular regulation of the neural crest would provide insight into the complexities underlying congenital eye diseases. Herein, we discuss the role of ocular neural crest cells in eye development, particularly focusing on congenital eye diseases associated with anterior segment defects and the interplay between three prominent molecules, Pitx2, Cyp1b1, and RA, which act in concert to specify a population of neural crest-derived mesenchymal progenitors for migration and differentiation to give rise to distinct anterior segment tissues. We also describe recent findings implicating this stem cell population in ocular coloboma formation and introduce recent evidence suggesting the involvement of NCCs in optic fissure closure and vascular angiogenesis..
Anterior segment dysgenesis: Ocular anomalies associated with NCC defects
The abnormal migration of NCCs and subsequent disruption of their derivative structures has been implicated in congenital eye diseases affecting the anterior segment of the eye. Anterior segment dysgenesis (ASD) encompasses a group of developmental disorders affecting the function of structures in front of the vitreous surface of the eye, including the cornea, iris, lens, ciliary body, trabecular meshwork, Schlemm’s canal, and sclera (Sowden, 2007). Increasing evidence has shown that transcriptional events are critical for many aspects of neural crest development, and the pathogenesis of several anterior segment disorders has been associated with defects in these genes.
Axenfeld-Rieger Syndrome and PITX2
Axenfeld-Rieger syndrome (ARS) describes a rare (1 in 200,0000) group of genetically and phenotypically heterologous disorders that primarily affect the eye and are also associated with systemic issues, including cardiovascular outflow malformations, craniofacial and dental defects, umbilical abnormalities, and pituitary anomalies with endocrine sequelae (Bohnsack et al., 2012; Evans and Gage, 2005; MacDonald et al., 2004). Ophthalmic manifestations of ARS are typically limited to varying degrees of ASD and include posterior embryotoxon (anteriorization of the angle structures into peripheral cornea) and iris hypoplasia (Bohnsack et al., 2012; Evans and Gage, 2005; MacDonald et al., 2004; Sowden, 2007). Maldevelopment of the iris results in corectopia (pupil displacement) and polycoria (multiple pupils) (Sowden, 2007). Further, abnormal iris strands can extend from the iris to the posterior embryotoxon, thereby covering the trabecular meshwork in the iridocorneal angle and resulting in glaucoma in approximately 50% of affected individuals (Bohnsack et al., 2011; Gage et al., 1999; MacDonald et al., 2004; Sowden, 2007). Molecular genetics studies have identified specific gene mutations, and one of the most commonly affected genes is paired-like homeodomain 2 (PITX2) on chromosome 4q25 (Semina et al., 1996; Semina et al., 1997; Vaux et al., 1992). More than 45 point and chromosomal mutations, including both gain- and loss-of-function of the PITX2 gene, have been identified. ARS is typically inherited in an autosomal dominant pattern, indicating that eye development is highly sensitive to alterations in PITX2 expression and function.
PITX2, a member of the homeobox protein family, is a transcription factor that plays a critical role in early development, particularly in the formation of structures in the anterior segment of the eye. Pitx2 is expressed in the cranial neural crest-derived POM, but not in ocular tissues derived from the neural and surface ectoderm (Evans and Gage, 2005; Gage et al., 1999). Pitx2 knockout mice die early in embryonic development, as a result of heart defects and display severe ocular defects, loss of extraocular muscles (EOMs), and jaw and pharyngeal arch abnormalities (Gage et al., 1999). Due to this severe lethal phenotype, conditional knockout studies in mice were used to demonstrate the specific requirement of Pitx2 in the cranial neural crest for optic stalk formation and development of the corneal endothelium and stroma and sclera (Evans and Gage, 2005). Similarly, in zebrafish, we have found that knockdown of Pitx2 or expression of a dominant negative mutant form of the gene disrupted neural crest migration into the craniofacial region (unpublished data) and caused malformation of the jaw, pharyngeal arches, corneal endothelium, and iris stroma (Bohnsack et al., 2012). Thus, Pitx2 is critical in the cranial neural crest, and disruption in gene function or expression results in craniofacial and ocular malformations.
