Abstract
Background
Carbapenem-resistant Enterobacteriaceae (CRE) have emerged as important health care-associated pathogens. Colonization precedes infection but the risk of developing infection amongst those colonized with CRE is not clear.
Methods
We searched multiple databases for studies reporting rates of CRE-colonized patients subsequently developing infection.
Results
Ten studies fulfilled our inclusion criteria, including 1,806 patients used in our analysis. All studies were observational and conducted among adult inpatients. The cumulative rate of infection was 16.5% in our study. The most common site of infection was the lung, identified in half of patients, followed in decreasing frequency by urinary tract; primary bloodstream; and skin and soft tissue, including surgical sites. Colonization or infection by CRE prolonged stay and was associated with a 10% overall mortality in our analysis.
Conclusion
Our study results suggest an overall 16.5% risk of infection with CRE amongst patients colonized with CRE. Given the high mortality rate observed with CRE infection and the difficulty in treating these infections, research to investigate and develop strategies to eliminate the colonization state are needed.
Keywords: Nonsusceptible, Carriage, Infection, Antibiotic, Antimicrobial
Since the early 1980s, carbapenems have been used with success as the last line of defense against multidrug-resistant gram-negative organisms. Widespread carbapenem-resistant Enterobacteriaceae (CRE) were unknown until the early 2000s.1 During recent years, CRE have emerged as an important family of health care-associated pathogens worldwide, including in the United States.2–5
In January 2015, the Centers for Disease Control and Prevention revised the definition of CRE as organisms that are nonsusceptible to imipenem, meropenem, doripenem, or ertapenem or a documented isolate that possesses a carbapenemase.6 A wide range of species in the Enterobacteriaceae family have been detected with carbapenemases, including Klebsiella pneumoniae, Citrobacter freundii, Escherichia coli, Acinetobacter baumannii, and Enterobacter aerogenes. K pneumonia carbapenemase and metallo-β–lactamase are the main mechanisms underlying resistance to carbapenems.7
Infections by CRE are considered a health care challenge because CRE isolates are usually extensively drug resistant and associated with high morbidity and mortality.8,9 Colonization is considered to be a prerequisite for infection,10 which suggests that prevention of CRE colonization is important in preventing the morbidity and mortality associated with these infections. However, the extent to which colonized patients develop infection with CRE is unclear. These data are important to guide decision making regarding infection-control interventions such as screening and contact precautions for colonized patients.
Therefore, we undertook a systematic review to understand the relationship between colonization with CRE and subsequent infection.
METHODS
During September 2014 and June 2015 we searched PubMed, Medline, Cochrane Library database, Cumulative Index to Nursing and Allied Health Literature, and Scielo databases from January 1, 1991, the year before the first reported case of CRE, for relevant publications. No language restrictions were used. Key words used in the search, alone or in combination, were: carbapenem-resistant En-terobacteriaceae, Klebsiella pneumonia carbapenemase, KPC, Verona integron-mediated metallo-beta-lactamase, CRE, carbapenem resistant, carbapenem nonsusceptible, carbapenemase, carbapenamase-producing, colonization, infection, and carriage. Reference lists of studies included in this review were also searched for relevant articles.
Inclusion criteria
To be included in our systematic review, studies needed to be clinical trials or observational studies. Data had to be provided to calculate rates of infection in patients initially colonized. Studies that reported data on only colonization or infection or did not allow for determination of infections arising from previous colonization were not included, and studies that reported numbers of cultures or isolates, not patients colonized or infected, were excluded. Review articles, abstracts, and editorials were excluded.
Outcome measures
The primary outcome of interest was rate of CRE infection amongst those colonized with CRE.
Data extraction
Two investigators independently extracted data using a standard data collection form. Data extracted included study sample size, demographic characteristics of the study sample, number of patients colonized, site of colonization, number of subsequent infections, site of infection, length of hospital stay, and mortality rate. The two investigators reviewed studies that met the inclusion criteria. Disagreement amongst investigators was resolved by discussion and arriving at consensus.
