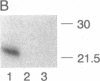
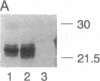
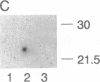
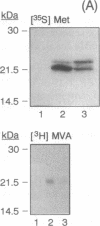
Abstract
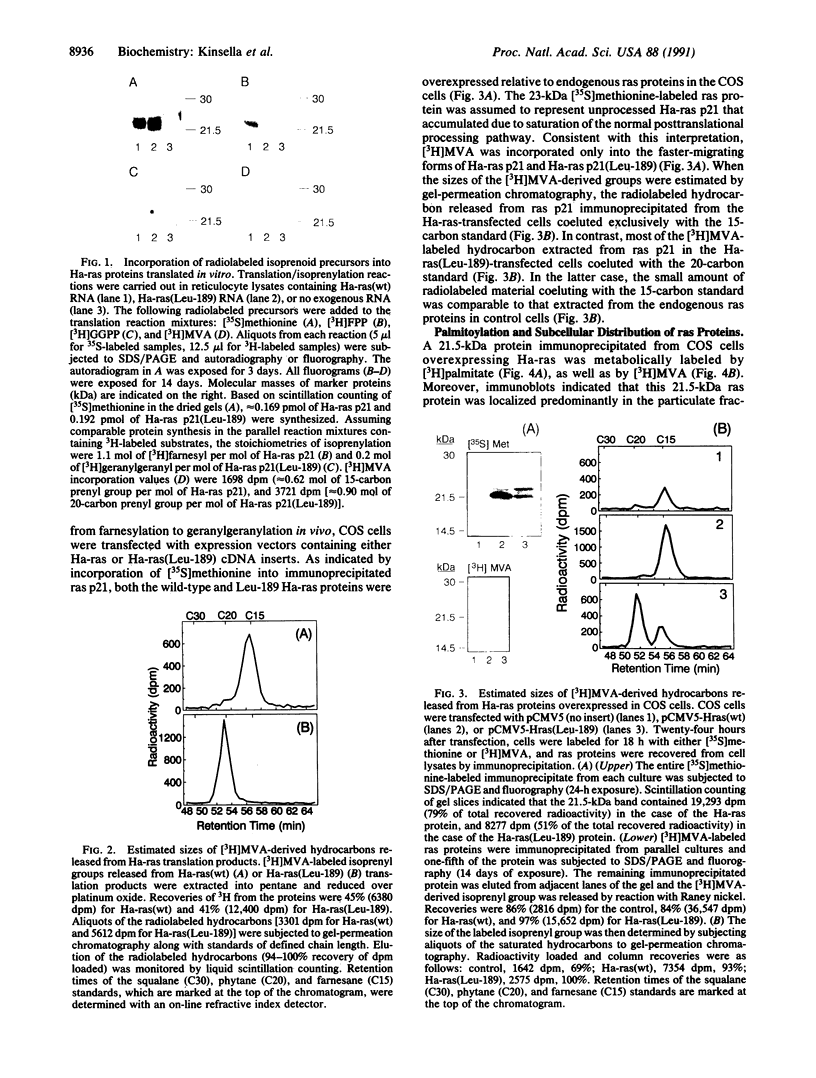
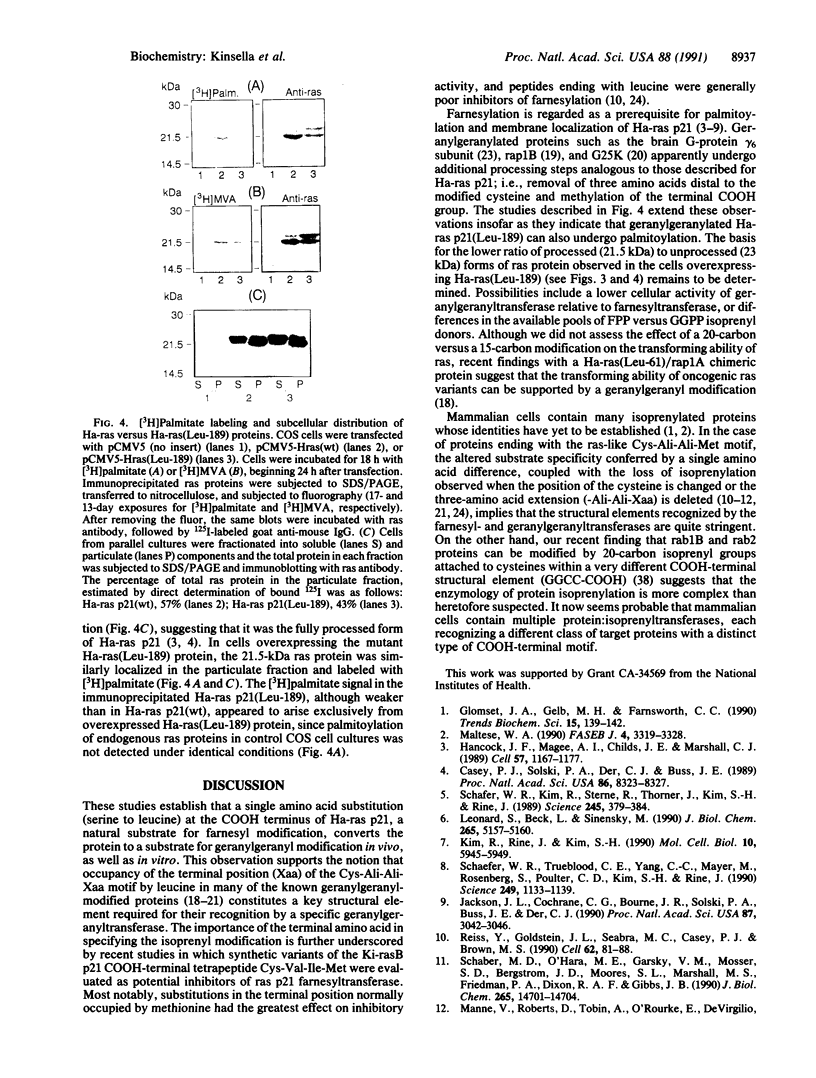
ras proteins undergo posttranslational modification by a 15-carbon farnesyl isoprenoid at a cysteine within a defined COOH-terminal amino acid motif; i.e., Cys-Ali-Ali-Ser/Met (where Ali represents an aliphatic residue). In other low molecular mass GTP-binding proteins, cysteines are modified by 20-carbon geranylgeranyl groups within a Cys-Ali-Ali-Leu motif. We changed the terminal Ser-189 of Ha-ras p21 to Leu-189 by site-directed mutagenesis and found that the protein was modified by [3H]geranylgeranyl instead of [3H]farnesyl in an in vitro assay. Gel-permeation chromatography of [3H]mevalonate-labeled hydrocarbons released from immunoprecipitated ras proteins overexpressed in COS cells indicated that Ha-ras p21(Leu-189) was also a substrate for 20-carbon isoprenyl modification in vivo. Additional steps in Ha-ras p21 processing, normally initiated by farnesylation, appear to be supported by geranylgeranylation, based on metabolic labeling of Ha-ras p21(Leu-189) with [3H]palmitate and its subcellular localization in a particulate fraction from COS cells. These observations indicate that the amino acid occupying the terminal position (Xaa) in the Cys-Ali-Ali-Xaa motif constitutes a key structural feature by which Ha-ras p21 and other proteins with ras-like COOH-terminal isoprenylation sites are distinguished as substrates for farnesyl- or geranylgeranyltransferases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Buss J. E., Quilliam L. A., Kato K., Casey P. J., Solski P. A., Wong G., Clark R., McCormick F., Bokoch G. M., Der C. J. The COOH-terminal domain of the Rap1A (Krev-1) protein is isoprenylated and supports transformation by an H-Ras:Rap1A chimeric protein. Mol Cell Biol. 1991 Mar;11(3):1523–1530. doi: 10.1128/mcb.11.3.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey P. J., Solski P. A., Der C. J., Buss J. E. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Clarke S., Vogel J. P., Deschenes R. J., Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4643–4647. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth C. C., Gelb M. H., Glomset J. A. Identification of geranylgeranyl-modified proteins in HeLa cells. Science. 1990 Jan 19;247(4940):320–322. doi: 10.1126/science.2296721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth C. C., Wolda S. L., Gelb M. H., Glomset J. A. Human lamin B contains a farnesylated cysteine residue. J Biol Chem. 1989 Dec 5;264(34):20422–20429. [PMC free article] [PubMed] [Google Scholar]