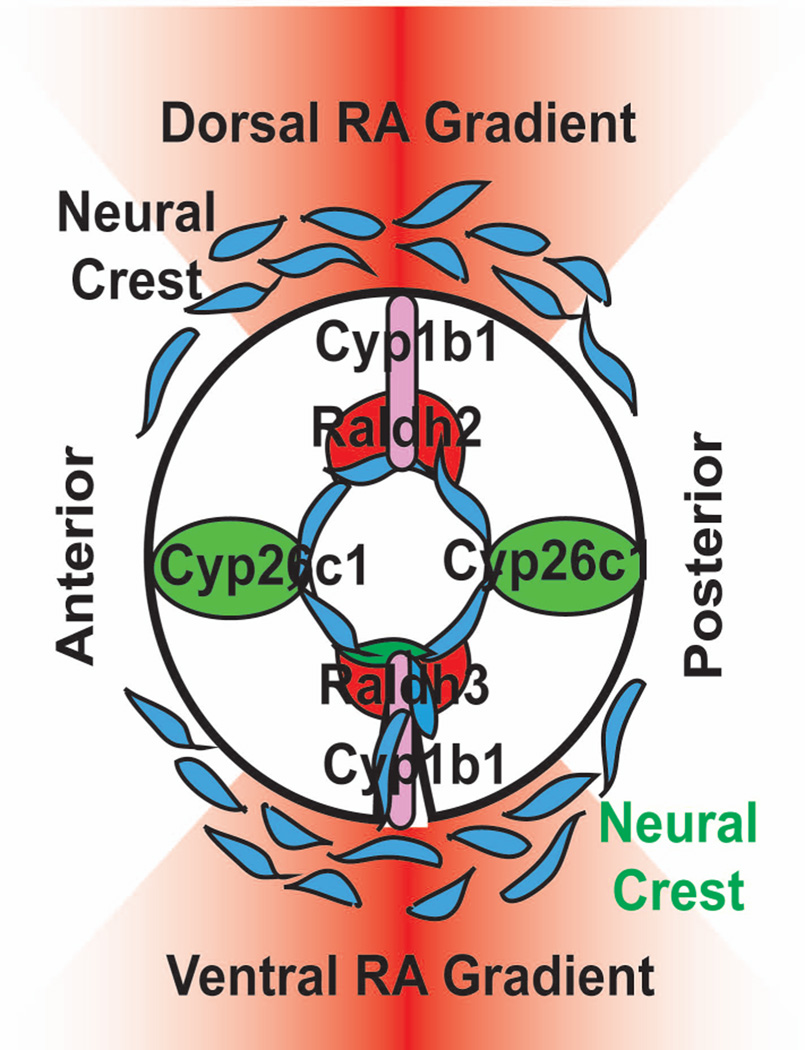
The regulation of PITX2 expression in the cranial neural crest is critical for ocular development. The essential morphogen, retinoic acid (RA), has been shown in both mice and zebrafish to regulate Pitx2 expression in the periocular mesenchyme (Duester, 2009). RA is derived from vitamin A (retinal) through a series of enzymatic reactions and exists as locally regulated gradients that control numerous embryonic processes (Bohnsack and Kahana, 2013; Bohnsack et al., 2012; Schier and Needleman, 2009). In the craniofacial region, the developing eye produces RA in a specific spatial and temporal pattern (Reijntjes et al., 2004). In mice, chick, and zebrafish, RA synthesis enzymes (e.g., RALDH2, RALDH3, RALDH4) are expressed in the developing dorsal and ventral retina, while RA degradation enzymes (e.g., CYP26A1 and CYP26C1) are localized to the cranial and caudal retina; thus, creating RA gradients that are centered around the dorsal and ventral axis of the eye (Cvekl and Wang, 2009; Duester, 2009; Molotkov et al., 2006) (Fig. 4). While systemic disruption of RA levels are embryonic lethal and cause severe pan-ocular malformations, localized alterations of RA signaling show that the POM is the primary target that regulates anterior segment development (Kumar and Duester, 2010). In the POM, RA binds to RA receptors (RARs β and γ subunits) which then heterodimerize with the retinoid X receptor (RXR) α subunit. This nuclear hormone receptor complex then upregulates the expression of Pitx2 in the POM (Diehl et al., 2006; Evans and Gage, 2005; Kumar and Duester, 2010; Lupo et al., 2011; Matt et al., 2005). In zebrafish, neural crest-derived corneal endothelial and iris stromal malformations induced by decreased RA synthesis were rescued by overexpression of human PITX2 (Bohnsack et al., 2012). Thus, PITX2 is a functional downstream target of RA in the POM, which mediates RA regulation of neural crest derivatives in the eye disrupted neural crest-derived corneal endothelial and iris stromal formation. Clinical mutations in PITX2 in individuals with ARS illustrate the essential role of the neural crest in ocular development (Acharya et al., 2009; Weisschuh et al., 2006). Animal studies demonstrate that Pitx2 along with RA signaling in the neural crest-derived POM is a critical regulator of anterior segment development and understanding these developmental mechanisms yield insight into the pathogenesis of this and other congenital eye diseases.
Fig. 4. Schematic showing the regulation of RA synthesis in ocular development.
At early stages of development, RA synthesis enzymes (e.g., RALDH2, RALDH3, RALDH4) are expressed in the developing dorsal and ventral retina, while RA degradation enzymes (e.g., CYP26A1 and CYP26C1) are localized to the cranial and caudal retina; thus, creating RA gradients centered around the dorsal and ventral axis of the eye. Interestingly, in zebrafish, cyp1b1 is expressed in a specific spatial and temporal pattern in the dorsal and ventral retina, similar to the RA synthesis enzymes, raldh2 and raldh3.
