Abstract
Hematospermia is a common complaint among patients seen in outpatient urology clinics. The differential diagnosis is broad and includes inflammatory, infectious, neoplastic, structural, systemic, and traumatic causes. The most common infectious causes are uropathogens and sexually transmitted infections. However, with increasing global travel, physicians must maintain a high clinical suspicion for pathogens not endemic to their region, including Echinococcus, Mycobacterium tuberculosis, and Schistosoma.1 We present a case of hematospermia in a traveler returning from Eastern Africa with exposure to Lake Malawi. The patient’s microscopic analysis of semen was positive for Schistosoma haematobium, revealing a rare presentation of S. haematobium infection.
Introduction
The parasitic Schistosoma trematode worms cause schistosomiasis in endemic regions, where transmission occurs through contact with contaminated fresh water. Schistosomiasis has been found in water sources of 78 countries, 52 of which are endemic with moderate-to-high transmission rates. Lake Malawi has been identified as an area of high endemicity. Schistosoma haematobium mainly affects the urinary tract and causes symptoms such as dysuria, urinary frequency, proteinuria, and hematuria. The organism can rarely invade the genital tract, causing hematospermia.2
Case report
A 24-year-old male was referred to our tertiary infectious diseases clinic by the urology service following a seven-month history of painful ejaculation and hematospermia. He had been originally referred to urology following the development of bright red blood and a white clot-like substance in his ejaculate in the winter of 2012. These symptoms lasted for two weeks and gradually improved, although they continued to remit and relapse to the point where he had “clots” in his ejaculate consistently by spring 2013. He denied dysuria, flank pain, and testicular swelling or tenderness.
The patient had traveled to Africa three months prior to the start of his symptoms. Over a 10-week period, he visited the countries of Mozambique, Ethiopia, Kenya, and Malawi. He reported swimming in Lake Malawi and he denied rash or skin symptoms thereafter. Just prior to returning to Canada in the summer of 2012, he developed a high fever with nausea and diarrhea. Stool testing revealed the presence of cryptosporidium and his symptoms resolved with supportive measures only.
The patient had recently completed a three-month course of terbinafine for a toenail fungus and denied any other medical history or medication usage. He was sexually active at the time, with two regular partners, one male and one female. A transrectal prostate ultrasound showed cystic dilation of the left seminal vesicle and to a lesser extent of the right seminal vesicle. A semen analysis was normal except for decreased morphology.
Physical exam was unremarkable. A complete blood count, renal panel, urinalysis, stool and urine culture were ordered. His hemoglobin was 161 g/L with a hematocrit of 45.7%, and a white blood cell count of 6.3×109/L. Eosinophils were normal at 0.4 ×109/L, neutrophils were 3.8×109/L. His serum electrolytes and liver function tests were within normal range. His urinalysis showed no abnormal findings. Urine and stool cultures were negative. HIV serology, hepatitis B surface antigen, and hepatitis C antibody were negative. Urine nucleic acid testing for chlamydia and gonorrhea, along with syphilis serology, were negative.
Given the travel history and clinical presentation, a fresh semen sample was submitted for microscopic analysis. The ova of Schistosoma haematobium were easily identified on direct wet mount (Fig. 1), confirming the diagnosis of hematospermia secondary to S. haematobium. Serology via enzyme-linked immunosorbent assay (ELISA) for S. mansoni/S. haematobium submitted to the Canadian National Reference Centre for Parasitology in Montreal, QC, Canada was strongly positive.
Fig. 1.
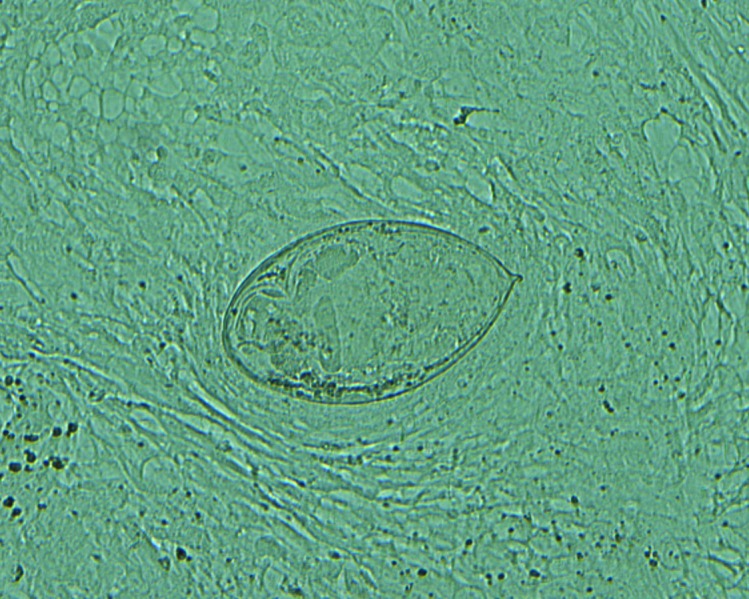
Schistosome egg visualized by direct wet mount microscopy (400× magnification).
The patient was treated with praziquantel 1800 mg (60 mg/kg) orally every four hours for three doses, for a total of 5400 mg in a single day. His symptoms resolved within a few days and the patient had a normal semen analysis and negative semen microscopy for parasites one month after treatment. No further symptoms were present one year following after treatment, suggesting durable cure.