- Fukada Y., Takao T., Ohguro H., Yoshizawa T., Akino T., Shimonishi Y. Farnesylated gamma-subunit of photoreceptor G protein indispensable for GTP-binding. Nature. 1990 Aug 16;346(6285):658–660. doi: 10.1038/346658a0. [DOI] [PubMed] [Google Scholar]
- Glomset J. A., Gelb M. H., Farnsworth C. C. Prenyl proteins in eukaryotic cells: a new type of membrane anchor. Trends Biochem Sci. 1990 Apr;15(4):139–142. doi: 10.1016/0968-0004(90)90213-u. [DOI] [PubMed] [Google Scholar]
- Goodman L. E., Judd S. R., Farnsworth C. C., Powers S., Gelb M. H., Glomset J. A., Tamanoi F. Mutants of Saccharomyces cerevisiae defective in the farnesylation of Ras proteins. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9665–9669. doi: 10.1073/pnas.87.24.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Jackson J. H., Cochrane C. G., Bourne J. R., Solski P. A., Buss J. E., Der C. J. Farnesol modification of Kirsten-ras exon 4B protein is essential for transformation. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3042–3046. doi: 10.1073/pnas.87.8.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata M., Farnsworth C. C., Yoshida Y., Gelb M. H., Glomset J. A., Takai Y. Posttranslationally processed structure of the human platelet protein smg p21B: evidence for geranylgeranylation and carboxyl methylation of the C-terminal cysteine. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8960–8964. doi: 10.1073/pnas.87.22.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. K. The mechanism and regulation of dolichyl phosphate biosynthesis in rat liver. J Biol Chem. 1986 Sep 15;261(26):12053–12059. [PubMed] [Google Scholar]
- Kim R., Rine J., Kim S. H. Prenylation of mammalian Ras protein in Xenopus oocytes. Mol Cell Biol. 1990 Nov;10(11):5945–5949. doi: 10.1128/mcb.10.11.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella B. T., Erdman R. A., Maltese W. A. Carboxyl-terminal isoprenylation of ras-related GTP-binding proteins encoded by rac1, rac2, and ralA. J Biol Chem. 1991 May 25;266(15):9786–9794. [PubMed] [Google Scholar]
- Kinsella B. T., Maltese W. A. rab GTP-binding proteins implicated in vesicular transport are isoprenylated in vitro at cysteines within a novel carboxyl-terminal motif. J Biol Chem. 1991 May 5;266(13):8540–8544. [PubMed] [Google Scholar]
- Lai R. K., Perez-Sala D., Cañada F. J., Rando R. R. The gamma subunit of transducin is farnesylated. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7673–7677. doi: 10.1073/pnas.87.19.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard S., Beck L., Sinensky M. Inhibition of isoprenoid biosynthesis and the post-translational modification of pro-p21. J Biol Chem. 1990 Mar 25;265(9):5157–5160. [PubMed] [Google Scholar]