Primary congenital glaucoma and Cyp1b1
Disruption of neural crest development as seen in ASD often results in abnormal iridocorneal angle and trabecular meshwork formation leading to early onset glaucoma (Sowden, 2007). Situations in which elevated intraocular pressure is directly due to the isolated finding of trabecular meshwork malformation are typically classified as primary congenital glaucoma (PCG), an uncommon (1:10,000) autosomal-recessive eye disease diagnosed between birth and 1 year of age (Vasiliou and Gonzalez, 2008). Elevated intraocular pressure in PCG damages the optic nerve, cornea, and sclera leading to vision loss. PCG is genetically heterologous, however, linkage studies and positional cloning have implicated the gene for cytochrome P4501B1 (CYP1B1) on chromosome 2p22.2, as the most commonly identified PCG-causing gene (Badeeb et al., 2014; Chavarria-Soley et al., 2008; Hollander et al., 2006; Mashima et al., 2001). Multiple missense and nonsense mutations, deletions, insertions and/or duplications, and silent mutations, in CYP1B1 account for 10–20% of cases of PCG (Vasiliou and Gonzalez, 2008). Despite its association with human disease, the definitive role of CYP1B1 in eye development remains unknown.
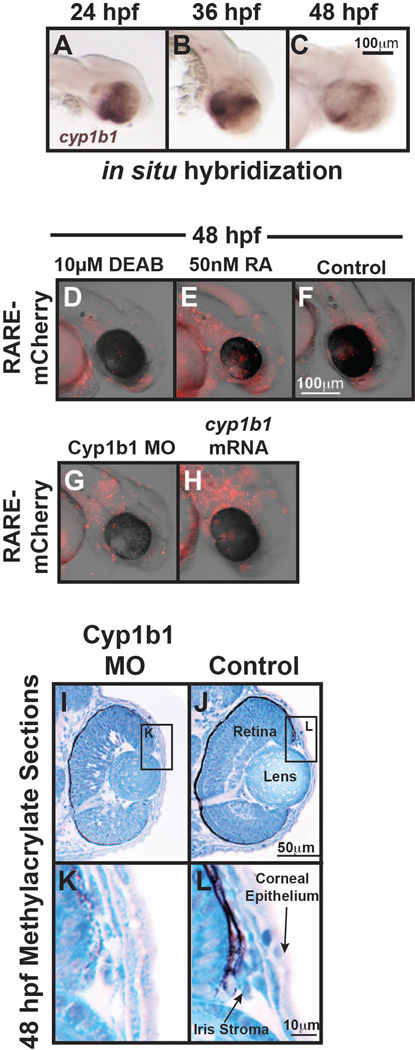
The CYP1B1 gene encodes a cytochrome p450 enzyme that catalyzes the monooxygenation of exogenous toxins, such as polycyclic aromatic hydrocarbons in zebrafish, and endogenous substrates, such as 17β estradiol in hormone-induced tumors. In zebrafish embryos, early cyp1b1 expression in the retina occurs independently of the toxin-induced activation of the aryl hydrocarbon receptor, suggesting an as yet unidentified endogenous substrate for cyp1b1 in the eye (Yin et al., 2008). In vitro studies have demonstrated that Cyp1b1 alone is sufficient to efficiently oxidize retinol to retinal and subsequently to RA in a dehydrogenase-independent pathway (Chambers et al., 2007). Further, expression of the Cyp1b1 ortholog in chick is associated with RA activity during early development (Chambers et al., 2007). In zebrafish, we have found that cyp1b1 was expressed in a specific spatial and temporal pattern in the dorsal and ventral retina in areas that overlapped with the RA synthesis enzymes, raldh2 and raldh3 (Fig. 4 and Fig. 5 A–C). Further, we found that alterations in Cyp1b1 expression correlated with response of a RA reporter in the periocular tissues (Fig. 5 D–H). Despite these findings, knockdown of Cyp1b1 in zebrafish embryos showed an early delay in neural crest-derived iris stromal formation (Fig. 5 I–L) and retinal development that recovered by the larval stage. Cyp1b1 deficient mice also did not exhibit a consistent phenotype as intraocular pressures were normal, despite mild angle abnormalities involving Schlemm’s canal, the trabecular meshwork, cornea, and iris. Thus, additional studies are required to determine whether RA mediates the molecular effects of CYP1B1 in neural crest and eye development.
Fig. 5.
A–C: Whole mount in situ hybridization of zebrafish embryos demonstrated that cyp1b1 was expressed in the developing eye. At 24 hpf (A), cyp1b1 was expressed in the dorsal and ventral retina and retinal pigment epithelium. Cyp1b1 expression peaked at 36 hpf (C), and at 48 hpf (E, F), cyp1b1 showed the highest expression in the region of the ocular fissures. D–H: Injection of the RARE::mCherry reporter construct in 1-cell stage zebrafish embryos showed RA activity in and around the developing eye at 48 hpf (F). Treatment with the pan-aldehyde dehydrogenase inhibitor, 10 µM diethylbenzaldehyde (DEAB) to decrease RA synthesis from 24–48 hpf decreased mCherry expression (D), while the application of exogenous 50 nM RA increased mCherry expression throughout the craniofacial region (E). The coinjection of Cyp1b1 MO and the RARE::mCherry reporter construct in 1-cell stage embryos decreased RA activity in the periocular region at 48 hpf (F). The injection of cyp1b1 mRNA diffusely increased mCherry expression at 48 hpf throughout the craniofacial region and developing heart (G). I–L: Methylacrylate sections of 48 hpf zebrafish embryos at 48 hpf demonstrated that MO knockdown of Cyp1b1 (I, K) mildly decreased eye size, inhibited retinal differentiation, and decreased iris stromal cellularity in the dorsal iridocorneal angle (K), compared with controls (J, L).