Data synthesis
Descriptive statistics were used to determine the percentage of patients initially colonized with CRE who went on to develop clinical infection. The mean age of subjects with infection and length of stay were also calculated for the subset of studies for which these data were available. For the subset of studies for which mortality rates were reported, we calculated cumulative in-hospital mortality, which is reported as the proportion of patients with CRE infection or colonization who died with all patients infected or colonized with CRE as the denominator. Because of the heterogeneity in included studies, we did not perform a meta-analysis.
RESULTS
Study characteristics
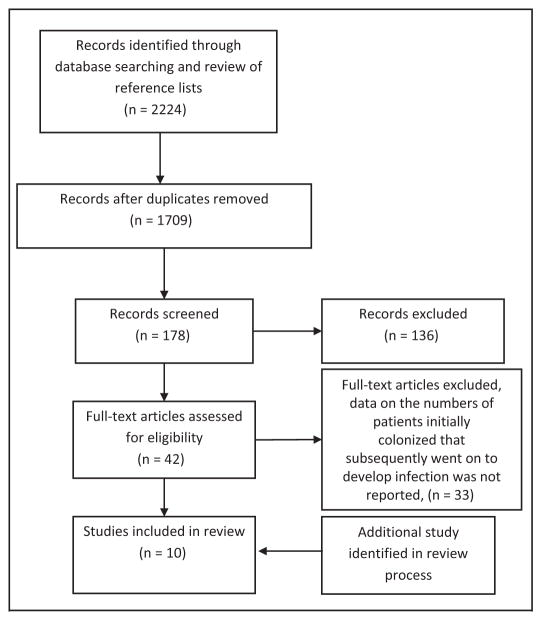
Our search strategy yielded 1,709 reports, of which 178 were considered potentially relevant and abstracts were reviewed. Of these, the full text of 42 studies was retrieved and reviewed; 33 were ultimately excluded because data on the numbers of patients initially colonized who subsequently went on to develop infection were not reported. In fact, many were point prevalence reports of those colonized or infected and not consistent with the aim of our review (Fig 1). One additional study11 was brought to the attention of the authors during the review process and was included in our analysis.
Fig 1.
Flowchart depicting the selection process of studies included in the review.
Ten studies fulfilled inclusion criteria; all were observational studies.11–20 Characteristics of the studies meeting inclusion criteria are reported in Table 1. Among these 10 studies, 1,806 colonized patients were used in our analysis. For 2 studies,13,14 only data on those patients with nosocomial acquisition of CRE were used for analysis because subsequent rates of infection were reported for only these patients. In a third instance,15 the authors reported on rates of infection for those patients infected with K pneumoniae alone; thus, only this subset of patients was used in our analysis.
Table 1.
Characteristics of studies included in the review
| Study, year | Patient population | Outbreak setting | Screening frequency | No. of patients colonized by CRE | No. of patients who developed CRE infection | Colonization site | Infection site | Type of CRE | Mortality |
|---|---|---|---|---|---|---|---|---|---|
| Borer et al, 201212 | Adults, hospital-wide | No | Rectal cultures performed in patients admitted for nursing or other outside facility and on patients in high-risk units | 464 | 42 (9.1%) | Rectal | Multiple | CRKP | 18.75%, colonized 30%, infected |
| Cho et al, 201413 | Adults, ICU | No | On admission, weekly thereafter | 530 with nosocomial acquisition included for analysis | 111 (20.9%) | Nares | Multiple | CRAB | Not included |
| Debby et al, 201214 | Adults, ICU | No | Within 72 h of ICU admission, then twice weekly | 48 with nosocomial acquisition included for analysis | 20 (41.7%) | Rectal | Multiple | CRKP | 17/48 (35.4%), patients with nosocomial acquisition |
| Wiener-Well et al, 201015 | Adults, hospital-wide | Yes | All hospitalized patients screened during 3 consecutive days | 42, 16 with CRKP included for analysis | 5 (31.3%) | Rectal, perianal, oral | Not included | CRKP | 1/16 (6%) of all CRKP carriers |
| Papadimitriou- Olivgeris et al, 201316 | Adults, ICU | No | Upon ICU admission, days 4 and 7, and weekly thereafter | 164 | 37 (22.6%) | Rectal | Not included | CRKP | 41/127 (32%), colonized 22/37 (59.5%), infected |
| Lübbert et al, 201417 | Liver transplant patients | Yes | — | 9 | 8 (89%) | Unclear | Multiple | CRKP | 1/1 (100%), colonized 6 out of 8 (75%), infected (2 of these determined “noninfectious causes”) |
| Pisney et al, 201418 | Adults, selected high-prevalence units | Yes | 7 rounds of point prevalence screening on affected units | 15 | 0 (0.0%) | Rectal | None | CRKP | Not included |
| Lowe et al, 201319 | Adults, hospital-wide | No | Known contact with CRE-infected patients were screened | 9 | 4 (44.4%) | Rectal | Bloodstream, urine | CRKP | Not included |
| Schechner et al, 201220 | Adults, hospital-wide | Yes | All patients hospitalized in the past year and those with CRE-infected contact | 502 | 38 (7.6%) | Rectal | Multiple | CRKP, Escherichia coli, Enterobacter spp, Citrobacter freundii | Not included |
| Latibeaudire, et al, 201511 | Adults, ICU | No | Upon ICU admission and weekly thereafter | 49 | 34 (69%) | Rectal, tracheal | Multiple | CRAB | Not included |
CRAB, carbapenemresistant Acinetobacter baumannii; CRE, carbapenem-resistant Enterobacteriaceae; CRKP, carbapenem resistant Klebsiella pneumoniae; ICU, intensive care unit.