- Maltese W. A. Posttranslational modification of proteins by isoprenoids in mammalian cells. FASEB J. 1990 Dec;4(15):3319–3328. doi: 10.1096/fasebj.4.15.2123808. [DOI] [PubMed] [Google Scholar]
- Maltese W. A., Robishaw J. D. Isoprenylation of C-terminal cysteine in a G-protein gamma subunit. J Biol Chem. 1990 Oct 25;265(30):18071–18074. [PubMed] [Google Scholar]
- Maltese W. A., Sheridan K. M. Isoprenoid modification of G25K (Gp), a low molecular mass GTP-binding protein distinct from p21ras. J Biol Chem. 1990 Oct 15;265(29):17883–17890. [PubMed] [Google Scholar]
- Manne V., Roberts D., Tobin A., O'Rourke E., De Virgilio M., Meyers C., Ahmed N., Kurz B., Resh M., Kung H. F. Identification and preliminary characterization of protein-cysteine farnesyltransferase. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7541–7545. doi: 10.1073/pnas.87.19.7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby S. M., Casey P. J., Gilman A. G., Gutowski S., Sternweis P. C. G protein gamma subunits contain a 20-carbon isoprenoid. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5873–5877. doi: 10.1073/pnas.87.15.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard K. M., Chan E. K., Grant B. J., Sullivan K. F., Tan E. M., Glass C. A. In vitro posttranslational modification of lamin B cloned from a human T-cell line. Mol Cell Biol. 1990 May;10(5):2164–2175. doi: 10.1128/mcb.10.5.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese J. H., Maltese W. A. Post-translational modification of proteins by 15-carbon and 20-carbon isoprenoids in three mammalian cell lines. 1991 May 29-Jun 12Mol Cell Biochem. 104(1-2):109–116. doi: 10.1007/BF00229810. [DOI] [PubMed] [Google Scholar]
- Reiss Y., Goldstein J. L., Seabra M. C., Casey P. J., Brown M. S. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell. 1990 Jul 13;62(1):81–88. doi: 10.1016/0092-8674(90)90242-7. [DOI] [PubMed] [Google Scholar]
- Reiss Y., Stradley S. J., Gierasch L. M., Brown M. S., Goldstein J. L. Sequence requirement for peptide recognition by rat brain p21ras protein farnesyltransferase. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):732–736. doi: 10.1073/pnas.88.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling H. C., Breunger E., Epstein W. W., Crain P. F. Prenylated proteins: the structure of the isoprenoid group. Science. 1990 Jan 19;247(4940):318–320. doi: 10.1126/science.2296720. [DOI] [PubMed] [Google Scholar]