3. Congenital ocular coloboma and the neural crest
During the early stages of eye formation, invagination of the optic vesicle generates a bilayered optic cup with a groove along the ventral margin. This groove, named the optic fissure (also known as choroidal fissure or embryonic fissure), is essential for the entry of mesenchyme cells that give rise to blood vesicles that nourish the eye throughout the life of the organism (Harada et al., 2007). Subsequently, the margins of the optic cup close around these vessels. In humans, the optic fissure closes during the 6–7th weeks of gestation (Barishak, 1992), while in mice and zebrafish, the fissure closes between 11–13 days post fertilization (dpf) and 1.5–2.5 dpf, respectively (Chang et al., 2006). Failure of fissure closure results in colobomas that affect the iris, lens, zonules, ciliary body, retina, choroid, and optic nerve of the eye, and occur in approximately 1 in 10,000 births (Shah et al., 2011; Shah et al., 2012). As this condition also affects the major tissues of the anterior segment of the developing eye, it is appropriate to discuss coloboma formation with respect to ASD, specifically highlighting the implications for the involvement of NCCs in choroid fissure closure.
Ocular coloboma can be seen in isolation or as part of a multisystem syndrome associated with other ocular abnormalities such as microphthalmia (small, disorganized globe), glaucoma, Peters, anomaly, and cataract in the absence of systemic findings (Chang et al., 2006; Shah et al., 2011; Shah et al., 2012). CHARGE, which is characterized by defects affecting the eyes, heart, brain, craniofacial structure, and genitourinary system, is the most common syndrome associated with colobomas (Chang et al., 2006; Eccles and Schimmenti, 1999; Gregory-Evans et al., 2004; Guirgis and Lueder, 2003; Morrison et al., 2000; D. Morrison et al., 2002; Porges et al., 1992). However, there are numerous chromosomal abnormalities and syndromes with unknown genetic causes that also exhibit colobomas. In these syndromic cases, the eye phenotype is observed concomitant with craniofacial abnormalities, further supporting the idea that NCCs play a role in the pathophysiology of colobomas (Gregory-Evans et al., 2004; Morrison et al., 2002).
Studies in model organisms, primarily mice and zebrafish, suggest that genetic lesions or toxic exposures can lead to abnormal dorsoventral patterning of the optic vesicle, reduced expression of ventral eye and POM genes, excessive cell proliferation, and tissue fusion defects (T. H. Kim et al., 2007; Lupo et al., 2011; McLaughlin et al., 2007; Mui et al., 2005; See and Clagett-Dame, 2009; Weiss et al., 2012). Hence, multiple mechanisms are involved in optic fissure closure, underscoring the complexity of this process. However, a mechanistic understanding of the process of optic fissure closure is still missing, and hence it is poorly understood why this process sometimes fails. As the neural crest gives rise to many of the tissues in the developed eye, it is reasonable to propose that these cells play an important and critical role in the cellular and molecular mechanisms underlying this step of ocular development. Recent studies indicate that POM cells play a critical role in choroid fissure closure (Etchevers et al., 2001; Weiss et al., 2012). However, what POM cells do, where POM cells go, or how POM cells function during this process remains unknown. To provide insight into this process, we must first consider the development of coloboma from the perspective of the potential role of NCCs in this ocular defect.
RA and PITX2 defects lead to failures in optic fissure closure
As previously described, RA has long been implicated in the development of the vertebrate eye. The requirement for RA signaling in eye development was first described in reports of severe congenital eye defects in vitamin A deficient (VAD) and/or RA-deficient human, pig, and rat fetuses (Warkany and Schraffenberger, 1946). Indeed, the ASD prominently observed in these individuals included agenesis of the lens, iris and corneal stroma, corneal endothelium, the absence of the anterior chamber, and optic nerve coloboma. These effects are phenocopied in mice where various combinations of RA-synthesis enzymes or RARs are deleted, indicating that RA is required during choroid fissure closure (See and Clagett-Dame, 2009). However, tight control of RA levels appears to be critical for this process as both RA deficiency and excess during embryogenesis induces colobomas in mice and zebrafish. In mice, treatment of pregnant dams with RA changes gene expression along the proximal-distal axis of the fissure, thereby altering specification of retina, retinal pigment epithelial, choroid, and optic nerve. Further, in zebrafish, local delivery of RA to the eye not only resulted in coloboma, but also induced ectopic fissure formation elsewhere in the retina (Bohnsack and Kahana, 2013). As previously described, RA targets the neural crest-derived POM that surrounds the developing eye (Matt et al., 2005). Interestingly, neural crest-specific knockout of all RAR genes in mice causes colobomas and eversion of the ventral optic cup, suggesting that RA acts via the POM to guide the morphogenesis of the optic cup and closure of the choroid fissure (Lohnes et al., 1994; Matt et al., 2008). In addition, the known RA-target in the POM, PITX2, has also been identified as playing a role in optic fissure closure (Gage et al., 1999). Optic fissure closure is defective or significantly delayed in Pitx2 null mutant mouse embryos (Kumar and Duester, 2010; See and Clagett-Dame, 2009). Further, conditional knockout of Pitx2 specifically in the neural crest also gives rise to colobomas (Gage et al., 2005; Sclafani et al., 2006). Thus, the effects of RA and PITX2 on not only anterior segment development, but also optic cup morphogenesis and specifically ocular fissure closure are via the neural crest.