All studies were conducted among adult inpatients, with 411,13,14,16 exclusively in an intensive care unit setting. One17 included liver transplant recipients. One study15 performed hospitalwide screening, whereas 412,18–20 performed screening on patients deemed to be at higher risk based on past acute- or long-term care admission, housing on a high-prevalence ward, or known contact with other patients infected or colonized with CRE. For the remaining studies, screening was performed at specified intervals for those patients admitted to an intensive care unit11,13,14,16 (Table 1).
Seven studies examined carbapenem-resistant K pneumoniae exclusively,12,14–19 2 reported prevalence of carbapenem-resistant A baumannii,11,13 and the remaining examined multiple CRE.20 Seven reported site of infection11–14,17,19,20 and 6 studies included data on length of stay amongst CRE cases,12,14–17,20 whereas only 5 included mortality data.12,14–17
For those studies that reported data on gender,11,14,17,18,20 men accounted for 33%–69.6% of patients colonized or infected with CRE. One study18 found a predominance among women. Patient age ranged from 45–76 years across studies.11,12,14,15,17,18,20 Four were conducted in the context of an outbreak.15,17,18,20
Risk of infection after colonization with CRE
Colonization was defined as gastrointestinal tract carriage determined by rectal swab in the majority of studies.11,12,14,15,18–20 Five studies cultured specimens on MacConkey agar.11,14,15,18–20 Alternative methods used included inoculation of brain-heart infusion broth14,15 and use of CHROMagar K pneumonia carbapenemase Petri dishes (CHROMagar, Paris, France) for some or all specimens.12,16,19 The study by Cho et al13 was unique in using nasal swabs cultured on 5% sheep’s blood agar plates. One study did not specify microbiologic techniques sufficiently to allow interpretation.17
Amongst the 1,806 initially colonized patients included in our analysis, 299 clinical infections were observed for a cumulative rate of 16.5%. Rates of infection in individual studies varied widely, from 0%18–89%,17 although with the majority falling in the range of 7.6%–44.4% (Table 1).
Quantitative data regarding site of infection was reported for 223 previously colonized patients in the studies.12–14,17,19,20 The most frequent clinical syndrome was pneumonia, identified in half of patients with CRE infection. Forty-four cases, or approximately 20%, of patients were diagnosed with urinary tract infection caused by CRE. In order of decreasing frequency, primary bloodstream infections, identified in 30 patients, and skin and soft tissue infections, including surgical site infection, identified in 16 patients, accounted for the remaining infections reported.
Risk of adverse outcomes following acquisition of CRE
There was no standardized method for reporting length of stay, so it is difficult to draw a meaningful conclusion regarding mean length of stay from currently available data. However, in studies that reported these data12,14–17,20 colonization or infection with CRE significantly prolonged hospital stay, with 2 studies reporting hospitalization nearly 2-fold longer in those patients with CRE.16,17
The overall mortality rate was 10% for all patients infected or colonized. Amongst the 3 studies that reported mortality in the subset of patients with clinical infection separate from those colonized,12,16,17 mortality rates ranged from 30%–75%.