Discussion
Schistosomiasis is a parasitic disease caused by flatworms or flukes. Transmission of the parasite occurs when fresh water sources are contaminated with eggs in the excreta of humans already infected. After an intermediate host stage involving snails, the larval form infects humans through penetration of the skin after contact with contaminated water, most commonly through swimming.3,4 According to the World Health Organization, 261 million people received preventive treatment for schistosomiasis and over 40 million people were treated for schistosomal infections in 2013.3
Schistosomiasis can cause both intestinal and urogenital symptoms. The species most commonly associated with urogenital symptoms is S. haematobium, which is the predominant species in Lake Malawi.4,5 It is estimated that at least 5000 expatriates and tourists acquire schistosomiasis from Lake Malawi annually, with the absolute risk of infection following one-day exposure being 52–74%.4
A previous retrospective analysis of travelers returning to Israel from Lake Malawi from 1997–1999 with Schistosomal infection showed that four of 70 (5.7%) patients infected complained of hematospermia, all without hematuria.2 Our patient complained of painful ejaculation, as well as a “clot-like” substance in his ejaculate, which has been documented previously.2,6 Eosinophilia is often present in acute schistosomiasis, however, was not present in this case.
Schistosomal eggs have been found in both the prostate and seminal vesicles of those affected.2,7 Transrectal ultra-sound of this patient’s prostate revealed cystic dilation of both seminal vesicles, which could have been secondary to schistosomiasis or, alternatively, could have represented simple congenital cysts. The latency period between exposure and development of clinical symptoms can range from 3–8 months, and it is important to maintain a high threshold of suspicion in the appropriate clinical setting with a relevant exposure history.5
The patient’s febrile illness just prior to his return to Canada may have been secondary to cryptosporidium or Katayama fever, which is a hypersensitivity reaction to circulating schistosomal antigens that can manifest 2–8 weeks following initial exposure. Katayama fever is associated with rash, fever, headache, myalgia, and diarrhea. Not all symptoms may be present.8
The patient was prescribed a higher dose of praziquantel based on previous reported experience suggesting that a 60 mg/kg dose is more efficacious than a traditional 40 mg/kg dose. Three patients with hematospermia caused by S. haematobium treated with an initial 40 mg/kg dose all relapsed within three months of treatment, but the fourth patient who received an initial 60mg/kg dose had no relapse in symptoms. Of the three reported patients who relapsed, all three received a second course of treatment consisting of a 40mg/kg dose. One of these three then required a third course of treatment consisting of 60 mg/kg of praziquantel in order to achieve cure.2 The higher dose of praziquantel was well-tolerated and effective in this case.
Footnotes
Competing interests: Dr. Wong has received consulting fees and honoraria from Abbvie, Boehringer-Ingelheim, Bristol Myers Squibb, Gilead Sciences, Janssen, Merck, and Pfizer; has received funding for regional and provincial programming from Abbvie, Bristol Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV; and has participated in clinical trials for Abbvie, Bristol Myers Squibb, Gilead Sciences, Merck, and ViiV. The remaining authors report no competing personal or financial interests.
This paper has been peer-reviewed.
References
- 1.Akhrer W, Khan F, Chinegwundoh F. Should every patient with hematospermai be investigated? A critical review. Cent European J Urol. 2013;66:79–82. doi: 10.5173/ceju.2013.01.art25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz E, Pick N, Shazberg G, et al. Hematospermia due to schistosome infection in travelers: Diagnostic and treatment challenges. Clin Infect Dis. 2002;35:1420–4. doi: 10.1086/344063. https://doi.org/10.1086/344063. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Schistosomiasis: Fact sheets. Geneva, Switzerland: World Health Organization; 2015. http://www.who.int/mediacentre/factsheets/fs115/en/. Accessed November 29, 2016. [Google Scholar]
- 4.Cetron MS, Chitsulo L, Sullivan JJ, et al. Schistosomiasis in Lake Malawi. Lancet. 1996;348:1274–8. doi: 10.1016/S0140-6736(96)01511-5. https://doi.org/10.1016/S0140-6736(96)01511-5. [DOI] [PubMed] [Google Scholar]
- 5.Van Delft F, Visser L, Polderman A, et al. Cough and alterations in semen after a tropical swim. Neth J Med. 2007;65:304–6. [PubMed] [Google Scholar]
- 6.Stevens JL, Schon K, Mukhtar H. An unusual case of rectal bleeding and hematospermia. Gastroenterology. 2010;138:826. doi: 10.1053/j.gastro.2009.06.066. https://doi.org/10.1053/j.gastro.2009.06.066. [DOI] [PubMed] [Google Scholar]
- 7.Corachan M, Valls ME, Gascon J, et al. Hematospermia: A new etiology of clinical interest. Am J Trop Med Hyg. 1994;50:580–4. doi: 10.4269/ajtmh.1994.50.580. [DOI] [PubMed] [Google Scholar]
- 8.Gryseels B, Polman K, Clerinx J, et al. Human schistosomiasis. Lancet. 2006;368:1106–18. doi: 10.1016/S0140-6736(06)69440-3. https://doi.org/10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]