Conclusions and Future perspectives
The most dynamic event in the morphogenesis of the developing eye is the migration and differentiation of neural crest-derived periocular mesenchymal cells, to provide multiple mature cell lineages necessary for normal ocular development and vision, including the corneal endothelium and stroma, trabecular meshwork, Schlemm’s canal, sclera, ciliary body muscles, and iris stroma (Cvekl and Tamm, 2004; Hay, 1980). The POM also provides the essential signals for the patterning of the neural ectoderm, including specification of the RPE from the optic cup and differentiation of the optic stalk from the neural ectoderm (Cvekl and Tamm, 2004; Hay, 1980). The derivation of these structures requires complex interactions between the neural crest, neural ectoderm, and surface ectoderm (Chow and Lang, 2001). Genetic or acquired defects in the development or function of the POM lead to debilitating vision loss and ocular disease, emphasizing the importance of these cells in every aspect of ocular development.
The use of animal models, particularly mice, chick, and zebrafish, has improved our understanding of neural crest development and importantly the pathogenesis of congenital eye diseases, such as ARS, congenital glaucoma, and colobomas. The next step is to use this information to create gene therapies or use stem cell technology to reverse or bypass the molecular defects to restore vision in children affected with these blinding diseases. Further, developmental studies will provide important insight into regenerative treatments for degenerative diseases of the cornea, such as keratoconus, Fuchs dystrophy, and adult-onset primary open-angle glaucoma.
Our understanding of neural crest contributions to the eye should continue to be expanded. Through ophthalmic genetics and congenital eye diseases, the knowledge regarding the molecular regulation of neural crest contributions to the anterior segment is growing. Another highly important area of research regarding ocular neural crest involves blood vessel formation. Studies in mice and quail have demonstrated that pericytes and vascular smooth muscle cells, which surround endothelial cells in the hyaloid, retinal, choroidal, and optic nerve vasculature, are in part, derived from NCCs (Etchevers et al., 2001; Gage et al., 2005; Trost et al., 2013). Impaired blood vessel development during eye development can lead to congenital eye diseases, including colobomas and microphthalmia. These vascular support cells play a key role in tissue homeostasis by stabilizing blood vessels, participating in blood flow regulation, and mediating the formation of the blood retina barrier (Gariano and Gardner, 2005; Hyoung Kim et al, 2011; Jeong et al., 2008; J. H. Kim et al., 2006; Saint-Geniez and D'Amore, 2004). In adults, loss of vascular pericytes in retinal blood vessels is a key element in the pathogenesis of diabetic retinopathy (Beltramo and Porta, 2013; Hammes, 2005; Hammes et al., 2002). Understanding the contributions of the neural crest to blood vessels in the developing eye holds promise for the treatment of both congenital and degenerative eye diseases.
The neural crest is a dynamic embryonic stem cell population that plays a critical role in ocular development. The neural crest interacts with the surrounding neural ectoderm, surface ectoderm, and mesoderm and gives rise to numerous components of the anterior segment of the eye. Studies delineating neural crest development are key for understanding the pathogenesis of congenital eye diseases, such as those we have outlined in this review. By elucidating these molecular signals, we unlock the potential for novel treatments for congenital diseases. Further, these pathways also have implications in regenerative therapies for adult degenerative diseases of neural crest-derivatives. Additional studies using animal models and genetic techniques will expand our knowledge of this unique population of stem cells and pioneer treatments.