DISCUSSION
Our results show that a substantial proportion of patients colonized the CRE go on to develop clinical infection with CRE. We summarized the results of the available literature and found an overall 16.5% risk of infection with CRE amongst patients colonized with CRE. Individual studies that have examined this question have found varying rates of infection, probably due to differences in type of organism, patient population, and clinical setting. Thus, the overall magnitude of risk was unclear. Our study provides an overall assessment of the risk of infection and has implications for infection preventionists and clinicians involved in the prevention and treatment of CRE.
Sites of screening and microbiologic methods varied across studies, contributing to the heterogeneity amongst studies. The most common site of screening for carriage was the gastrointestinal tract, specifically, rectal swabs. Wiener-Well et al15 examined the sensitivity of 3 screening sites and found rectal swabs to be more sensitive that perianal or oral swabs. MacConkey agar was used most commonly for culturing CRE; however, a limited number of other methods were used in some studies. Future studies should compare the different types of selective media available to determine the best way to recover CRE from surveillance specimens. Studies also varied in the approach to screening for CRE; 9 of the 10 included studies reported screening method. Screening approach varied from hospitalwide point prevalence screening15 to screening of those only with known contact with a CRE-infected patient.19
CRE infections are associated with considerable mortality with rates reported up to 50% in the literature.9,21 Our review found similar rates, ranging from 30%–75% for those infected amongst the included studies. There are many contributors to this high rate of mortality, including longer length of stay,12,14–17,20 poorer overall health status, site of infection, comorbid illnesses, and most importantly the limited antimicrobial options for treating these infections. Panresistant Enterobacteriaceae have been reported,22,23 highlighting the challenges in treating these infections and underscoring the importance of antimicrobial discovery and development targeted to these organisms.
Given the heightened risk of mortality associated with CRE infection, >30% for patients in our review, our results emphasize the importance of identifying and eliminating the carrier state to prevent development of clinical infection with CRE. Studies have examined the role for oral decontamination therapy in decolonizing patients with CRE. In a modestly sized randomized controlled trial of 40 patients colonized the carbapenem-resistant K pneumoniae, the utility of treatment with combination of gentamicin and colistin in both oral, nonabsorbable solution and nasal gel delivery method was examined.24 In the 20 patients randomized to treatment, the rate of rectal colonization at 2 weeks was significantly reduced, with 61.1% of patients decolonized in the treatment group versus 16.1% in the placebo arm. This difference persisted at 6 weeks, with 58.5% of patients remaining decolonized in the treatment arm versus 33.3% in the placebo arm. In a larger study,25 50 patients colonized with carbapenem-resistant K pneumoniae strains with demonstrated susceptibility to gentamicin and colistin were randomized to treatment with gentamicin, colistin, or combination therapy. Compared with the spontaneous eradication rate of 7% in their study, all 3 treatment arms demonstrated a significantly greater decolonization rate. The rate of decolonization with colistin was 50%, with gentamicin, 42%. Similar to the study by Saidel-Oades,24 no significant adverse effects were noted. Interestingly, combination therapy yielded the lowest rate of decontamination, only 37.5%, although the differences amongst groups did not reach statistical significance. These promising results should be confirmed in larger studies and tested in both outbreak and endemic situations with close attention to analysis of gut microbiome changes as a result of the antibiotic agents.
Our study has several limitations. First, given the methodology of a systematic review, our results have the limitations of the included studies. Secondly, the studies were quite heterogeneous in their study methods and patient populations. We did develop a priori inclusion and exclusion criteria and did not include studies that did not clearly establish patients as colonized before developing clinical infection. Third, we were not able to study risk factors for development of clinical infection and mortality because of limited available data; this should be an area for future research. Fourth, the included studies used varying approaches to identifying cases of colonization and/or infection, potentially contributing to misclassification of colonization and infection. Similarly, many studies did not include data on those patients developing infection without preceding colonization, which precluded our ability to comment on the relative risk of infection in those patients with colonization. Limited by reported data, we were not able to comment on the time from colonization to the development of infection. Finally, publication bias is a concern.