- Schaber M. D., O'Hara M. B., Garsky V. M., Mosser S. C., Bergstrom J. D., Moores S. L., Marshall M. S., Friedman P. A., Dixon R. A., Gibbs J. B. Polyisoprenylation of Ras in vitro by a farnesyl-protein transferase. J Biol Chem. 1990 Sep 5;265(25):14701–14704. [PubMed] [Google Scholar]
- Schafer W. R., Kim R., Sterne R., Thorner J., Kim S. H., Rine J. Genetic and pharmacological suppression of oncogenic mutations in ras genes of yeast and humans. Science. 1989 Jul 28;245(4916):379–385. doi: 10.1126/science.2569235. [DOI] [PubMed] [Google Scholar]
- Schafer W. R., Trueblood C. E., Yang C. C., Mayer M. P., Rosenberg S., Poulter C. D., Kim S. H., Rine J. Enzymatic coupling of cholesterol intermediates to a mating pheromone precursor and to the ras protein. Science. 1990 Sep 7;249(4973):1133–1139. doi: 10.1126/science.2204115. [DOI] [PubMed] [Google Scholar]
- Seabra M. C., Reiss Y., Casey P. J., Brown M. S., Goldstein J. L. Protein farnesyltransferase and geranylgeranyltransferase share a common alpha subunit. Cell. 1991 May 3;65(3):429–434. doi: 10.1016/0092-8674(91)90460-g. [DOI] [PubMed] [Google Scholar]
- Stephenson R. C., Clarke S. Identification of a C-terminal protein carboxyl methyltransferase in rat liver membranes utilizing a synthetic farnesyl cysteine-containing peptide substrate. J Biol Chem. 1990 Sep 25;265(27):16248–16254. [PubMed] [Google Scholar]
- Vorburger K., Kitten G. T., Nigg E. A. Modification of nuclear lamin proteins by a mevalonic acid derivative occurs in reticulocyte lysates and requires the cysteine residue of the C-terminal CXXM motif. EMBO J. 1989 Dec 20;8(13):4007–4013. doi: 10.1002/j.1460-2075.1989.tb08583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane H. K., Farnsworth C. C., Xie H. Y., Evans T., Howald W. N., Gelb M. H., Glomset J. A., Clarke S., Fung B. K. Membrane-binding domain of the small G protein G25K contains an S-(all-trans-geranylgeranyl)cysteine methyl ester at its carboxyl terminus. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):286–290. doi: 10.1073/pnas.88.1.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane H. K., Farnsworth C. C., Xie H. Y., Howald W., Fung B. K., Clarke S., Gelb M. H., Glomset J. A. Brain G protein gamma subunits contain an all-trans-geranylgeranylcysteine methyl ester at their carboxyl termini. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5868–5872. doi: 10.1073/pnas.87.15.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Kawata M., Katayama M., Horiuchi H., Kita Y., Takai Y. A geranylgeranyltransferase for rhoA p21 distinct from the farnesyltransferase for ras p21S. Biochem Biophys Res Commun. 1991 Mar 15;175(2):720–728. doi: 10.1016/0006-291x(91)91625-m. [DOI] [PubMed] [Google Scholar]