Literature Cited
- Acharya M, Lingenfelter DJ, Huang L, Gage PJ, Walter MA. Human PRKC apoptosis WT1 regulator is a novel PITX2-interacting protein that regulates PITX2 transcriptional activity in ocular cells. J Biol Chem. 2009;284(50):34829–34838. doi: 10.1074/jbc.M109.006684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badeeb OM, Micheal S, Koenekoop RK, den Hollander AI, Hedrawi MT. CYP1B1 mutations in patients with primary congenital glaucoma from Saudi Arabia. BMC Med Genet. 2014;15:109. doi: 10.1186/s12881-014-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barishak YR. Embryology of the eye and its adnexae. Dev Ophthalmol. 1992;24:1–142. [PubMed] [Google Scholar]
- Beebe DC, Coats JM. The lens organizes the anterior segment: specification of neural crest cell differentiation in the avian eye. Dev Biol. 2000;220(2):424–431. doi: 10.1006/dbio.2000.9638. [DOI] [PubMed] [Google Scholar]
- Beltramo E, Porta M. Pericyte loss in diabetic retinopathy: mechanisms and consequences. Curr Med Chem. 2013;20(26):3218–3225. doi: 10.2174/09298673113209990022. [DOI] [PubMed] [Google Scholar]
- Bohnsack BL, Gallina D, Kahana A. Phenothiourea sensitizes zebrafish cranial neural crest and extraocular muscle development to changes in retinoic acid and IGF signaling. PLoS One. 2011;6(8):e22991. doi: 10.1371/journal.pone.0022991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack BL, Kahana A. Thyroid hormone and retinoic acid interact to regulate zebrafish craniofacial neural crest development. Dev Biol. 2013;373(2):300–309. doi: 10.1016/j.ydbio.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack BL, Kasprick DS, Kish PE, Goldman D, Kahana A. A zebrafish model of axenfeld-rieger syndrome reveals that pitx2 regulation by retinoic acid is essential for ocular and craniofacial development. Invest Ophthalmol Vis Sci. 2012;53(1):7–22. doi: 10.1167/iovs.11-8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers D, Wilson L, Maden M, Lumsden A. RALDH-independent generation of retinoic acid during vertebrate embryogenesis by CYP1B1. Development. 2007;134(7):1369–1383. doi: 10.1242/dev.02815. [DOI] [PubMed] [Google Scholar]
- Chang L, Blain D, Bertuzzi S, Brooks BP. Uveal coloboma: clinical and basic science update. Curr Opin Ophthalmol. 2006;17(5):447–470. doi: 10.1097/01.icu.0000243020.82380.f6. [DOI] [PubMed] [Google Scholar]
- Chavarria-Soley G, Sticht H, Aklillu E, Ingelman-Sundberg M, Pasutto F, Reis A, Rautenstrauss B. Mutations in CYP1B1 cause primary congenital glaucoma by reduction of either activity or abundance of the enzyme. Hum Mutat. 2008;29(9):1147–1153. doi: 10.1002/humu.20786. [DOI] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Creuzet S, Vincent C, Couly G. Neural crest derivatives in ocular and periocular structures. Int J Dev Biol. 2005;49(2–3):161–171. doi: 10.1387/ijdb.041937sc. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Tamm ER. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays. 2004;26(4):374–386. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A, Wang WL. Retinoic acid signaling in mammalian eye development. Exp Eye Res. 2009;89(3):280–291. doi: 10.1016/j.exer.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl AG, Zareparsi S, Qian M, Khanna R, Angeles R, Gage PJ. Extraocular muscle morphogenesis and gene expression are regulated by Pitx2 gene dose. Invest Ophthalmol Vis Sci. 2006;47(5):1785–1793. doi: 10.1167/iovs.05-1424. [DOI] [PubMed] [Google Scholar]
- Duester G. Keeping an eye on retinoic acid signaling during eye development. Chem Biol Interact. 2009;178(1–3):178–181. doi: 10.1016/j.cbi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles MR, Schimmenti LA. Renal-coloboma syndrome: a multi-system developmental disorder caused by PAX2 mutations. Clin Genet. 1999;56(1):1–9. doi: 10.1034/j.1399-0004.1999.560101.x. [DOI] [PubMed] [Google Scholar]
- Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128(7):1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- Evans AL, Gage PJ. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Hum Mol Genet. 2005;14(22):3347–3359. doi: 10.1093/hmg/ddi365. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S, Levine EM, Reh TA. Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development. 2000;127(21):4599–4609. doi: 10.1242/dev.127.21.4599. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Rhoades W, Prucka SK, Hjalt T. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci. 2005;46(11):4200–4208. doi: 10.1167/iovs.05-0691. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126(20):4643–4651. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438(7070):960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- Gregory-Evans CY, Williams MJ, Halford S, Gregory-Evans K. Ocular coloboma: a reassessment in the age of molecular neuroscience. J Med Genet. 2004;41(12):881–891. doi: 10.1136/jmg.2004.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirgis MF, Lueder GT. Choroidal neovascular membrane associated with optic nerve coloboma in a patient with CHARGE association. Am J Ophthalmol. 2003;135(6):919–920. doi: 10.1016/s0002-9394(02)02293-6. [DOI] [PubMed] [Google Scholar]