CONCLUSIONS
We found that colonization with CRE poses a 16.5% risk of subsequent CRE infection. With a high rate of mortality associated with CRE infection, pending further research, eradication of colonization with CRE should be considered in select situations such as outbreaks. Future research should attempt to determine the utility of widespread routine surveillance for CRE amongst hospitalized patients as an infection control strategy as well as determine strategies of eradication of CRE colonization.
Acknowledgments
Supported by Agency for Healthcare Research and Quality grant No. 11670428, Department of Veterans Affairs Quality Enhancement Research Initiative 11901470, and a MERIT award from the Department of Veterans Affairs.
Footnotes
Conflicts of Interest: None to report.
References
- 1.Temkin E, Adler A, Lerner A, Carmeli Y. Carbapenem-resistant Enterobacteriaceae: biology, epidemiology, and management. Ann N Y Acad Sci. 2014;1323:22–42. doi: 10.1111/nyas.12537. [DOI] [PubMed] [Google Scholar]
- 2.Schwaber MJ, Carmeli Y. Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA. 2008;300:2911–3. doi: 10.1001/jama.2008.896. [DOI] [PubMed] [Google Scholar]
- 3.Lledo W, Hernandez M, Lopez E, Molinari OL, Soto RQ, Hernandez E, et al. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR Morb Mortal Wkly Rep. 2009;58:256–60. [PubMed] [Google Scholar]
- 4.Antoniadou A, Kontopidou F, Poulakou G, Koratzanis E, Galani I, Papadomichelakis E, et al. Colistin-resistant isolates of Klebsiella pneumoniae emerging in intensive care unit patients: first report of a multiclonal cluster. J Antimicrob Chemother. 2007;59:786–90. doi: 10.1093/jac/dkl562. [DOI] [PubMed] [Google Scholar]
- 5.Kitchel B, Rasheed JK, Patel JB, Srinivasan A, Navon-Venezia S, Carmeli Y, et al. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob Agents Chemother. 2009;53:3365–70. doi: 10.1128/AAC.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. [Accessed June 18, 2015];Carbapenem resistant Enterobacteriaceae: CRE definition. Available from: http://www.cdc.gov/hai/organisms/cre/definition.html.
- 7.Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362:1804–13. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother. 2008;52:1028–33. doi: 10.1128/AAC.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borer A, Saidel-Odes L, Riesenberg K, Eskira S, Peled N, Nativ R, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol. 2009;30:972–6. doi: 10.1086/605922. [DOI] [PubMed] [Google Scholar]
- 10.Bonten MJ, Weinstein RA. The role of colonization in the pathogenesis of nosocomial infections. Infect Control Hosp Epidemiol. 1996;17:193–200. doi: 10.1086/647274. [DOI] [PubMed] [Google Scholar]
- 11.Latibeaudiere R, Rosa R, Laowansiri P, Arheart K, Namias N, Silvia Munoz-Price L. Surveillance cultures growing carbapenem-resistant Acinetobacter baumannii predict the development of clinical infections: a retrospective cohort study. Clin Infect Dis. 2015;60:415–22. doi: 10.1093/cid/ciu847. [DOI] [PubMed] [Google Scholar]
- 12.Borer A, Saidel-Odes L, Eskira S, Nativ R, Riesenberg K, Livshiz-Riven I, et al. Risk factors for developing clinical infection with carbapenem-resistant Klebsiella pneumoniae in hospital patients initially only colonized with carbapenem-resistant K pneumoniae. Am J Infect Control. 2012;40:421–5. doi: 10.1016/j.ajic.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Cho OH, Bak MH, Baek EH, Park KH, Kim S, Bae IG. Successful control of carbapenem-resistant Acinetobacter baumannii in a Korean university hospital: a 6-year perspective. Am J Infect Control. 2014;42:976–9. doi: 10.1016/j.ajic.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Debby BD, Ganor O, Yasmin M, David L, Nathan K, Ilana T, et al. Epidemiology of carbapenem resistant Klebsiella pneumoniae colonization in an intensive care unit. Eur J Clin Microbiol Infect Dis. 2012;31:1811–7. doi: 10.1007/s10096-011-1506-5. [DOI] [PubMed] [Google Scholar]
- 15.Wiener-Well Y, Rudensky B, Yinnon AM, Kopuit P, Schlesinger Y, Broide E, et al. Carriage rate of carbapenem-resistant Klebsiella pneumoniae in hospitalised patients during a national outbreak. J Hosp Infect. 2010;74:344–9. doi: 10.1016/j.jhin.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Papadimitriou-Olivgeris M, Marangos M, Fligou F, Christofidou M, Sklavou C, Vamvakopoulou S, et al. KPC-producing Klebsiella pneumoniae enteric colonization acquired during intensive care unit stay: the significance of risk factors for its development and its impact on mortality. Diagn Microbiol Infect Dis. 2013;77:169–73. doi: 10.1016/j.diagmicrobio.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Lübbert C, Becker-Rux D, Rodloff AC, Laudi S, Busch T, Bartels M, Kalsers UX. Colonization of liver transplant recipients with KPC-producing Klebsiella pneumoniae is associated with high infection rates and excess mortality: a case-control analysis. Infection. 2014;42:309–16. doi: 10.1007/s15010-013-0547-3. [DOI] [PubMed] [Google Scholar]
- 18.Pisney LM, Barron MA, Kassner E, Havens D, Madinger NE. Carbapenem-resistant Enterobacteriaceae rectal screening during an outbreak of New Delhi Metallo-β-Lactamase-producing Klebsiella pneumoniae at an acute care hospital. Infect Control Hosp Epidemiol. 2014;35:434–6. doi: 10.1086/675597. [DOI] [PubMed] [Google Scholar]
- 19.Lowe CF, Kus JV, Salt N, Callery S, Louie L, Khan MA, et al. Nosocomial transmission of New Delhi Metallo-β-Lactamase-1-producing Klebsiella pneumoniae in Toronto, Canada. Infect Control Hosp Epidemiol. 2013;34:49–55. doi: 10.1086/668778. [DOI] [PubMed] [Google Scholar]
- 20.Schechner V, Kotlovsky T, Kazma M, Mishali H, Schwartz D, Navon-Venezia S, et al. Asymptomatic rectal carriage of blaKPC producing carbapenem-resistant Enterobacteriaceae: who is prone to become clinically infected? Clin Microbiol Infect. 2012;19:451–6. doi: 10.1111/j.1469-0691.2012.03888.x. [DOI] [PubMed] [Google Scholar]
- 21.Chitnis AS, Caruthers PS, Rao AK, Lamb J, Lurvey R, Beau De Rochars V, et al. Outbreak of carbapenem-resistant Enterobacteriaceae at a long-term acute care hospital: sustained reductions in transmission through active surveillance and targeted interventions. Infect Control Hosp Epidemiol. 2012;33:984–92. doi: 10.1086/667738. [DOI] [PubMed] [Google Scholar]
- 22.Elemam A, Rahimian J, Mandell W. Infection with panresistant Klebsiella pneumoniae: a report of 2 cases and brief review of the literature. Clin Infect Dis. 2009;49:271–4. doi: 10.1086/600042. [DOI] [PubMed] [Google Scholar]
- 23.Marchaim D, Chopra T, Perez F, Hayakawa K, Lephart PR, Beheemreddy S, et al. Outcomes and genetic relatedness of carbapenem-resistant Enterobacteriaceae at Detroit Medical Center. Infect Control Hosp Epidemiol. 2011;32:861–71. doi: 10.1086/661597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saidel-Odes L, Polachek H, Peled N, Reisenberg K, Schlaeferr F, Travelsi Y. A randomized, double-blind, placebo-controlled trial of selective digestive decontamination using oral gentamicin and oral polymixin E for eradication of carbapenem-resistant Klebsiella pneumoniae carriage. Infect Control Hosp Epidemiol. 2012;33:14–9. doi: 10.1086/663206. [DOI] [PubMed] [Google Scholar]
- 25.Oren I, Sprecher H, Finkelstein R, Hadad S, Neuberger A, Hussein K, et al. Eradication of carbapenem-resistant Enterobacteriaceae gastrointestinal colonization with nonabsorbable oral antibiotic treatment: a prospective controlled trial. Am J Infect Control. 2013;42:1167–72. doi: 10.1016/j.ajic.2013.04.018. [DOI] [PubMed] [Google Scholar]