- Hammes HP. Pericytes and the pathogenesis of diabetic retinopathy. Horm Metab Res. 2005;37(Suppl 1):39–43. doi: 10.1055/s-2005-861361. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Deutsch U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51(10):3107–3112. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- Harada T, Harada C, Parada LF. Molecular regulation of visual system development: more than meets the eye. Genes Dev. 2007;21(4):367–378. doi: 10.1101/gad.1504307. [DOI] [PubMed] [Google Scholar]
- Hay ED. Development of the vertebrate cornea. Int Rev Cytol. 1980;63:263–322. doi: 10.1016/s0074-7696(08)61760-x. [DOI] [PubMed] [Google Scholar]
- Hollander DA, Sarfarazi M, Stoilov I, Wood IS, Fredrick DR, Alvarado JA. Genotype and phenotype correlations in congenital glaucoma: CYP1B1 mutations, goniodysgenesis, and clinical characteristics. Am J Ophthalmol. 2006;142(6):993–1004. doi: 10.1016/j.ajo.2006.07.054. [DOI] [PubMed] [Google Scholar]
- Hyoung Kim J, Suk Yu Y, Kim KW, Hun Kim J. Investigation of barrier characteristics in the hyaloid-retinal vessel of zebrafish. J Neurosci Res. 2011;89(6):921–928. doi: 10.1002/jnr.22607. [DOI] [PubMed] [Google Scholar]
- Jeong JY, Kwon HB, Ahn JC, Kang D, Kwon SH, Park JA, Kim KW. Functional and developmental analysis of the blood-brain barrier in zebrafish. Brain Res Bull. 2008;75(5):619–628. doi: 10.1016/j.brainresbull.2007.10.043. [DOI] [PubMed] [Google Scholar]
- Johnston MC, Noden DM, Hazelton RD, Coulombre JL, Coulombre AJ. Origins of avian ocular and periocular tissues. Exp Eye Res. 1979;29(1):27–43. doi: 10.1016/0014-4835(79)90164-7. [DOI] [PubMed] [Google Scholar]
- Kao WW, Liu H, Zhang J. Wakayama symposium: challenges of future research in ocular surface cell biology. Ocul Surf. 2013;11(1):19–24. doi: 10.1016/j.jtos.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim JH, Park JA, Lee SW, Kim WJ, Yu YS, Kim KW. Blood-neural barrier: intercellular communication at glio-vascular interface. J Biochem Mol Biol. 2006;39(4):339–345. doi: 10.5483/bmbrep.2006.39.4.339. [DOI] [PubMed] [Google Scholar]
- Kim TH, Goodman J, Anderson KV, Niswander L. Phactr4 regulates neural tube and optic fissure closure by controlling PP1-, Rb-, and E2F1-regulated cell-cycle progression. Dev Cell. 2007;13(1):87–102. doi: 10.1016/j.devcel.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Kish PE, Bohnsack BL, Gallina D, Kasprick DS, Kahana A. The eye as an organizer of craniofacial development. Genesis. 2011;49(4):222–230. doi: 10.1002/dvg.20716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Duester G. Retinoic acid signaling in perioptic mesenchyme represses Wnt signaling via induction of Pitx2 and Dkk2. Dev Biol. 2010;340(1):67–74. doi: 10.1016/j.ydbio.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Lievre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34(1):125–154. [PubMed] [Google Scholar]
- Lohnes D, Mark M, Mendelsohn C, Dolle P, Dierich A, Gorry P, Chambon P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120(10):2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- Lupo G, Gestri G, O'Brien M, Denton RM, Chandraratna RA, Ley SV, Wilson SW. Retinoic acid receptor signaling regulates choroid fissure closure through independent mechanisms in the ventral optic cup and periocular mesenchyme. Proc Natl Acad Sci U S A. 2011;108(21):8698–8703. doi: 10.1073/pnas.1103802108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald IM, Tran M, Musarella MA. Ocular genetics: current understanding. Surv Ophthalmol. 2004;49(2):159–196. doi: 10.1016/j.survophthal.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Mashima Y, Suzuki Y, Sergeev Y, Ohtake Y, Tanino T, Kimura I, Araie M. Novel cytochrome P4501B1 (CYP1B1) gene mutations in Japanese patients with primary congenital glaucoma. Invest Ophthalmol Vis Sci. 2001;42(10):2211–2216. [PubMed] [Google Scholar]
- Matt N, Dupe V, Garnier JM, Dennefeld C, Chambon P, Mark M, Ghyselinck NB. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development. 2005;132(21):4789–4800. doi: 10.1242/dev.02031. [DOI] [PubMed] [Google Scholar]
- Matt N, Ghyselinck NB, Pellerin I, Dupe V. Impairing retinoic acid signalling in the neural crest cells is sufficient to alter entire eye morphogenesis. Dev Biol. 2008;320(1):140–148. doi: 10.1016/j.ydbio.2008.04.039. [DOI] [PubMed] [Google Scholar]
- McLaughlin ME, Kruger GM, Slocum KL, Crowley D, Michaud NA, Huang J, Jacks T. The Nf2 tumor suppressor regulates cell-cell adhesion during tissue fusion. Proc Natl Acad Sci U S A. 2007;104(9):3261–3266. doi: 10.1073/pnas.0700044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development. 2006;133(10):1901–1910. doi: 10.1242/dev.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison DA, FitzPatrick DR, Fleck BW. Iris coloboma with iris heterochromia: a common association. Arch Ophthalmol. 2000;118(11):1590–1591. doi: 10.1001/archopht.118.11.1590. [DOI] [PubMed] [Google Scholar]
- Morrison DA, FitzPatrick DR, Fleck BW. Iris coloboma and a microdeletion of chromosome 22: del(22)(q11.22) Br J Ophthalmol. 2002;86(11):1316. doi: 10.1136/bjo.86.11.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D, FitzPatrick D, Hanson I, Williamson K, van Heyningen V, Fleck B, Campbell H. National study of microphthalmia, anophthalmia, and coloboma (MAC) in Scotland: investigation of genetic aetiology. J Med Genet. 2002;39(1):16–22. doi: 10.1136/jmg.39.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mui SH, Kim JW, Lemke G, Bertuzzi S. Vax genes ventralize the embryonic eye. Genes Dev. 2005;19(10):1249–1259. doi: 10.1101/gad.1276605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges Y, Gershoni-Baruch R, Leibu R, Goldscher D, Zonis S, Shapira I, Miller B. Hereditary microphthalmia with colobomatous cyst. Am J Ophthalmol. 1992;114(1):30–34. doi: 10.1016/s0002-9394(14)77409-4. [DOI] [PubMed] [Google Scholar]
- Reijntjes S, Gale E, Maden M. Generating gradients of retinoic acid in the chick embryo: Cyp26C1 expression and a comparative analysis of the Cyp26 enzymes. Dev Dyn. 2004;230(3):509–517. doi: 10.1002/dvdy.20025. [DOI] [PubMed] [Google Scholar]
- Saint-Geniez M, D'Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004;48(8–9):1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- Schier AF, Needleman D. Developmental biology: Rise of the source-sink model. Nature. 2009;461(7263):480–481. doi: 10.1038/461480a. [DOI] [PubMed] [Google Scholar]
- Sclafani AM, Skidmore JM, Ramaprakash H, Trumpp A, Gage PJ, Martin DM. Nestin-Cre mediated deletion of Pitx2 in the mouse. Genesis. 2006;44(7):336–344. doi: 10.1002/dvg.20220. [DOI] [PubMed] [Google Scholar]
- See AW, Clagett-Dame M. The temporal requirement for vitamin A in the developing eye: mechanism of action in optic fissure closure and new roles for the vitamin in regulating cell proliferation and adhesion in the embryonic retina. Dev Biol. 2009;325(1):94–105. doi: 10.1016/j.ydbio.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Semina EV, Reiter R, Leysens NJ, Alward WL, Small KW, Datson NA, Murray JC. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet. 1996;14(4):392–399. doi: 10.1038/ng1296-392. [DOI] [PubMed] [Google Scholar]
- Semina EV, Reiter RS, Murray JC. Isolation of a new homeobox gene belonging to the Pitx/Rieg family: expression during lens development and mapping to the aphakia region on mouse chromosome 19. Hum Mol Genet. 1997;6(12):2109–2116. doi: 10.1093/hmg/6.12.2109. [DOI] [PubMed] [Google Scholar]
- Shah SP, Taylor AE, Sowden JC, Ragge NK, Russell-Eggitt I, Rahi JS, Surveillance of Eye Anomalies Special Interest, Group Anophthalmos, microphthalmos, and typical coloboma in the United Kingdom: a prospective study of incidence and risk. Invest Ophthalmol Vis Sci. 2011;52(1):558–564. doi: 10.1167/iovs.10-5263. [DOI] [PubMed] [Google Scholar]
- Shah SP, Taylor AE, Sowden JC, Ragge N, Russell-Eggitt I, Rahi JS, Surveillance of Eye Anomalies Special Interest, Group Anophthalmos, microphthalmos, and Coloboma in the United kingdom: clinical features, results of investigations, and early management. Ophthalmology. 2012;119(2):362–368. doi: 10.1016/j.ophtha.2011.07.039. [DOI] [PubMed] [Google Scholar]
- Sowden JC. Molecular and developmental mechanisms of anterior segment dysgenesis. Eye (Lond) 2007;21(10):1310–1318. doi: 10.1038/sj.eye.6702852. [DOI] [PubMed] [Google Scholar]
- Trost A, Schroedl F, Lange S, Rivera FJ, Tempfer H, Korntner S, Reitsamer HA. Neural crest origin of retinal and choroidal pericytes. Invest Ophthalmol Vis Sci. 2013;54(13):7910–7921. doi: 10.1167/iovs.13-12946. [DOI] [PubMed] [Google Scholar]
- Vasiliou V, Gonzalez FJ. Role of CYP1B1 in glaucoma. Annu Rev Pharmacol Toxicol. 2008;48:333–358. doi: 10.1146/annurev.pharmtox.48.061807.154729. [DOI] [PubMed] [Google Scholar]
- Vaux C, Sheffield L, Keith CG, Voullaire L. Evidence that Rieger syndrome maps to 4q25 or 4q27. J Med Genet. 1992;29(4):256–258. doi: 10.1136/jmg.29.4.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warkany J, Schraffenberger E. Congenital malformations induced in rats by maternal vitamin A deficiency; defects of the eye. Arch Ophthal. 1946;35:150–169. doi: 10.1001/archopht.1946.00890200155008. [DOI] [PubMed] [Google Scholar]
- Weiss O, Kaufman R, Michaeli N, Inbal A. Abnormal vasculature interferes with optic fissure closure in lmo2 mutant zebrafish embryos. Dev Biol. 2012;369(2):191–198. doi: 10.1016/j.ydbio.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Weisschuh N, Dressler P, Schuettauf F, Wolf C, Wissinger B, Gramer E. Novel mutations of FOXC1 and PITX2 in patients with Axenfeld-Rieger malformations. Invest Ophthalmol Vis Sci. 2006;47(9):3846–3852. doi: 10.1167/iovs.06-0343. [DOI] [PubMed] [Google Scholar]
- Whikehart, D R. Corneal Endothelium: overview. 2010:424–434. [Google Scholar]
- Yin HC, Tseng HP, Chung HY, Ko CY, Tzou WS, Buhler DR, Hu CH. Influence of TCDD on zebrafish CYP1B1 transcription during development. Toxicol Sci. 2008;103(1):158–168. doi: 10.1093/toxsci/kfn035. [DOI] [PubMed] [Google Scholar]